TiO2 Modification with Transition Metallic Species (Cr, Co, Ni, and Cu) for Photocatalytic Abatement of Acetic Acid in Liquid Phase and Propene in Gas Phase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of M/P25 Samples
2.2. Characterization
2.3. Photocatalytic Activity Measurements
2.3.1. Photocatalytic Decomposition of Acetic Acid
2.3.2. Photocatalytic Oxidation of Propene
3. Results
3.1. Textural and Morphological Properties
3.2. X-ray Diffraction
3.3. UV-Vis Diffuse Reflectance Spectroscopy
3.4. XPS
3.5. Photocatalytic Activity
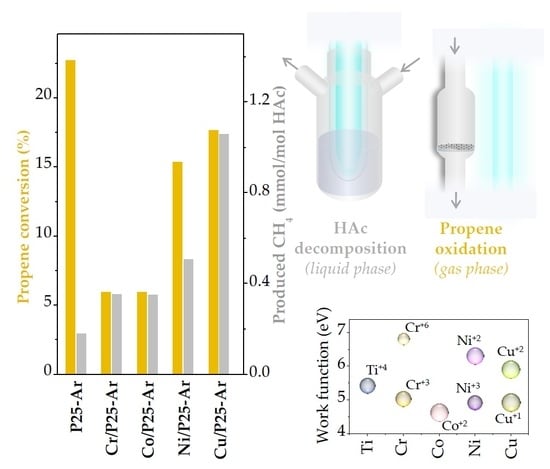
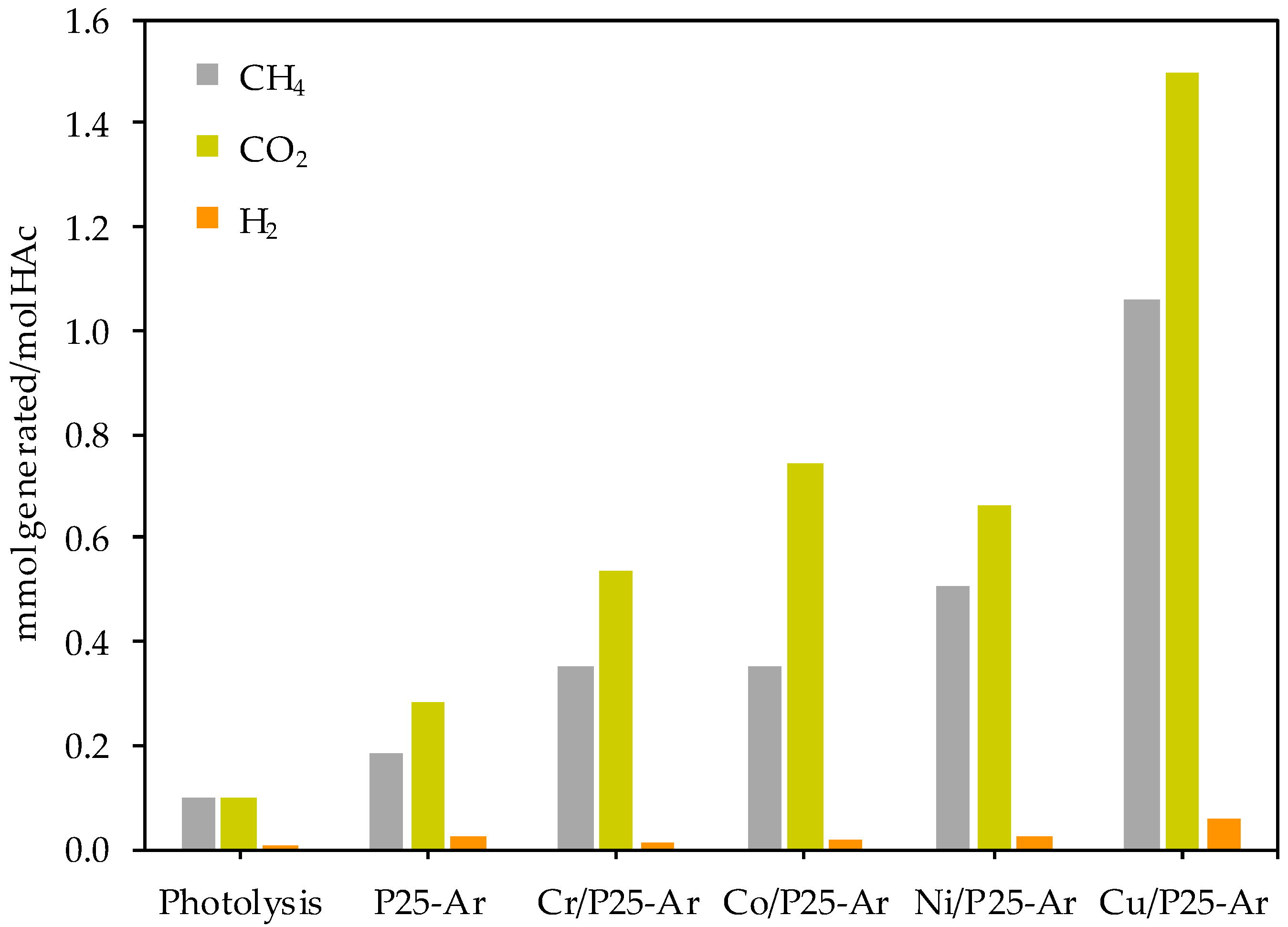
3.5.1. Photocatalytic Decomposition of Acetic Acid
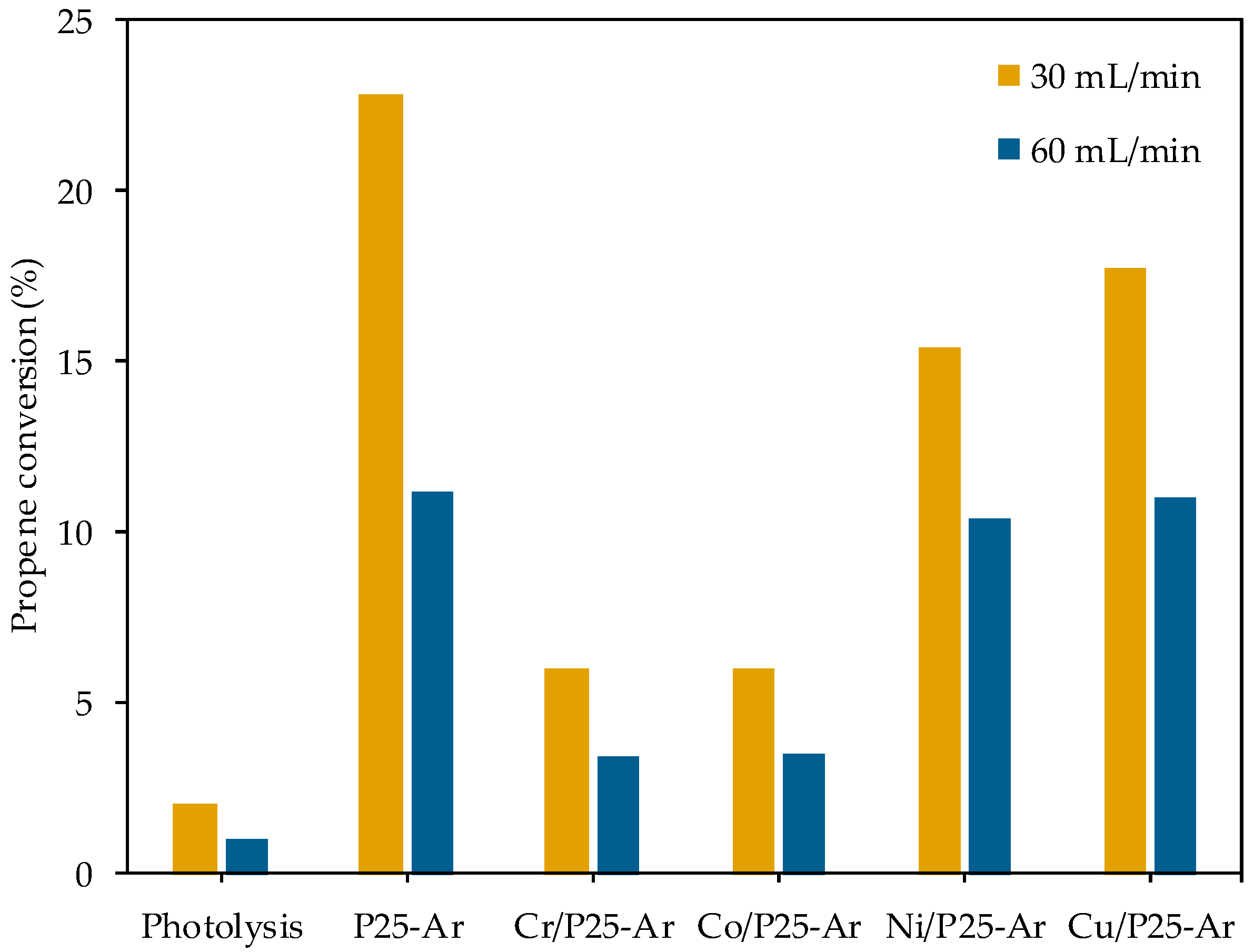
3.5.2. Propene Oxidation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Litter, M.I. Heterogeneous photocatalysis: Transition metal ions in photocatalytic systems. Appl. Catal. B Environ. 1999, 23, 89–114. [Google Scholar] [CrossRef]
- MiarAlipour, S.; Friedmann, D.; Scott, J.; Amal, R. TiO2/porous adsorbents: Recent advances and novel applications. J. Hazard. Mater. 2018, 341, 404–423. [Google Scholar] [CrossRef] [PubMed]
- Betts, L.M.; Dappozze, F.; Guillard, C. Understanding the photocatalytic degradation by P25 TiO2 of acetic acid and propionic acid in the pursuit of alkane production. Appl. Catal. A Gen. 2018, 554, 35–43. [Google Scholar] [CrossRef]
- Hassan, M.; Zhao, Y.; Xie, B. Employing TiO2 photocatalysis to deal with landfill leachate: Current status and development. Chem. Eng. J. 2016, 285, 264–275. [Google Scholar] [CrossRef]
- Ding, C.; Sun, Y.; Lin, Y.; Sun, W.; Liu, H.; Zhu, X.; Dai, Y.; Luo, C. Magnetically separable functionalized TiO2 nanotubes: Synthesis, characterization, and photocatalysis. J. Photochem. Photobiol. A Chem. 2018, 356, 123–131. [Google Scholar] [CrossRef]
- Heciak, A.; Morawski, A.W.; Grzmil, B.; Mozia, S. Cu-modified TiO2 photocatalysts for decomposition of acetic acid with simultaneous formation of C1–C3 hydrocarbons and hydrogen. Appl. Catal. B Environ. 2013, 140, 108–114. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.Á.; Bouazza, N.; Berenguer-Murcia, Á.; Linares-Salinas, J.J.; Soto, P.; Linares-Solano, Á. Photocatalytic oxidation of propene at low concentration. Appl. Catal. B Environ. 2007, 71, 298–309. [Google Scholar] [CrossRef]
- Bacsik, Z.; McGregor, J.; Mink, J. FTIR analysis of gaseous compounds in the mainstream smoke of regular and light cigarettes. Food Chem. Toxicol. 2007, 45, 266–271. [Google Scholar] [CrossRef]
- Centi, G.; Quadrelli, E.A.; Perathoner, S. Catalysis for CO2 conversion: A key technology for rapid introduction of renewable energy in the value chain of chemical industries. Energy Environ. Sci. 2013, 6, 1711–1731. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Coronado, J.M.; Fresno, F.; Hernández-Alonso, M.D.; Portela, R. The Keys of Succes: TiO2 as a Benchmark Photocatalyst. In Design of Advanced Photocatalytic Materials for Energy and Environmental Applications; Coronado, J.M., Hernández-Alonso, M.D., Fresno, F., Portela, R., Eds.; Springer: London, UK, 2013; pp. 85–102. ISBN 978-1-4471-5061-9. [Google Scholar]
- Katsumata, H.; Sada, M.; Nakaoka, Y.; Kaneco, S.; Suzuki, T.; Ohta, K. Photocatalytic degradation of diuron in aqueous solution by platinized TiO2. J. Hazard. Mater. 2009, 171, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Kerkez-Kuyumcu, Ö.; Kibar, E.; Dayıoğlu, K.; Gedik, F.; Akın, A.N.; Özkara-Aydınoğlu, Ş. A comparative study for removal of different dyes over M/TiO2 (M = Cu, Ni, Co, Fe, Mn and Cr) photocatalysts under visible light irradiation. J. Photochem. Photobiol. A Chem. 2015, 311, 176–185. [Google Scholar] [CrossRef]
- Rauf, M.A.; Meetani, M.A.; Hisaindee, S. An overview on the photocatalytic degradation of azo dyes in the presence of TiO2 doped with selective transition metals. Desalination 2011, 276, 13–27. [Google Scholar] [CrossRef]
- Inturi, S.N.R.; Boningari, T.; Suidan, M.; Smirniotis, P.G. Visible-light-induced photodegradation of gas phase acetonitrile using aerosol-made transition metal (V, Cr, Fe, Co, Mn, Mo, Ni, Cu, Y, Ce, and Zr) doped TiO2. Appl. Catal. B Environ. 2014, 144, 333–342. [Google Scholar] [CrossRef]
- Sahu, M.; Biswas, P. Single-step processing of copper-doped titania nanomaterials in a flame aerosol reactor. Nanoscale Res. Lett. 2011, 6, 441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashby, M.; Messler, R.; Asthana, R.; Furlani, E.; Smallman, R.E.; Ngan, A.H.W.; Crawford, R.J.; Mills, N. Engineering Materials and Processes Desk Reference, 1st ed.; Butterworth-Heinemann: Oxford, UK, 2009; ISBN 9780080878393. [Google Scholar]
- Tayade, R.J.; Kulkarni, R.G.; Jasra, R.V. Transition Metal Ion Impregnated Mesoporous TiO2 for Photocatalytic Degradation of Organic Contaminants in Water. Ind. Eng. Chem. Res. 2006, 45, 5231–5238. [Google Scholar] [CrossRef]
- Devi, L.G.; Kottam, N.; Murthy, B.N.; Kumar, S.G. Enhanced photocatalytic activity of transition metal ions Mn2+, Ni2+ and Zn2+ doped polycrystalline titania for the degradation of Aniline Blue under UV/solar light. J. Mol. Catal. A Chem. 2010, 328, 44–52. [Google Scholar] [CrossRef]
- Halas, S. 100 years of work function. Mater. Sci. 2016, 24, 951–968. [Google Scholar]
- Ola, O.; Maroto-Valer, M.M. Review of material design and reactor engineering on TiO2 photocatalysis for CO2 reduction. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 16–42. [Google Scholar] [CrossRef]
- Wang, L.; Egerton, T. The Effect of Transition Metal on the Optical Properties and Photoactivity of Nano-particulate Titanium Dioxide. J. Mater. Sci. Res. 2012, 1, 19–27. [Google Scholar] [CrossRef]
- Choi, W.; Termin, A.; Hoffmann, M.R. The role of metal ion dopants in quantum-sized TiO2: Correlation between photoreactivity and charge carrier recombination dynamics. J. Phys. Chem. 1994, 98, 13669–13679. [Google Scholar] [CrossRef]
- Al-Azri, Z.H.N.; Chen, W.-T.; Chan, A.; Jovic, V.; Ina, T.; Idriss, H.; Waterhouse, G.I.N. The roles of metal co-catalysts and reaction media in photocatalytic hydrogen production: Performance evaluation of M/TiO2 photocatalysts (M = Pd, Pt, Au) in different alcohol–water mixtures. J. Catal. 2015, 329, 355–367. [Google Scholar] [CrossRef]
- Lin, H.; Shih, C. Efficient one-pot microwave-assisted hydrothermal synthesis of M (M = Cr, Ni, Cu, Nb) and nitrogen co-doped TiO2 for hydrogen production by photocatalytic water splitting. J. Mol. Catal. A Chem. 2016, 411, 128–137. [Google Scholar] [CrossRef]
- Di Paola, A.; Marcì, G.; Palmisano, L.; Schiavello, M.; Uosaki, K.; Ikeda, S.; Ohtani, B. Preparation of polycrystalline TiO2 photocatalysts impregnated with various transition metal ions: Characterization and photocatalytic activity for the degradation of 4-nitrophenol. J. Phys. Chem. B 2002, 106, 637–645. [Google Scholar] [CrossRef]
- Devi, L.G.; Kavitha, R. A review on non metal ion doped titania for the photocatalytic degradation of organic pollutants under UV/solar light: Role of photogenerated charge carrier dynamics in enhancing the activity. Appl. Catal. B Environ. 2013, 140–141, 559–587. [Google Scholar] [CrossRef]
- Amorós-Pérez, A.; Cano-Casanova, L.; Lillo-Ródenas, M.Á.; Román-Martínez, M.C. Cu/TiO2 photocatalysts for the conversion of acetic acid into biogas and hydrogen. Catal. Today 2016. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Rodriguez-Reinoso, F.; Linares-Solano, A. Microporous Structure of Activated Carbons as Revealed by Adsorption Methods; Thrower, P.A., Ed.; Dekker, Marcel: New York, NY, USA, 1989; Volume 21, ISBN 0-8247-7939-8. [Google Scholar]
- Joyner, L.G.; Barrett, E.P.; Skold, R. The Determination of Pore Volume and Area Distributions in Porous Substances. II. Comparison between Nitrogen Isotherm and Mercury Porosimeter Methods. J. Am. Ceram. Soc. 1951, 73, 3155–3158. [Google Scholar] [CrossRef]
- Jenkins, R.; Snyder, R.L. Introduction to X-ray Powder Diffractometry; John Wiley and Sons: New York, NY, USA, 1996; ISBN 978-0-471-51339-1. [Google Scholar]
- Jensen, H.; Joensen, K.D.; Jørgensen, J.E.; Pedersen, J.S.; Søgaard, E.G. Characterization of nanosized partly crystalline photocatalysts. J. Nanopart. Res. 2004, 6, 519–526. [Google Scholar] [CrossRef]
- Olsen, E.D. Modern Optical Methods of Analysis; McGraw-Hill: New York, NY, USA, 1975. [Google Scholar]
- Valencia, S.; Marin, J.M.; Restrepo, G. Study of the bandgap of synthesized titanium dioxide nanoparticules using the sol-gel method and a hydrothermal treatment. Open Mater. Sci. J. 2010, 4, 9–14. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Bouazza, N.; Lillo-Ródenas, M.A.; Linares-Solano, A. Enhancement of the photocatalytic activity of pelletized TiO2 for the oxidation of propene at low concentration. Appl. Catal. B Environ. 2008, 77, 284–293. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- The International Centre for Diffraction Data. Available online: http://www.icdd.com/ (accessed on 30 October 2018).
- Lopez, T.; Cuevas, J.L.; Ilharco, L.; Ramírez, P.; Rodríguez-Reinoso, F.; Rodríguez-Castellón, E. XPS characterization and E. Coli DNA degradation using functionalized Cu/TiO2 nanobiocatalysts. Mol. Catal. 2018, 449, 62–71. [Google Scholar] [CrossRef]
- Tseng, L.-T.; Luo, X.; Bao, N.; Ding, J.; Li, S.; Yi, J. Structures and properties of transition-metal-doped TiO2 nanorods. Mater. Lett. 2016, 170, 142–146. [Google Scholar] [CrossRef]
- Behzad, H.; Ghodsi, F.E.; Peksu, E.; Karaagac, H. The effect of Cu content on structural, optical and photo-electrical properties of sol-gel derived CuxCo3-xO4 thin films. J. Alloys Compd. 2018, 744, 470–480. [Google Scholar] [CrossRef]
- López, R.; Gómez, R.; Oros-Ruiz, S. Photophysical and photocatalytic properties of TiO2-Cr sol–gel prepared semiconductors. Catal. Today 2011, 166, 159–165. [Google Scholar] [CrossRef]
- Dong, Z.; Ding, D.; Li, T.; Ning, C. Ni-doped TiO2 nanotubes photoanode for enhanced photoelectrochemical water splitting. Appl. Surf. Sci. 2018, 443, 321–328. [Google Scholar] [CrossRef]
- Fu, Z.; Hu, J.; Hu, W.; Yang, S.; Luo, Y. Quantitative analysis of Ni2+/Ni3+ in Li[NixMnyCoz]O2 cathode materials: Non-linear least-squares fitting of XPS spectra. Appl. Surf. Sci. 2018, 441, 1048–1056. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Mozia, S.; Heciak, A.; Morawski, A.W. Photocatalytic acetic acid decomposition leading to the production of hydrocarbons and hydrogen on Fe-modified TiO2. Catal. Today 2011, 161, 189–195. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, P.; Ji, H.; Ma, W.; Chen, C.; Zhao, J. Enhancement of photocatalytic decarboxylation on TiO2 by water-induced change in adsorption-mode. Appl. Catal. B Environ. 2018, 224, 376–382. [Google Scholar] [CrossRef]
- Ngo, S.; Betts, L.M.; Dappozze, F.; Ponczek, M.; George, C.; Guillard, C. Kinetics and mechanism of the photocatalytic degradation of acetic acid in absence or presence of O2. J. Photochem. Photobiol. A Chem. 2017, 339, 80–88. [Google Scholar] [CrossRef]
- Ouzzine, M.; Lillo-Ródenas, M.A.; Linares-Solano, A. Photocatalytic oxidation of propene in gas phase at low concentration by optimized TiO2 nanoparticles. Appl. Catal. B Environ. 2013, 134–135, 333–343. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef] [Green Version]
- Greiner, M.T.; Chai, L.; Helander, M.G.; Tang, W.M.; Lu, Z.H. Transition metal oxide work functions: The influence of cation oxidation state and oxygen vacancies. Adv. Funct. Mater. 2012, 22, 4557–4568. [Google Scholar] [CrossRef]
- Hinojosa-Reyes, M.; Camposeco-Solís, R.; Zanella, R.; Rodríguez González, V. Hydrogen production by tailoring the brookite and Cu2O ratio of sol-gel Cu-TiO2 photocatalysts. Chemosphere 2017, 184, 992–1002. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Helaïli, N.; Bessekhouad, Y.; Bouguelia, A.; Trari, M. Visible light degradation of Orange II using xCuyOz/TiO2 heterojunctions. J. Hazard. Mater. 2009, 168, 484–492. [Google Scholar] [CrossRef]
- Rimoldi, L.; Giordana, A.; Cerrato, G.; Falletta, E.; Meroni, D. Insights on the photocatalytic degradation processes supported by TiO2/WO3 systems. The case of ethanol and tetracycline. Catal. Today 2018. [Google Scholar] [CrossRef]
- Sabatini, V.; Rimoldi, L.; Tripaldi, L.; Meroni, D.; Farina, H.; Ortenzi, M.; Ardizzone, S. TiO2-SiO2-PMMA Terpolymer Floating Device for the Photocatalytic Remediation of Water and Gas Phase Pollutants. Catalysts 2018, 8, 568. [Google Scholar] [CrossRef]
- Piera, E.; Ayllón, J.; Doménech, X.; Peral, J. TiO2 deactivation during gas-phase photocatalytic oxidation of ethanol. Catal. Today 2002, 76, 259–270. [Google Scholar] [CrossRef]
- Peral, J.; Domènech, X.; Ollis, D.F. Review Heterogeneous Photocatal y sis for Purification, Decontamination and Deodorization of Air. J. Chem. Technol. Biotechnol. 1997, 70, 117–140. [Google Scholar] [CrossRef]
- Alberici, R.M.; Jardim, W.F. Photocatalytic destruction of VOCs in the gas-phase using titanium dioxide. Appl. Catal. B Environ. 1997, 14, 55–68. [Google Scholar] [CrossRef]
- Dibble, L.A.; Raupp, G.B. Fluidized-Bed Photocatalytic Oxidation of Trichloroethylene in Contaminated Air Streams. Environ. Sci. Technol. 1992, 26, 492–495. [Google Scholar] [CrossRef]
- Howe, R.F. Recent Developments in Photocatalysis. Dev. Chem. Eng. Miner. Process. 1998, 6, 55–84. [Google Scholar] [CrossRef]
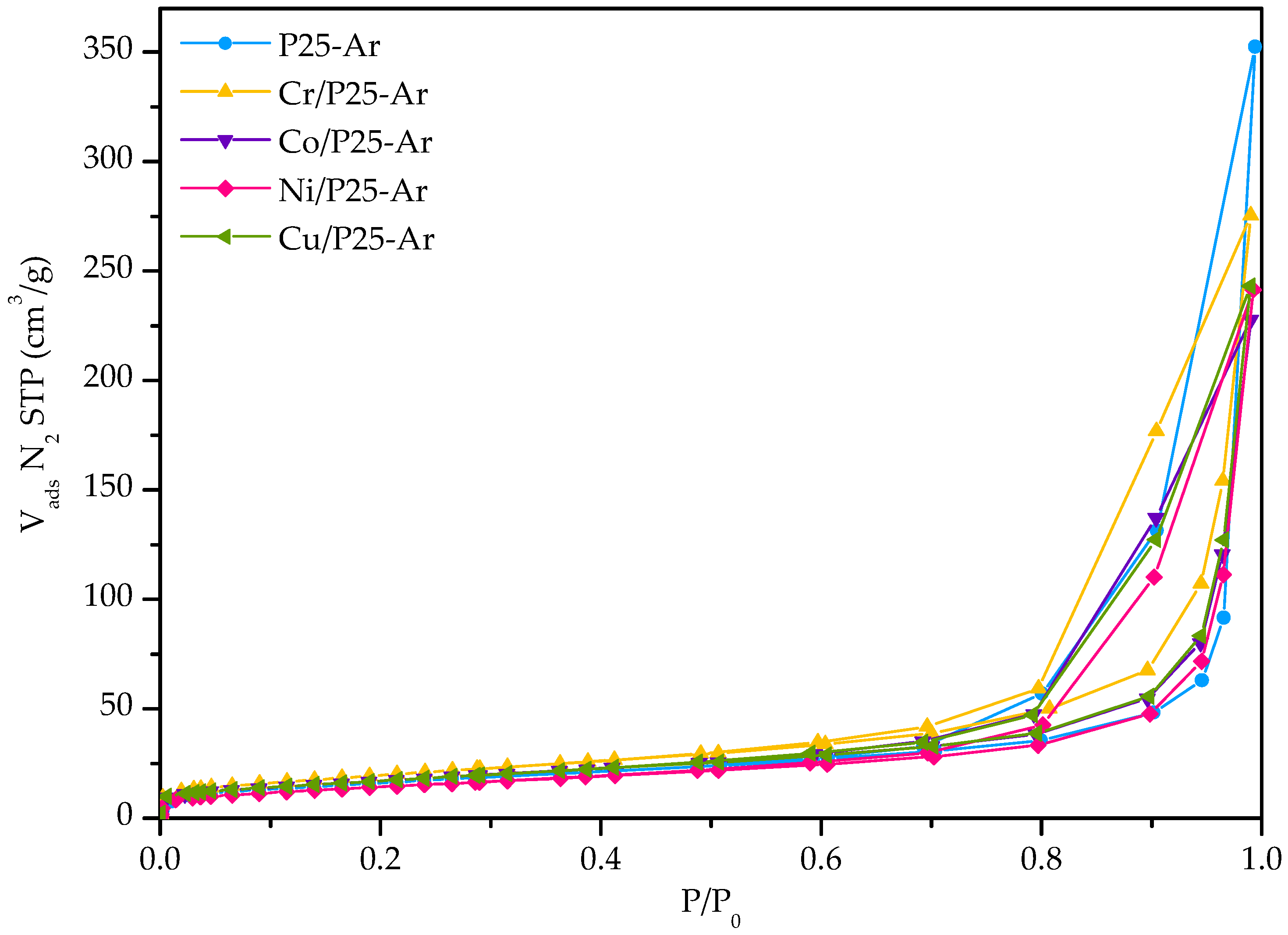
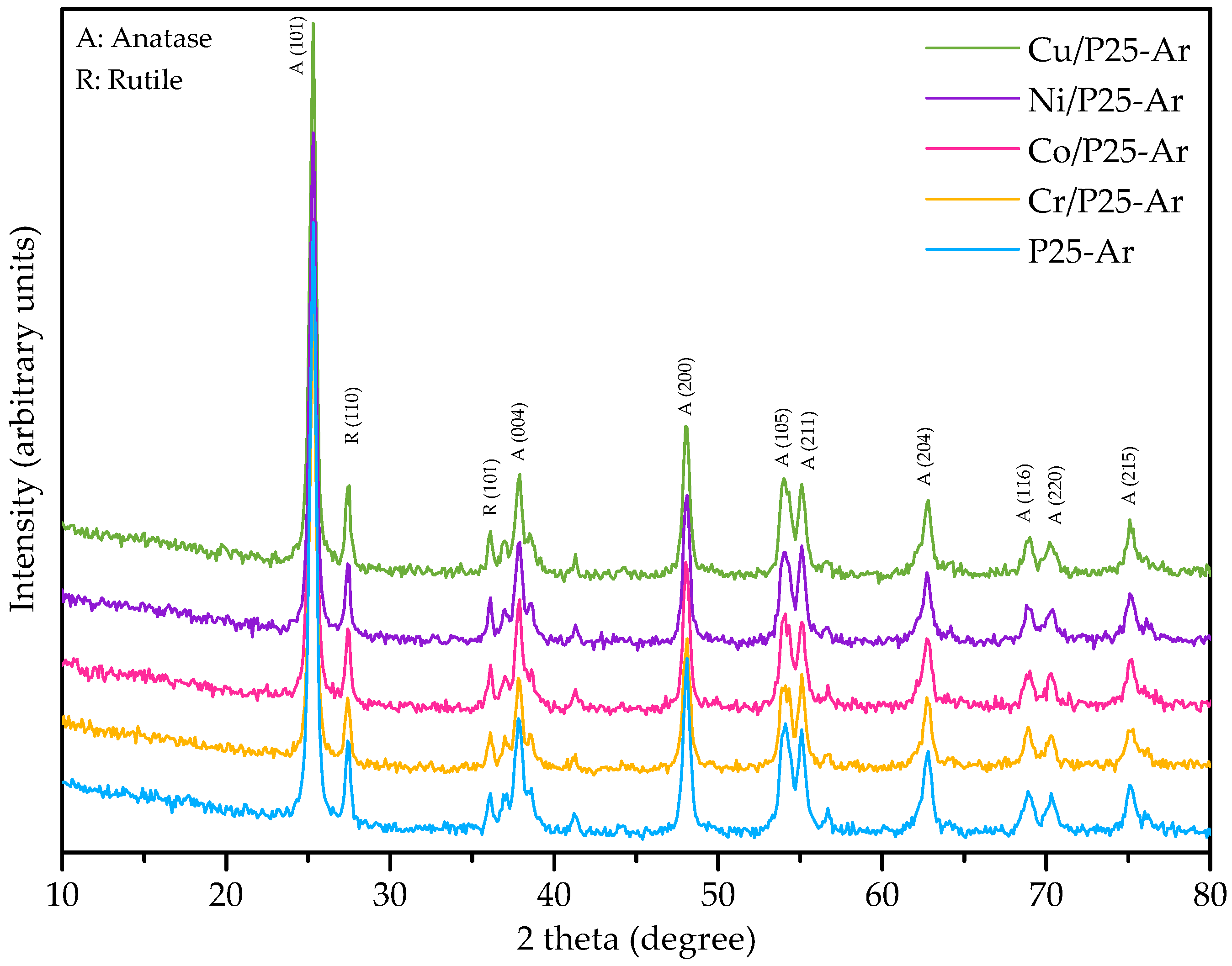
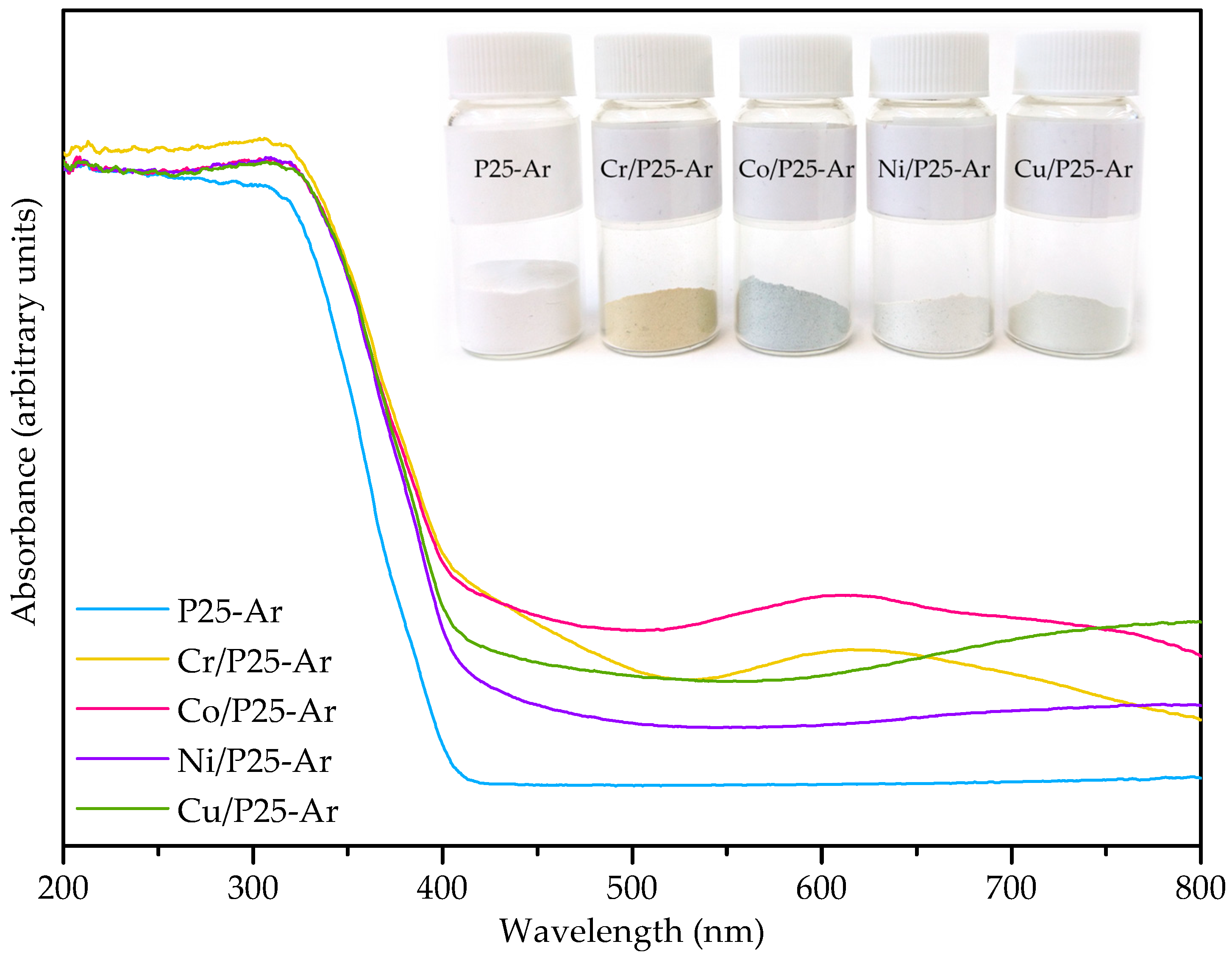


| Photocatalyst | SBET (m2/g) | VN2 (cm3/g) | Vmeso (cm3/g) | VT (cm3/g) | Ø 1 (nm) |
|---|---|---|---|---|---|
| P25-Ar | 58 | 0.02 | 0.48 | 0.53 | 36 |
| Cr/P25-Ar | 73 | 0.03 | 0.37 | 0.43 | 23 |
| Co/P25-Ar | 62 | 0.02 | 0.31 | 0.35 | 23 |
| Ni/P25-Ar | 53 | 0.02 | 0.34 | 0.37 | 28 |
| Cu/P25-Ar | 63 | 0.02 | 0.33 | 0.38 | 24 |
| Photocatalyst | Average Crystal Size (nm) | Crystalline TiO2 (wt.%) | Amorphous TiO2 (%) | ||
|---|---|---|---|---|---|
| Anatase | Rutile | Anatase | Rutile | ||
| P25-Ar | 19 | 32 | 77 | 11 | 12 |
| Cr/P25-Ar | 19 | 25 | 76 | 13 | 11 |
| Co/P25-Ar | 17 | 30 | 74 | 14 | 12 |
| Ni/P25-Ar | 20 | 32 | 76 | 12 | 12 |
| Cu/P25-Ar | 19 | 31 | 76 | 12 | 12 |
| Photocatalysts | Absorption Edge Wavelength (nm) | Eg 1 (eV) | Eg 2 (eV) | Eg 3 (eV) |
|---|---|---|---|---|
| P25-Ar | 403 | 3.08 | 3.53 | 3.11 |
| Cr/P25-Ar | 436 | 2.85 | 3.40 | 2.86 |
| Co/P25-Ar | 436 | 2.84 | 3.40 | 2.88 |
| Ni/P25-Ar | 422 | 2.93 | 3.41 | 2.95 |
| Cu/P25-Ar | 426 | 2.91 | 3.39 | 2.92 |
| Photocatalyst | Binding Energy (eV) | Indentified Metal Oxidation States | Proportion 1 (%) | ||
|---|---|---|---|---|---|
| Ti 2p3/2 | O 1s | M 2p3/2 | |||
| P25-Ar | 458.6 | 529.8 | - | - | - |
| Cr/P25-Ar | 458.4 | 529.7 | 576.6 | Cr (III) | 74 |
| 578.6 | Cr (VI) | 26 | |||
| Co/P25-Ar | 458.6 | 529.9 | 780.7 | Co (II) | 100 |
| Ni/P25-Ar | 458.6 | 529.8 | 855.7 | Ni (II) | 66 |
| 857.3 | Ni (III) | 34 | |||
| Cu/P25-Ar | 458.7 | 529.9 | 932.4 | Cu (I) | 86 |
| 934.1 | Cu (II) | 14 | |||
| Identified Metal Oxidation States 1 | Ionic Radius 2 (Å) | Work Function 3 (eV) |
|---|---|---|
| Ti (IV) | 0.75 | 5.4 ± 0.2 |
| Cr (III) | 0.76 | 5.0 ± 0.2 |
| Cr (VI) | 0.58 | 6.8 ± 0.2 |
| Co (II) | 0.88 | 4.6 ± 0.2 |
| Ni (II) | 0.83 | 6.3 ± 0.2 |
| Ni (III) | 0.74 | 4.9 ± 0.1 |
| Cu (I) | 0.91 | 4.9 ± 0.1 |
| Cu (II) | 0.87 | 5.9 ± 0.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amorós-Pérez, A.; Cano-Casanova, L.; Castillo-Deltell, A.; Lillo-Ródenas, M.Á.; Román-Martínez, M.d.C. TiO2 Modification with Transition Metallic Species (Cr, Co, Ni, and Cu) for Photocatalytic Abatement of Acetic Acid in Liquid Phase and Propene in Gas Phase. Materials 2019, 12, 40. https://doi.org/10.3390/ma12010040
Amorós-Pérez A, Cano-Casanova L, Castillo-Deltell A, Lillo-Ródenas MÁ, Román-Martínez MdC. TiO2 Modification with Transition Metallic Species (Cr, Co, Ni, and Cu) for Photocatalytic Abatement of Acetic Acid in Liquid Phase and Propene in Gas Phase. Materials. 2019; 12(1):40. https://doi.org/10.3390/ma12010040
Chicago/Turabian StyleAmorós-Pérez, Ana, Laura Cano-Casanova, Ana Castillo-Deltell, María Ángeles Lillo-Ródenas, and María del Carmen Román-Martínez. 2019. "TiO2 Modification with Transition Metallic Species (Cr, Co, Ni, and Cu) for Photocatalytic Abatement of Acetic Acid in Liquid Phase and Propene in Gas Phase" Materials 12, no. 1: 40. https://doi.org/10.3390/ma12010040