Monoclinic 122-Type BaIr2Ge2 with a Channel Framework: A Structural Connection between Clathrate and Layered Compounds
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis of Monoclinic BaIr2Ge2
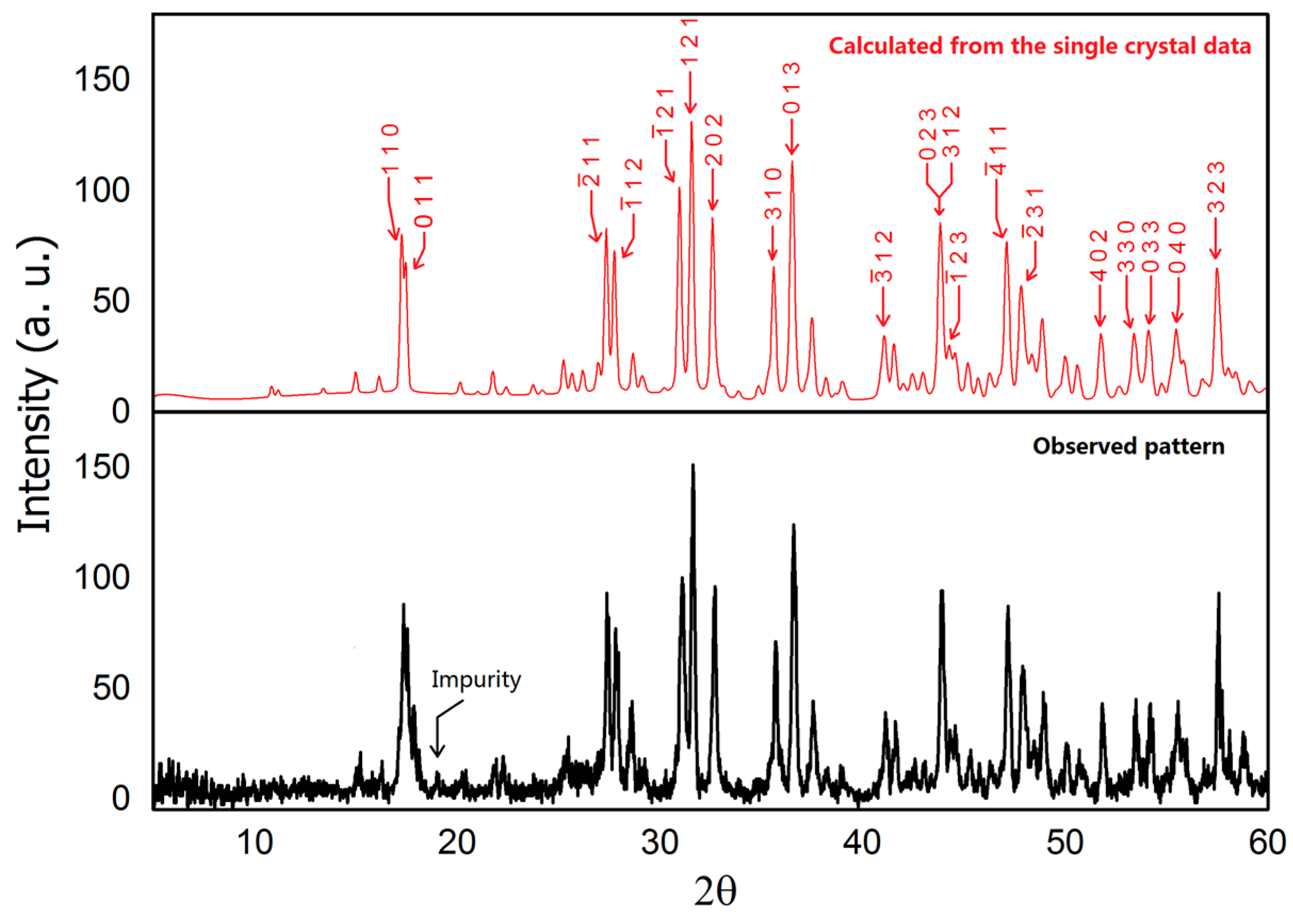
2.2. Phase Identification
2.3. Single Crystal Structure Determination
2.4. Magnetic Property Measurements
2.5. Electronic Structure Calculations
3. Results and Discussion
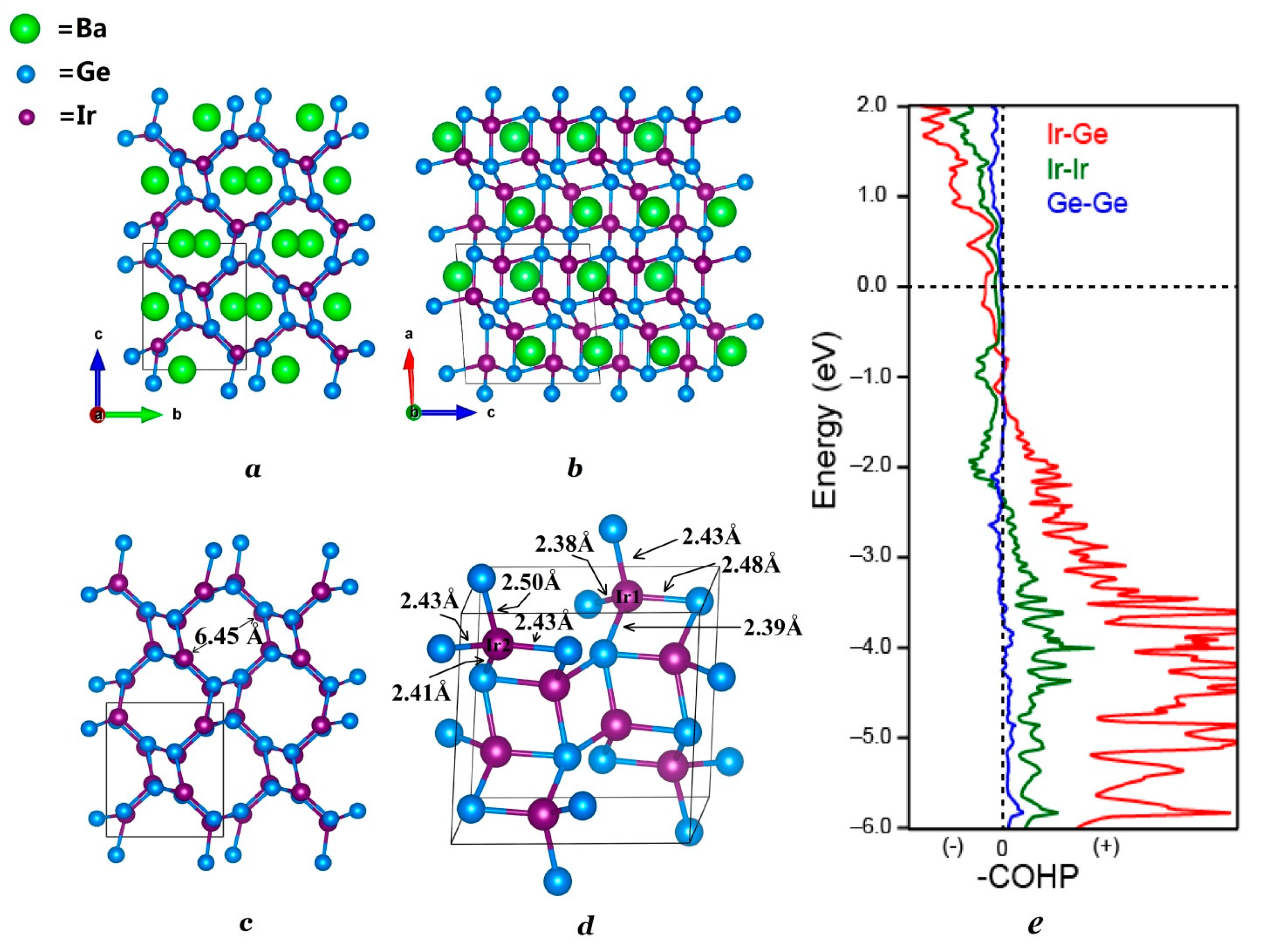
3.1. Phase Identification and Structure Determination of BaIr2Ge2
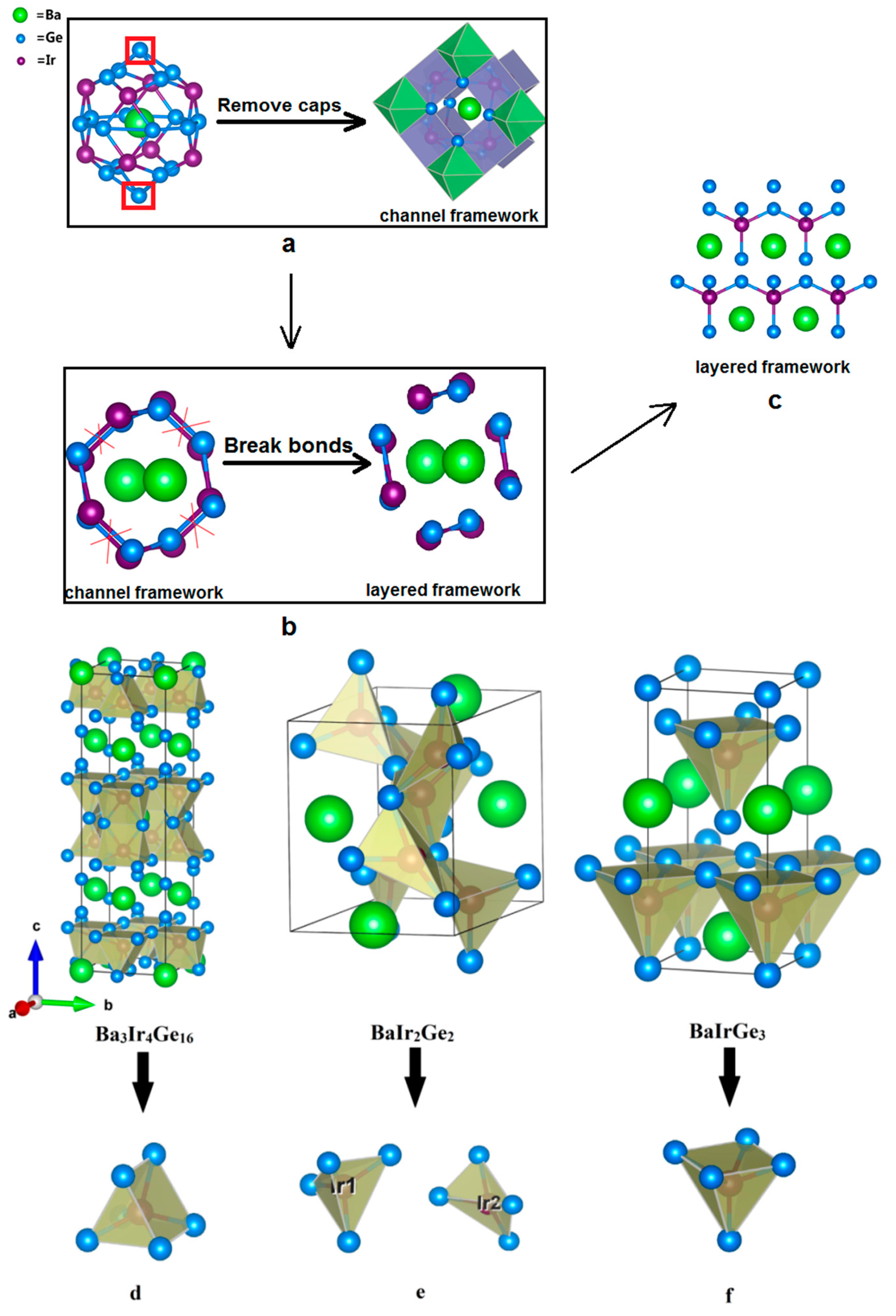
3.2. Structural Comparison of the Different 122 Phases
3.3. Structural Connections between Clathrate and Layered Compounds
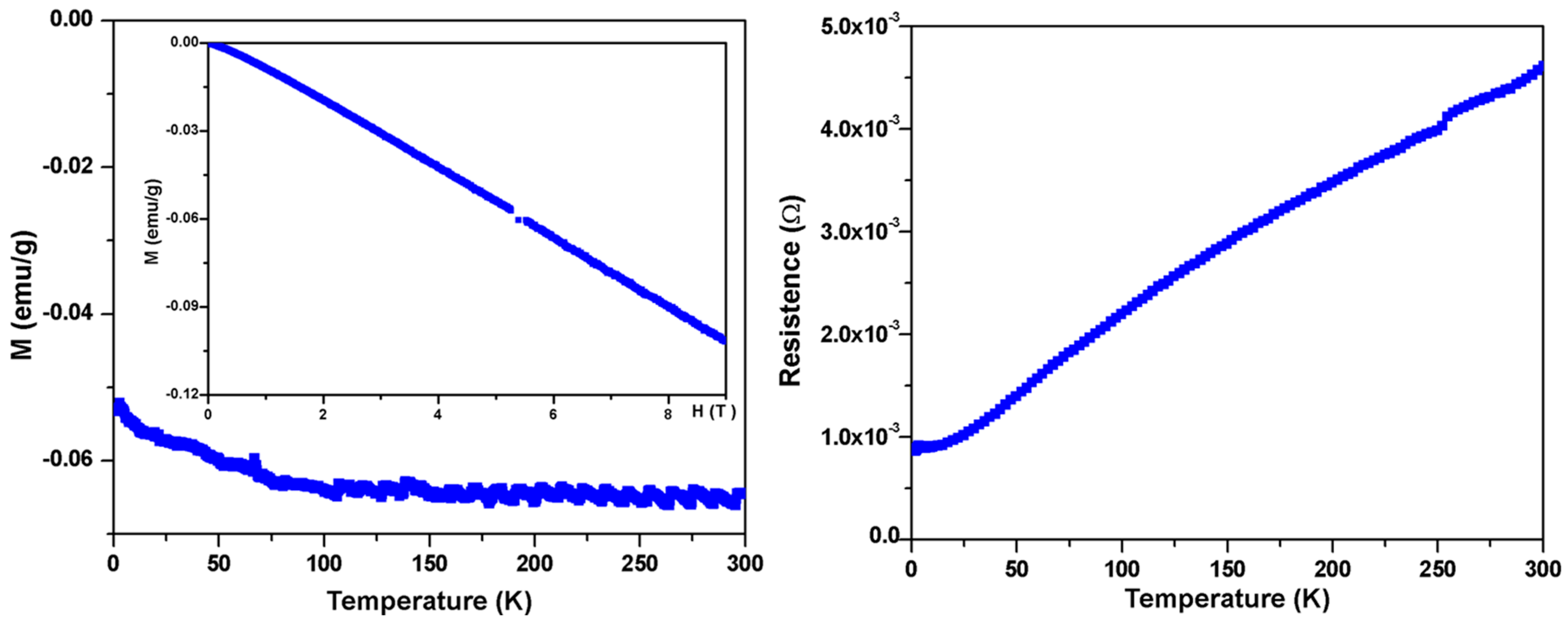
3.4. Magnetic Properties of Monoclinic BaIr2Ge2
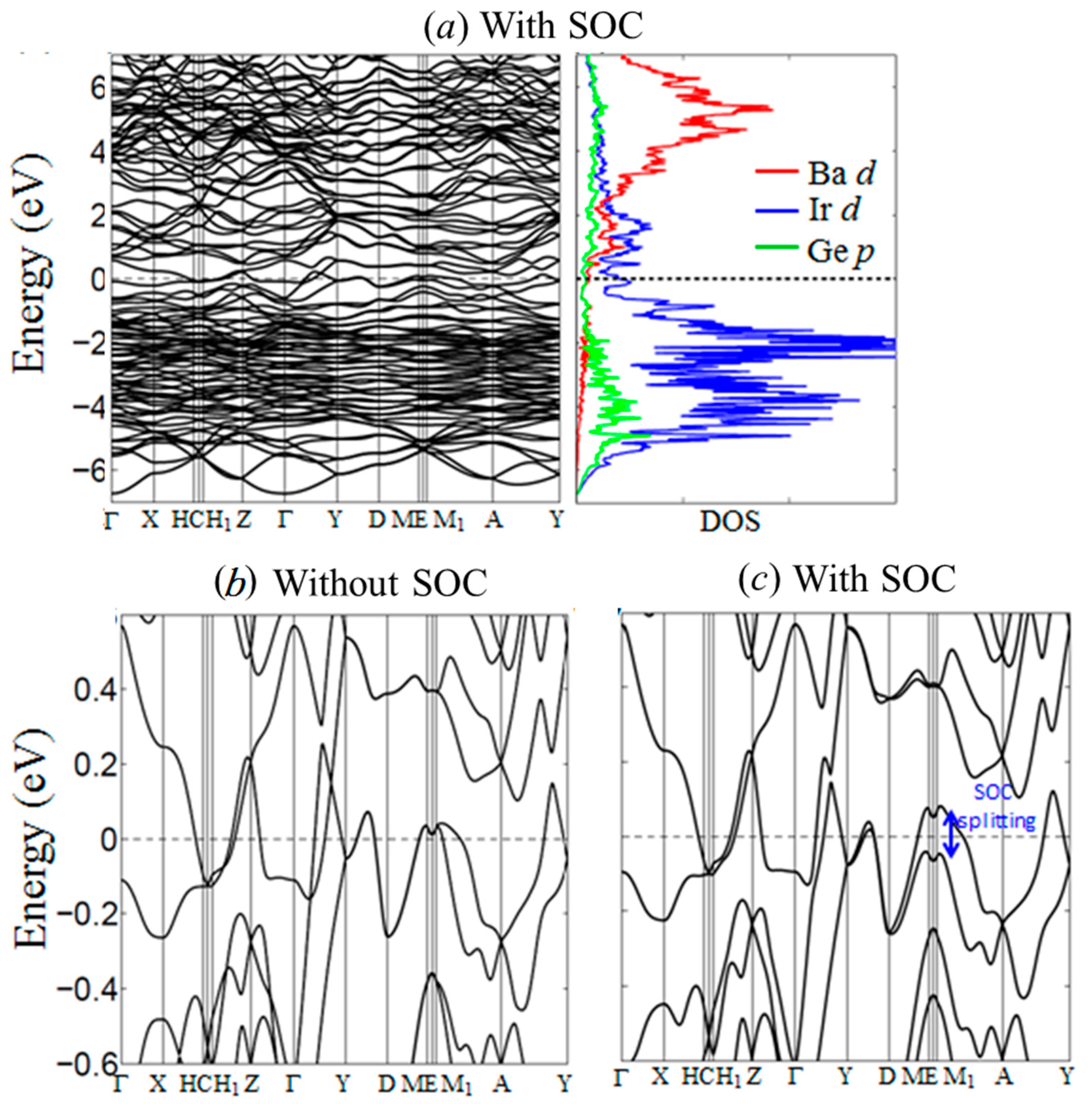
3.5. Electronic Structure Calculations
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Margadonna, S.; Takabayashi, Y.; Mcdonald, M.T.; Kasperkiewicz, K.; Mizuguchi, Y.; Takano, Y.; Fitch, A.N.; Suard, E.; Prassides, K. Crystal structure of the new FeSe1−x superconductor. Chem. Commun. 2008, 43, 5607–5609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.-W.; Pardo, V.; Pickett, W.E. Magnetism driven by anion vacancies in superconducting α-FeSe1−x. Phys. Rev. B 2008, 78, 174502. [Google Scholar] [CrossRef]
- Okada, H.; Igawa, K.; Takahashi, H.; Kamihara, Y.; Hirano, M.; Hosono, H.; Matsubayashi, K.; Uwatoko, Y. Superconductivity under high pressure in LaFeAsO. J. Phys. Soc. Jpn. 2008, 77, 113712. [Google Scholar] [CrossRef]
- Rotter, M.; Tegel, M.; Johrendt, D. Superconductivity at 38 K in the iron arsenide (Ba1−xKx)Fe2As2. Phys. Rev. Lett. 2008, 101, 107006. [Google Scholar] [CrossRef] [PubMed]
- Gor’kov, L.P.; Grüner, G. Charge Density Waves in Solids, 1st ed.; North Holland: Amsterdam, The Netherlands, 1989; pp. 1–494. ISBN 9780444600738. [Google Scholar]
- Gruner, G. Density Waves in Solids; reprint; Westview Press: Boulder, CO, USA, 2009; pp. 1–288. ISBN 9780786747795. [Google Scholar]
- Lin, Q.; Miller, G.J.; Corbett, J.D. Ordered BaAl4-type variants in the BaAuxSn4−x system: A unified view on their phase stabilities versus valence electron counts. Inorg. Chem. 2014, 53, 5875–5877. [Google Scholar] [CrossRef] [PubMed]
- Häussermann, U.; Amerioun, S.; Eriksson, L.; Lee, C.-S.; Miller, G.J. The s–p bonded representatives of the prominent BaAl4 structure type: A case study on structural stability of polar intermetallic network structures. J. Am. Chem. Soc. 2002, 124, 4371–4383. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, R.; Zheng, C. Making and breaking bonds in the solid state: The thorium chromium silicide (ThCr2Si2) structure. J. Phys. Chem. 1985, 89, 4175–4181. [Google Scholar] [CrossRef]
- Zheng, C.; Hoffmann, R. Donor-acceptor layer formation and lattice site preference in the solid: The CaBe2Ge2 structure. J. Am. Chem. Soc. 1986, 108, 3078–3088. [Google Scholar] [CrossRef]
- Tan, X.; Fabbris, G.; Haskel, D.; Yaroslavtsev, A.A.; Cao, H.; Thompson, C.M.; Kovnir, K.; Menushenkov, A.P.; Chernikov, R.V.; Garlea, V.O.; et al. Transition from localized to strongly correlated electron behavior and mixed valence driven by physical or chemical pressure in ACo2As2 (A = Eu and Ca). J. Am. Chem. Soc. 2016, 138, 2724–2731. [Google Scholar] [CrossRef] [PubMed]
- Kudo, K.; Nishikubo, Y.; Nohara, M. Coexistence of superconductivity and charge density wave in SrPt2As2. J. Phys. Soc. Jpn. 2010, 79, 123710. [Google Scholar] [CrossRef]
- Settaia, R.; Sugitania, I.; Okudaa, Y.; Thamizhavela, A.; Nakashimab, M.; Onukia, Y.; Harimac, H. Pressure-induced superconductivity in CeCoGe3 without inversion symmetry. J. Magn. Magn. Mater. 2007, 310, 844–846. [Google Scholar] [CrossRef]
- Samokhin, K.V.; Zijlstra, E.S.; Bose, S.K. CePt3Si: An unconventional superconductor without inversion center. Phys. Rev. B 2004, 69, 094514. [Google Scholar] [CrossRef]
- Kovnir, K.A.; Zaikina, J.V.; Reshetova, L.N.; Olenev, A.V.; Dikarev, E.V.; Shevelkov, A.V. Unusually high chemical compressibility of normally rigid type-I clathrate framework: Synthesis and structural study of Sn24P19.3BrxI8−x solid solution, the prospective thermoelectric material. Inorg. Chem. 2004, 43, 3230–3236. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Hu, S.; Uher, C.; Hogan, T.; Huang, B.; Corbett, J.D.; Kanatzidis, M.G. Structure and thermoelectric properties of Ba6Ge25−x, Ba6Ge23Sn2, and Ba6Ge22In3: Zintl phases with a chiral clathrate structure. J. Solid State Chem. 2000, 153, 321–329. [Google Scholar] [CrossRef]
- Fukuoka, H.; Kiyoto, J.; Yamanaka, S. Superconductivity and crystal structure of the solid solutions of Ba8−dSi46−xGex (0 ≤ x ≤ 23) with Type I clathrate structure. J. Solid State Chem. 2003, 175, 237–244. [Google Scholar] [CrossRef]
- Klemm, W. Centenary-Lecture-Metalloids and their Compounds with the Alkali Metals. Proc. Chem. Soc. Lond. 1958, 12, 329–341. [Google Scholar]
- Mott, N.F. Metal-Insulator Transitions, 2nd ed.; Taylor & Francis Inc.: London, UK, 1990; pp. 1–34. ISBN 0-85066-783-6. [Google Scholar]
- Kakihana, M.; Akamine, H.; Tomori, K.; Nishimura, K.; Teruya, A.; Nakamura, A.; Honda, F.; Aoki, D.; Nakashima, M.; Amako, Y.; et al. Superconducting, Fermi surface, and magnetic properties in SrTGe3 and EuTGe3 (T: Transition metal) with the Rashba-type tetragonal structure. J. Alloys Compd. 2017, 694, 439–451. [Google Scholar] [CrossRef]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic computing system JANA2006: General features. Z. Kristallogr. Cryst. Mater. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A: Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Bruker. Smart; Bruker AXS Inc.: Madison, WI, USA, 2012; Available online: https://www.bruker.com/products/x-ray-diffraction-and-elemental-analysis/single-crystal-x-ray-diffraction/sc-xrd-software/overview/sc-xrd-software/apex3.html (accessed on 12 July 2017).
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. 1964, 136, B864. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Sato, A. Crystal structure of ternary germanides SrM2Ge2 (M = Ni and Ir). J. Alloys Compd. 2009, 487, 198–201. [Google Scholar] [CrossRef]
- Siggelkow, L.; Hlukhyy, V.; Fässler, T.F. Synthesis, Structure and chemical bonding of CaCo2Si2 and BaCo2Ge2—Two new compounds with ThCr2Si2 Structure type. Z. Anorg. Allg. Chem. 2010, 636, 378–384. [Google Scholar] [CrossRef]
- Venturini, G.; Malaman, B. X-ray single crystal refinements on some RT2Ge2 compounds (R = Ca, Y, La, Nd, U; T = Mn-Cu, Ru-Pd): Evolution of the chemical bonds. J. Alloys Compd. 1996, 235, 201–209. [Google Scholar] [CrossRef]
- Venturini, G.; Malaman, B.; Roques, B. Contribution a la cristallochimie des isotypes de ThCr2Si2 et CaBe2Ge2: II. Variation des distances interatomiques dans des germaniures MM'2Ge2 de type ThCr2Si2 (M = Y, Nd, Ca; M’ = Mn, Cu, Ru, Rh, Pd, Ir). J. Solid State Chem. 1989, 79, 136–145. [Google Scholar] [CrossRef]
- González, J.; Kessens, R.; Schuster, H.U. Darstellung und kristallstruktur neuer AM2X2-verbindungen in den systemen erdalkalimetall-platinmetall-germanium. Z. Anorg. Allg. Chem. 1993, 619, 13–16. [Google Scholar] [CrossRef]
- Hlukhyy, V.; Hoffmann, A.V.; Fässler, T.F. Synthesis, structure and chemical bonding of CaFe2−xRhxSi2 (x = 0, 1.32, and 2) and SrCo2Si2. J. Solid State Chem. 2013, 203, 232–239. [Google Scholar] [CrossRef]
- Doerrscheidt, W.; Niess, N.; Schaefer, H. Neue verbindungen AB2X2 (A = Erdalkalimetall, B = Uebergangselement, X = Element (IV)) im ThCr2Si2-Typ. Z. Naturforsch. B 1976, 31, 890–891. [Google Scholar]
- May, N.; Mueller, W.; Schaefer, H. Ternäre erdalkali-beryllium-silicide und -germanide mit AlB2-struktur. ternary alkaline-earth-beryllium-silicides and -germanides of AlB2-structure. Z. Naturforsch. B 1974, 29, 325–327. [Google Scholar] [CrossRef]
- Langen, D.; Schoolaert, S.; Ploss, H.; Jung, W. Die isotypen verbindungen BaRh2Si2, BaIr2Si2 und BaPt2Ga2-eine monokline verzerrungsvariante der CaRh2B2-strukur. Z. Anorg. Allg. Chem. 1997, 623, 1561–1566. [Google Scholar] [CrossRef]
- Falmbigl, M.; Grytsiv, A.; Rogl, P.; Giester, G. Clathrate formation in the systems Ba-Ir-Ge and Ba-{Rh, Ir}-Si: Crystal chemistry and phase relations. Intermetallics 2013, 36, 61–72. [Google Scholar] [CrossRef]
- Nasir, N.; Melnychenko-Koblyuk, N.; Grytsiv, A.; Rogl, P.; Giester, G.; Wosik, J.; Nauer, G.E. Ternary systems Sr-{Ni, Cu}-Si: Phase equilibria and crystal structure of ternary phases. J. Solid State Chem. 2010, 183, 565–574. [Google Scholar] [CrossRef]
- Baelus, B.J.; Peeters, F.M.; Schweigert, V.A. Saddle-point states and energy barriers for vortex entrance and exit in superconducting disks and rings. Phys. Rev. B 2001, 63, 144517. [Google Scholar] [CrossRef]
| Refined Formula | BaIr2Ge2 |
|---|---|
| Formula weight (F.W.) (g/mol) | 666.92 |
| Space group; Z | P21/c (No. 14); 4 |
| a (Å) | 8.204 (5) |
| b (Å) | 6.625 (4) |
| c (Å) | 7.959 (5) |
| β (°) | 94.27 (1) |
| V (Å3) | 431.4 (4) |
| Extinction Coefficient | 0.00061 (9) |
| θ range (deg) | 2.489–32.085 |
| hkl ranges | −12 ≤ h ≤ 12 |
| −9 ≤ k ≤ 9 | |
| −11 ≤ l ≤ 10 | |
| No. reflections; Rint | 9731; 0.0925 |
| No. independent reflections | 1483 |
| No. parameters | 47 |
| R1: ωR2 (all I) | 0.0503; 0.0872 |
| Goodness of fit | 0.954 |
| Diffraction peak and hole (e−/Å3) | 3.812; −3.705 |
| Atom | Wyckoff. | Occ. | x | y | z | Ueq |
|---|---|---|---|---|---|---|
| Ba1 | 4e | 1 | 0.2318 (1) | 0.8818 (2) | 0.4993 (2) | 0.0116 (3) |
| Ir2 | 4e | 1 | 0.6260 (1) | 0.8959 (1) | 0.1069 (1) | 0.0074 (2) |
| Ir3 | 4e | 1 | 0.8532 (1) | 0.6648 (1) | 0.3334 (1) | 0.0077 (2) |
| Ge4 | 4e | 1 | 0.5560 (2) | 0.8515 (3) | 0.8069 (3) | 0.0088 (4) |
| Ge5 | 4e | 1 | 0.9282 (2) | 0.9171 (3) | 0.1356 (3) | 0.0091 (4) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gui, X.; Chang, T.-R.; Kong, T.; Pan, M.T.; Cava, R.J.; Xie, W. Monoclinic 122-Type BaIr2Ge2 with a Channel Framework: A Structural Connection between Clathrate and Layered Compounds. Materials 2017, 10, 818. https://doi.org/10.3390/ma10070818
Gui X, Chang T-R, Kong T, Pan MT, Cava RJ, Xie W. Monoclinic 122-Type BaIr2Ge2 with a Channel Framework: A Structural Connection between Clathrate and Layered Compounds. Materials. 2017; 10(7):818. https://doi.org/10.3390/ma10070818
Chicago/Turabian StyleGui, Xin, Tay-Rong Chang, Tai Kong, Max T. Pan, Robert J. Cava, and Weiwei Xie. 2017. "Monoclinic 122-Type BaIr2Ge2 with a Channel Framework: A Structural Connection between Clathrate and Layered Compounds" Materials 10, no. 7: 818. https://doi.org/10.3390/ma10070818





