Mechanical Response and Shear-Induced Initiation Properties of PTFE/Al/MoO3 Reactive Composites
Abstract
:1. Introduction
2. Experimental
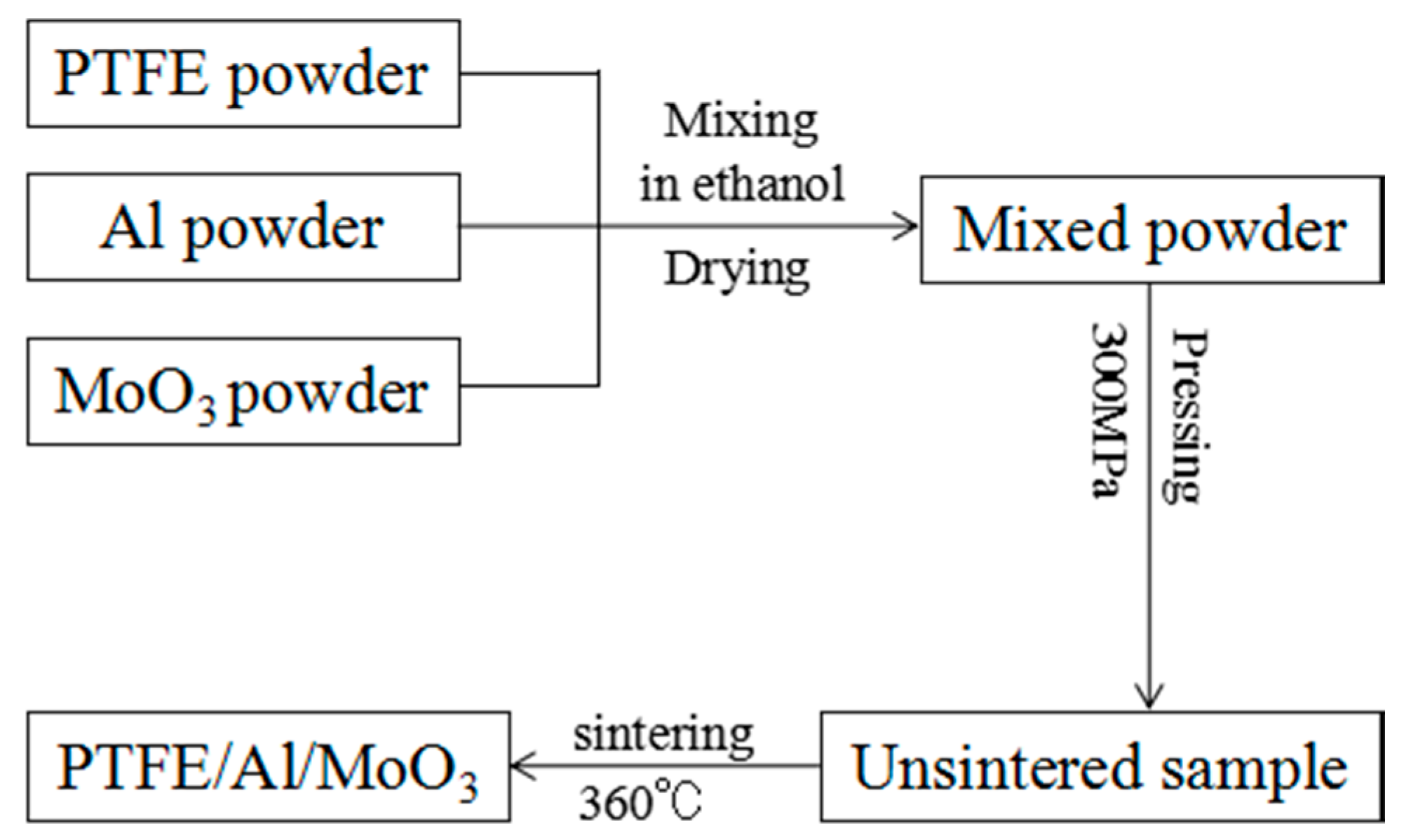
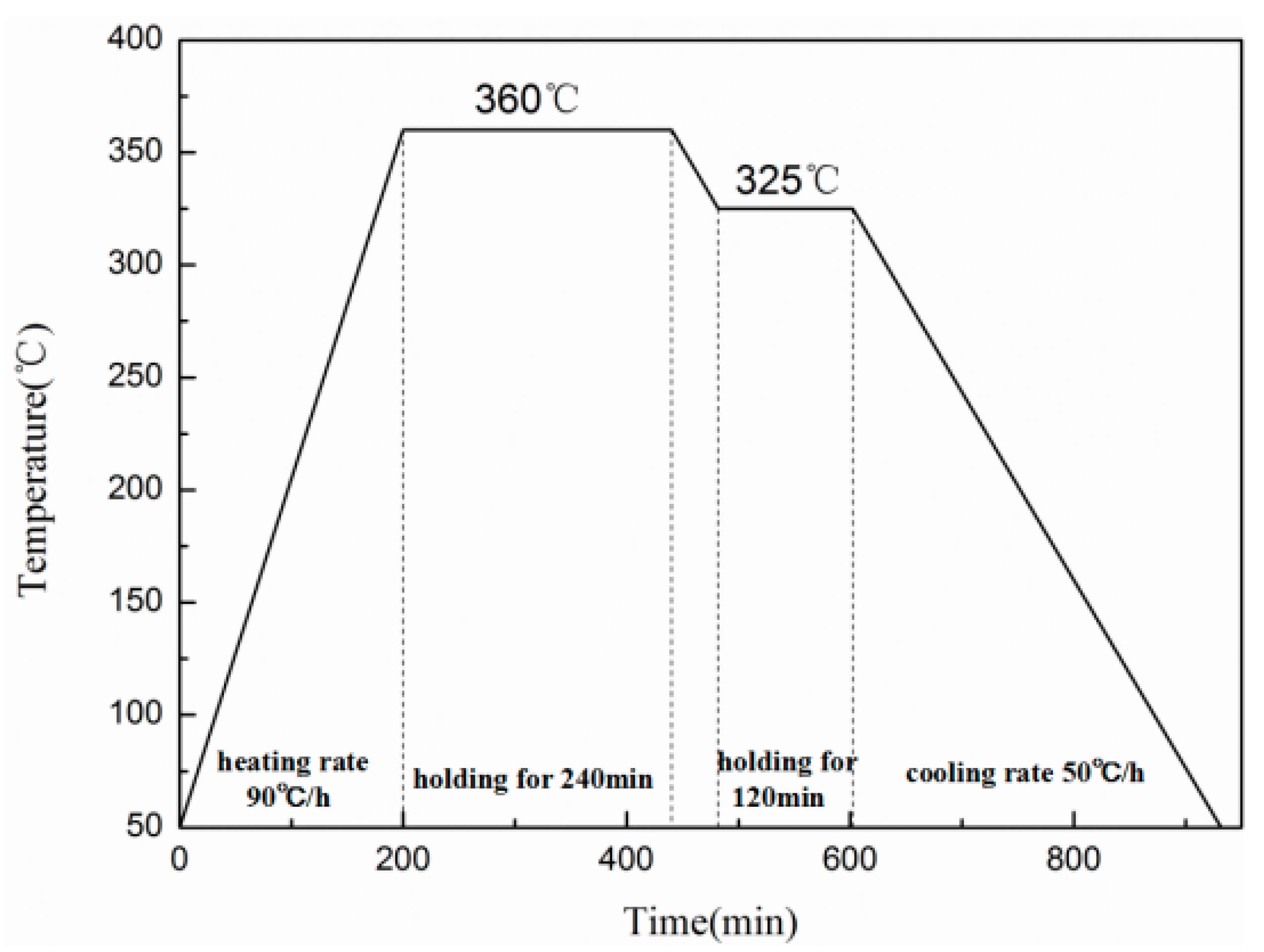
2.1. Specimens Preparation
2.2. Quasi-Static Compression Experiments
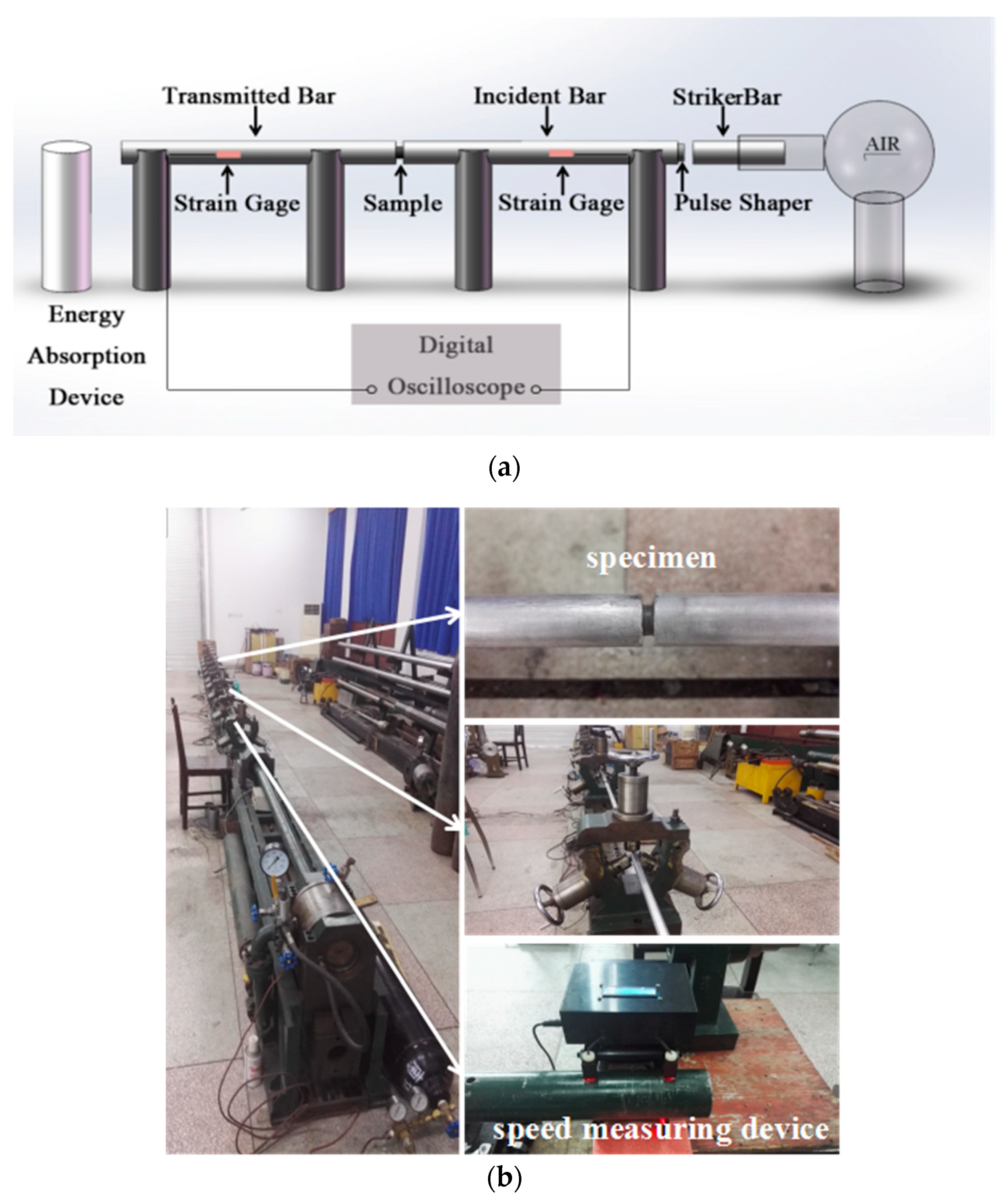
2.3. SHPB Experiments
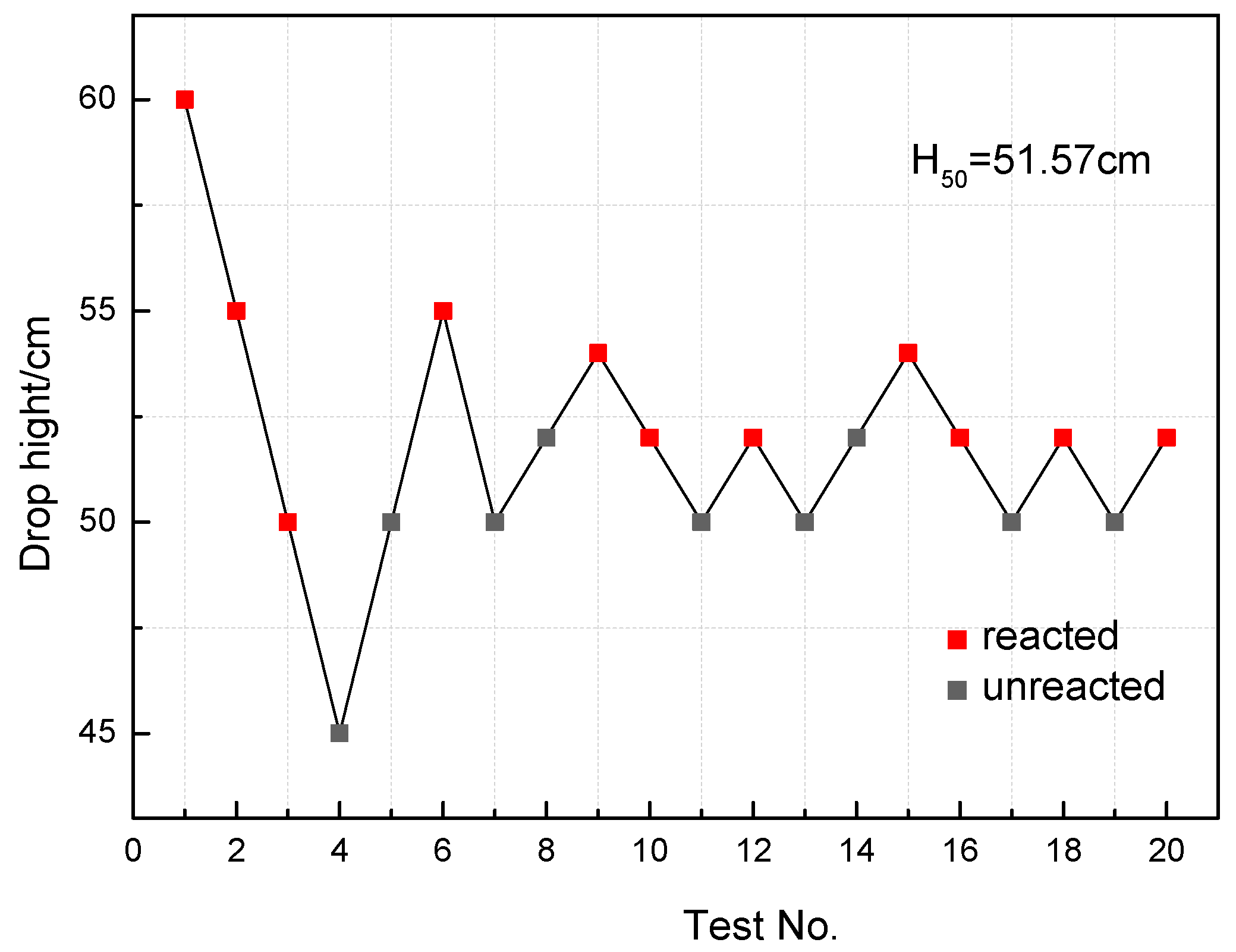
2.4. Drop-Weight Experiments
3. Results and Discussion
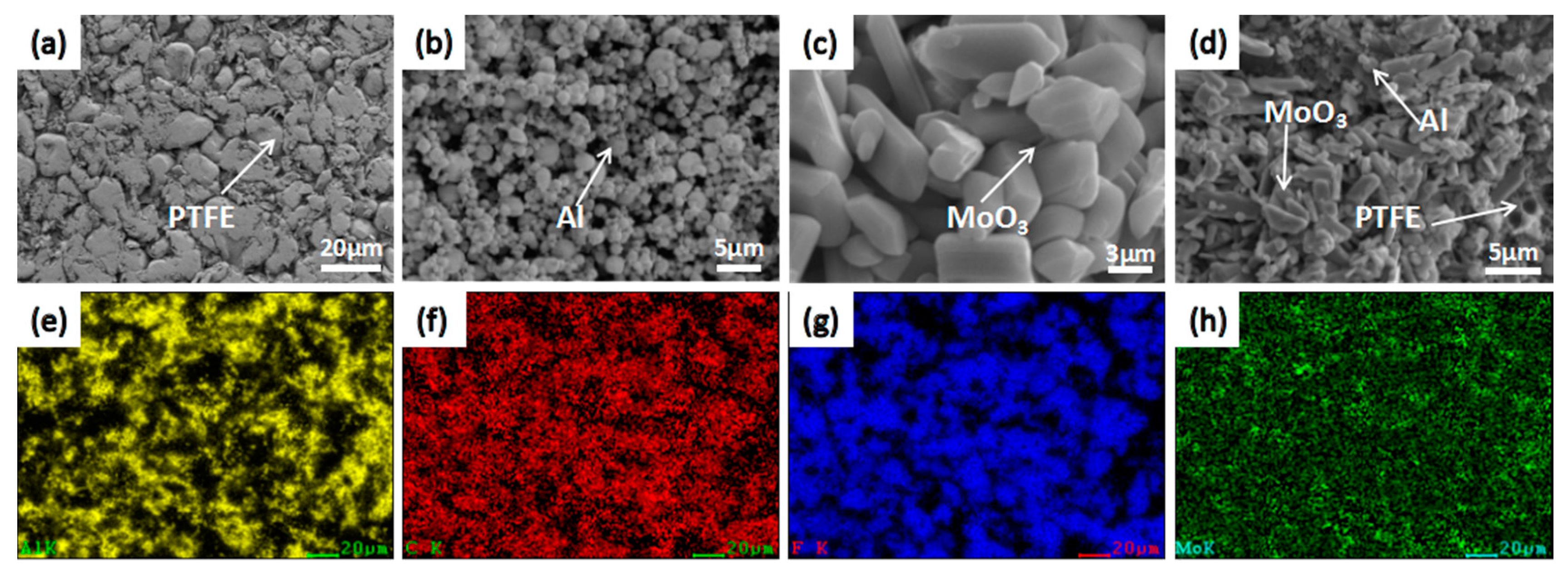
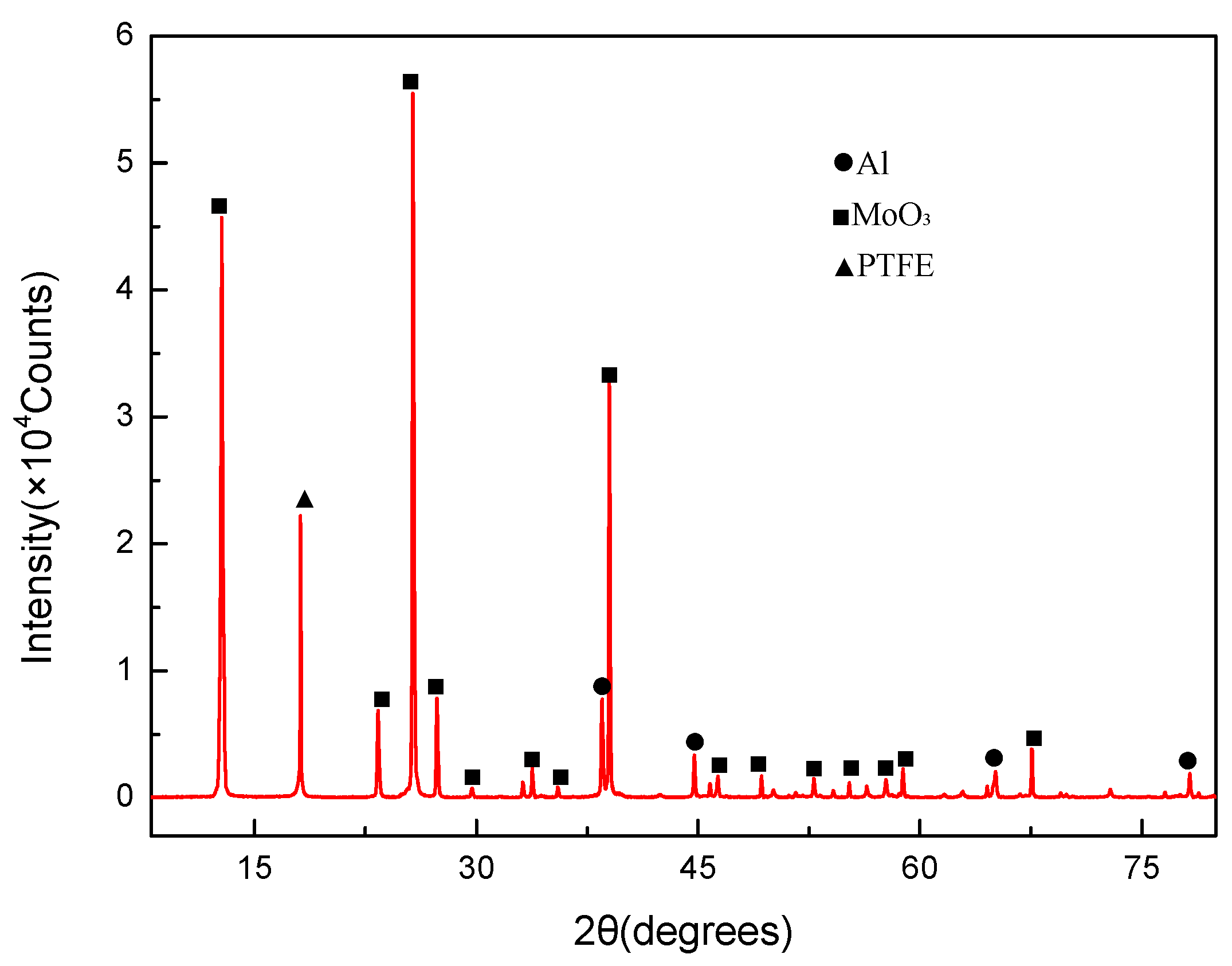
3.1. Mesoscale Characteristics
3.2. Mechanical Responses under Quasi-Static Compression
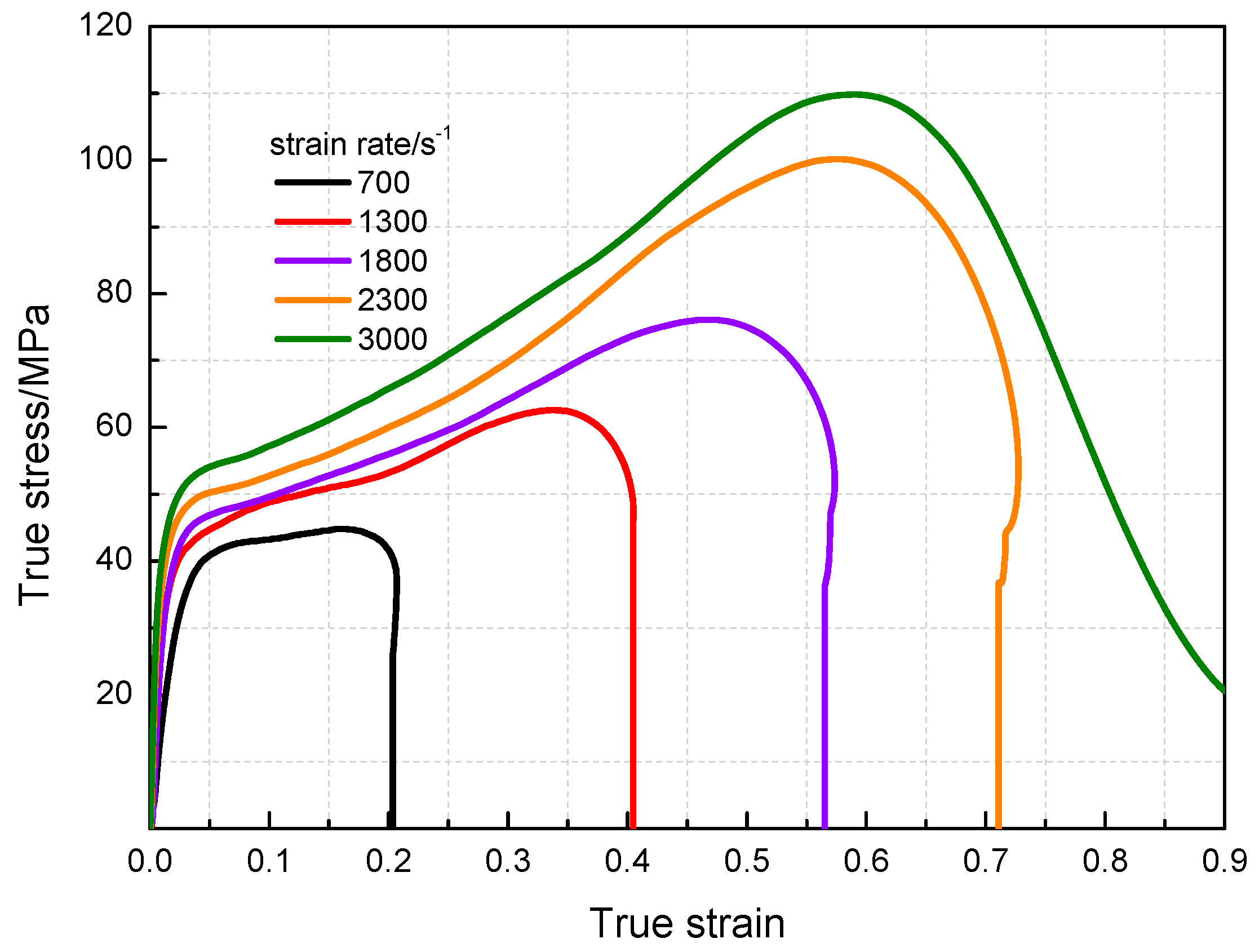
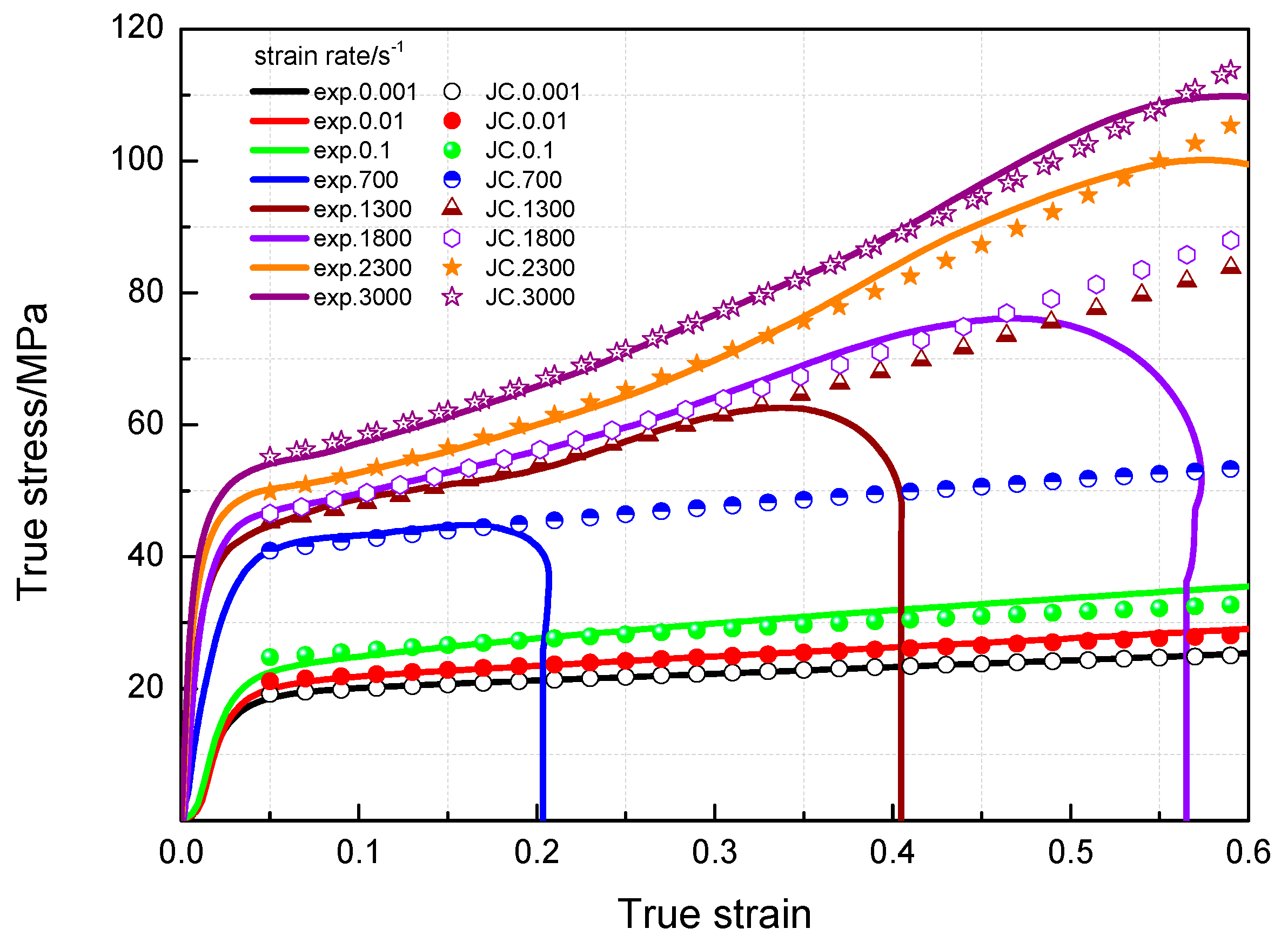
3.3. Dynamic Compression Performance
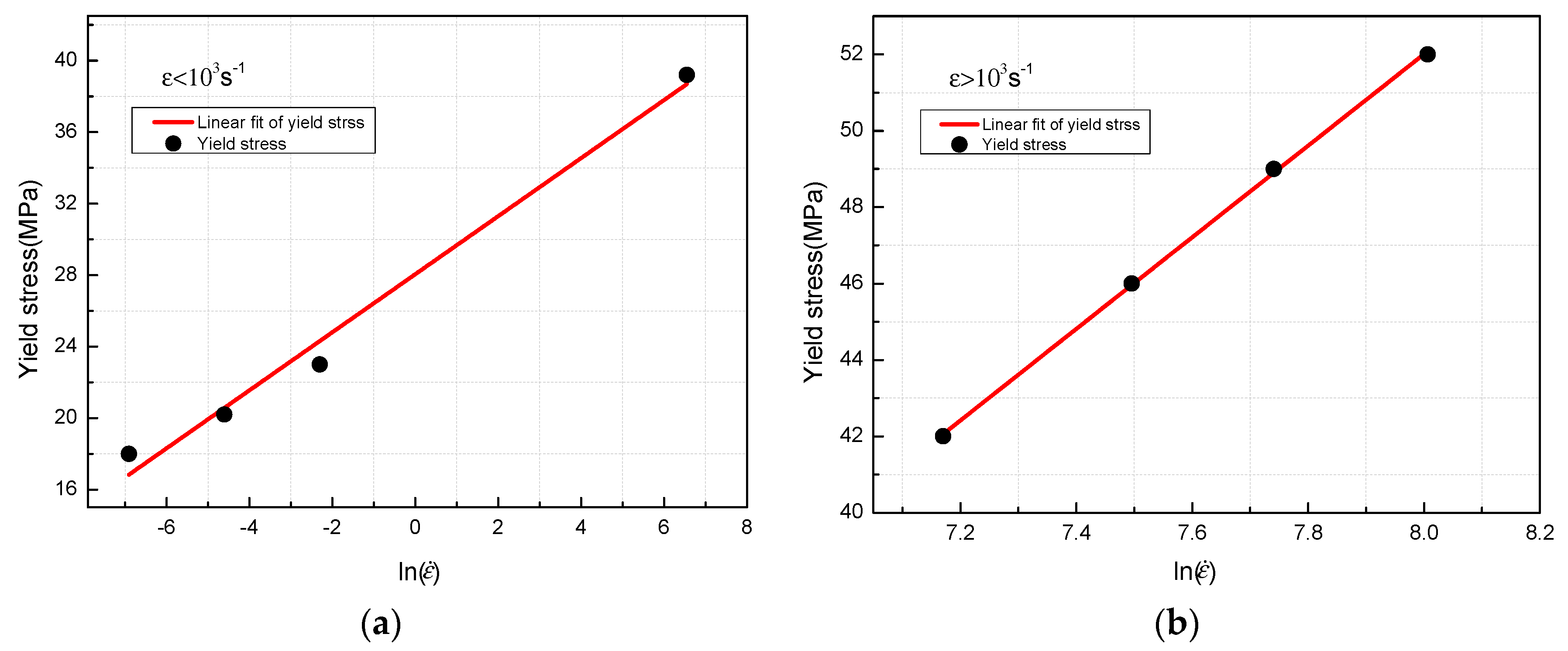
3.4. Johnson-Cook Constitutive Model
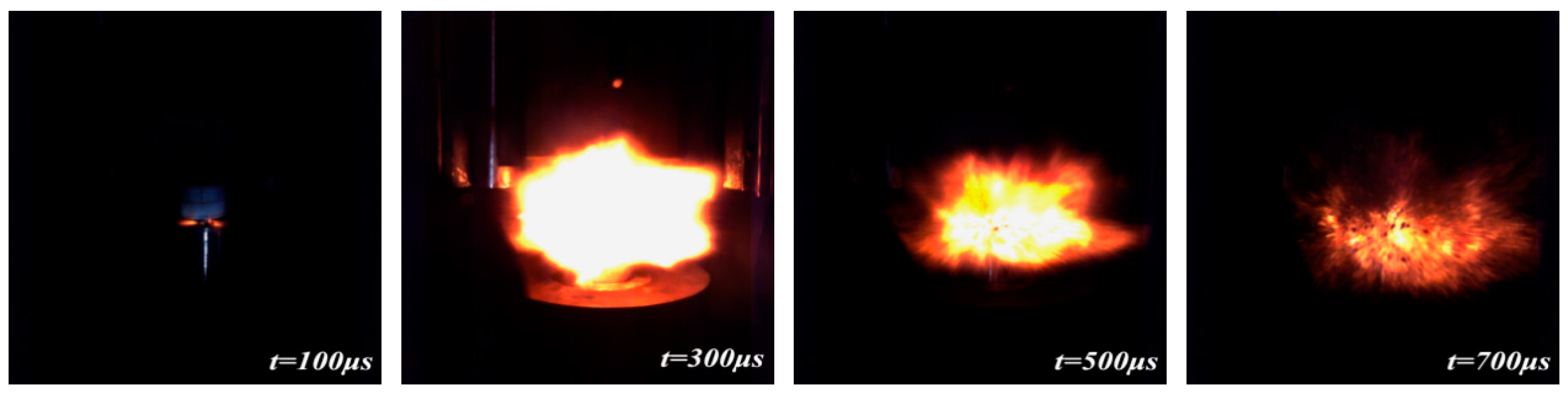
3.5. Drop-Weight Tests
4. Conclusions
- (1)
- Mesoscale images of the specimens after sintering obtained by SEM demonstrate that PTFE, Al, and MoO3 particles were evenly mixed. And no chemical reaction occurred after the material was stirred, pressed and sintered.
- (2)
- The yield stress and compressive strength of PTFE/Al/MoO3 specimens are sensitive to strain rate within the range of 10−3~3 × 103 s−1. The elastic modulus is insensitive to the strain rate at low strain rate, but shows significant strain rate dependence at high strain rate.
- (3)
- The yield stress shows a bilinear dependence on the logarithm values of strain rate, and the slope has an increase at the strain rate of 103 s−1. The established JC constitutive model can describe the mechanical response of PTFE/Al/MoO3 material well and can provide certain references for the practical applications of this material.
- (4)
- The recovered specimens show that the reaction started from the outer edge of the specimen with the most concentrated shear deformation, indicating a shear-induced initiation mechanism. The characteristic drop height of impact sensitivity (H50) of the PTFE/Al/MoO3 specimens was calculated as 51.57 cm.
- (5)
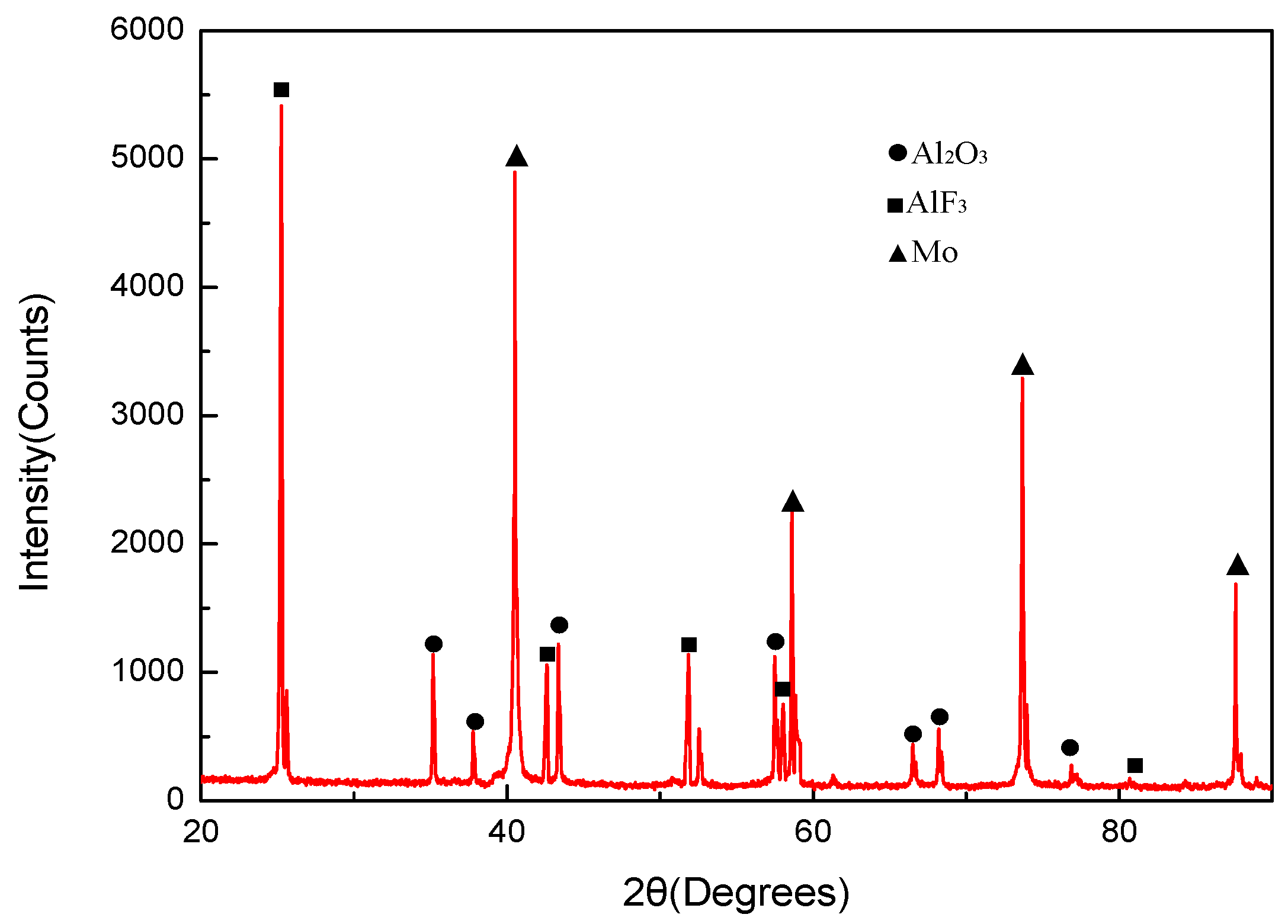
- The reaction products of PTFE/Al/MoO3 specimens were AlF3, Al2O3, Mo and C, indicating that redox reaction occurred between PTFE and Al, and between Al and MoO3.
Author Contributions
Funding
Conflicts of Interest
References
- National Research Council. Advanced Energetic Materials; National Academies Press: Pittsburgh, PA, USA, 2004; pp. 20–23. [Google Scholar]
- Thadhani, N.N. Shock-induced and shock-assisted solid-state chemical reactions in powder mixtures. J. Appl. Phys. 1994, 76, 2129–2138. [Google Scholar] [CrossRef]
- Cai, J.; Walley, S.; Hunt, R.; Proud, W.; Nesterenko, V.; Meyers, M. High-strain, high-strain-rate flow and failure in PTFE/Al/W granular composites. Mater. Sci. Eng. A 2008, 472, 308–315. [Google Scholar] [CrossRef]
- Raftenberg, M.N.; Mock, W., Jr.; Kirby, G.C. Modeling the impact deformation of rods of a pressed PTFE/Al composite mixture. Int. J. Impact Eng. 2008, 35, 1735–1744. [Google Scholar] [CrossRef]
- Ge, C.; Dong, Y.X.; Maimaitituersun, W. Microscale simulation on mechanical properties of Al/PTFE composite based on real microstructures. Materials 2016, 9, 590. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Nesterenko, V.F.; Vecchio, K.S.; Jiang, F.; Herbold, E.B.; Benson, D.J.; Addiss, J.W.; Walley, S.M.; Proud, W.G. The influence of metallic particle size on the mechanical properties of polytetrafluoroethylene-Al-W powder composites. Appl. Phys. Lett. 2008, 92, 031903. [Google Scholar] [CrossRef]
- Herbold, E.; Nesterenko, V.; Benson, D.; Cai, J.; Vecchio, K.; Jiang, F.; Addiss, J.; Walley, S.; Proud, W. Particle size effect on strength, failure, and shock behavior in Polytetrafluoroethylene-Al-W granular composite materials. J. Appl. Phys. 2008, 104, 103903. [Google Scholar] [CrossRef]
- Herbold, E.B.; Cai, J.; Benson, D.J.; Nesterenko, V.F. Simulation of particle size effect on dynamic properties and fracture of PTFE-W-Al composites. In Proceedings of the 15th Biennial International Conference of the APS Topical Group on Advances in Materials Science and Engineering 9 Shock Compression of Condensed Matter (SCCM ’07), Waikoloa, HI, USA, 24–29 June 2007; Volume 955, pp. 785–788. [Google Scholar]
- Zhang, X.; Zhang, J.; Qiao, L.; Shi, A.; Zhang, Y.; He, Y.; Guan, Z. Experimental study of the compression properties of Al/W/PTFE granular composites under elevated strain rates. Mater. Sci. Eng. A 2013, 581, 48–55. [Google Scholar] [CrossRef]
- Xu, F.Y.; Liu, S.B.; Zheng, Y.F.; Yu, Q.B.; Wang, H.F. Quasi-static compression properties and failure of PTFE/Al/W reactive materials. Adv. Eng. Mater. 2016, 19, 1600350. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J.; Li, S.; Zhang, X. Investigation on reaction energy, mechanical behavior and impact insensitivity of W/PTFE/Al composites with different w percentage. Mater. Des. 2016, 92, 397–404. [Google Scholar] [CrossRef]
- Addiss, J.; Cai, J.; Walley, S.; Proud, E.; Nesterenko, V. High strain and strain-rate behaviour of PTFE/aluminuim/tungsten mixtures. In Proceedings of the 15th Biennial International Conference of the APS Topical Group on Shock Compression of Condensed Matter (SCCM ’07), Waikoloa, HI, USA, 24–29 June 2007; pp. 773–776. [Google Scholar]
- Cai, J.; Jiang, F.; Vecchio, K.S.; Meyers, M.A.; Nesterenko, V.F. Mechanical and microstructural properties of PTFE/Al/W system. In Proceedings of the 15th Biennial International Conference of the APS Topical Group on Shock Compression of Condensed Matter (SCCM ’07), Waikoloa, HI, USA, 24–29 June 2007; pp. 723–726. [Google Scholar]
- Fischer, S.H.; Grubelich, M.C. Theoretical energy release of thermites, intermetallics, and combustible metals. In Australian-American Joint Conference on the Technologies of Mines and Mine Countermeasures; Sandia National Laboratories (SNL): Albuquerque, NM, USA; Livermore, CA, USA, 1999. [Google Scholar]
- Watson, K.W.; Pantoya, M.L.; Levitas, V.I. Fast reactions with nano- and micrometer aluminum: A study on oxidation versus fluorination. Combust. Flame 2008, 155, 619–634. [Google Scholar] [CrossRef]
- Nielson, D.B.; Truitt, R.M.; Ashcroft, B.N. Reactive Material Enhanced Projectiles and Related Methods. U.S. Patent US 2008/0035007 A1, 14 February 2008. [Google Scholar]
- Wang, P. PTFE sintering technology. Eng. Plast. Appl. 2001, 2, 19–21. [Google Scholar]
- Feng, B.; Li, Y.C.; Wu, S.Z. A crack-induced initiation mechanism of Al-PTFE under quasi-static compression and the investigation of influencing factors. Mater. Des. 2016, 108, 411–417. [Google Scholar]
- Xu, S.; Yang, S.; Zhang, W. The mechanical behaviors of polytetrafluorethylene/Al/W energetic composites. J. Phys. Condens. Mater. 2009, 21, 285401. [Google Scholar] [CrossRef] [PubMed]
- Hopkinson, B. A method of measuring the pressure produced in the detonation of high explosives or by the impact of bullets. Philos. Trans. R. Soc. Lond. 1914, 213, 437–456. [Google Scholar] [CrossRef]
- Kolsky, H. An investigation of the mechanical properties of materials at very high rates of loading. Proc. Phys. Soc. B 1949, 62, 676–681. [Google Scholar] [CrossRef]
- Blackstone, W.R.; Baber, B.B.; Ku, P.M. New test techniques for evaluating the compatibility of materials with liquid oxygen under impact. Tribol. Trans. 1968, 11, 216–227. [Google Scholar] [CrossRef]
- Johnson, G.R.; Cook, W.H. A constitutive model and data for metals subjected to large strains, high strain rates, and high temperatures. In Proceedings of the 7th International Symposium on Ballistics, The Hague, The Netherlands, 19–21 April 1983; pp. 541–547. [Google Scholar]
- Holmquist, T.J.; Johnson, G.R. Determination of constants and comparison of results for various constitutive models. J. Phys. IV 1991, 1, 299–306. [Google Scholar] [CrossRef]
- Feng, B.; Fang, X.; Li, Y.C.; Wang, H.X. An initiation phenomenon of Al-PTFE under quasi-static compression. Chem. Phys. Lett. 2015, 637, 38–41. [Google Scholar] [CrossRef]
- Feng, B.; Fang, X.; Wang, H.X.; Dong, W.; Li, Y.C. The effect of crystallinity on compressive properties of Al-PTFE. Polymers 2016, 8, 356. [Google Scholar] [CrossRef]
- Ames, R.G. Vented chamber calorimetry for impact-initiated energetic materials. In Proceedings of the 43rd AIAA Aerospace Sciences Meeting and Exhibit, Reno, NV, USA, 10–13 January 2005. [Google Scholar]
- Lee, R.J.; Mock, W., Jr.; Carney, J.R.; Holt, W.H.; Pangilinan, G.I.; Gamache, R.M.; Boteler, J.M.; Bohl, D.G.; Drotar, J.; Lawrence, G.W. Reactive materials studies. AIP Conf. Proc. 2006, 845, 169–174. [Google Scholar]
- Feng, B.; Fang, X.; Li, Y.C. Influence of processing techniques on mechanical properties and impact initiation of an Al-PTFE reactive material. Cent. Eur. J. Energ. Mater. 2016, 13, 989–1004. [Google Scholar] [CrossRef]
- Granier, J.J.; Pantoya, M.L. Ignition and combustion behaviors of nanocomposite Al/MoO3. MRS Online Proc. Libr. Arch. 2003, 800. [Google Scholar] [CrossRef]
- Koch, E.C. Metal-Fluorocarbon Based Energetic Materials; Wiley-VCH Verlag Gmbh & Co. KGa A: Weinheim, Germany, 2012; pp. 6–17. [Google Scholar]
- Sanders, V.E.; Asay, B.W.; Foley, T.J. Reaction propagation of four nanoscale energetic composites (Al/MoO3, Al/WO3, Al/CuO, and Bi2O3). J. Propul. Power 2012, 23, 707–714. [Google Scholar] [CrossRef]

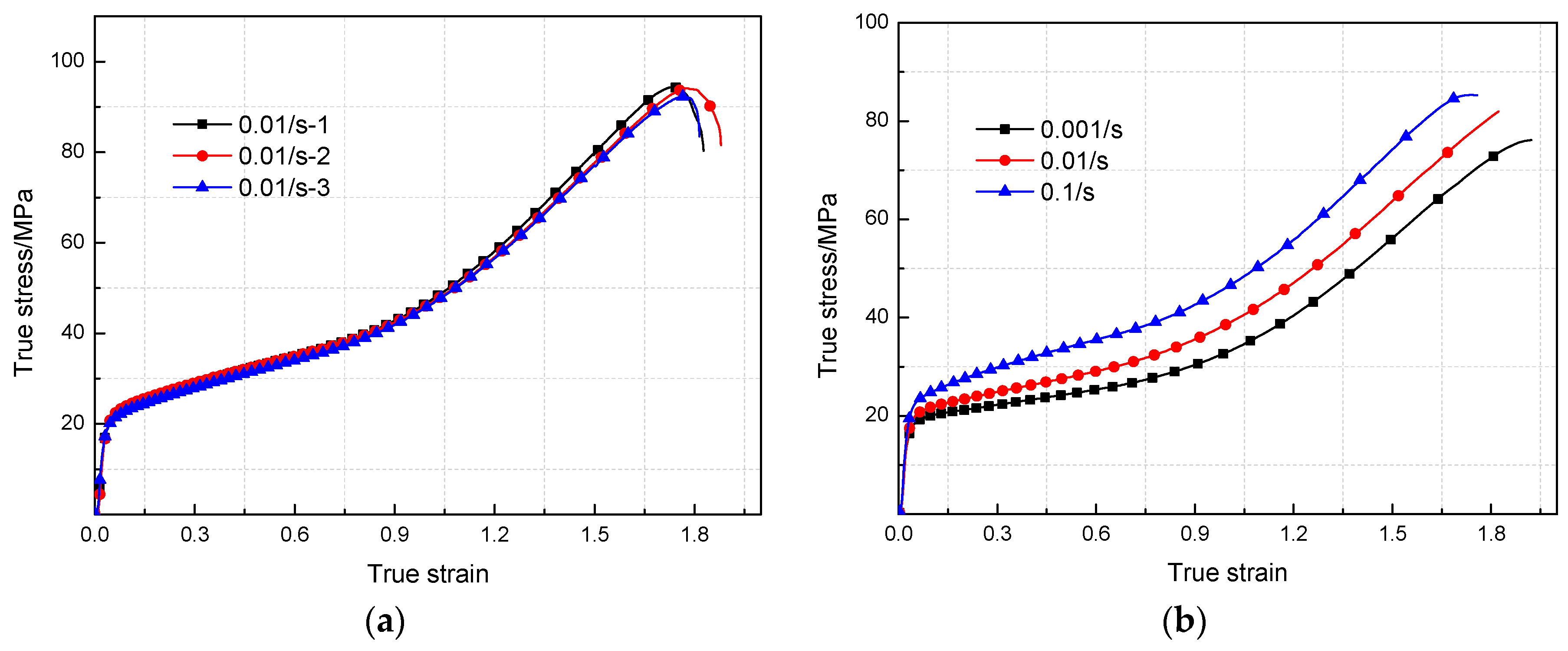


| Strain Rate/s−1 | Elastic Modulus/MPa | Yield Stress/MPa | Compression Strength/MPa | Failure Strain |
|---|---|---|---|---|
| 0.001 | 851 | 18 | 76 | 1.91 |
| 0.01 | 852 | 21 | 82 | 1.82 |
| 0.1 | 854 | 24 | 85 | 1.75 |
| Strain Rate/s−1 | Elastic Modulus/MPa | Yield Stress/MPa | Compression Strength/MPa | Critical Strain |
|---|---|---|---|---|
| 700 | 1226 | 39.2 | 44.9 | 0.17 |
| 1300 | 1456 | 43.2 | 62.8 | 0.35 |
| 1800 | 1895 | 46 | 76.3 | 0.49 |
| 2300 | 2568 | 48 | 100.4 | 0.59 |
| 3000 | 3569 | 52 | 110.2 | 0.60 |
| Fitting Values | Standard Error | R2 | Fitting Values | Standard Error | R2 | |
|---|---|---|---|---|---|---|
| A | 18 | 0 | 1 | 43.2 | 0 | 1 |
| B | 10.2354 | 0.00492 | 0.99633 | 77.0012 | 0.0062 | 0.96842 |
| n | 0.7107 | 0.00387 | 0.99633 | 1.21353 | 0.00326 | 0.96842 |
| C | 0.084 | 0.12302 | 0.98039 | 0.2255 | 0.71825 | 0.98387 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Fang, X.; Wu, S.; Yang, L.; Yu, Z.; Li, Y. Mechanical Response and Shear-Induced Initiation Properties of PTFE/Al/MoO3 Reactive Composites. Materials 2018, 11, 1200. https://doi.org/10.3390/ma11071200
Huang J, Fang X, Wu S, Yang L, Yu Z, Li Y. Mechanical Response and Shear-Induced Initiation Properties of PTFE/Al/MoO3 Reactive Composites. Materials. 2018; 11(7):1200. https://doi.org/10.3390/ma11071200
Chicago/Turabian StyleHuang, Junyi, Xiang Fang, Shuangzhang Wu, Li Yang, Zhongshen Yu, and Yuchun Li. 2018. "Mechanical Response and Shear-Induced Initiation Properties of PTFE/Al/MoO3 Reactive Composites" Materials 11, no. 7: 1200. https://doi.org/10.3390/ma11071200
APA StyleHuang, J., Fang, X., Wu, S., Yang, L., Yu, Z., & Li, Y. (2018). Mechanical Response and Shear-Induced Initiation Properties of PTFE/Al/MoO3 Reactive Composites. Materials, 11(7), 1200. https://doi.org/10.3390/ma11071200





