Phenolic Modified Ceramic Coating on Biodegradable Mg Alloy: The Improved Corrosion Resistance and Osteoblast-Like Cell Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of MAO and Phenolic Monolayer on Coating Morphology
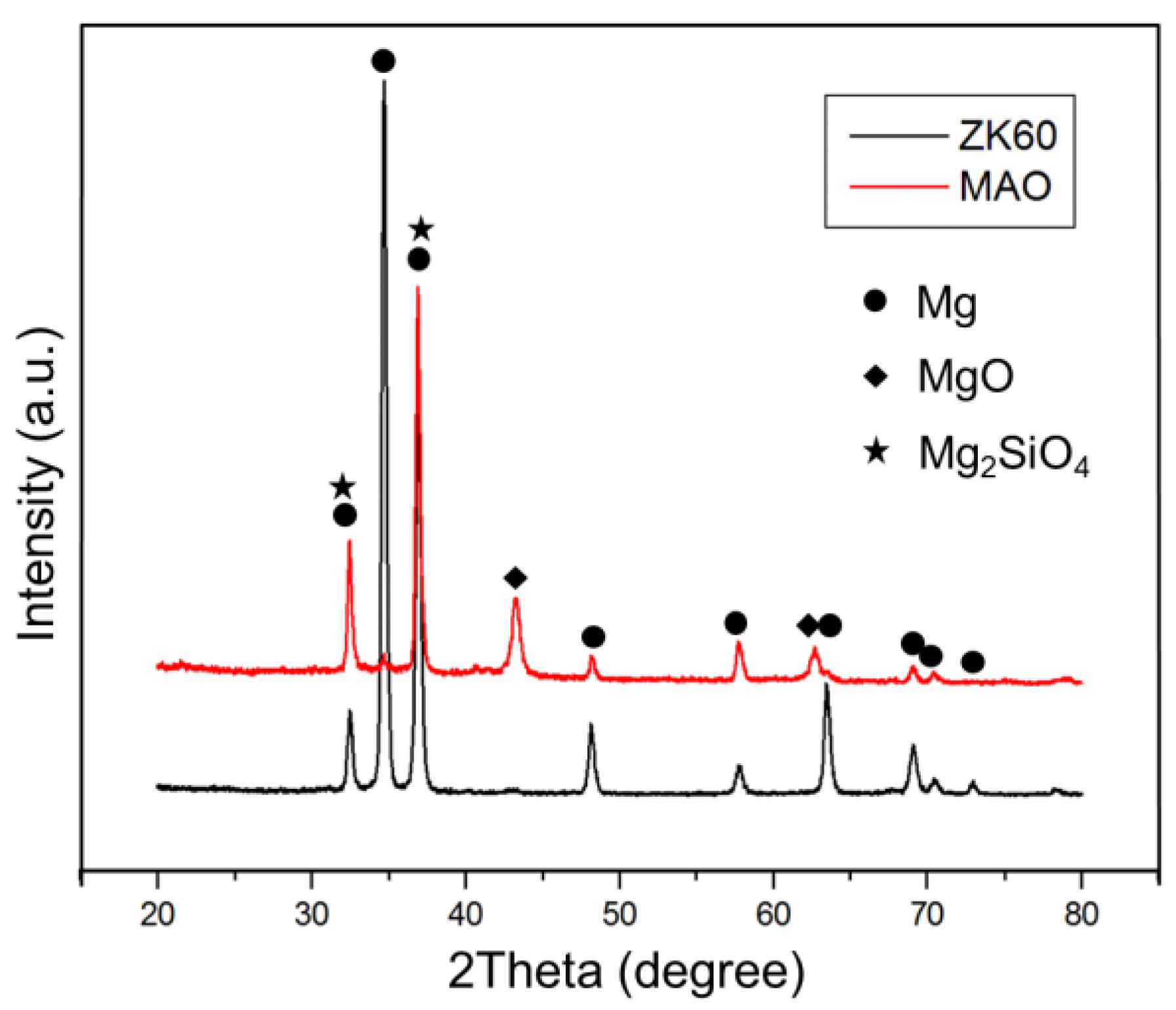
2.2. The Coating Composition Analysis
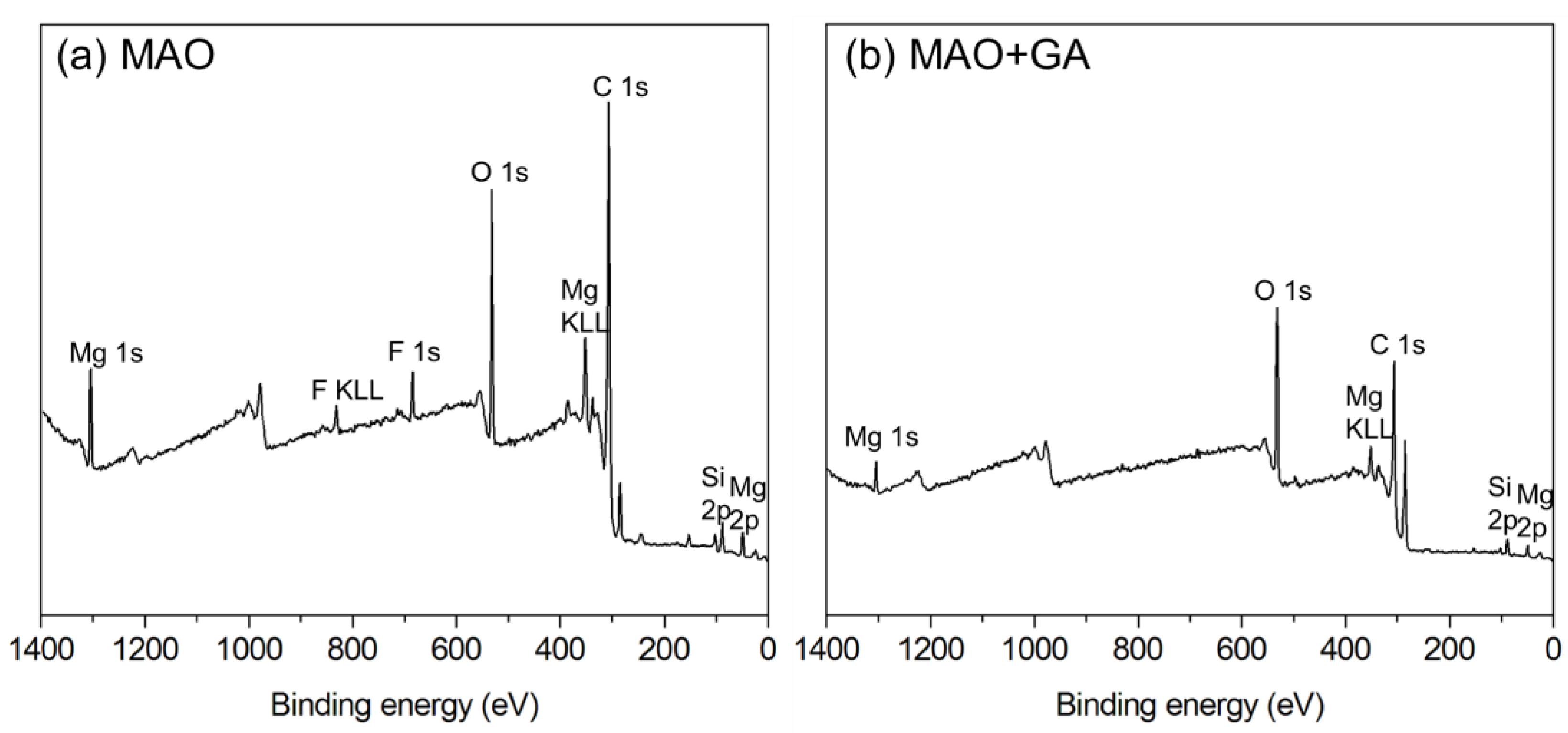
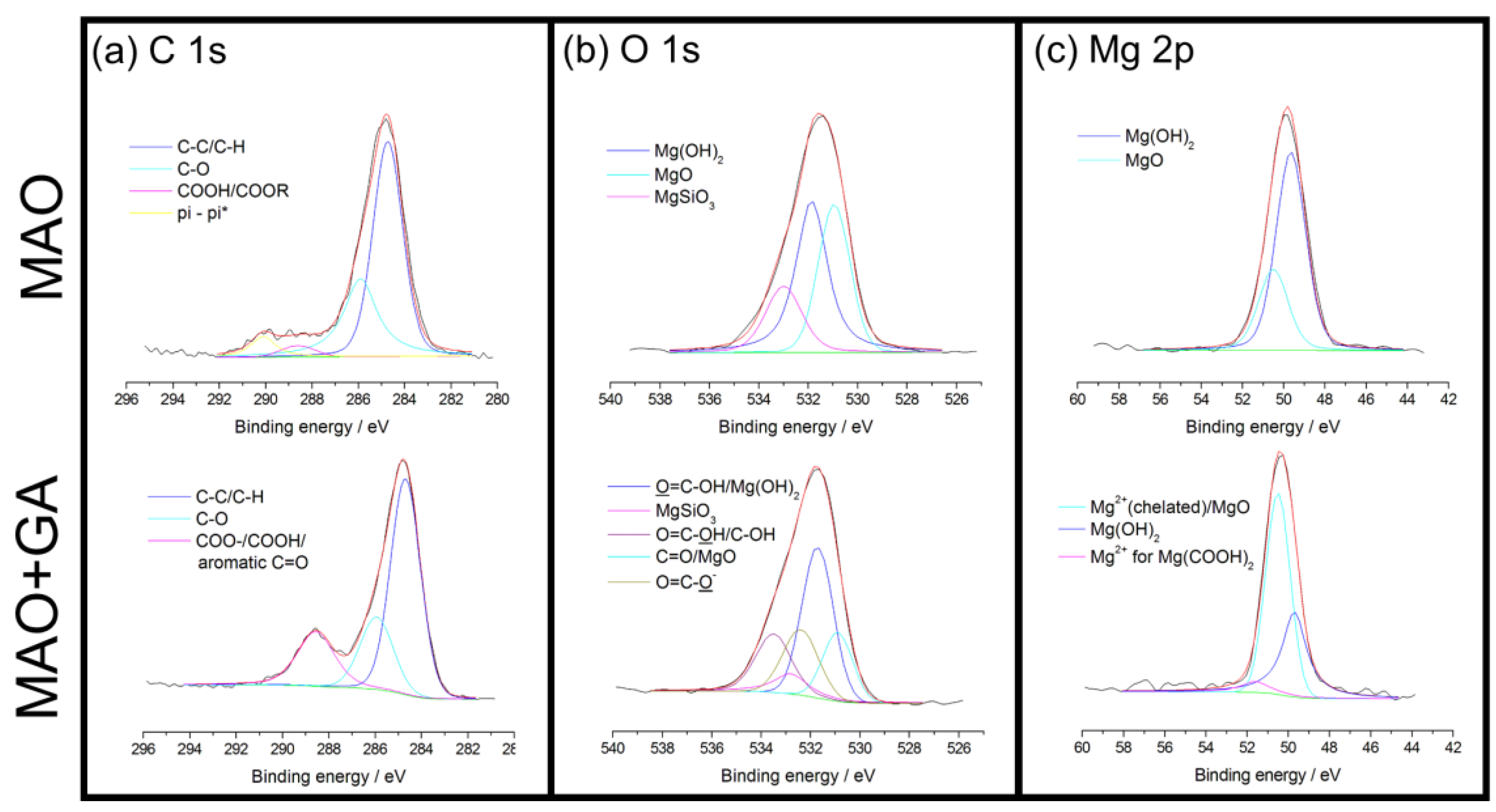
2.3. The Surface Chemical Bonding Characteristics
2.4. The Electrochemical Tests
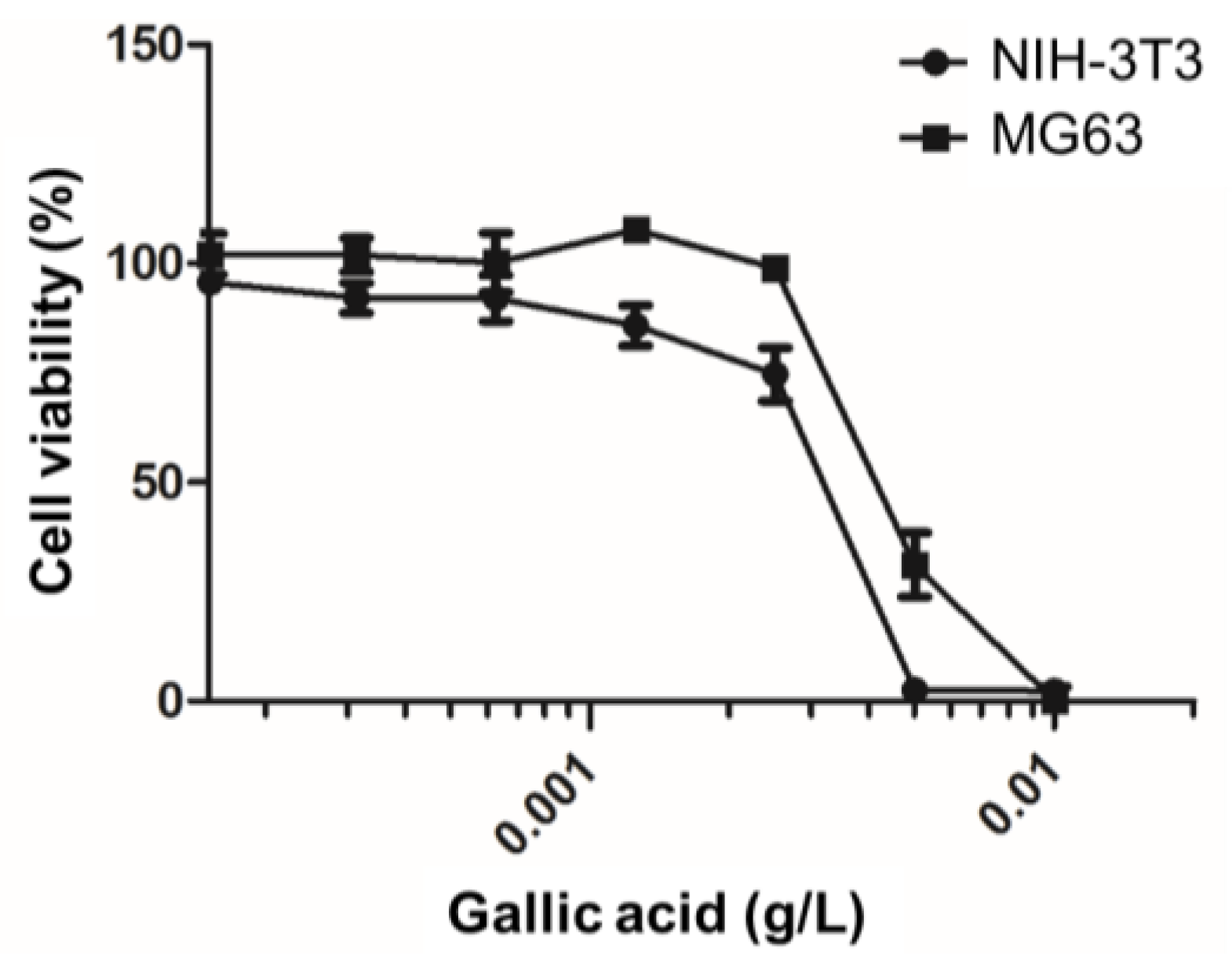
2.5. Effects of Phenolic Monolayer on Cell Viability
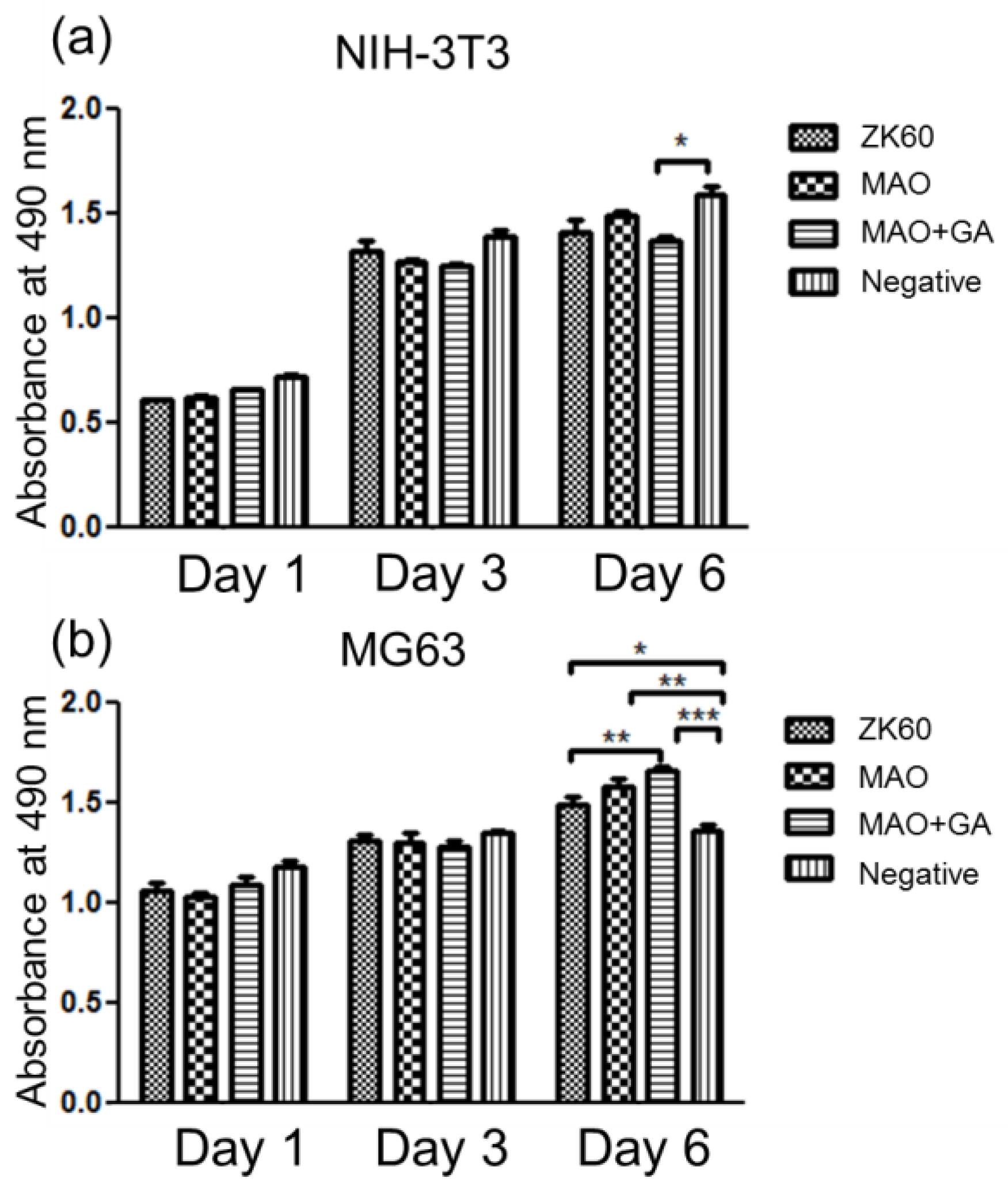
2.6. The Cytocompatibility Evaluation
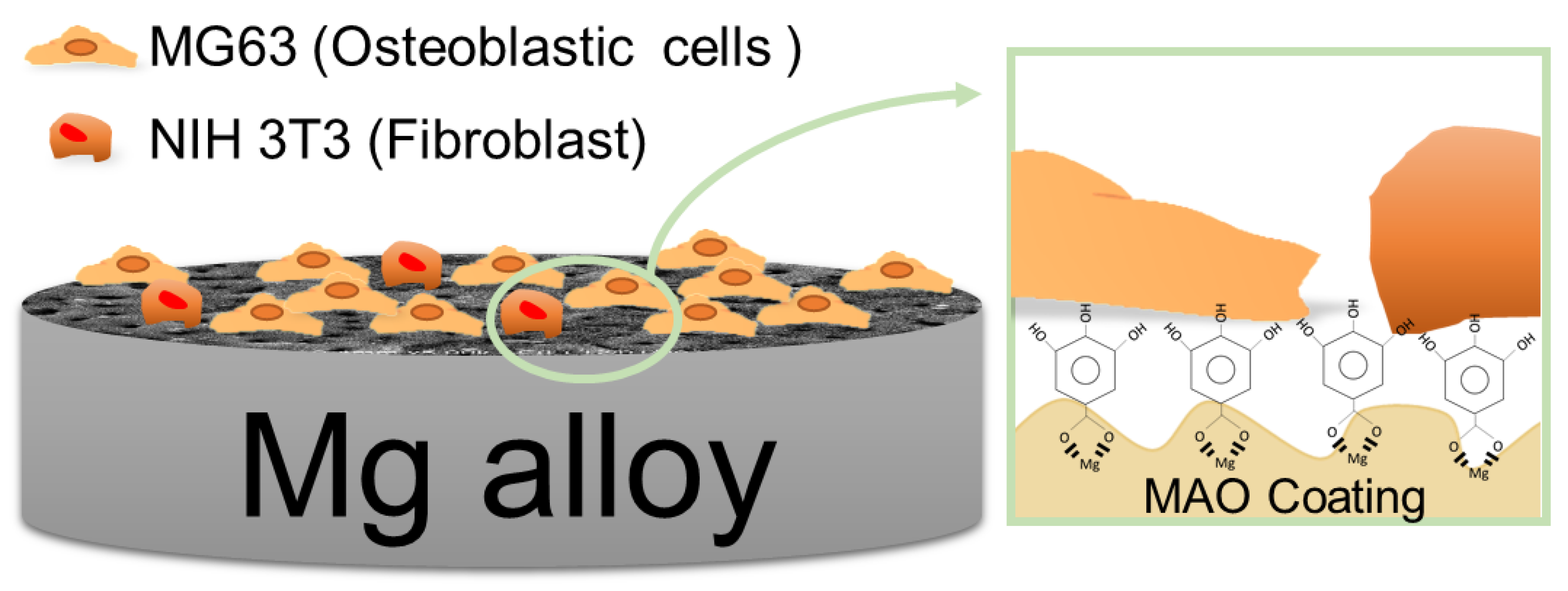
2.7. The Cell Attachment Tests
3. Materials and Methods
3.1. Specimen Preparation
3.2. Characterization of the Surface Properties
3.3. Electrochemical Test
3.4. Cell Culture and the Cell Viability Test
3.5. Cell Adhesion Tests
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Hung, F.; Lui, T.; Yeh, M. Heat treatment mechanism and biodegradable characteristics of ZAX1330 Mg alloy. Mater. Sci. Eng. C 2015, 51, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.J.; Hung, F.Y.; Jakfar, S.; Yeh, M.L. Tailored coating chemistry and interfacial properties for construction of bioactive ceramic coatings on magnesium biomaterial. Mater. Des. 2016, 89, 235–244. [Google Scholar] [CrossRef]
- Zreiqat, H.; Howlett, C.R.; Zannettino, A.; Evans, P.; Knabe, C.; Shakibaei, M. Mechanisms of magnesium-stimulated adhesion of osteoblastic cells to commonly used orthopaedic implants. J. Biomed. Mater. Res. 2002, 62, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Bornapour, M.; Celikin, M.; Cerruti, M.; Pekguleryuz, M. Magnesium implant alloy with low levels of strontium and calcium: The third element effect and phase selection improve bio-corrosion resistance and mechanical performance. Mater. Sci. Eng. C 2014, 35, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wan, P.; Ge, Y.; Fan, X.; Tan, L.; Li, J.; Yang, K. Tailoring the degradation and biological response of a magnesium-strontium alloy for potential bone substitute application. Mater. Sci. Eng. C 2016, 58, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ren, L.; Li, L.; He, P.; Lan, G.; Zhang, Y.; Yang, K. Cytotoxic effect on osteosarcoma MG-63 cells by degradation of magnesium. J. Mater. Sci. Technol. 2014, 30, 888–893. [Google Scholar] [CrossRef]
- Wagener, V.; Schilling, A.; Mainka, A.; Hennig, D.; Gerum, R.; Kelch, M.-L.; Keim, S.F.; Fabry, B.; Virtanen, S. Cell adhesion on surface-functionalized magnesium. ACS Appl. Mater. Interfaces 2016. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.J.; Hou, Y.; Wang, Y.N.; Gao, F.; Liu, T.; Hou, Y.H.; Zhu, Y.F.; Ye, W.; Wang, L.R. Effects of self-assembly of 3-phosphonopropionic acid, 3-aminopropyltrimethoxysilane and dopamine on the corrosion behaviors and biocompatibility of a magnesium alloy. Mater. Sci. Eng. C 2016, 67, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.R.; Guo, Y.; Huang, W.D. Study on the corrosion resistance of phytic acid conversion coating for magnesium alloys. Surf. Coat. Technol. 2006, 201, 1536–1541. [Google Scholar]
- Liu, Q.; Chen, D.; Kang, Z. One-Step Electrodeposition Process To Fabricate Corrosion-Resistant Superhydrophobic Surface on Magnesium Alloy. ACS Appl. Mater. Interfaces 2015, 7, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Sankara Narayanan, T.S.N.; Park, I.S.; Lee, M.H. Strategies to improve the corrosion resistance of microarc oxidation (MAO) coated magnesium alloys for degradable implants: Prospects and challenges. Prog. Mater. Sci. 2014, 60, 1–71. [Google Scholar] [CrossRef]
- Lin, X.; Tan, L.; Wang, Q.; Zhang, G.; Zhang, B.; Yang, K. In vivo degradation and tissue compatibility of ZK60 magnesium alloy with micro-arc oxidation coating in a transcortical model. Mater. Sci. Eng. C 2013, 33, 3881–3888. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Tan, L.; Zhang, Q.; Yang, K.; Hu, Z.; Qiu, J.; Cai, Y. The in vitro degradation process and biocompatibility of a ZK60 magnesium alloy with a forsterite-containing micro-arc oxidation coating. Acta Biomater. 2013, 9, 8631–8642. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.J.; Lin, X.Z.; Liu, C.H.; Yang, R.S.; Zheng, X.W.; Gong, M. Fabrication and corrosion resistance of a hydrophobic micro-arc oxidation coating on AZ31 Mg alloy. Corros. Sci. 2015, 90, 402–412. [Google Scholar] [CrossRef]
- Liu, P.; Pan, X.; Yang, W.; Cai, K.; Chen, Y. Improved anticorrosion of magnesium alloy via layer-by-layer self-assembly technique combined with micro-arc oxidation. Mater. Lett. 2012, 75, 118–121. [Google Scholar] [CrossRef]
- Sileika, T.S.; Barrett, D.G.; Zhang, R.; Lau, K.H.A.; Messersmith, P.B. Colorless multifunctional coatings inspired by polyphenols found in tea, chocolate, and wine. Angew. Chem. Int. Ed. 2013, 52, 10766–10770. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H. Gallic Acid Inhibits Histamine Release and Pro-inflammatory Cytokine Production in Mast Cells. Toxicol. Sci. 2006, 91, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Saavedra, M.J.; Simões, M. The activity of ferulic and gallic acids in biofilm prevention and control of pathogenic bacteria. Biofouling 2012, 28, 755–767. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, J.; Yan, W.; Huang, N. Gallic acid and gallic acid-loaded coating involved in selective regulation of platelet, endothelial and smooth muscle cell fate. RSC Adv. 2014, 4, 212–221. [Google Scholar] [CrossRef]
- Ueno, T.; Ikeda, T.; Tsukimura, N.; Ishijima, M. Biomaterials Novel antioxidant capability of titanium induced by UV light treatment. Biomaterials 2016, 108, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.J.; Hung, F.Y.; Yeh, M.L.; Lui, T.S. Microstructure-modified biodegradable magnesium alloy for promoting cytocompatibility and wound healing in vitro. J. Mater. Sci. Mater. Med. 2015, 26, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.-J.; Hung, F.-Y.; Liu, H.-J.; Yeh, M.-L. Dynamic Corrosion and Material Characteristics of Mg-Zn-Zr Mini-Tubes: The Influence of Microstructures and Extrusion Parameters. Adv. Eng. Mater. 2017. [Google Scholar] [CrossRef]
- Liang, J.; Guo, B.; Tian, J.; Liu, H.; Zhou, J.; Xu, T. Effect of potassium fluoride in electrolytic solution on the structure and properties of microarc oxidation coatings on magnesium alloy. Appl. Surf. Sci. 2005, 252, 345–351. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, M.; Yang, X.; Huang, P.; Xu, K. Effect of Na2SiO3 solution concentration of micro-arc oxidation process on lap-shear strength of adhesive-bonded magnesium alloys. Appl. Surf. Sci. 2014, 314, 447–452. [Google Scholar] [CrossRef]
- Chen, X.; Li, G.; Lian, J.; Jiang, Q. An organic chromium-free conversion coating on AZ91D magnesium alloy. Appl. Surf. Sci. 2008, 255, 2322–2328. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, J.; Chen, Y.; Zhao, S.; Chen, M.; Li, X.; Maitz, M.F.; Wang, J.; Huang, N. Application of Phenol/Amine Copolymerized Film Modified Magnesium Alloys: Anticorrosion and Surface Biofunctionalization. ACS Appl. Mater. Interfaces 2015, 7, 24510–24522. [Google Scholar] [CrossRef] [PubMed]
- Grubac, Z.; Kugor Roncević, I.S.; Metikos-Huković, M.; Babić, R.; Petravić, M.; Peter, R. Surface Modification of Biodegradable Magnesium Alloys. J. Electrochem. Soc. 2012, 159, C253–C258. [Google Scholar] [CrossRef]
- Chen, X.; Li, G.; Lian, J.; Jiang, Q. Study of the formation and growth of tannic acid based conversion coating on AZ91D magnesium alloy. Surf. Coat. Technol. 2009, 204, 736–747. [Google Scholar] [CrossRef]
- Tóth, I.Y.; Szekeres, M.; Turcu, R.; Sáringer, S.; Illés, E.; Nesztor, D.; Tombácz, E. Mechanism of in situ surface polymerization of gallic acid in an environmental-inspired preparation of carboxylated core-shell magnetite nanoparticles. Langmuir 2014, 30, 15451–15461. [Google Scholar] [CrossRef] [PubMed]
- Araujo, P.Z.; Morando, P.J.; Blesa, M.A. Interaction of catechol and gallic acid with titanium dioxide in aqueous suspensions. 1. Equilibrium studies. Langmuir 2005, 21, 3470–3474. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Du, K.; Yan, C.; Wang, F. Electrochemical corrosion behavior of composite coatings of sealed MAO film on magnesium alloy AZ91D. Electrochim. Acta 2006, 51, 2898–2908. [Google Scholar] [CrossRef]
- Yang, Z.; Xiong, K.; Qi, P.; Yang, Y.; Tu, Q.; Wang, J.; Huang, N. Gallic acid tailoring surface functionalities of plasma-polymerized allylamine-coated 316L SS to selectively direct vascular endothelial and smooth muscle cell fate for enhanced endothelialization. ACS Appl. Mater. Interfaces 2014, 6, 2647–2656. [Google Scholar] [CrossRef] [PubMed]
- Payra, D.; Naito, M.; Fujii, Y.; Nagao, Y. Hydrophobized plant polyphenols: Self-assembly and promising antibacterial, adhesive, and anticorrosion coatings. Chem. Commun. 2016, 52, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Xu, L.; Yu, G.; Pan, F.; Yang, K. In vivo evaluation of biodegradable magnesium alloy bone implant in the first 6 months implantation. J. Biomed. Mater. Res. 2008. [Google Scholar] [CrossRef]
- Yang, X.; Li, M.; Lin, X.; Tan, L.; Lan, G.; Li, L.; Yin, Q.; Xia, H.; Zhang, Y.; Yang, K. Enhanced in vitro biocompatibility/bioactivity of biodegradable Mg-Zn-Zr alloy by micro-arc oxidation coating contained Mg2SiO4. Surf. Coat. Technol. 2013, 233, 65–73. [Google Scholar] [CrossRef]
- Li, G.; Cao, H.; Zhang, W.; Ding, X.; Yang, G.; Qiao, Y.; Liu, X.; Jiang, X. Enhanced Osseointegration of Hierarchical Micro/Nanotopographic Titanium Fabricated by Microarc Oxidation and Electrochemical Treatment. ACS Appl. Mater. Interfaces 2016, 8, 3840–3852. [Google Scholar] [CrossRef] [PubMed]
- Kunzler, T.P.; Drobek, T.; Schuler, M.; Spencer, N.D. Systematic study of osteoblast and fibroblast response to roughness by means of surface-morphology gradients. Biomaterials 2007, 28, 2175–2182. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.Y.; Schwartz, Z.; Hummert, T.W.; Schraub, D.M.; Simpson, J.; Lankford, J.; Dean, D.D.; Cochran, D.L.; Boyan, B.D. Effect of titanium surface roughness on proliferation, differentiation, and protein synthesis of human osteoblast-like cells. J. Biomed. Mater. Res. Part A 1995, 29, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.J.; Hung, F.Y.; Yeh, M.L.; Lee, H.P.; Lui, T.-S. Development of a novel micro-textured surface using duplex surface modification for biomedical Mg alloy applications. Mater. Lett. 2017. [Google Scholar] [CrossRef]
- Chen, W.; Shen, X.; Hu, Y.; Xu, K.; Ran, Q.; Yu, Y.; Dai, L.; Yuan, Z.; Huang, L.; Shen, T.; et al. Biomaterials Surface functionalization of titanium implants with chitosan-catechol conjugate for suppression of ROS-induced cells damage and improvement of osteogenesis. Biomaterials 2017, 114, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Marino, T.; Galano, A.; Russo, N. Radical Scavenging Ability of Gallic Acid toward OH and OOH Radicals. Reaction Mechanism and Rate Constants from the Density Functional Theory. J. Phys. Chem. B 2014, 118, 10380–10389. [Google Scholar] [CrossRef] [PubMed]
- Symons, M.C.R. Radicals generated by bone cutting and fracture. Free Radic. Biol. Med. 1996, 20, 831–835. [Google Scholar] [CrossRef]
- Banfi, G.; Iorio, E.L.; Corsi, M.M. Oxidative stress, free radicals and bone remodeling. Clin. Chem. Lab. Med. 2008, 46, 1550–1555. [Google Scholar] [CrossRef] [PubMed]
- Nuss, K.M.R.; VonRechenberg, B. Biocompatibility Issues with Modern Implants in Bone—A Review for Clinical Orthopedics. Open Orthop. J. 2008, 2, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yang, Y.; Yan, W.; Tu, Q.; Wang, J.; Huang, N. Construction of polyfunctional coatings assisted by gallic acid to facilitate Co-immobilization of diverse biomolecules. ACS Appl. Mater. Interfaces 2013, 5, 10495–10501. [Google Scholar] [CrossRef] [PubMed]
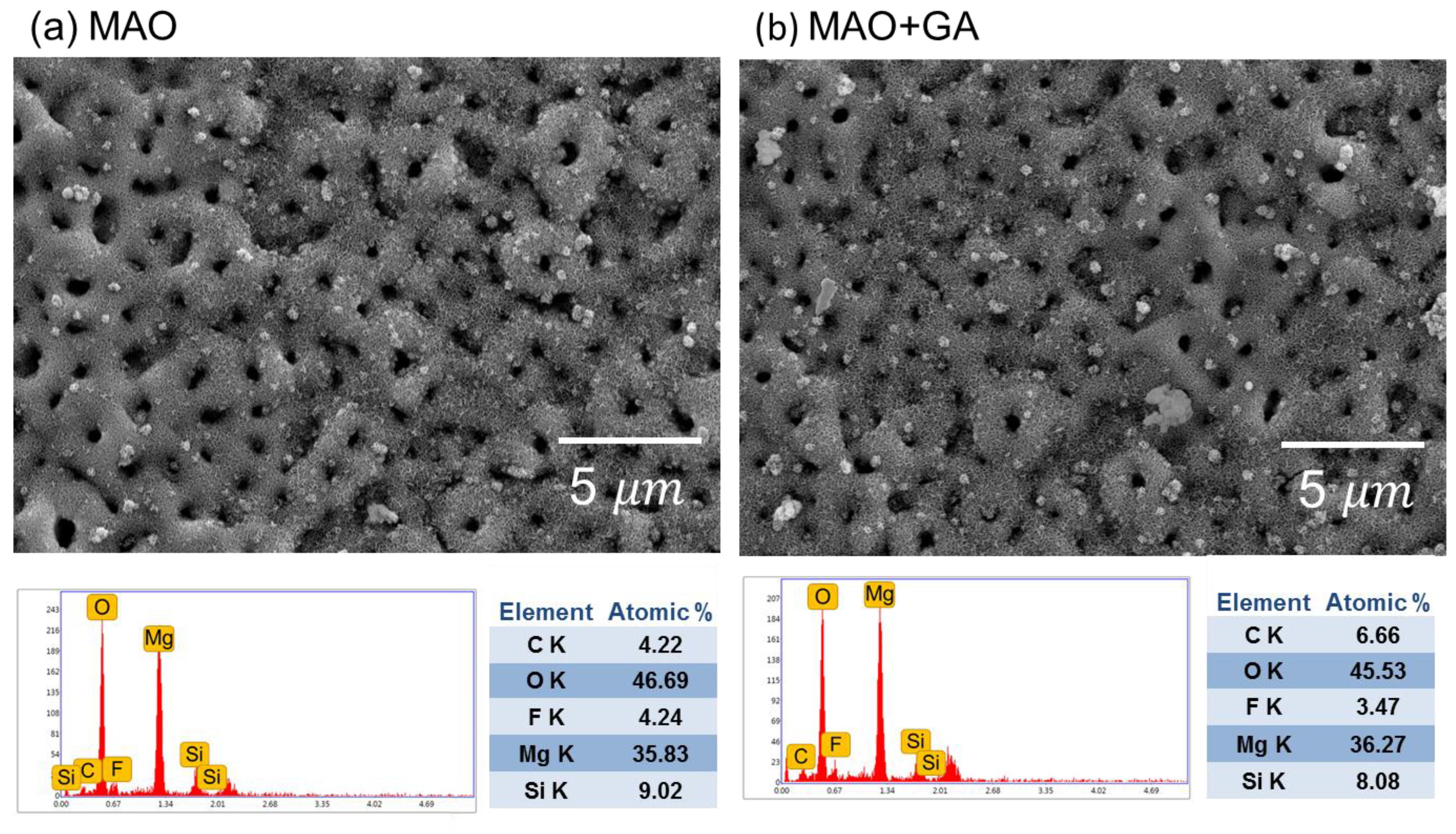


| Group | Element concentration (at.%) | ||||
|---|---|---|---|---|---|
| O | C | Mg | Si | F | |
| MAO | 42.6 | 26.6 | 19 | 6.2 | 5.6 |
| MAO+GA | 33.3 | 55.3 | 8.2 | 1.9 | 1.3 |
| Group | Ecorr (V) | Icorr () |
|---|---|---|
| ZK60 | −1.564 | 96.025 |
| MAO | −1.485 | 0.583 |
| MAO+GA | −1.453 | 0.372 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-P.; Lin, D.-J.; Yeh, M.-L. Phenolic Modified Ceramic Coating on Biodegradable Mg Alloy: The Improved Corrosion Resistance and Osteoblast-Like Cell Activity. Materials 2017, 10, 696. https://doi.org/10.3390/ma10070696
Lee H-P, Lin D-J, Yeh M-L. Phenolic Modified Ceramic Coating on Biodegradable Mg Alloy: The Improved Corrosion Resistance and Osteoblast-Like Cell Activity. Materials. 2017; 10(7):696. https://doi.org/10.3390/ma10070696
Chicago/Turabian StyleLee, Hung-Pang, Da-Jun Lin, and Ming-Long Yeh. 2017. "Phenolic Modified Ceramic Coating on Biodegradable Mg Alloy: The Improved Corrosion Resistance and Osteoblast-Like Cell Activity" Materials 10, no. 7: 696. https://doi.org/10.3390/ma10070696
APA StyleLee, H.-P., Lin, D.-J., & Yeh, M.-L. (2017). Phenolic Modified Ceramic Coating on Biodegradable Mg Alloy: The Improved Corrosion Resistance and Osteoblast-Like Cell Activity. Materials, 10(7), 696. https://doi.org/10.3390/ma10070696





