Effect of Alternating Current on the Cathodic Protection and Interface Structure of X80 Steel
Abstract
:1. Introduction
2. Experimental
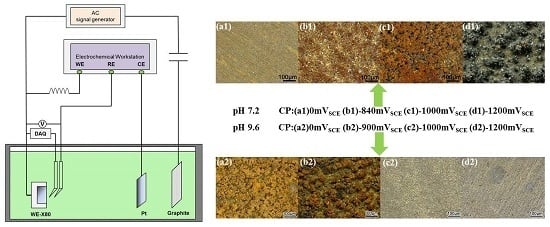
2.1. Electrodes and Solutions
2.2. Immersion Tests
2.3. DC and Real-Time Potential Measurement
2.4. Cyclic Voltammetry Measurements
2.5. AC Voltammetry Technique
3. Results and Discussion
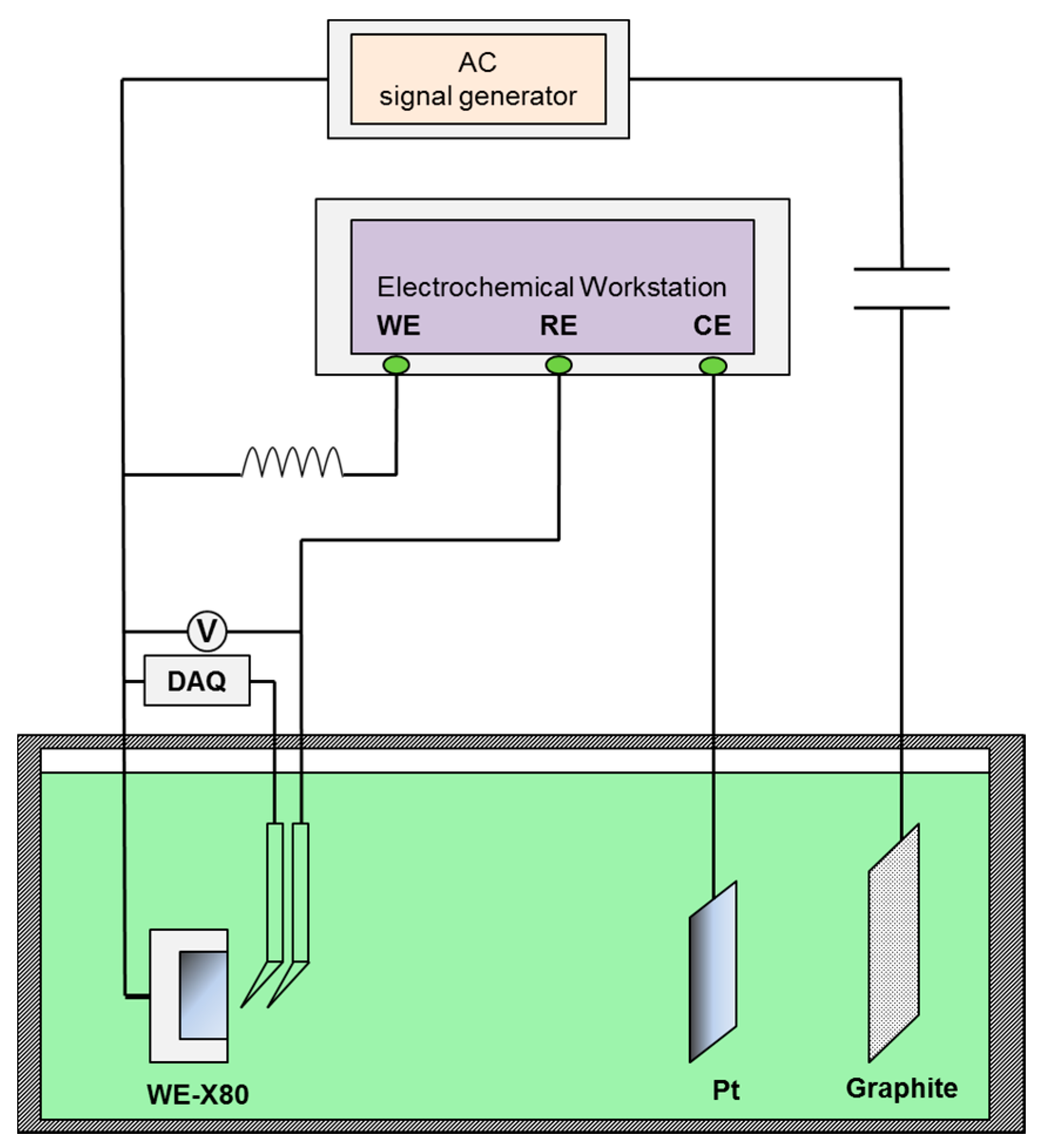
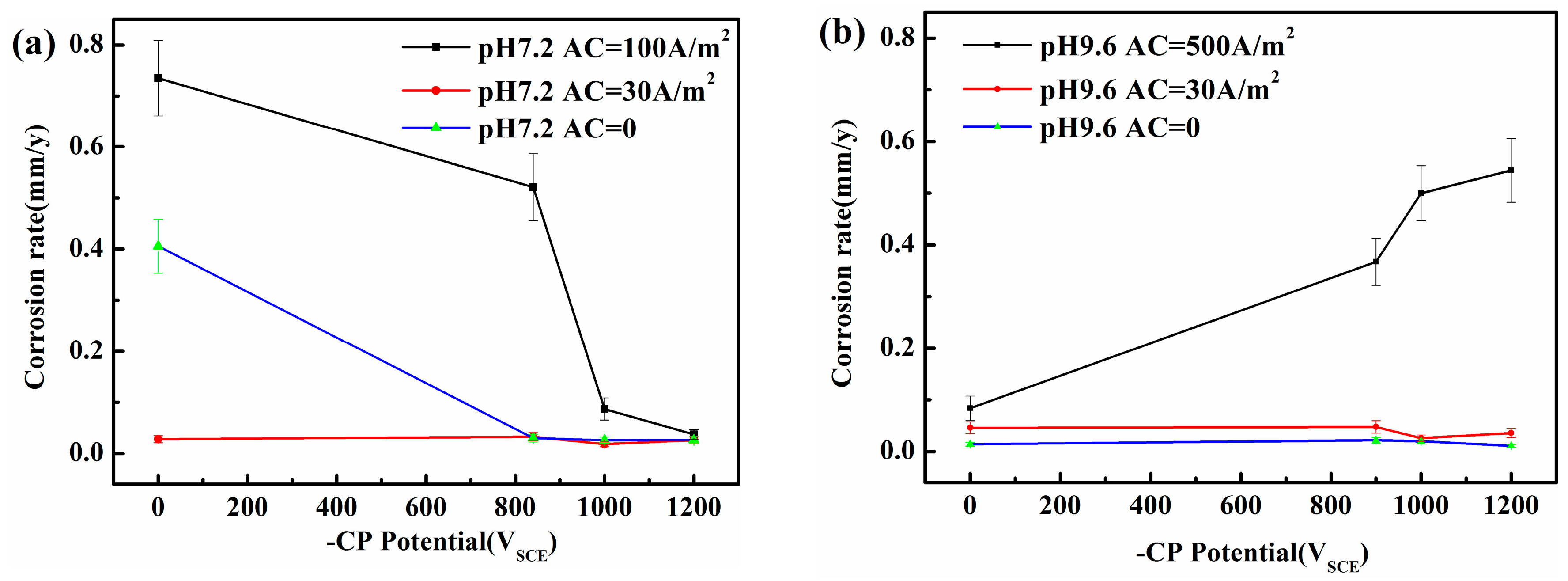
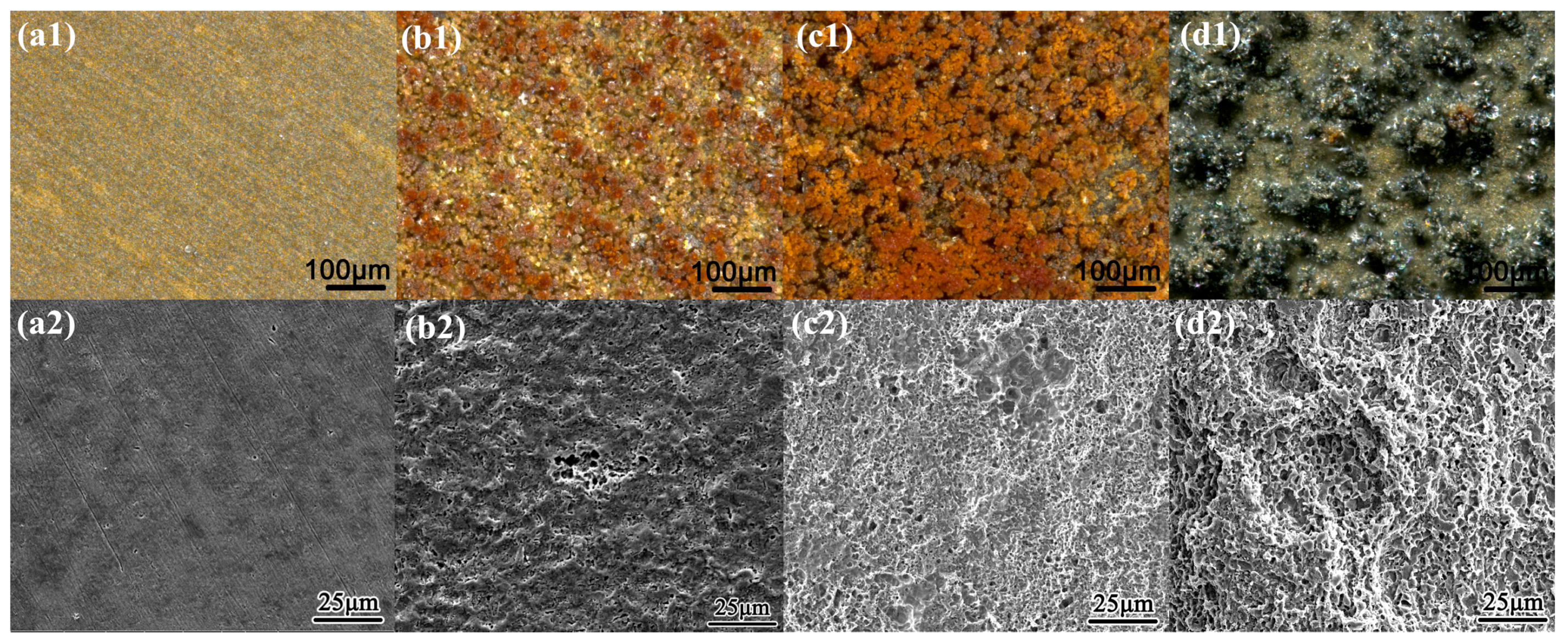
3.1. Weight Loss Measurement and Surface Morphology Characterization
3.2. Electrochemical Measurements
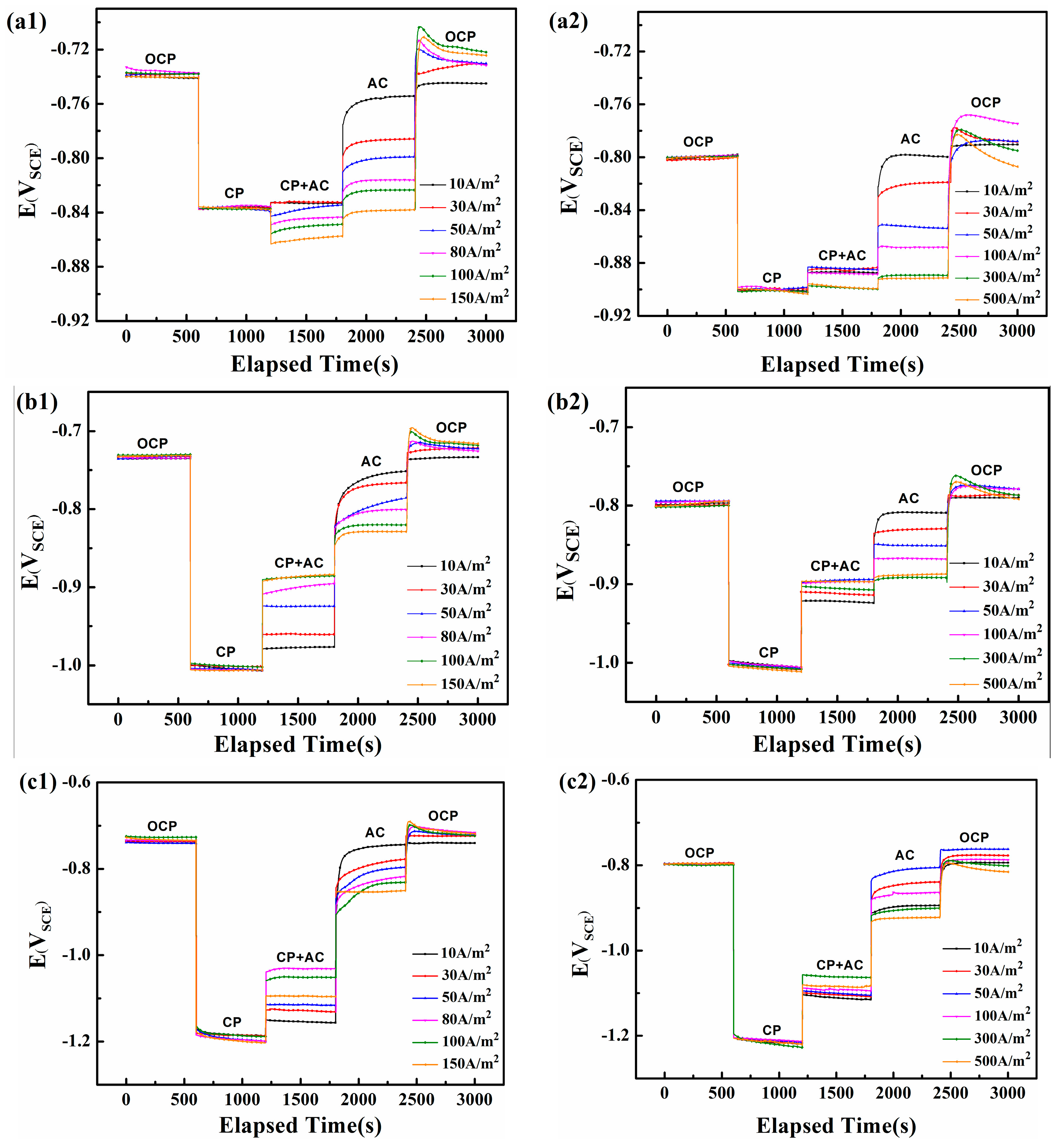
3.2.1. Analysis of DC Potential
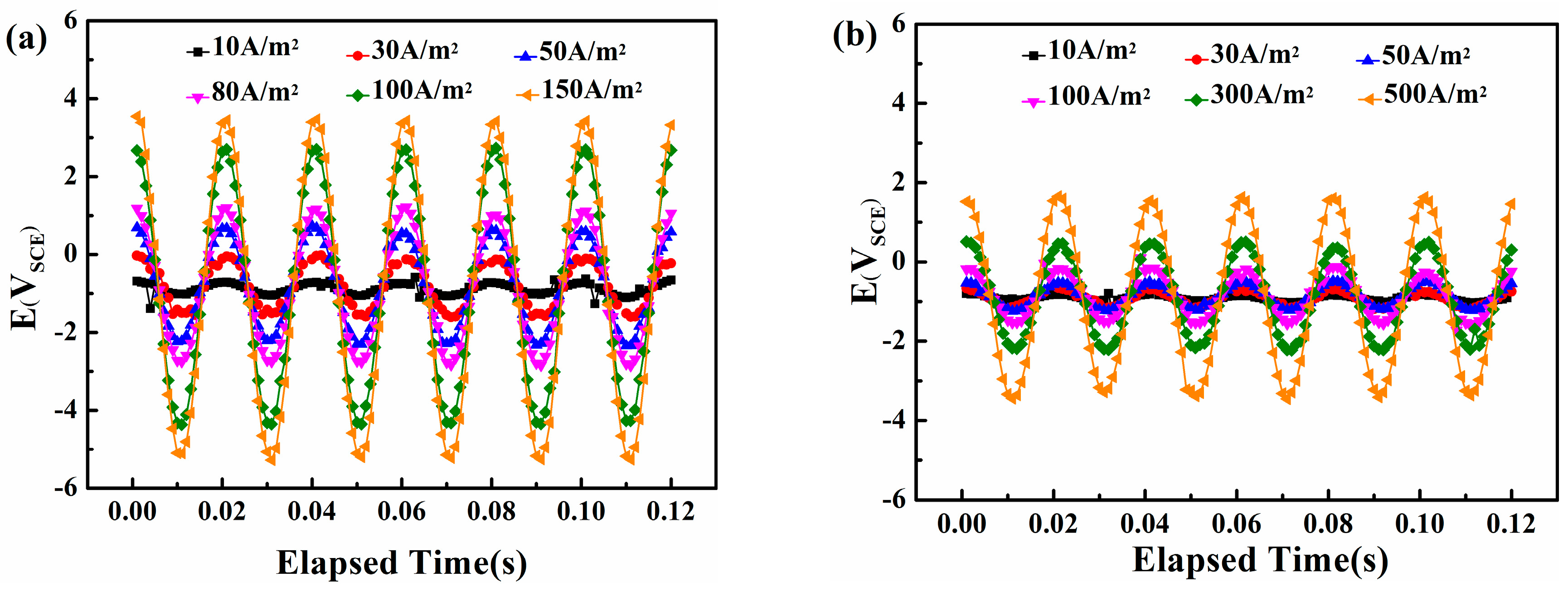
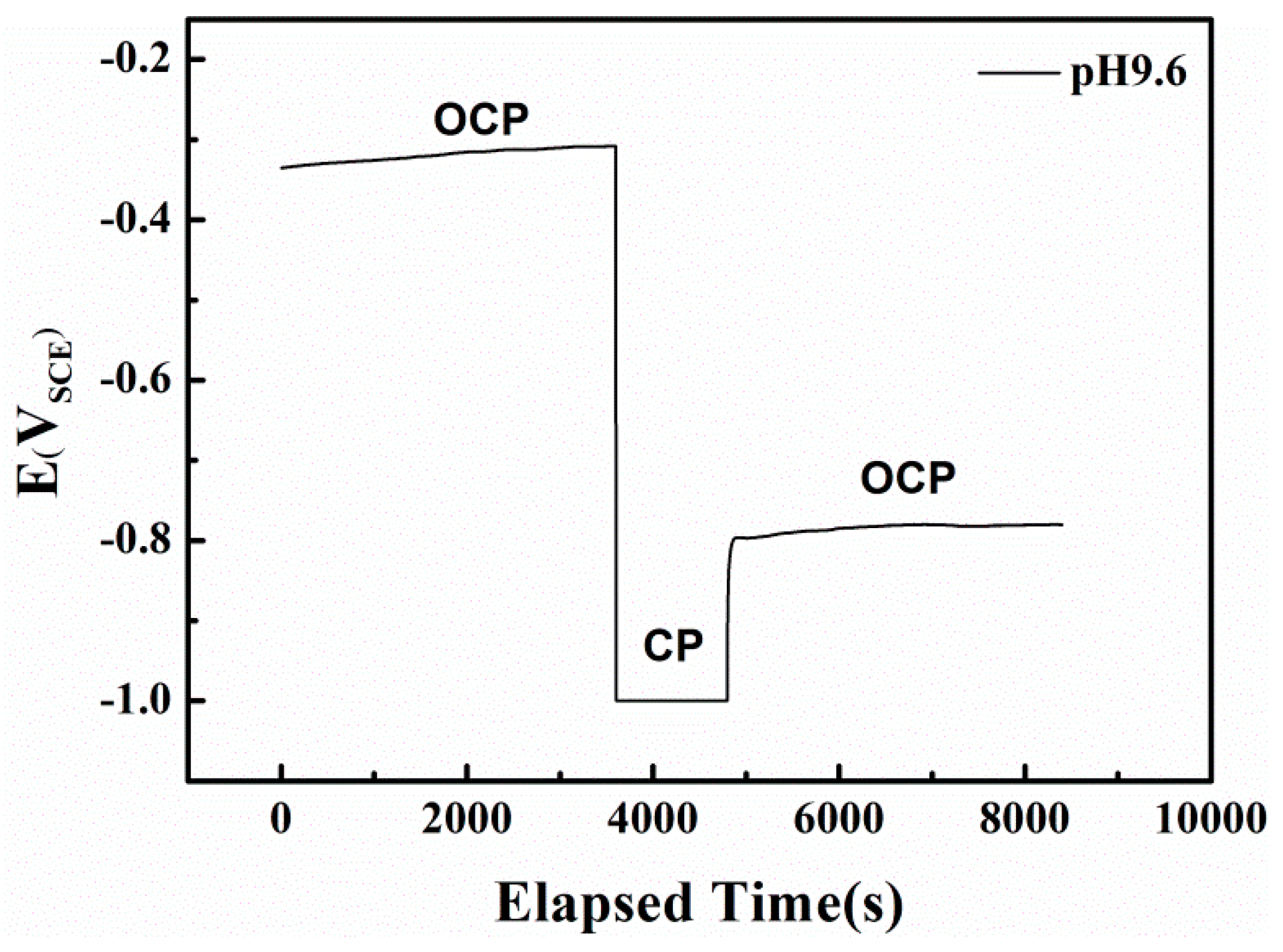
3.2.2. Real Time Potential Measurement
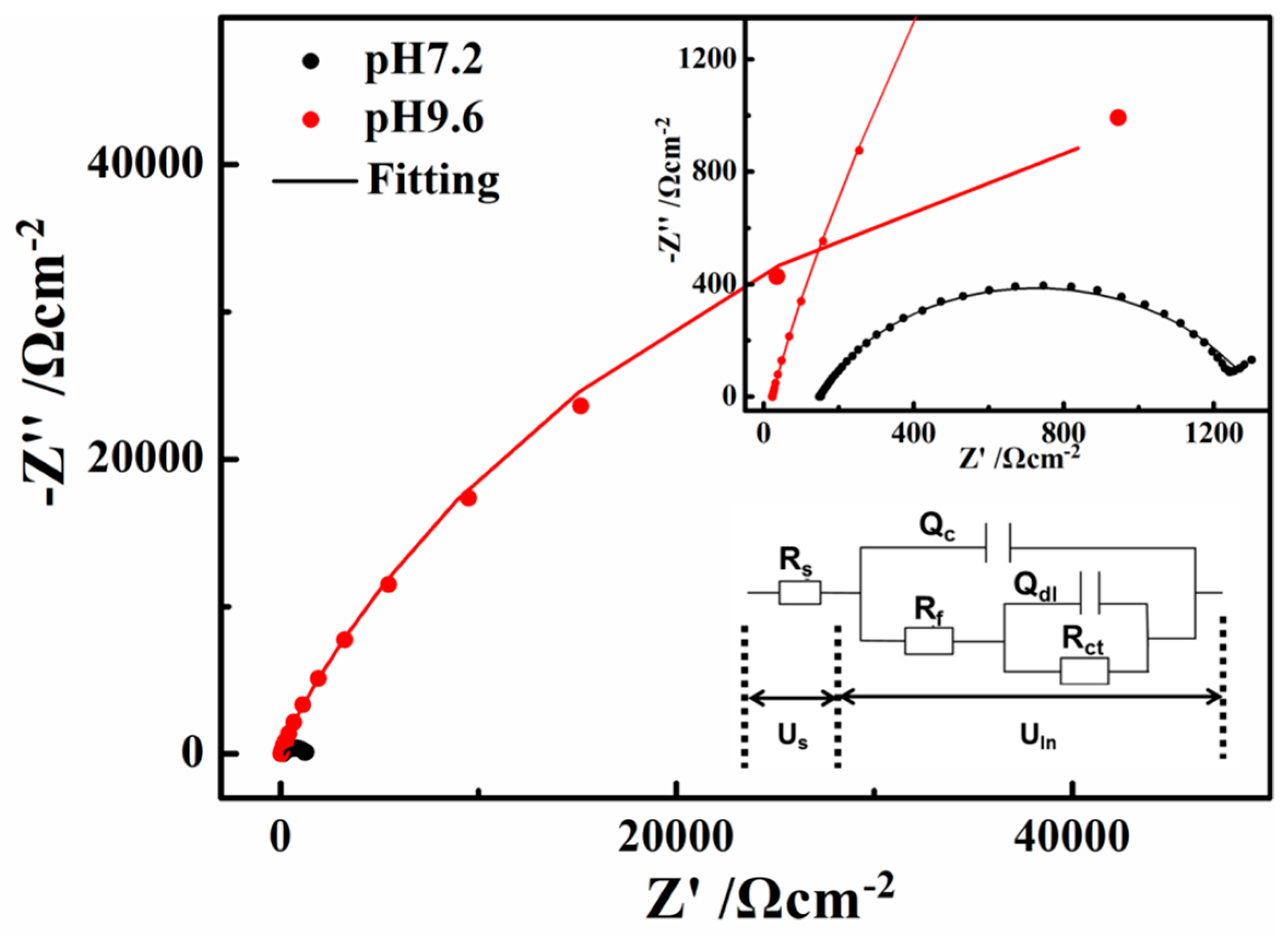
3.2.3. EIS and Potentiodynamic Polarization Studies
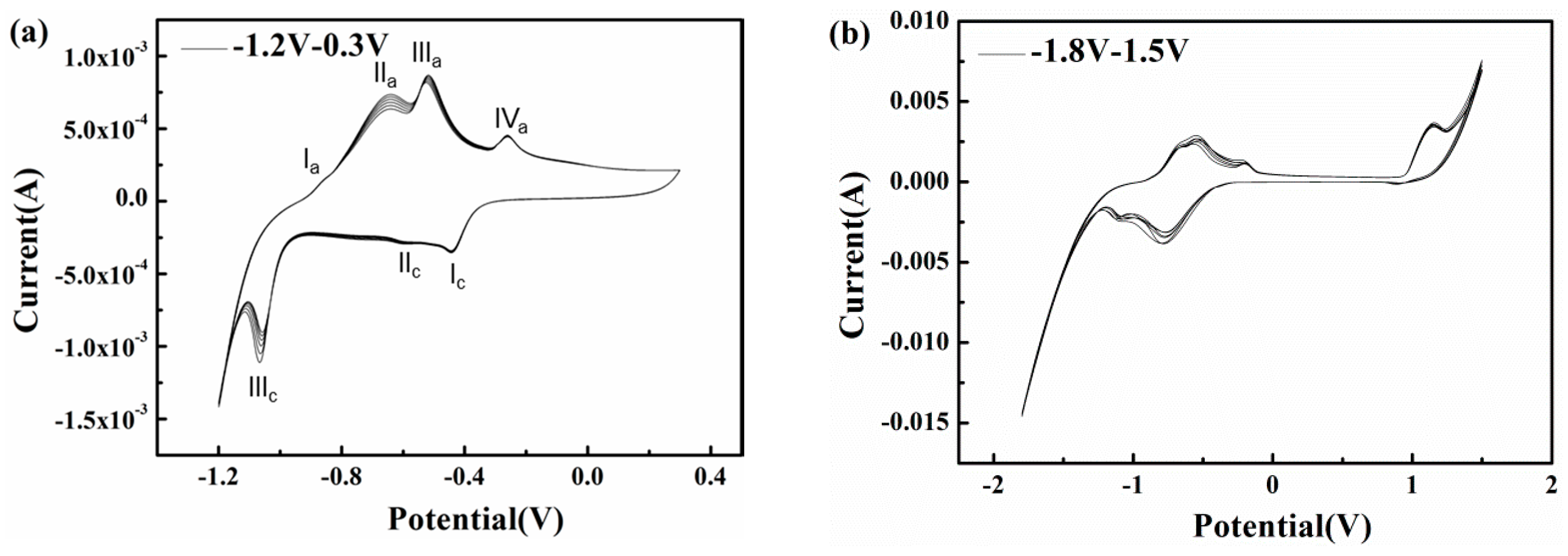
3.3. Short and Long-Range Cyclic Voltammetry Analysis
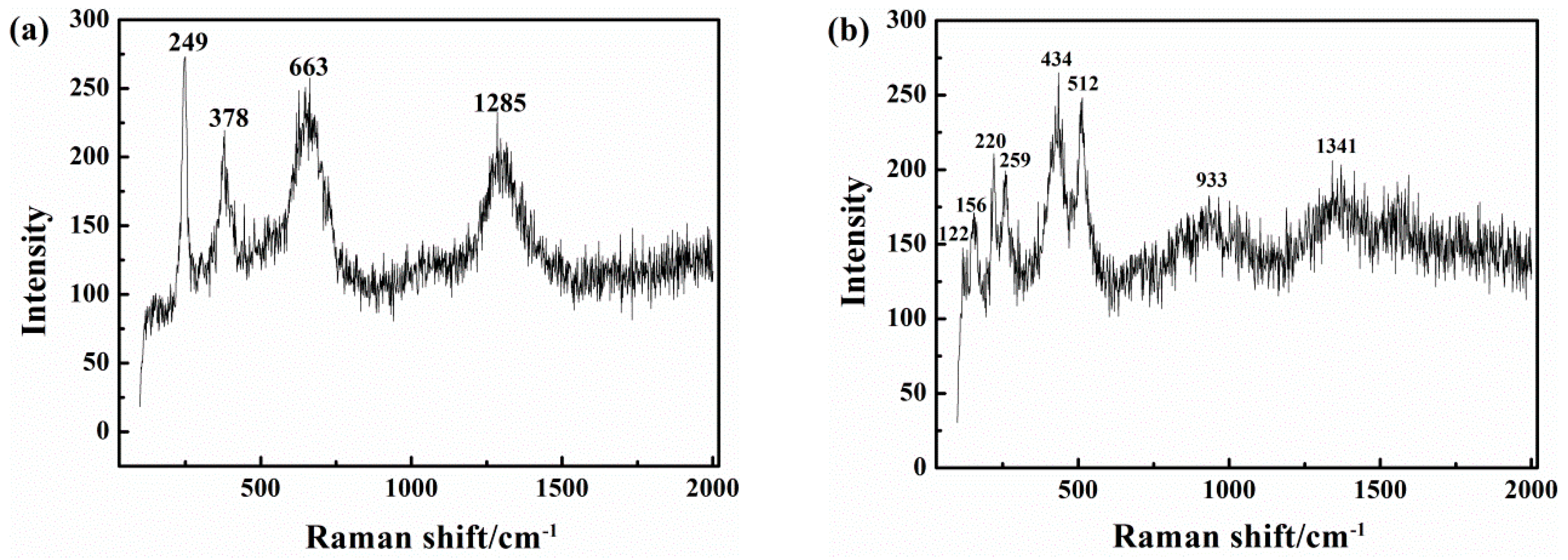
3.4. Analysis of Raman Spectroscopy
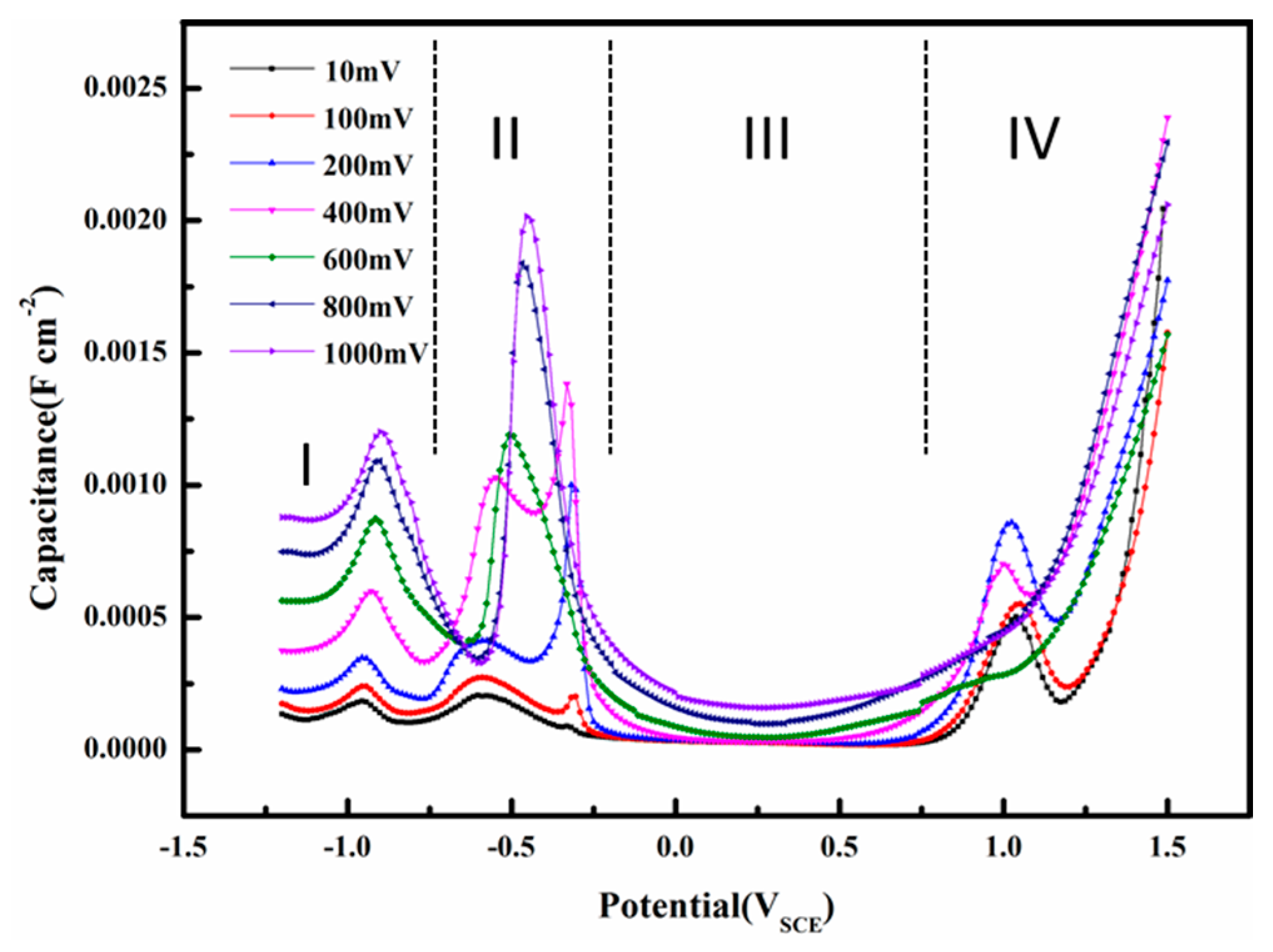
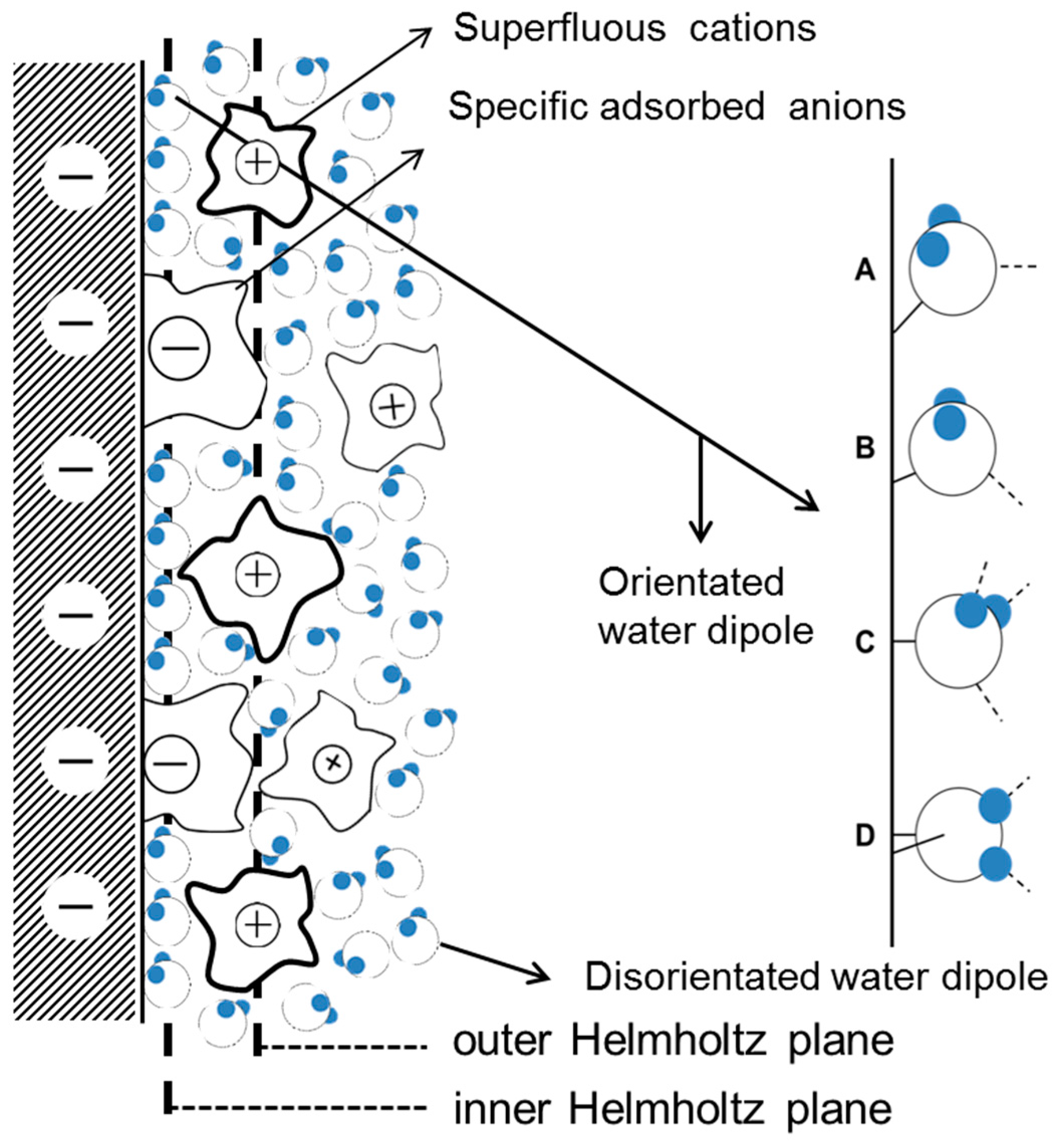
3.5. Interface Properties
4. Conclusions
- (1)
- In the non-passive system with pH 7.2, AC could change the CP potential from the original value and the shifting direction of the CP potential depends on the applied cathodic potential. The minimum CP criterion −840 mVSCE cannot fully protect the steel in the presence of AC, but the steel can remain intact in the presence of iAC 100 A/m2 (Vp+ 2.69 VSCE and Vp− −4.34 VSCE) when the CP potential decreases negatively enough, such as −1000 mVSCE and −1200 mVSCE.
- (2)
- In the alkaline environment with pH 9.6, AC corrosion is negligible without CP, but accelerates dramatically as the CP potential becomes more negative when iAC is 500 A/m2 (Vp+ 1.63 VSCE and Vp− −3.39 VSCE).
- (3)
- The dielectric constant of water molecules absorbed on the electrode surface in the inner Helmholtz layer increases with the increase of AC voltage amplitude, resulting in the increase of interfacial capacitance between the electrode and solution.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, D.K.; Muralidharan, S.; Ha, T.H.; Bae, J.H.; Ha, Y.C.; Lee, H.G.; Scantlebury, J.D. Electrochemical studies on the alternating current corrosion of mild steel under cathodic protection condition in marine environments. Electrochim.Acta 2006, 51, 5259–5267. [Google Scholar] [CrossRef]
- Zhu, M.; Du, C.; Li, X.; Liu, Z.; Wang, S.; Li, J.; Zhang, D. Effect of AC current density on stress corrosion cracking behavior of X80 pipeline steel in high pH carbonate/bicarbonate solution. Electrochim. Acta 2014, 117, 351–359. [Google Scholar] [CrossRef]
- Kioupis, N.; Kouloumbi, N.; Batis, G. Simulation of cathodically protected pipeline with capacitive AC mitigation devices for interpretation of misleading instant Eoff readings. Corros. Eng. Sci. Technol. 2013, 48, 166–172. [Google Scholar] [CrossRef]
- Tang, D.Z.; Du, Y.X.; Lu, M.X.; Jiang, Z.T.; Dong, L.; Wang, J.J. Effect of AC current on corrosion behavior of cathodically protected Q235 steel. Mater. Corros. 2015, 66, 278–285. [Google Scholar] [CrossRef]
- Xu, L.Y.; Su, X.; Cheng, Y.F. Effect of alternating current on cathodic protection on pipelines. Corros. Sci. 2013, 66, 263–268. [Google Scholar] [CrossRef]
- Fu, A.Q.; Cheng, Y.F. Effect of alternating current on corrosion and effectiveness of cathodic protection of pipelines. Can. Metall. Q. 2012, 51, 81–90. [Google Scholar] [CrossRef]
- Buchler, M.; Schoneich, H. Investigation of alternating current corrosion of cathodically protected pipelines: Development of a detection method, mitigation measures, and a model for the mechanism. Corrosion 2009, 65, 578–586. [Google Scholar] [CrossRef]
- Nielsen, L.; Baumgarten, B.; Cohn, P.; Rosenberg, H. A Field Study of Line Currents and Corrosion Rate Measurements in a Pipeline Critically Interfered with AC and DC Stray Currents; CEOCOR: Brussels, Belgium, 2006. [Google Scholar]
- Nielsen, L.V.; Nielsen, K.; Baumgarten, B.; Breuning-Madsen, H.; Cohn, P.; Rosenberg, H. AC Induced Corrosion in Pipelines: Detection, Characterization and Mitigation; CORROSION 2004; NACE International: New Orleans, LA, USA, 2004. [Google Scholar]
- Bolzoni, F.; Beretta, S.; Brenna, A.; Diamanti, M.V.; Lazzari, L.; Ormellese, M.; Pedeferri, M.P. Evaluation of Additional Protection Methods to Control Reinforcement Corrosion. Key Eng. Mater. 2016, 711, 37–44. [Google Scholar] [CrossRef]
- Zhang, R.; Vairavanathan, P.R.; Lalvani, S.B. Perturbation method analysis of AC-induced corrosion. Corros. Sci. 2008, 50, 1664–1671. [Google Scholar] [CrossRef]
- Ibrahim, I.; Meyer, M.; Takenouti, H.; Tribollet, B. AC Induced Corrosion of Underground Steel Pipelines. Faradaic Rectification under Cathodic Protection: II. Theoretical Approach with Electrolyte Resistance and Double Layer Capacitance for Bi-Tafelian Corrosion Mechanism. J. Braz. Chem. Soc. 2016, 27, 605–615. [Google Scholar] [CrossRef]
- Bosch, R.-W.; Bogaerts, W. Harmonic analysis of corroding systems considering diffusion phenomena. J. Electrochem. Soc. 1996, 143, 4033–4039. [Google Scholar] [CrossRef]
- Bueno, V.; Lazzari, L.; Ormellese, M.; Spinelli, P. Interaction between Alternating Current and Cathodic Protection over Nano Sized/Structured Surface Metals. Available online: http://www.nsti.org/publications/Nanotech/2008/pdf/569.pdf (accessed on 20 July 2017).
- Wang, L.; Wang, X.; Cui, Z.; Liu, Z.; Du, C.; Li, X. Effect of alternating voltage on corrosion of X80 and X100 steels in a chloride containing solution–Investigated by AC voltammetry technique. Corros. Sci. 2014, 86, 213–222. [Google Scholar] [CrossRef]
- Sher, A.A.; Bond, A.M.; Gavaghan, D.J.; Harriman, K.; Feldberg, S.W.; Duffy, N.W.; Guo, S.-X.; Zhang, J. Resistance, capacitance, and electrode kinetic effects in fourier-transformed large-amplitude sinusoidal voltammetry: Emergence of powerful and intuitively obvious tools for recognition of patterns of behavior. Anal. Chem. 2004, 76, 6214–6228. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.G.; Anastassiou, C.A.; O’Hare, D.; Parker, K.H.; Siggers, J.H. Theory of large-amplitude sinusoidal voltammetry for reversible redox reactions. Electrochim. Acta 2011, 56, 8492–8508. [Google Scholar] [CrossRef]
- Committee, G. Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. ASTM Int. 2011. [Google Scholar] [CrossRef]
- Panossian, Z.; Sérgio Filho, E.; de Almeida, N.L.; Pereira Filho, M.L.; de L Silva, D.; Laurino, E.W.; Oliver, L.; Hipólito, J.; de S Pimenta, G.; Albertini, C. Effect of Alternating Current by High Power Lines Voltage and Electric Transmission Systems in Pipelines Corrosion; CORROSION 2009; NACE International: Atlanta, GA, USA, 2009. [Google Scholar]
- Kuang, D.; Cheng, Y. Understand the AC induced pitting corrosion on pipelines in both high pH and neutral pH carbonate/bicarbonate solutions. Corros. Sci. 2014, 85, 304–310. [Google Scholar] [CrossRef]
- Bordbar, S.; Alizadeh, M.; Hashemi, S.H. Effects of microstructure alteration on corrosion behavior of welded joint in API X70 pipeline steel. Mater. Des. 2013, 45, 597–604. [Google Scholar] [CrossRef]
- Yuan, J.; Wu, X.; Wang, W.; Zhu, S.; Wang, F. Investigation on the enhanced oxidation of ferritic/martensitic steel P92 in pure steam. Materials 2014, 7, 2772–2783. [Google Scholar] [CrossRef]
- Raddaha, N.S.; Cordero-Arias, L.; Cabanas-Polo, S.; Virtanen, S.; Roether, J.A.; Boccaccini, A.R. Electrophoretic deposition of chitosan/h-BN and chitosan/h-BN/TiO2 composite coatings on stainless steel (316L) substrates. Materials 2014, 7, 1814–1829. [Google Scholar] [CrossRef]
- Standard Recommended Practice: Control of External Corrosion on Underground or Submerged Metallic Piping Systems; NACE International: Houston, TX, USA, 1996.
- Ghanbari, E.; Iannuzzi, M.; Lillard, R. The Mechanism of Alternating Current Corrosion of API Grade X65 Pipeline Steel. Corrosion 2016, 72, 1196–1210. [Google Scholar] [CrossRef]
- Ghanbari, E.; Lillard, S.; Iannuzzi, M.; Ortiz, M.R. Corrosion Behavior of Buried Pipeline in Presence of AC Stray Current in Controlled Environment; CORROSION 2015; NACE International: Dallas, TX, USA, 2015. [Google Scholar]
- Brenna, A.; Lazzari, L.; Pedeferri, M.; Ormellese, M. Monitoring cathodic protection of buried pipeline by means of a potential probe with an embedded zinc reference electrode. Mater. Des. 2017, 114, 194–201. [Google Scholar] [CrossRef]
- Xiao, H.; Lalvani, S.B. A linear model of alternating voltage-induced corrosion. J. Electrochem. Soc. 2008, 155, C69–C74. [Google Scholar] [CrossRef]
- Bosch, R.W.; Bogaerts, W.F. A theoretical study of AC-induced corrosion considering diffusion phenomena. Corros. Sci. 1998, 40, 323–336. [Google Scholar] [CrossRef]
- Goidanich, S.; Lazzari, L.; Ormellese, M. AC corrosion¨CPart 1: Effects on overpotentials of anodic and cathodic processes. Corros. Sci. 2010, 52, 491–497. [Google Scholar] [CrossRef]
- Lalvani, S.; Lin, X. A revised model for predicting corrosion of materials induced by alternating voltages. Corros. Sci. 1996, 38, 1709–1719. [Google Scholar] [CrossRef]
- Zhang, D.; Qian, H.; Wang, L.; Li, X. Comparison of barrier properties for a superhydrophobic epoxy coating under different simulated corrosion environments. Corros. Sci. 2016, 103, 230–241. [Google Scholar] [CrossRef]
- Mccollum, B.; Ahlborn, G.H. The influence of frequency of alternating or infrequently reversed current on electrolytic corrosion. J. Frankl. Inst. 1916, 182, 108–110. [Google Scholar] [CrossRef]
- Bertocci, U. AC Induced Corrosion. The Effect of an Alternating Voltage on Electrodes Under Charge-Transfer Control. Med. Sci. Sports Exerc. 2000, 32, 1281–1287. [Google Scholar] [CrossRef]
- Ibrahim, I.; Takenouti, H.; Tribollet, B.; Campaignolle, X.; Fontaine, S.; Schoeneich, H.G.; Ibrahim, I.; Takenouti, H.; Tribollet, B.; Campaignolle, X. Harmonic Analysis Study of the AC Corrosion of Buried Pipelines Under Cathodic Protection; NACE International: Nashville, TN, USA, 2007. [Google Scholar]
- Jiang, Z.; Du, Y.; Lu, M.; Zhang, Y.; Tang, D.; Dong, L. New findings on the factors accelerating AC corrosion of buried pipeline. Corros. Sci. 2014, 81, 1–10. [Google Scholar] [CrossRef]
- Luo, H.; Dong, C.F.; Li, X.G.; Xiao, K. The electrochemical behaviour of 2205 duplex stainless steel in alkaline solutions with different pH in the presence of chloride. Electrochim. Acta 2012, 64, 211–220. [Google Scholar] [CrossRef]
- Guitián, B.; Nóvoa, X.R.; Puga, B. Electrochemical Impedance Spectroscopy as a tool for materials selection: Water for haemodialysis. Electrochim. Acta 2011, 56, 7772–7779. [Google Scholar] [CrossRef]
- Geng, S.; Sun, J.; Guo, L. Effect of sandblasting and subsequent acid pickling and passivation on the microstructure and corrosion behavior of 316L stainless steel. Mater. Des. 2015, 88, 1–7. [Google Scholar] [CrossRef]
- Vignal, V.; Richoux, V.; Suzon, E.; Thiébaut, S.; Tabaleiv, K. The use of potentiostatic pulse testing to study the corrosion behavior of welded stainless steels in sodium chloride solution. Mater. Des. 2015, 88, 186–195. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, D.; Xue, P.; Wu, L.H.; Ni, D.R.; Ma, Z.Y. Microstructural evolution and pitting corrosion behavior of friction stir welded joint of high nitrogen stainless steel. Mater. Des. 2016, 110, 802–810. [Google Scholar] [CrossRef]
- Nielsen, L.V.; Galsgaard, F. A Method and a System of Diagnosing Corrosion Risk of a Pipe or a Pipeline in Soil. U.S. Patent No. 7,541,817, 2 June 2009. [Google Scholar]
- Liang, P.; Li, X.; Du, C.; Chen, X. Stress corrosion cracking of X80 pipeline steel in simulated alkaline soil solution. Mater. Des. 2009, 30, 1712–1717. [Google Scholar] [CrossRef]
- Alcántara, J.; de la Fuente, D.; Chico, B.; Simancas, J.; Díaz, I.; Morcillo, M. Marine Atmospheric Corrosion of Carbon Steel: A Review. Materials 2017, 10, 406. [Google Scholar] [CrossRef]
- Chin, D.T.; Venkatesh, S. A study of alternating voltage modulation on the polarization of mild steel. J. Electrochem. Soc. 1979, 126, 1908–1913. [Google Scholar] [CrossRef]
- Eliyan, F.F.; Alfantazi, A. Effect of bicarbonate concentration on corrosion of high strength steel. Corros. Eng. Sci. Technol. 2014, 50, 178–185. [Google Scholar] [CrossRef]
- Eliyan, F.F.; Alfantazi, A. On the theory of CO2 corrosion reactions—Investigating their interrelation with the corrosion products and API-X100 steel microstructure. Corros. Sci. 2014, 85, 380–393. [Google Scholar] [CrossRef]
- Eliyan, F.F.; Alfantazi, A. Corrosion cyclic voltammetry of two types of heat-affected zones (HAZs) of API-X100 steel in bicarbonate solutions. Metall. Mater. Trans. B 2014, 45, 2464–2474. [Google Scholar] [CrossRef]
- Gadala, I.M.; Wahab, M.A.; Alfantazi, A. Numerical simulations of soil physicochemistry and aeration influences on the external corrosion and cathodic protection design of buried pipeline steels. Mater. Des. 2016, 97, 287–299. [Google Scholar] [CrossRef]
- Jiang, J.-Y.; Wang, D.; Chu, H.-Y.; Ma, H.; Liu, Y.; Gao, Y.; Shi, J.; Sun, W. The Passive Film Growth Mechanism of New Corrosion-Resistant Steel Rebar in Simulated Concrete Pore Solution: Nanometer Structure and Electrochemical Study. Materials 2017, 10, 412. [Google Scholar] [CrossRef]
- Panossian, Z.; Mariaca, L.; Morcillo, M.; Flores, S.; Rocha, J.; Peña, J.J.; Herrera, F.; Corvo, F.; Sanchez, M.; Rincon, O.T.; et al. Steel cathodic protection afforded by zinc, aluminium and zinc/aluminium alloy coatings in the atmosphere. Surf. Coat. Technol. 2005, 190, 244–248. [Google Scholar] [CrossRef]
- Ha, H.-Y.; Lee, C.-H.; Lee, T.-H.; Kim, S. Effects of Nitrogen and Tensile Direction on Stress Corrosion Cracking Susceptibility of Ni-Free FeCrMnC-Based Duplex Stainless Steels. Materials 2017, 10, 294. [Google Scholar] [CrossRef]
- Niu, L.; Cheng, Y. Corrosion behavior of X-70 pipe steel in near-neutral pH solution. Appl. Surf. Sci. 2007, 253, 8626–8631. [Google Scholar] [CrossRef]
- Ejaz, A.; Lu, Z.; Shoji, T.; Chen, J.; Zhou, B. The Effects of Hydrogen on Reactivation of Iron in Alkaline Solutions. ECS Trans. 2014, 59, 421–428. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Sasaki, Y.; Hyono, A. AC Potential Modulation Reflectance of Iron Electrode Covered by Thin Passive Oxide. Electrochim. Acta 2014, 131, 116–122. [Google Scholar] [CrossRef]
- Larsen, K.R.; Chmilar, J.; Hevle, D.; Lauber, M.; Moriber, N. Updating NACE SP0169 for Controlling External Corrosion on Underground or Submerged Metallic Piping Systems. Mater. Perform. 2014, 53, 24–29. [Google Scholar]
- Refait, P.; Bourdoiseau, J.A.; Jeannin, M.; Nguyen, D.D.; Romaine, A.; Sabot, R. Electrochemical formation of carbonated corrosion products on carbon steel in deaerated solutions. Electrochim. Acta 2012, 79, 210–217. [Google Scholar] [CrossRef]
- Meng, G.; Zhang, C.; Cheng, Y. Effects of corrosion product deposit on the subsequent cathodic and anodic reactions of X-70 steel in near-neutral pH solution. Corros. Sci. 2008, 50, 3116–3122. [Google Scholar] [CrossRef]
- Albu, C.; Van Damme, S.; Abodi, L.; Demeter, A.; Deconinck, J.; Topa, V. Influence of the applied potential and pH on the steady-state behavior of the iron oxide. Electrochim. Acta 2012, 67, 119–126. [Google Scholar] [CrossRef]
- El-Naggar, M. Cyclic voltammetric studies of carbon steel in deaerated NaHCO3 solution. J. Appl. Electrochem. 2004, 34, 911–918. [Google Scholar] [CrossRef]
- Tanupabrungsun, T.; Young, D.; Brown, B.; Nešić, S. Construction and Verification of Pourbaix Diagrams for CO2 Corrosion of Mild Steel Valid up to 250 °C; CORROSION 2012; NACE International: Salt Lake City, UT, USA, 2012. [Google Scholar]
- Xu, L.; Su, X.; Yin, Z.; Tang, Y.; Cheng, Y. Development of a real-time AC/DC data acquisition technique for studies of AC corrosion of pipelines. Corros. Sci. 2012, 61, 215–223. [Google Scholar] [CrossRef]
- Büchler, M. Alternating current corrosion of cathodically protected pipelines: Discussion of the involved processes and their consequences on the critical interference values. Mater. Corros. 2012, 63, 1181–1187. [Google Scholar] [CrossRef]
- Das, N.K.; Shoji, T. An atomic study of hydrogen effect on the early stage oxidation of transition metal surfaces. Int. J. Hydrogen Energy 2013, 38, 1644–1656. [Google Scholar] [CrossRef]
- Lvovich, V.F. Impedance Spectroscopy: Applications to Electrochemical and Dielectric Phenomena; John Wiley & Sons: Houston, TX, USA, 2012. [Google Scholar]
- Bard, A.J.; Stratmann, M.; Calvo, E. Encyclopedia of Electrochemistry, Volume 2, Interfacial Kinetics and Mass Transport; Wiley-VCH Weinheim: Berlin, Germany, 2003. [Google Scholar]
- Fawcett, W.; Levine, S.; McDonald, A. A molecular model for the dielectric properties of the inner layer at the mercury/aqueous solution interface. J. Electroanal. Chem. Interfacial Electrochem. 1980, 111, 163–180. [Google Scholar] [CrossRef]
- Ataka, K.; Yotsuyanagi, T.; Osawa, M. Potential-Dependent Reorientation of Water Molecules at an Electrode/Electrolyte Interface Studied by Surface-Enhanced Infrared Absorption Spectroscopy. J. Phys. Chem. 1996, 100, 10664–10672. [Google Scholar] [CrossRef]
- Cao, C.N.; Zhang, J.Q. An Introduction of Electrochemical Impedance Spectroscopy. Science 2002, 21, 86–106. [Google Scholar]
- Tian, Y.; Xue, R.; Zhou, X.; Liu, Z.; Huang, L. Double layer capacitor based on active carbon and its improved capacitive properties using redox additive electrolyte of anthraquinonedisulphonate. Electrochim. Acta 2015, 152, 135–139. [Google Scholar] [CrossRef]
- Zhou, J.-L.; Li, X.-G.; Du, C.-W.; Pan, Y.; Li, T.; Liu, Q. Passivation process of X80 pipeline steel in bicarbonate solutions. Int. J. Miner. Metall. Mater. 2011, 18, 178–185. [Google Scholar] [CrossRef]




| Material | Fe | C | Mn | Si | S | Cr | Ni | Cu | Al | Ti | Mo | V | Nb | N |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X80 | 96.933 | 0.036 | 1.771 | 0.197 | 0.002 | 0.223 | 0.278 | 0.22 | 0.021 | 0.019 | 0.184 | 0.001 | 0.11 | 0.005 |
| Test Condition | Raman Shift (Wave number/cm−1) | Species |
|---|---|---|
| pH 9.6 AC = 500 A/m2 CP = 0 | 249, 378, 1285 | γFeOOH |
| 663 | Fe3O4 | |
| pH 9.6 AC = 500 A/m2 CP = −1000 mVSCE | 122, 155, 220 | γFe2O3 |
| 259 | γFeOOH | |
| 434 | Fe(OH)2 | |
| 512 | γFe2O3 | |
| 1341 | Fe(OH)3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Du, C.; Liu, Z.; Wang, L.; Ding, D. Effect of Alternating Current on the Cathodic Protection and Interface Structure of X80 Steel. Materials 2017, 10, 851. https://doi.org/10.3390/ma10080851
Wang H, Du C, Liu Z, Wang L, Ding D. Effect of Alternating Current on the Cathodic Protection and Interface Structure of X80 Steel. Materials. 2017; 10(8):851. https://doi.org/10.3390/ma10080851
Chicago/Turabian StyleWang, Huiru, Cuiwei Du, Zhiyong Liu, Luntao Wang, and De Ding. 2017. "Effect of Alternating Current on the Cathodic Protection and Interface Structure of X80 Steel" Materials 10, no. 8: 851. https://doi.org/10.3390/ma10080851