4,2’:6’,4”- and 3,2’:6’,3”-Terpyridines: The Conflict between Well-Defined Vectorial Properties and Serendipity in the Assembly of 1D-, 2D- and 3D-Architectures
Abstract
:1. Introduction
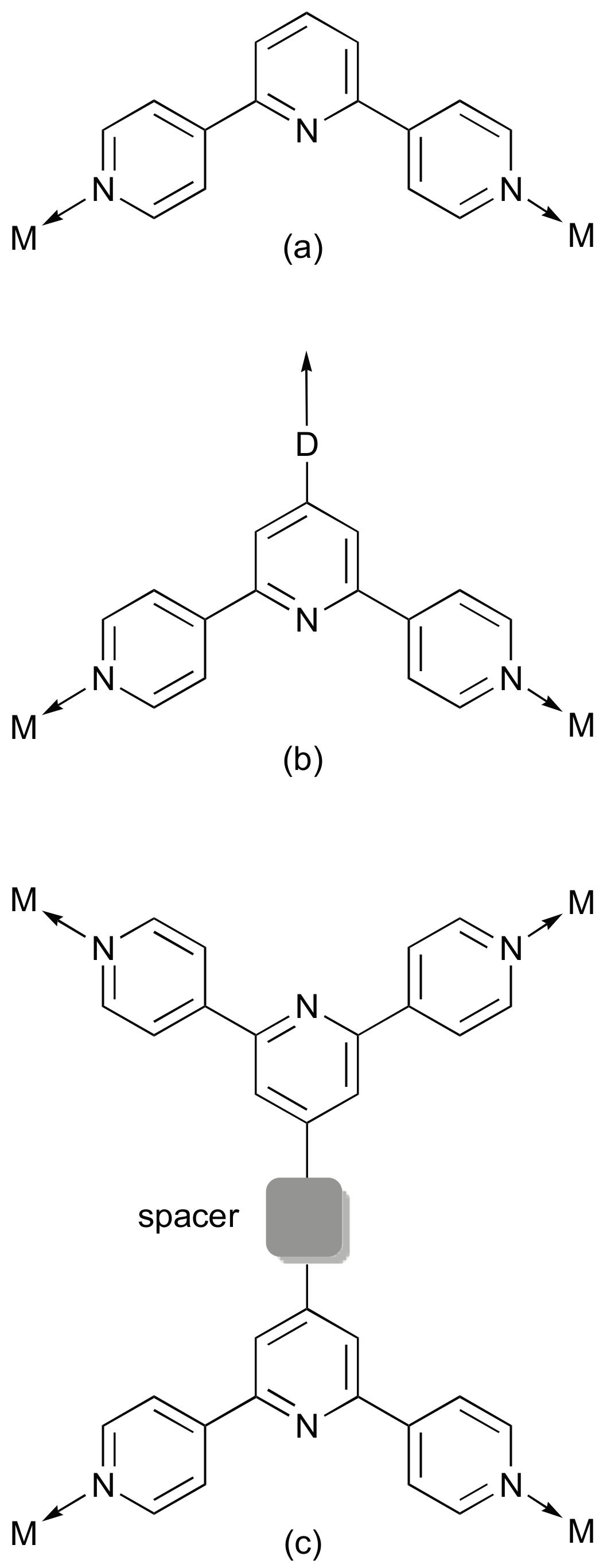
2. Results and Discussion
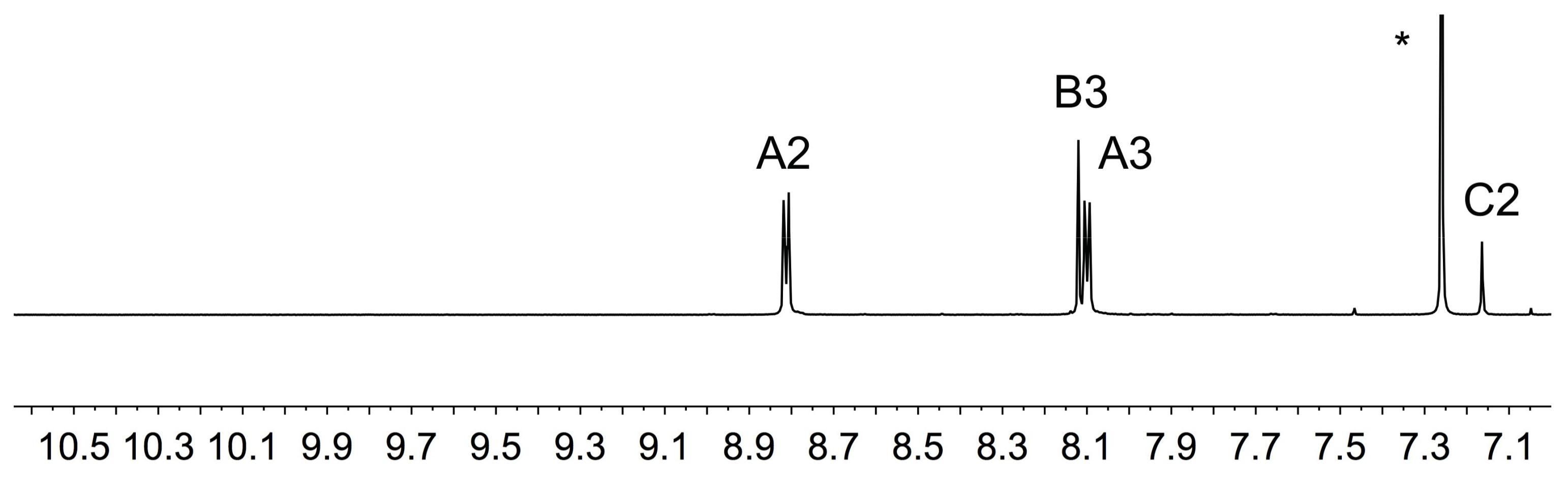
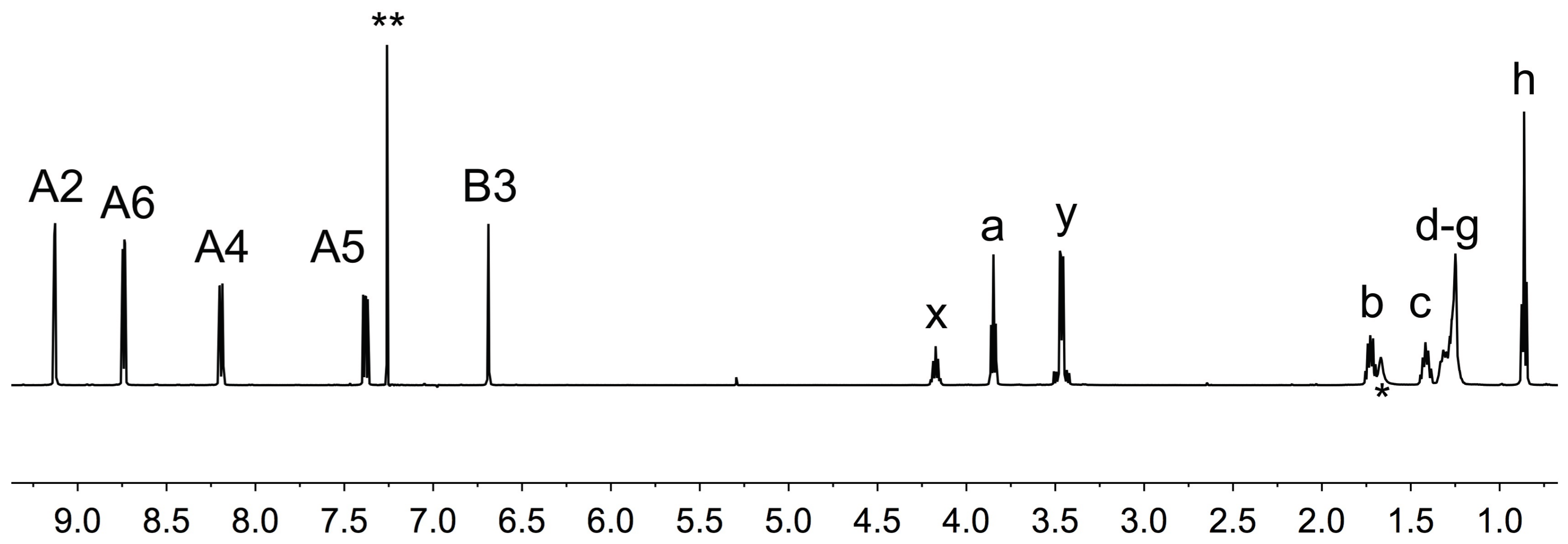
2.1. Ligand Syntheses and Characterization
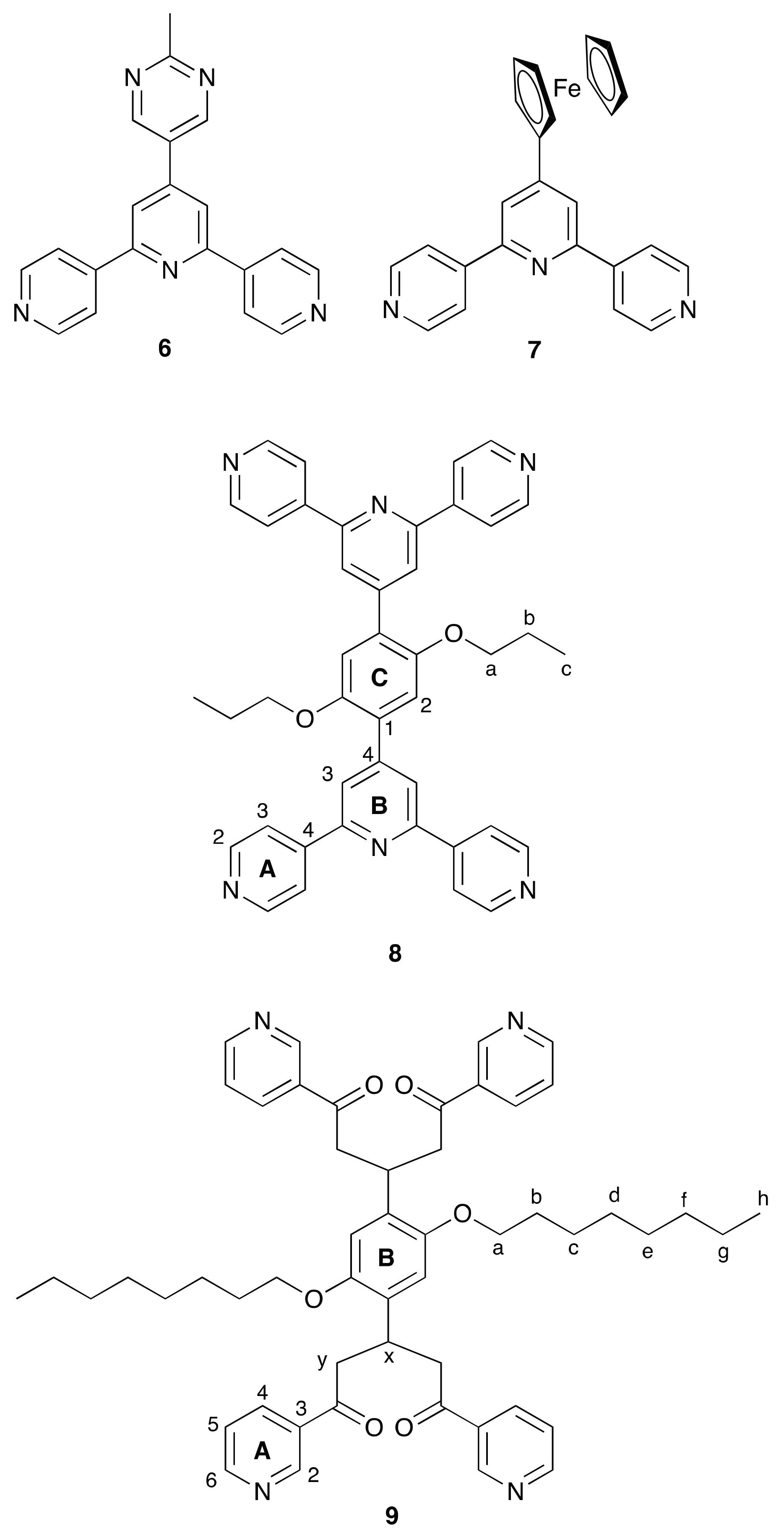
2.2. 4’-(2-Methylpyrimidin-5-yl)-Functionalization: Assembly of a 1D-Chain
2.3. Ferrocenyl-Functionalization: Assembly of a 2D-Network
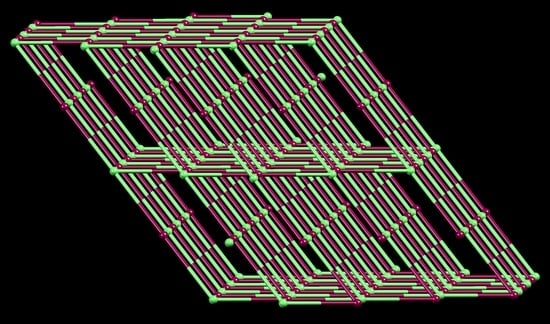
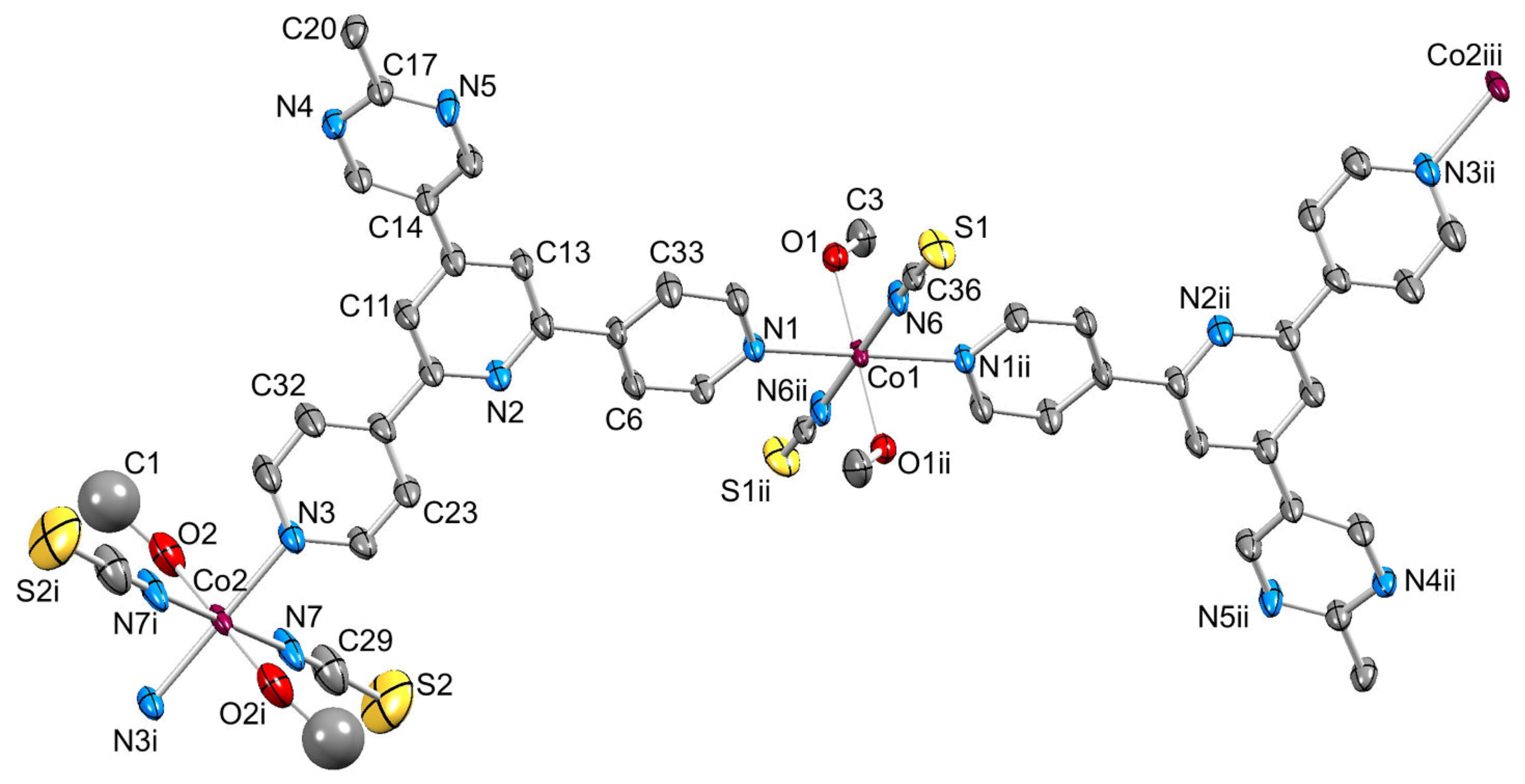
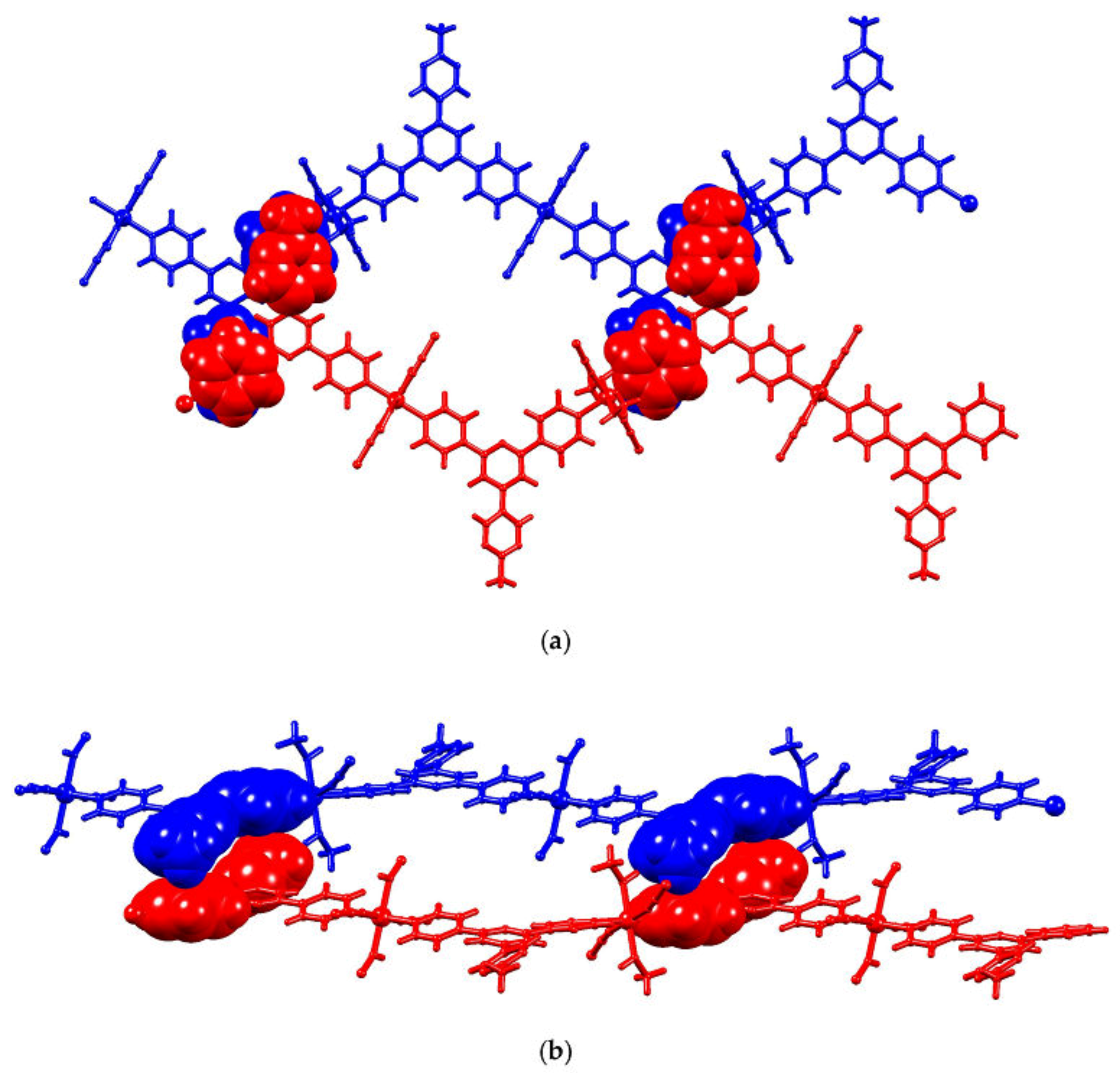
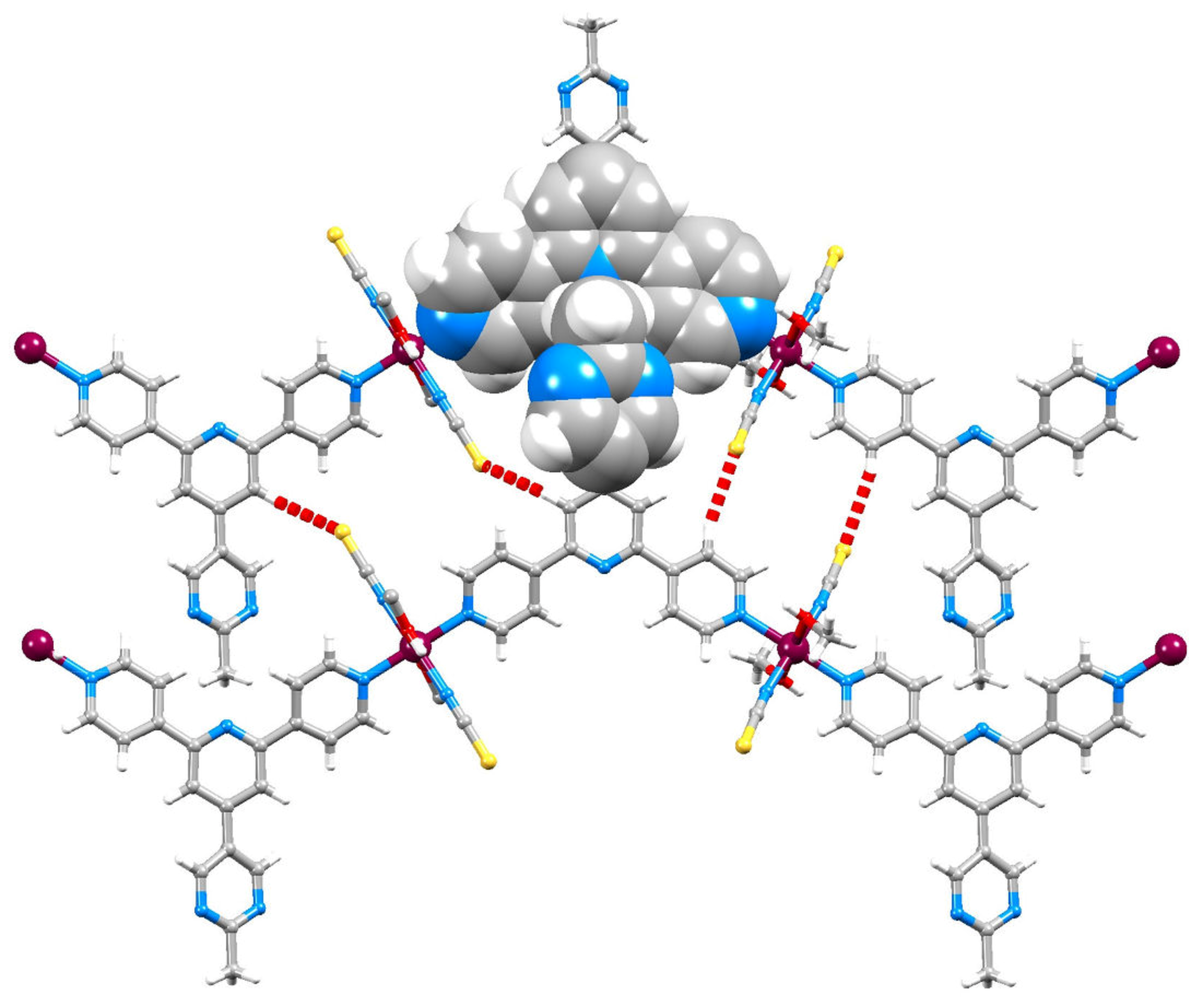
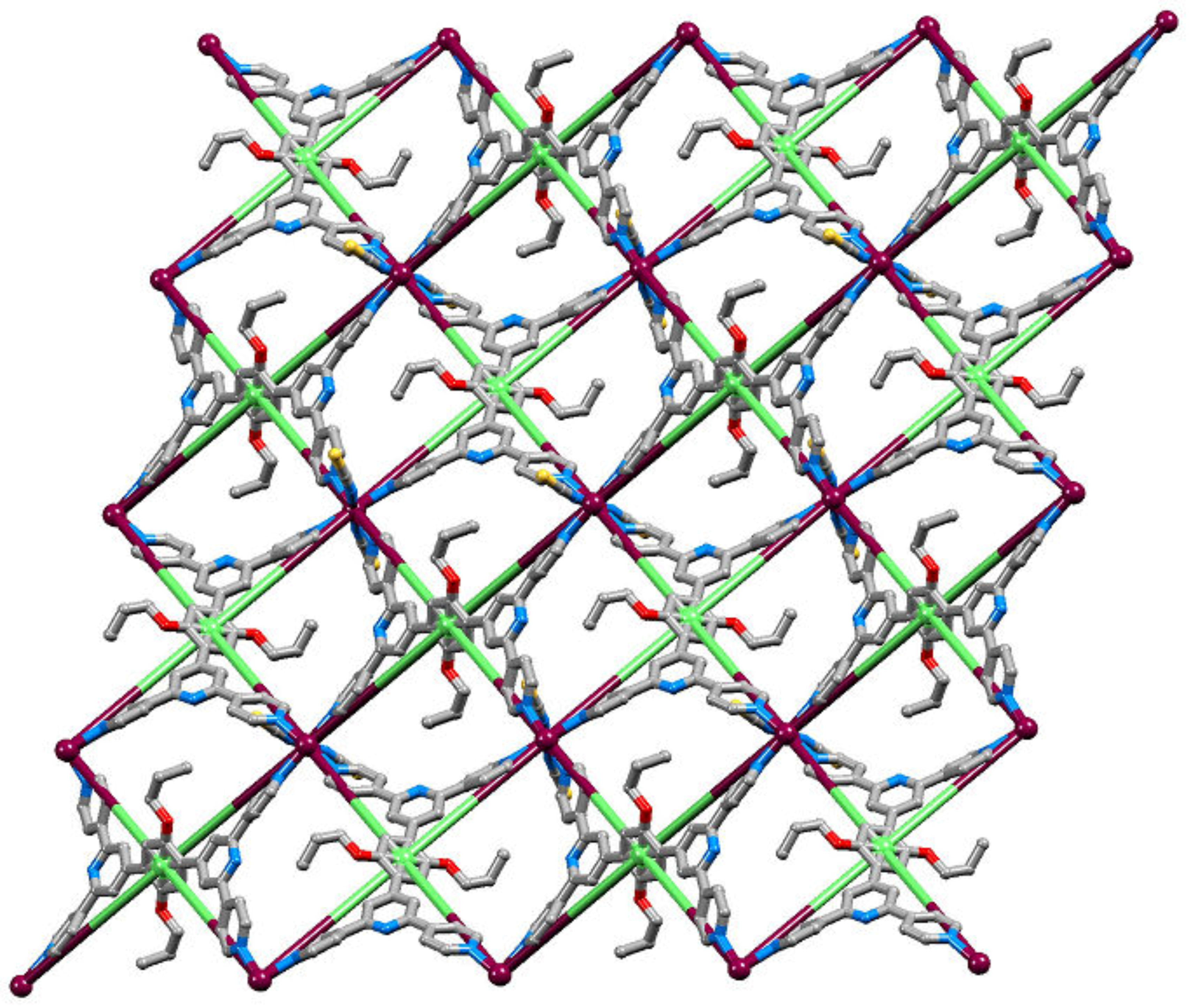
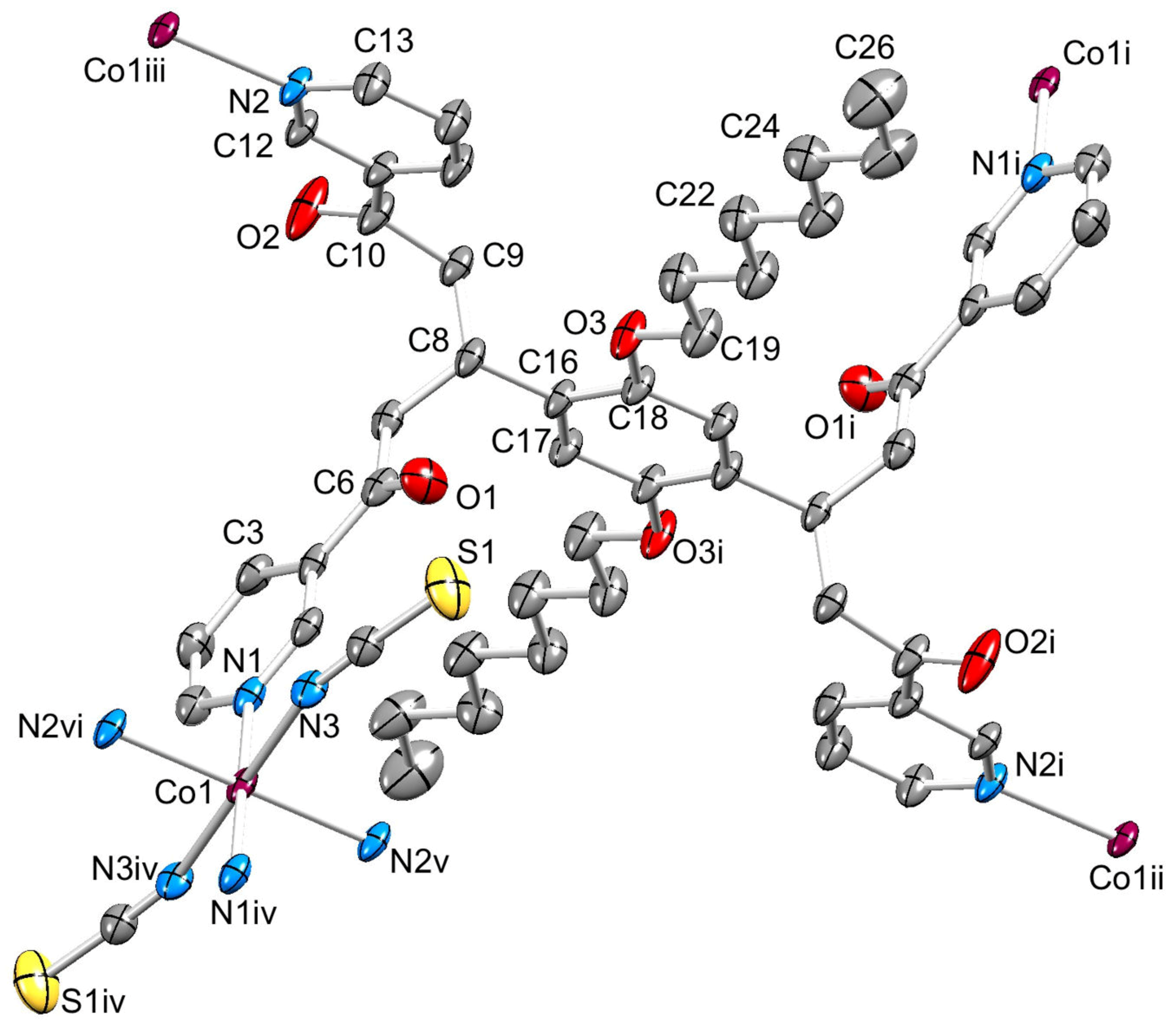
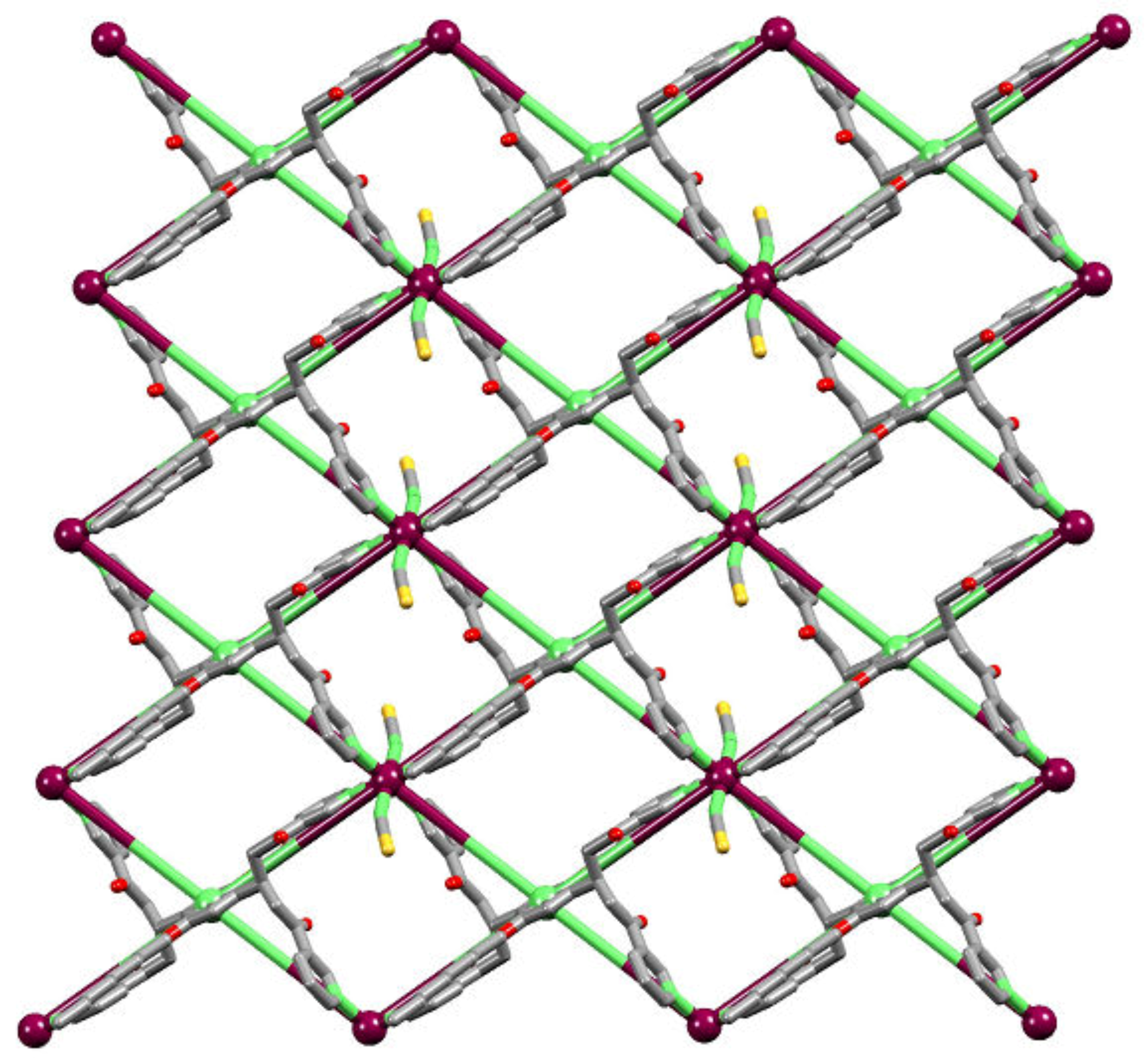
2.4. To a 3D-Network: {[Co(NCS)2(8)2].2C6H4Cl2}n
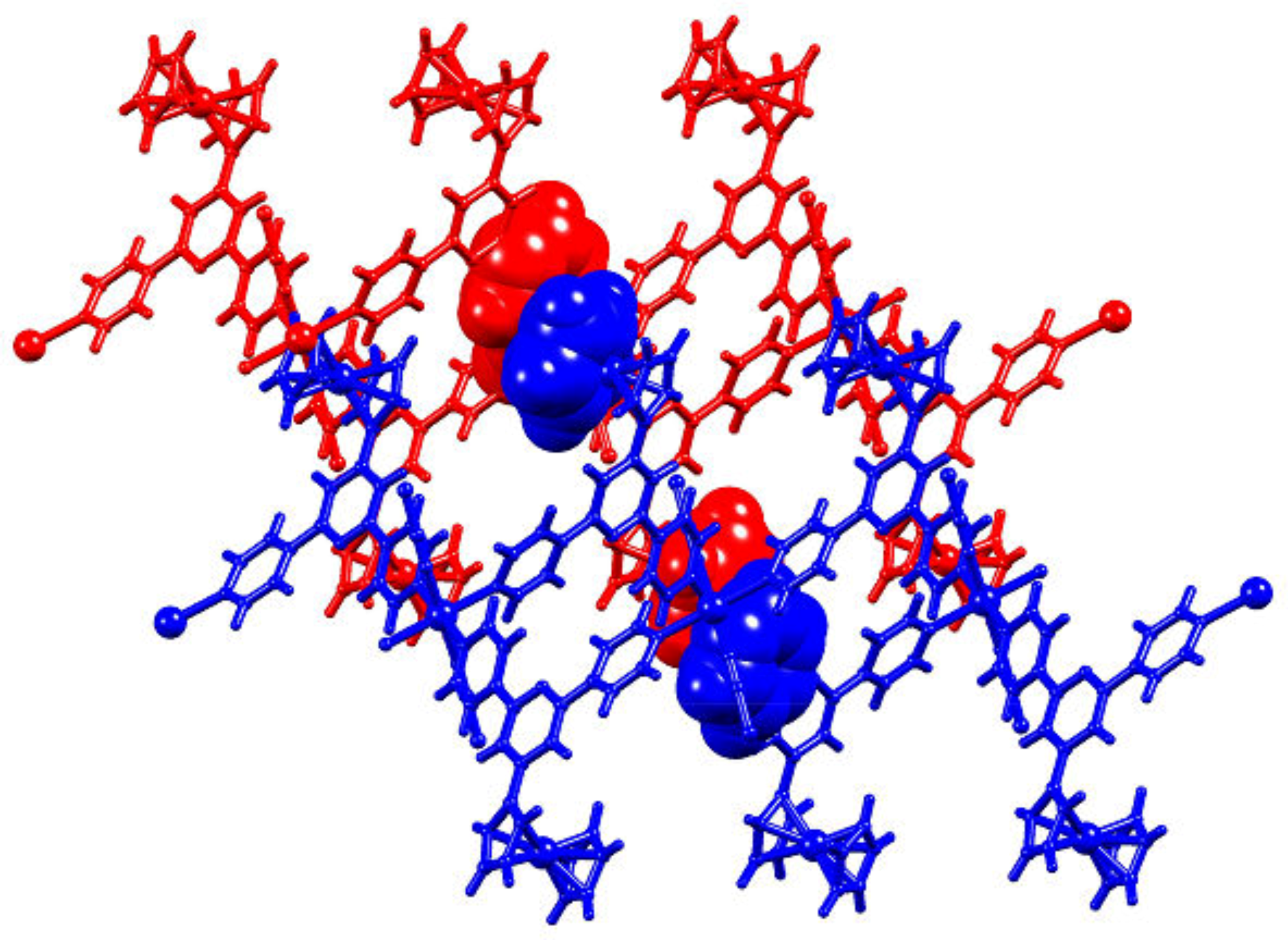
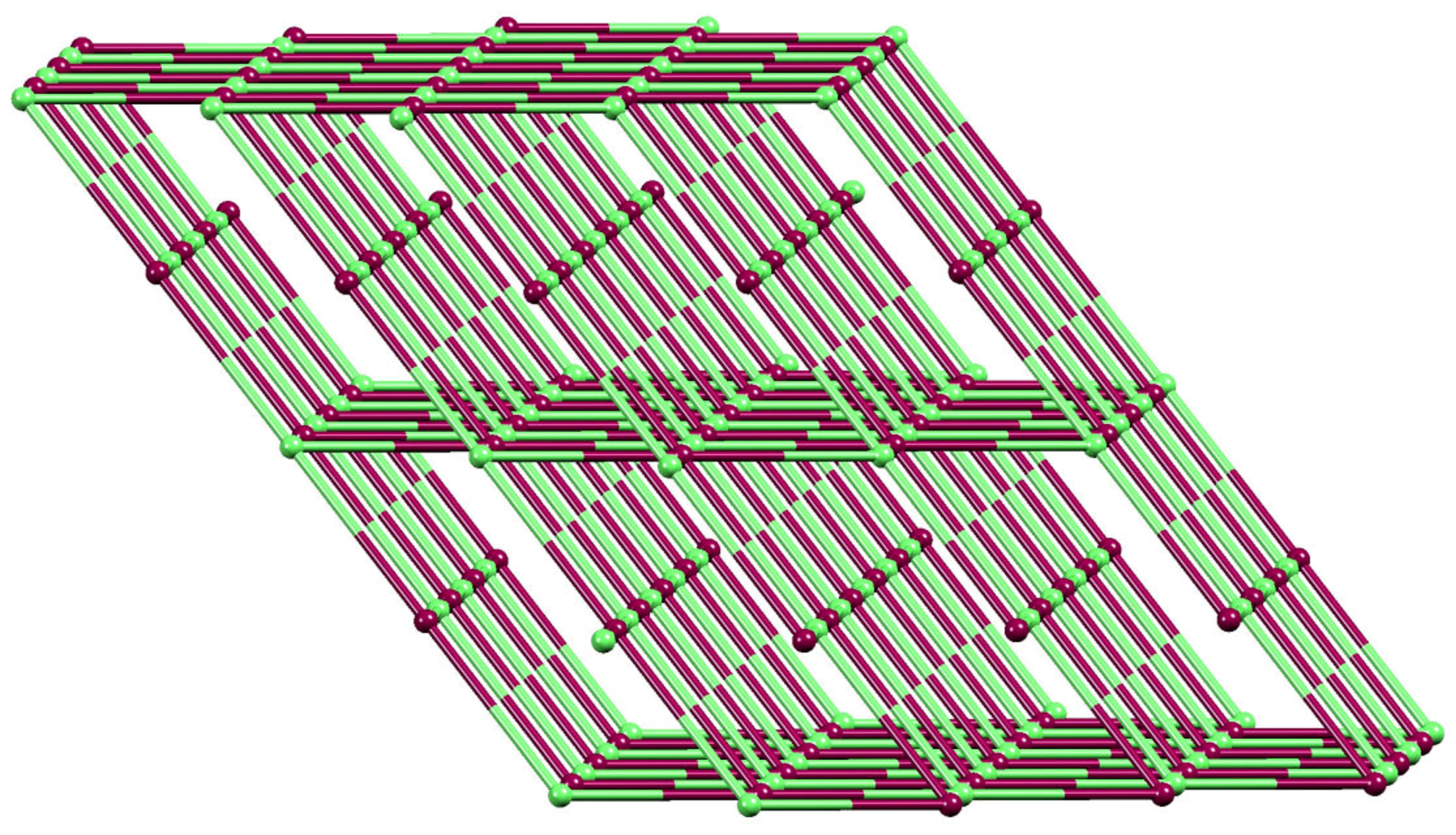
2.5. Relaxing the Backbone: Going from an lvt Net to a 2D-Sheet
3. Materials and Methods
3.1. General
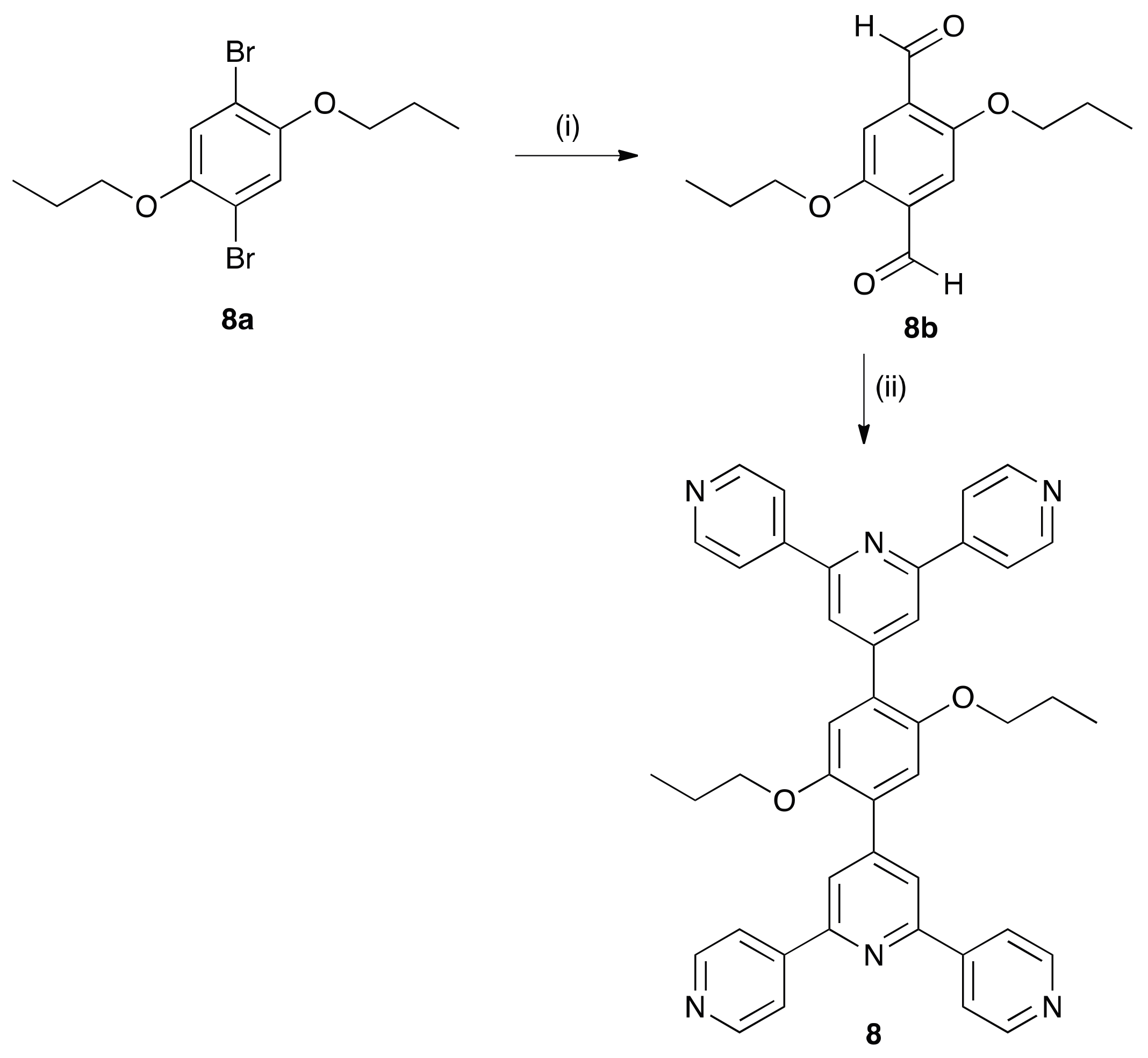
3.2. Synthesis of 8a
3.3. Synthesis of 8b
3.4. Synthesis of 8
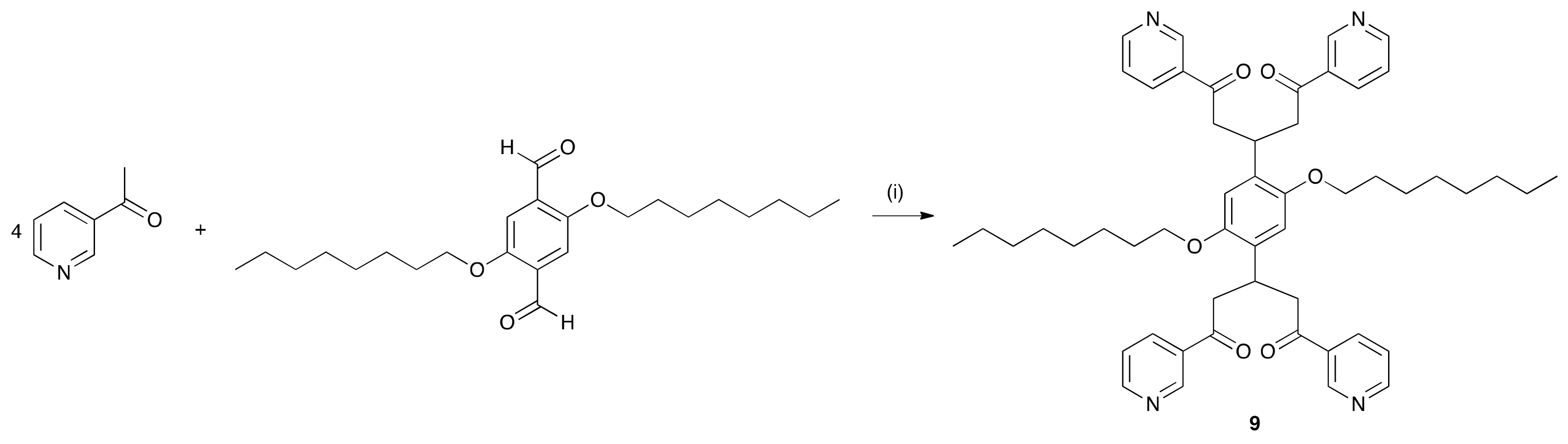
3.5. Synthesis of 9
3.6. {[Co2(NCS)4(MeOH)4(6)2]∙2MeOH∙8H2O}n
3.7. {[Co(NCS)2(7)2]∙4CHCl3}n
3.8. {[Co(NCS)2(8)2]·2C6H4Cl2}n
3.9. {[Co(NCS)2(9)]∙2CHCl3}n
3.10. Crystallography
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Housecroft, C.E. 4,2′:6′,4′′-Terpyridines: Diverging and diverse building blocks in coordination polymers and metallomacrocycles. Dalton Trans. 2014, 43, 6594–6604. [Google Scholar] [CrossRef] [PubMed]
- Housecroft, C.E. Divergent 4,2′:6′,4′′- and 3,2′:6′,3′′-terpyridines as linkers in 2- and 3-dimensional architectures. CrystEngComm 2015, 17, 7461–7468. [Google Scholar] [CrossRef]
- Granifo, J.; Gaviño, R.; Freire, E.; Baggio, R. Monodentate and bridging behaviour of the sulfur-containing ligand 4′-[4-(methyl-sulfan-yl)phen-yl]-4,2′:6′,4′′-terpyridine in two discrete zinc(II) complexes with acetyl-acetonate. Acta Crystallogr. Sect. C 2012, 68, m269–m274. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.-S.; Bai, C.; Hu, H.-M.; Yuan, F.; Wang, X.; Xue, G. Syntheses, structures, and luminescent properties of two cadmium(II) coordination compounds based on a sulfonate functionalized terpyridine ligand. Z. Anorg. Allg. Chem. 2015, 641, 1772–1776. [Google Scholar] [CrossRef]
- Granifo, J.; Gaviño, R.; Freire, E.; Baggio, R. A novel hybrid terpyridine–pyrimidine ligand and the supramolecular structures of two of its complexes with Zn(II) and acetylacetonato: The underlying role of non-covalent π...π contacts and C–H...X(O, N, π) hydrogen bonds. J. Mol. Struct. 2014, 1063, 102–108. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, S.; Zheng, S.; Golen, J.A.; Rheingold, A.L.; Zhang, G. Cobalt(II) coordination polymers versus discrete complex with 4,2′:6′,4′′-terpyridine ligands: The role of a pyrenyl substituent. Polyhedron 2015, 101, 139–145. [Google Scholar] [CrossRef]
- Klein, Y.M.; Constable, E.C.; Housecroft, C.E.; Zampese, J.A.; Crochet, A. Greasy tails switch 1D-coordination [Zn2(OAc)4(4′-(4-ROC6H4)-4,2′:6′,4′′-tpy)]n polymers to discrete [Zn2(OAc)4(4′-(4-ROC6H4)- 4,2′:6′,4′′-tpy)2] complexes. CrystEngComm 2014, 16, 9915–9929. [Google Scholar] [CrossRef]
- Kröhnke, F. The specific synthesis of pyridines and oligopyridines. Synthesis 1976, 1975, 1–24. [Google Scholar] [CrossRef]
- Wang, J.; Hanan, G.S. A facile route to sterically hindered and non-hindered 4’-Aryl-2,2’:6’,2’’-terpyridines. Synlett 2005, 2005, 1251–1254. [Google Scholar] [CrossRef]
- Cave, G.W.V.; Raston, C.L. Efficient synthesis of pyridines via a sequential solventless aldol condensation and Michael addition. J. Chem. Soc. Perkin Trans. 1 2001, 3258–3264. [Google Scholar] [CrossRef]
- Yoshida, J.; Nishikiori, S.-I.; Yuge, H. Bis(3-cyano-pentane-2,4-dionato) Co(II) as a linear building block for coordination polymers: Combinations with two polypyridines. J. Coord. Chem. 2013, 66, 2191–2200. [Google Scholar] [CrossRef]
- Ghozlan, S.A.S.; Hassanien, A.Z.A. β-Amino-β-(pyrid-4-yl)acrylonitrile (I) in heterocyclic synthesis: Synthesis of some new pyridine, pyridone, pyrazole, thiophene, fused pyrimidine and triazine derivatives. Tetrahedron 2002, 58, 9423–9429. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E.; Vujovic, S.; Zampese, J.A. 2D→2D Parallel interpenetration of (4,4) sheets constructed from a ditopic bis(4,2′:6′,4′′-terpyridine). CrystEngComm 2014, 16, 3494–3497. [Google Scholar] [CrossRef]
- Vujovic, S.; Constable, E.C.; Housecroft, C.E.; Morris, C.D.; Neuburger, M.; Prescimone, A. Engineering 2D→2D parallel interpenetration using long alkoxy-chain substituents. Polyhedron 2015, 92, 77–83. [Google Scholar] [CrossRef]
- Klein, Y.M.; Prescimone, A.; Neuburger, M.; Constable, E.C.; Housecroft, C.E. What a difference a tail makes: 2D→2D parallel interpenetration of sheets to interpenetrated nbo networks using ditopic-4,2′:6′,4′′-terpyridine ligands. CrystEngComm 2017, 19, 2894–2902. [Google Scholar] [CrossRef]
- Klein, Y.M.; Constable, E.C.; Housecroft, C.E.; Prescimone, A. A 3-dimensional {42.84} lvt net built from a ditopic bis(3,2’:6’,3”-terpyridine) tecton bearing long alkyl tails. CrystEngComm 2015, 17, 2070–2073. [Google Scholar] [CrossRef]
- Klein, Y.M.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. A double-stranded 1D-coordination polymer assembled using the tetravergent ligand 1,1′-bis(4,2′:6′,4′′-terpyridin-4′-yl)ferrocene. Inorg. Chem. Commun. 2016, 70, 118–120. [Google Scholar] [CrossRef]
- Klein, Y.M.; Prescimone, A.; Pitak, M.B.; Coles, S.J.; Constable, E.C.; Housecroft, C.E. Constructing chiral MOFs by functionalizing 4,2′:6′,4′′-terpyridine with long-chain alkoxy domains: Rare examples of neb nets. CrystEngComm 2016, 18, 4704–4707. [Google Scholar] [CrossRef]
- Klein, Y.M.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. 2-Dimensional networks assembled using 4’-functionalized 4,2′:6′,4′′-terpyridines and Co(NCS)2. Polyhedron 2016, 103, 58–65. [Google Scholar] [CrossRef]
- Mondal, A.K.; Khatua, S.; Tomar, K.; Konar, S. Field-induced single-ion-magnetic behavior of octahedral CoII in a two-dimensional coordination polymer. Eur. J. Inorg. Chem. 2016, 2016, 3545–3552. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E.; Neuburger, M.; Vujovic, S.; Zampese, J.A.; Zhang, G. Cobalt(II) coordination polymers with 4′-substituted 4,2′:6′,4′′- and 3,2′:6′,3”-terpyridines: Engineering a switch from planar to undulating chains and sheets. CrystEngComm 2012, 14, 3554–3563. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E.; Kopecky, P.; Neuburger, M.; Zampese, J.A.; Zhang, G. Coordination polymers with divergent 4′-tert-butyl-4,2′:6′,4′′-terpyridine linkers: From aryl-aryl to ball-and-socket packing. CrystEngComm 2012, 14, 446–452. [Google Scholar] [CrossRef]
- Klein, Y.M.; Constable, E.C.; Housecroft, C.E.; Zampese, J.A. 4′-(Pyrimidin-5-yl)- and 4′-(2-methylpyrimidin-5-yl)-4,2′:6′,4′′-terpyridines: Selective coordination to zinc(II) through the 4,2′:6′,4′′-terpyridine domain. Polyhedron 2014, 81, 98–104. [Google Scholar] [CrossRef]
- Klein, Y.M.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Coordination behaviour of 1-(4,2′:6′,4′′-terpyridin-4′-yl)ferrocene and 1-(3,2’:6’,3”-terpyridin-4′-yl)ferrocene: Predictable and unpredictable assembly algorithms. Aust. J. Chem. 2017, 70, 468–477. [Google Scholar] [CrossRef]
- Xiao, L.; Zhu, L.; Zeng, Q.; Liu, Q.; Zhang, J.; Li, S.; Zhou, H.; Zhang, S.; Wu, J.; Tian, Y. Novel metal-organic hybrid materials constructed by ferrocenyl terpyridine derivatives and ZnIIX2 (X = Cl–, Br–, I–, SCN– and CH3COO–). J. Organomet. Chem. 2015, 789–790, 22–28. [Google Scholar] [CrossRef]
- Kuhnert, N.; Lopez-Periago, A.; Rossignolo, G.M. The synthesis and conformation of oxygenated trianglimine macrocycles. Org. Biomol. Chem. 2005, 3, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Domagała, M.; Grabowski, S.J. C–H...N and C–H...S hydrogen bonds Influence of hybridization on their strength. J. Phys. Chem. A 2005, 109, 5683–5688. [Google Scholar] [CrossRef] [PubMed]
- Bondi, A. van der Waals volumes and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Klein, R.A. Modified van der Waals atomic radii for hydrogen bonding based on electron density topology. Chem. Phys. Lett. 2006, 425, 128–133. [Google Scholar] [CrossRef]
- Rowland, R.S.; Taylor, R. Intermolecular nonbonded contact distances in organic crystal structures: Comparison with distances expected from van der Waals Radii. J. Phys. Chem. 1996, 100, 7384–7391. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P. TOPOS Professional v. 4.0; Samara State University: Samara, Russia, 2010. [Google Scholar]
- Li, D.-S.; Wu, Y.-P.; Zhao, J.; Zhang, J.; Lu, J.Y. Metal-organic frameworks based upon non-zeotype 4-connected topology. Coord. Chem. Rev. 2014, 261, 1–27. [Google Scholar] [CrossRef]
- Batten, S.R.; Neville, S.M.; Turner, D.R. Coordination Polymers: Design, Analysis and Application; RSC Publishing: Cambridge, UK, 2009; ISBN 978-0-85404-837-3. [Google Scholar]
- Nishio, M. CH/π hydrogen bonds in crystals. CrystEngComm 2004, 6, 130–158. [Google Scholar] [CrossRef]
- Bruker Analytical X-ray Systems, Inc. APEX2, Version 2 User Manual, M86-E01078; Bruker Analytical X-ray Systems, Inc.: Madison, WI, USA, 2006. [Google Scholar]
- Palatinus, L.; Chapuis, G. SUPERFLIP—A computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Cryst. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Betteridge, P.W.; Carruthers, J.R.; Cooper, R.I.; Prout, K.; Watkin, D.J. CRYSTALS version 12: Software for guided crystal structure analysis. J. Appl. Cryst. 2003, 36, 1487. [Google Scholar] [CrossRef]
- Bruno, I.J.; Cole, J.C.; Edgington, P.R.; Kessler, M.K.; Macrae, C.F.; McCabe, P.; Pearson, J.; Taylor, R. New software for searching the Cambridge Structural Database and visualizing crystal structures. Acta Cryst. B 2002, 58, 389–397. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Cryst. C 2015, 71, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Constable, E.C.; Zhang, G.; Housecroft, C.E.; Zampese, J.A. Zinc(II) coordination polymers, metallohexacycles and metallocapsules—Do we understand self-assembly in metallosupramolecular chemistry: Algorithms or serendipity? CrystEngComm 2011, 13, 6864–6870. [Google Scholar] [CrossRef]





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klein, Y.M.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. 4,2’:6’,4”- and 3,2’:6’,3”-Terpyridines: The Conflict between Well-Defined Vectorial Properties and Serendipity in the Assembly of 1D-, 2D- and 3D-Architectures. Materials 2017, 10, 728. https://doi.org/10.3390/ma10070728
Klein YM, Prescimone A, Constable EC, Housecroft CE. 4,2’:6’,4”- and 3,2’:6’,3”-Terpyridines: The Conflict between Well-Defined Vectorial Properties and Serendipity in the Assembly of 1D-, 2D- and 3D-Architectures. Materials. 2017; 10(7):728. https://doi.org/10.3390/ma10070728
Chicago/Turabian StyleKlein, Y. Maximilian, Alessandro Prescimone, Edwin C. Constable, and Catherine E. Housecroft. 2017. "4,2’:6’,4”- and 3,2’:6’,3”-Terpyridines: The Conflict between Well-Defined Vectorial Properties and Serendipity in the Assembly of 1D-, 2D- and 3D-Architectures" Materials 10, no. 7: 728. https://doi.org/10.3390/ma10070728