Biomedical Application of Electroactive Polymers in Electrochemical Sensors: A Review
Abstract
:1. Introduction
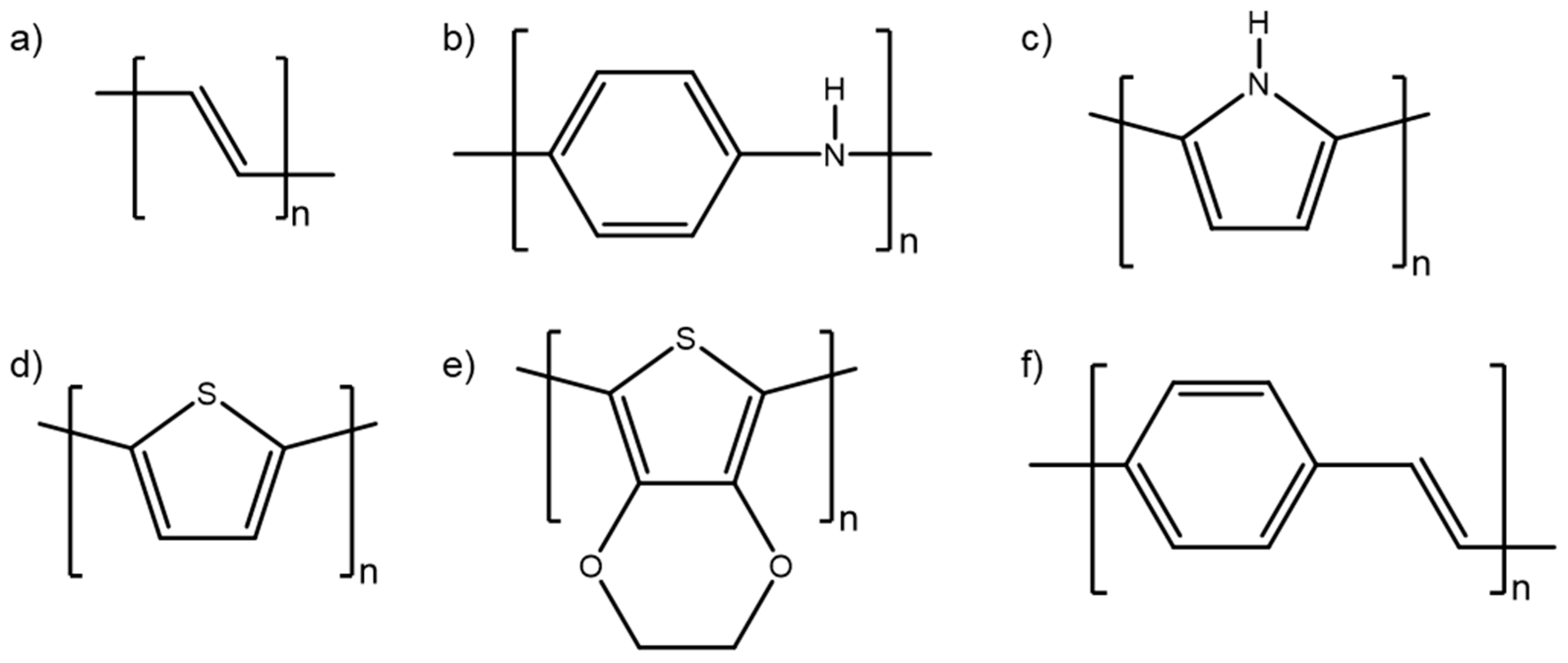
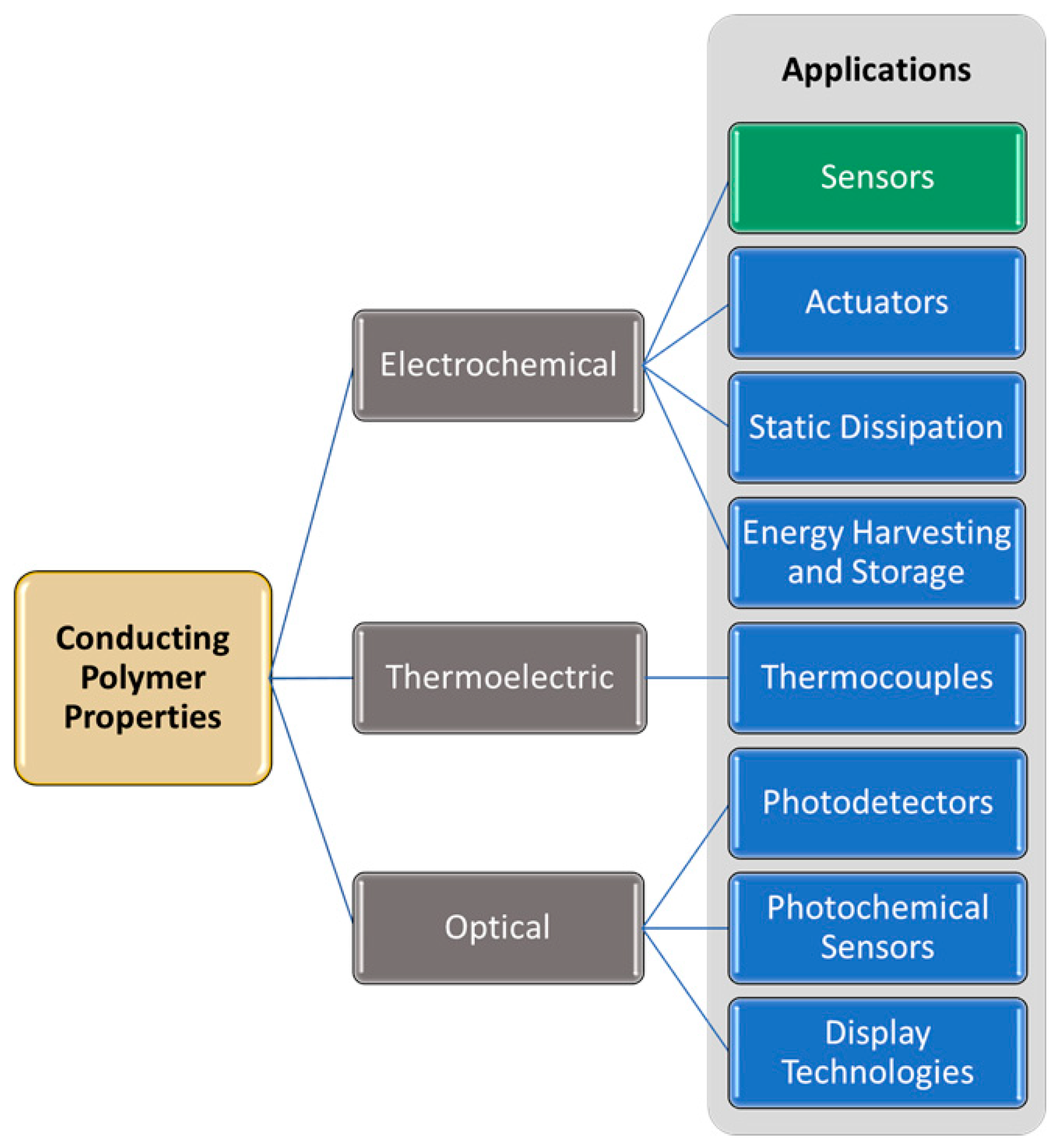
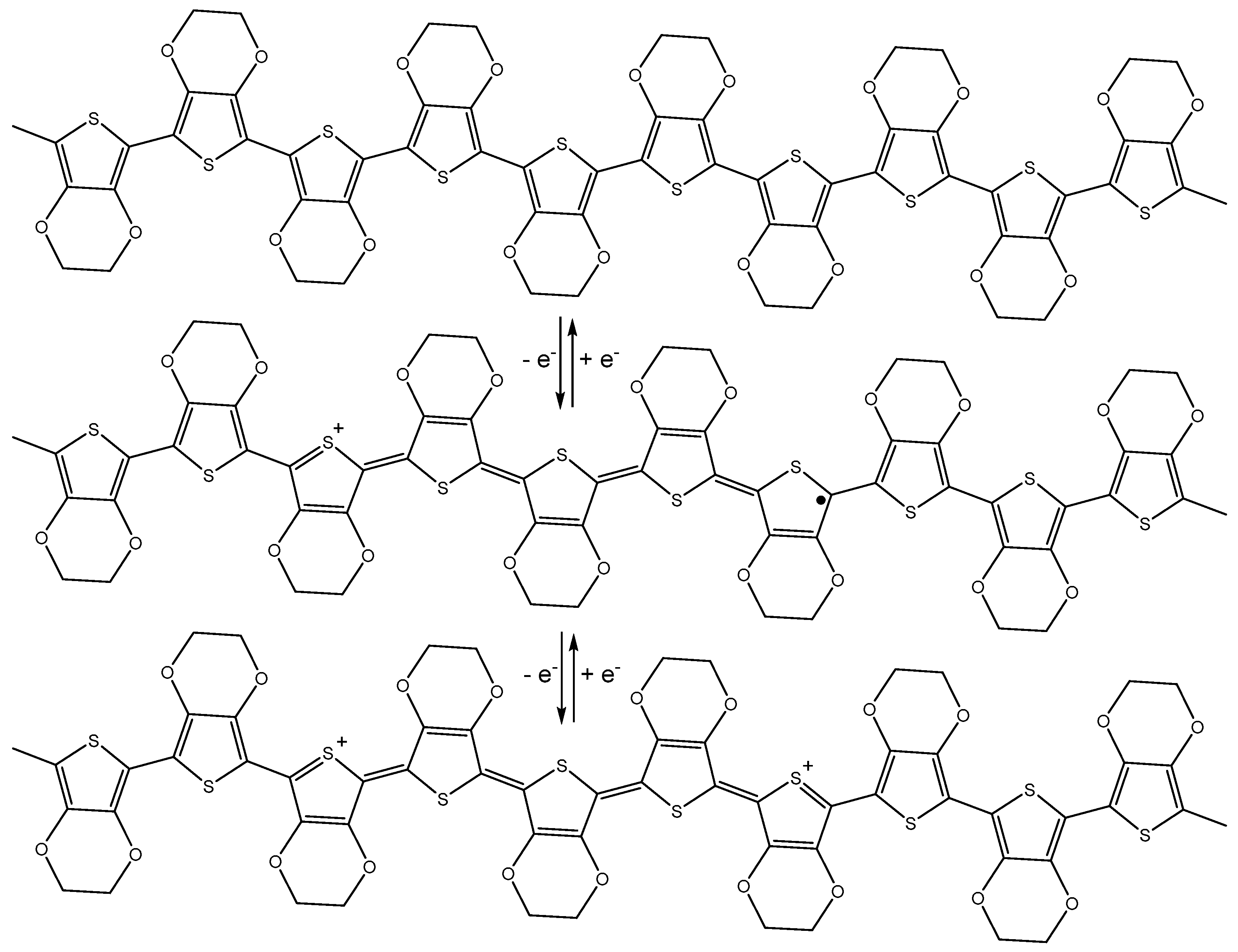
2. Properties of Conducting Polymers
3. Conducting Polymer-Based Electrochemical Sensors
3.1. Electrochemical Characterization Methods
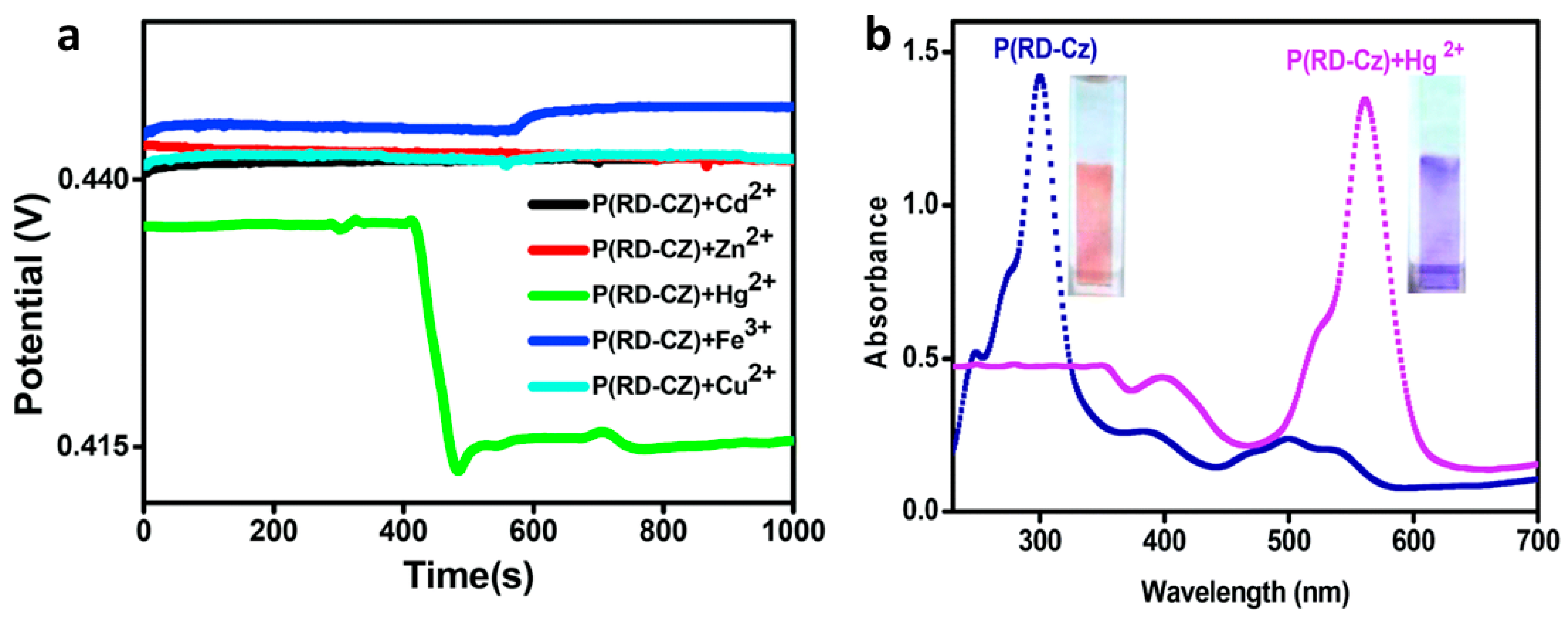
3.1.1. Potentiometry
3.1.2. Amperometry
3.1.3. Conductometry
3.1.4. Voltammetry
3.2. Biorecognition Molecules
- enzyme-based recognition
- antibody-based recognition
- aptamer-based recognition
- peptide-and nucleic acid-based recognition.
3.2.1. Enzyme-Based Recognition
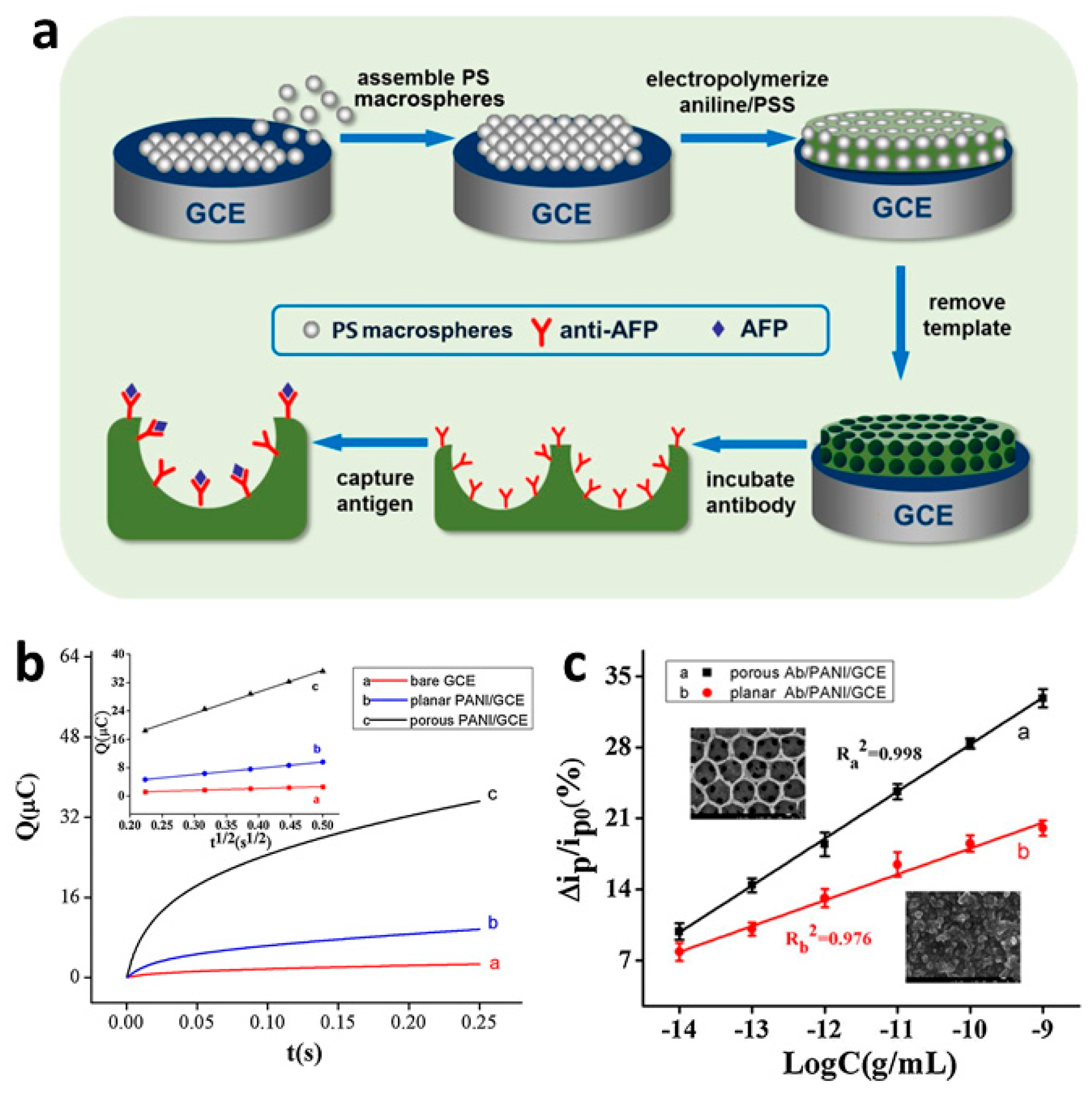
3.2.2. Antibody-Based Recognition
3.2.3. Nucleic Acid-Based Recognition
3.2.4. Aptamer-Based Recognition
3.2.5. Biosensors Based on Other Recognition Molecules
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, W.S.; Humphrey, B.D.; MacDiarmid, A.G. Polyaniline, a Novel Conducting Polymer. Morphology and Chemistry of Its Oxidation and Reduction in Aqueous Electrolytes. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1986, 82, 2385–2400. [Google Scholar] [CrossRef]
- Nambiar, S.; Yeow, J.T.W. Conductive Polymer-Based Sensors for Biomedical Applications. Biosens. Bioelectron. 2011, 26, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Rivers, T.J.; Hudson, T.W.; Schmidt, C.E. Synthesis of a Novel, Biodegradable Electrically Conducting Polymer for Biomedical Applications. Adv. Funct. Mater. 2002, 12, 33–37. [Google Scholar] [CrossRef]
- Abidian, M.R.; Kim, D.H.; Martin, D.C. Conducting-Polymer Nanotubes for Controlled Drug Release. Adv. Mater. 2006, 18, 405–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collier, J.H.; Camp, J.P.; Hudson, T.W.; Schmidt, C.E. Synthesis and Characterization of Polypyrrole-Hyaluronic Acid Composite Biomaterials for Tissue Engineering Applications. J. Biomed. Mater. Res. 2000, 50, 574–584. [Google Scholar] [CrossRef]
- Wang, C.Y.; Ballantyne, A.M.; Hall, S.B.; Too, C.O.; Officer, D.L.; Wallace, G.G. Functionalized Polythiophene-Coated Textile: A New Anode Material for a Flexible Battery. J. Power Sources 2006, 156, 610–614. [Google Scholar] [CrossRef]
- Chiang, C.K.; Fincher, C.R., Jr.; Park, Y.W.; Heeger, A.J.; Shirakawa, H.; Louis, E.J.; Gau, S.C.; MacDiarmid, A.G. No TitleElectrical Conductivity in Doped Polyacetylene. Phys. Rev. Lett. 1977, 39, 1098–1101. [Google Scholar] [CrossRef]
- Le, T.-H.; Kim, Y.; Yoon, H. Electrical and Electrochemical Properties of Conducting Polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef]
- Tsukamoto, J. Recent Advances in Highly Conductive Polyacetylene. Adv. Phys. 1992, 41, 509–546. [Google Scholar] [CrossRef]
- Tsukamoto, J.; Takahashi, A.; Kawasaki, K. Structure and Electrical Properties of Polyacetylene Yielding a Conductivity of 105 S/Cm. Jpn. J. Appl. Phys. 1990, 29, 125–130. [Google Scholar] [CrossRef]
- Guay, J.; Diaz, A.; Wu, R.; Tour, J.M.; Dao, L.H. Electrooxidation of Soluble a, a-Coupled Thiophene Oligomers. Chem. Mater. 1992, 4, 254–255. [Google Scholar] [CrossRef]
- Cao, Y.; Andreatta, A.; Heeger, A.J.; Smith, P. Influence of Chemical Polymerization Conditions on the Properties of Polyaniline. Polymers 1989, 30, 2305–2311. [Google Scholar] [CrossRef]
- Loewe, R.S.; Ewbank, P.C.; Liu, J.; Zhai, L.; Mccullough, R.D. Regioregular, Head-to-Tail Coupled Poly(3-Alkylthiophenes) Made Easy by the GRIM Method: Investigation of the Reaction and the Origin of Regioselectivity. Macromolecules 2001, 34, 4324–4333. [Google Scholar] [CrossRef]
- Tamba, S.; Fuji, K.; Meguro, H.; Okamoto, S.; Tendo, T.; Komobuchi, R.; Sugie, A.; Nishino, T.; Mori, A. Synthesis of High-Molecular-Weight Head-to-Tail-Type Poly(3-Substituted-Thiophene)s by Cross-Coupling Polycondensation with [CpNiCl(NHC)] as a Catalyst. Chem. Lett. 2013, 42, 281–283. [Google Scholar] [CrossRef]
- Moliton, A.; Hiorns, R.C. Review of Electronic and Optical Properties of Semiconducting π-Conjugated Polymers: Applications in Optoelectronics. Polym. Int. 2004, 53, 1397–1412. [Google Scholar] [CrossRef]
- Beaujuge, P.M.; Reynolds, J.R. Color Control in π-Conjugated Organic Polymers for Use in Electrochromic Devices. Chem. Rev. 2010, 110, 268–320. [Google Scholar] [CrossRef]
- Bharti, M.; Singh, A.; Samanta, S.; Aswal, D.K. Conductive Polymers for Thermoelectric Power Generation. Prog. Mater. Sci. 2018, 93, 270–310. [Google Scholar] [CrossRef]
- Irvin, J.A.; Irvin, D.J.; Stenger-Smith, J.D. Electroactive Polymers for Batteries and Supercapacitors. In Handbook of Conducting Polymers: Processing and Applications; Skotheim, T.A., Reynolds, J.R., Eds.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Katz, H.E.; Searson, P.C.; Poehler, T.O. Batteries and Charge Storage Devices Based on Electronically Conducting Polymers. J. Mater. Res. 2010, 25, 1561–1574. [Google Scholar] [CrossRef]
- Li, Y.; Zou, Y. Conjugated Polymer Photovoltaic Materials with Broad Absorption Band and High Charge Carrier Mobility. Adv. Mater. 2008, 20, 2952–2958. [Google Scholar] [CrossRef]
- Boudreault, P.L.T.; Najari, A.; Leclerc, M. Processable Low-Bandgap Polymers for Photovoltaic Applications. Chem. Mater. 2011, 23, 456–469. [Google Scholar] [CrossRef]
- Muccini, M.; Nazionale, C. A Bright Future for Organic Field-Effect Transistors. Nat. Mater. 2006, 5, 605–613. [Google Scholar] [CrossRef]
- Bhuvana, K.P.; Joseph Bensingh, R.; Abdul Kader, M.; Nayak, S.K. Polymer Light Emitting Diodes: Materials, Technology and Device. Polym. Plast. Technol. Eng. 2018, 57, 1784–1800. [Google Scholar] [CrossRef]
- Yoon, H. Current Trends in Sensors Based on Conducting Polymer Nanomaterials. Nanomaterials 2013, 3, 524–549. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.A.; Kumar, P.; Park, D.-S.; Shim, Y.B. Electrochemical Sensors Based on Organic Conjugated Polymers. Sensors 2008, 8, 118–141. [Google Scholar] [CrossRef]
- Anquetil, P.A.; Yu, H.; Madden, J.D.; Swager, T.M.; Hunter, I.W. Recent Advances in Thiophene-Based Molecular Actuators. In Smart Structures and Materials 2003: Electroactive Polymer Actuators and Devices (EAPAD); International Society for Optics and Photonics: Bellingham, WA, USA, 2003; Volume 5051, pp. 42–53. [Google Scholar]
- Kongahage, D.; Foroughi, J. Actuator Materials: Review on Recent Advances and Future Outlook for Smart Textiles. Fibers 2019, 7, 21. [Google Scholar] [CrossRef]
- Bredas, J.L.; Street, G.B. Polarons, Bipolarons, and Solitons in Conducting Polymers. Acc. Chem. Res. 1985, 18, 309–315. [Google Scholar] [CrossRef]
- Bredas, J.L.; Chance, R.R.; Silbey, R. Theoretical Studies of Charged Defect States in Doped Polyacetylene and Polyparaphenylene. Mol. Cryst. Liq. Cryst. 1981, 77, 319–332. [Google Scholar] [CrossRef]
- Scrosati, B. Electrochemical Properties of Conducting Polymers. Prog. Solid State Chem. 1988, 18, 1–77. [Google Scholar] [CrossRef]
- Irvin, J.A.; Carberry, J.R. Dominant Ion Transport Processes of Ionic Liquid Electrolyte in Poly(3,4-Ethylenedioxythiophene). J. Polym. Sci. Part B Polym. Phys. 2013, 51, 337–342. [Google Scholar] [CrossRef]
- Heeger, A.J. Conjugated Polymers: The Interconnection of Chemical and Electronic Structure. In Conjugated Polymers and Related Materials: The Interconnection of Chemical and Electronic Structure: Proceedings of the Eighty-first Nobel Symposium; Salanek, W.R., Lundstrom, I., Ranby, B., Eds.; Oxford University Press: New York, NY, USA, 1993; pp. 27–62. [Google Scholar]
- Svirskis, D.; Travas-Sejdic, J.; Rodgers, A.; Garg, S. Electrochemically Controlled Drug Delivery Based on Intrinsically Conducting Polymers. J. Control. Release 2010, 146, 6–15. [Google Scholar] [CrossRef]
- Tourillon, G.; Garnier, F. Effect of Dopant on the Physicochemical and Electrical Properties of Organic Conducting Polymers. J. Phys. Chem. 1983, 87, 2289–2292. [Google Scholar] [CrossRef]
- Tanaka, Y.; Hasegawa, T.; Shimamura, T.; Ukeda, H.; Ueda, T. Potentiometric Evaluation of Antioxidant Capacity Using Polyoxometalate-Immobilized Electrodes. J. Electroanal. Chem. 2018, 828, 102–107. [Google Scholar] [CrossRef]
- Ayranci, R.; Demirkol, D.O.; Timur, S.; Ak, M. Rhodamine-Based Conjugated Polymers: Potentiometric, Colorimetric and Voltammetric Sensing of Mercury Ions in Aqueous Medium. Analyst 2017, 142, 3407–3415. [Google Scholar] [CrossRef]
- Heller, A.; Feldman, B. Electrochemical Glucose Sensors and Their Applications in Diabetes Management. Chem. Rev. 2008, 108, 2482–2505. [Google Scholar] [CrossRef] [Green Version]
- Sanna, G.; Spano, N.; Panzanelli, A.; Farre, R.; Senes, N.; Sobral, A.; Lachowicz, J.I.; Masolo, E.; Pilo, M. Design of Amperometric Biosensors for the Detection of Glucose Prepared by Immobilization of Glucose Oxidase on Conducting (Poly)Thiophene Films. J. Anal. Methods Chem. 2018, 2018, 1849439. [Google Scholar] [CrossRef]
- Marquitan, M.; Bobrowski, T.; Ernst, A.; Wilde, P.; Clausmeyer, J.; Ruff, A.; Schuhmann, W. Miniaturized Amperometric Glucose Sensors Based on Polymer/ Enzyme Modified Carbon Electrodes in the Sub-Micrometer Scale. J. Electrochem. Soc. 2018, 165, G3008–G3014. [Google Scholar] [CrossRef] [Green Version]
- Gurudatt, N.G.; Kim, J.-I.; Mir, T.A.; Choi, C.S.; Shim, Y.-B.; Akhtar, M.H. An Amperometric Nanobiosensor for the Selective Detection of K + -Induced Dopamine Released from Living Cells. Biosens. Bioelectron. 2015, 68, 421–428. [Google Scholar] [CrossRef]
- Bilgi, M.; Ayranci, E. Development of Amperometric Biosensors Using Screen-Printed Carbon Electrodes Modified with Conducting Polymer and Nanomaterials for the Analysis of Ethanol, Methanol and Their Mixtures. J. Electroanal. Chem. 2018, 823, 588–592. [Google Scholar] [CrossRef]
- Cevik, E.; Cerit, A.; Gazel, N.; Yildiz, H.B. Construction of an Amperometric Cholesterol Biosensor Based on DTP(Aryl)Aniline Conducting Polymer Bound Cholesterol Oxidase. Electroanalysis 2018, 30, 2445–2453. [Google Scholar] [CrossRef]
- Braik, M.; Barsan, M.M.; Dridi, C.; Ben Ali, M.; Brett, C.M.A. Highly Sensitive Amperometric Enzyme Biosensor for Detection of Superoxide Based on Conducting Polymer/CNT Modified Electrodes and Superoxide Dismutase. Sens. Actuators B Chem. 2016, 236, 574–582. [Google Scholar] [CrossRef]
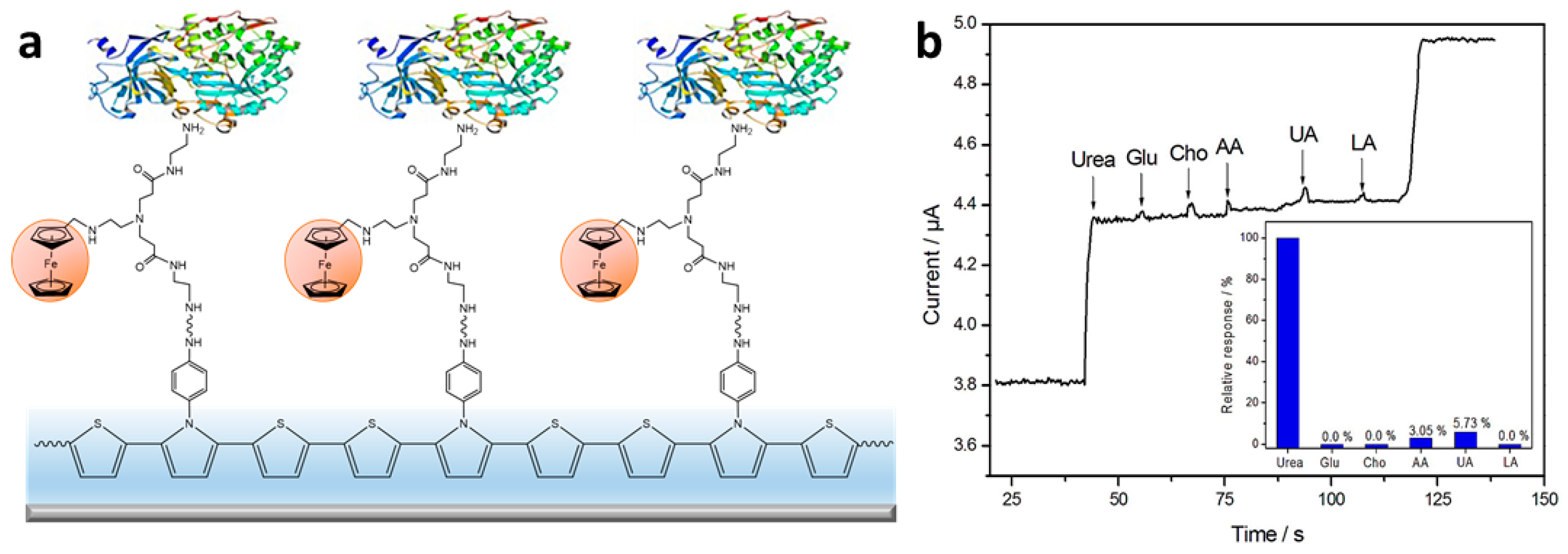
- Dervisevic, M.; Dervisevic, E.; Senel, M.; Cevik, E.; Yildiz, H.B.; Camurlu, P. Construction of Ferrocene Modified Conducting Polymer Based Amperometric Urea Biosensor. Enzym. Microb. Technol. 2017, 102, 53–59. [Google Scholar] [CrossRef]
- Symanski, J.S.; Martinchek, G.A.; Bruckenstein, S. Conductometric Sensor for Atmospheric Carbon Dioxide Determination. Anal. Chem. 1983, 55, 1152–1156. [Google Scholar] [CrossRef]
- Ogasawara, K.; Mitsubayashi, K.; Tsuru, T.; Karube, I. Electrical Conductivity of Tear Fluid in Healthy Persons and Keratoconjunctivitis Sicca Patients Measured by a Flexible Conductimetric Sensor. Graefe’s Arch. Clin. Exp. Ophthalmol. 1996, 234, 542–546. [Google Scholar] [CrossRef]
- Le Ouay, B.; Boudot, M.; Kitao, T.; Yanagida, T.; Kitagawa, S.; Uemura, T. Nanostructuration of PEDOT in Porous Coordination Polymers for Tunable Porosity and Conductivity. J. Am. Chem. Soc. 2016, 138, 10088–10091. [Google Scholar] [CrossRef]
- Patil, S.; Ghadi, H.; Ramgir, N.; Adhikari, A.; Rao, V.R. Monitoring Soil PH Variation Using Polyaniline/SU-8 Composite Film Based Conductometric Microsensor. Sens. Actuators B Chem. 2019, 286, 583–590. [Google Scholar] [CrossRef]
- Campbell, M.G.; Sheberla, D.; Liu, S.F.; Swager, T.M.; Dincə, M. Cu3(Hexaiminotriphenylene)2: An Electrically Conductive 2D Metal-Organic Framework for Chemiresistive Sensing. Angew. Chem. Int. Ed. 2015, 54, 4349–4352. [Google Scholar] [CrossRef]
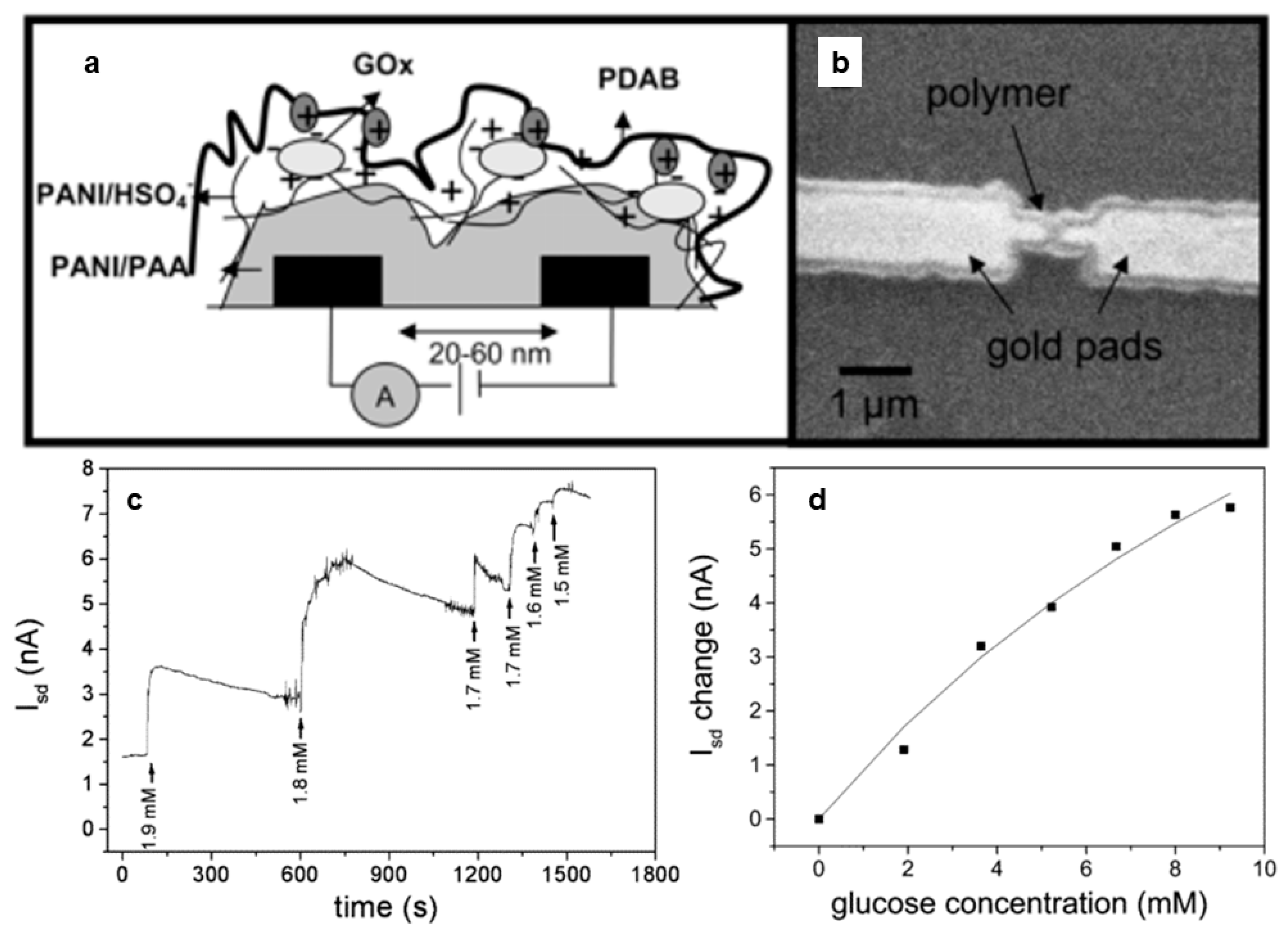
- Forzani, E.S.; Zhang, H.; Nagahara, L.A.; Amlani, I.; Tsui, R.; Tao, N. A Conducting Polymer Nanojunction Sensor for Glucose Detection. Nano Lett. 2004, 4, 1785–1788. [Google Scholar] [CrossRef]
- Fabre, B.; Taillebois, L. Poly(Aniline Boronic Acid)-Based Conductimetric Sensor of Dopamine. Chem. Commun. 2003, 3, 2982–2983. [Google Scholar] [CrossRef]
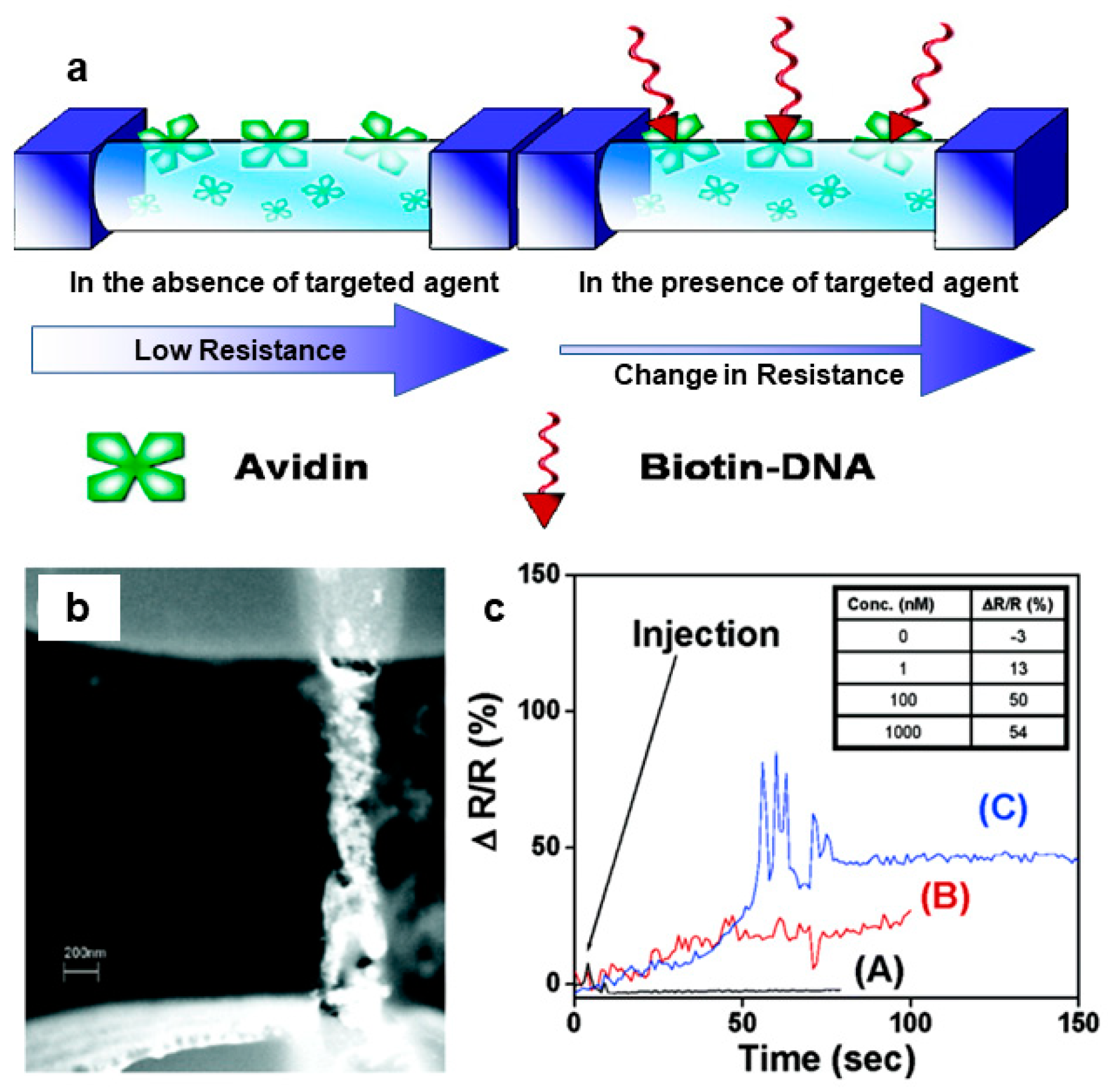
- Ramanathan, K.; Bangar, M.A.; Yun, M.; Chen, W.; Myung, N.V.; Mulchandani, A. Bioaffinity Sensing Using Biologically Functionalized Conducting-Polymer Nanowire. J. Am. Chem. Soc. 2005, 127, 496–497. [Google Scholar] [CrossRef]
- Pillalamarri, S.K.; Blum, F.D.; Tokuhiro, A.T.; Bertino, M.F. One-Pot Synthesis of Polyaniline—Metal Nanocomposites. Chem. Mater. 2005, 17, 5941–5944. [Google Scholar] [CrossRef]
- Liu, Y.; Turner, A.P.F.; Zhao, M.; Mak, W.C. Processable Enzyme-Hybrid Conductive Polymer Composites for Electrochemical Biosensing. Biosens. Bioelectron. 2018, 100, 374–381. [Google Scholar] [CrossRef]
- Fayemi, O.E.; Adekunle, A.S.; Kumara Swamy, B.E.; Ebenso, E.E. Electrochemical Sensor for the Detection of Dopamine in Real Samples Using Polyaniline/NiO, ZnO, and Fe3O4 Nanocomposites on Glassy Carbon Electrode. J. Electroanal. Chem. 2018, 818, 236–249. [Google Scholar] [CrossRef]
- Arrieta, A.A.; Apetrei, C.; Rodriguez-Mendez, M.L.; de Saja, J. Voltammetric Sensor Array Based on Conducting Polymer-Modified Electrodes for the Discrimination of Liquids. Electrochm. Acta 2004, 49, 4543–4551. [Google Scholar] [CrossRef]
- Hamid, H.H.; Harb, M.E.; Elshaer, A.M.; Erahim, S.; Soliman, M.M. Electrochemical Preparation and Electrical Characterization of Polyaniline as a Sensitive Biosensor. Microsyst. Technol. 2018, 24, 1775–1781. [Google Scholar] [CrossRef]
- Shahrokhian, S.; Azimzadeh, M.; Amini, M.K. Modification of Glassy Carbon Electrode with a Bilayer of Multiwalled Carbon Nanotube/Tiron-Doped Polypyrrole: Application to Sensitive Voltammetric Determination of Acyclovir. Mater. Sci. Eng. C 2015, 53, 134–141. [Google Scholar] [CrossRef]
- Shirane, M.; Nakamura, K. Aniracetam Enhances Cortical Dopamine and Serotonin Release via Cholinergic and Glutamatergic Mechanisms in SHRSP. Brain Res. 2001, 916, 211–221. [Google Scholar] [CrossRef]
- Raj, M.; Gupta, P.; Goyal, R.N.; Shim, Y.-B. Graphene/Conducting Polymer Nano-Composite Loaded Screen Printed Carbon Sensor for Simultaneous Determination of Dopamine and 5-Hydroxytryptamine. Sens. Actuators B Chem. 2017, 239, 993–1002. [Google Scholar] [CrossRef]
- Updike, S.J.; Hicks, G.P. The Enzyme Electrode. Nature 1967, 214, 986–988. [Google Scholar] [CrossRef]
- Chambers, J.P.; Arulanandam, B.P.; Matta, L.L.; Weis, A.; Valdes, J.J. Biosensor Recognition Elements. Curr. Issues Mol. Biol. 2008, 10, 1–12. [Google Scholar]
- Barhoumi, H.; Maaref, A.; Rammah, M.; Martelet, C.; Jaffrezic, N.; Mousty, C.; Vial, S.; Forano, C. Urea Biosensor Based on Zn3Al-Urease Layered Double Hydroxides Nanohybrid Coated on Insulated Silicon Structures. Mater. Sci. Eng. C 2006, 26, 328–333. [Google Scholar] [CrossRef]
- Heller, A. Implanted Electrochemical Glucose Sensors for the Management of Diabetes. Annu. Rev. Biomed. Eng. 2002, 1, 153–175. [Google Scholar] [CrossRef]
- Smoller, B.R.; Roe, J.N. Bloodless Glucose Measurements. Crit. Rev. Drug Carr. Syst. 2012, 15, 43. [Google Scholar] [CrossRef]
- Wang, H. Label-Free Hybridization Detection of a Single Nucleotide Mismatch by Immobilization of Molecular Beacons on an Agarose Film. Nucleic Acids Res. 2002, 30, e61. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X. Needle-Type Dual Microsensor for the Simultaneous Monitoring of Glucose and Insulin. Anal. Chem. 2001, 73, 844–847. [Google Scholar] [CrossRef]
- Wang, J.; Chatrathi, M.P.; Tian, B.; Polsky, R. Microfabricated Electrophoresis Chips for Simultaneous Bioassays of Glucose, Uric Acid, Ascorbic Acid, and Acetaminophen. Anal. Chem. 2000, 72, 2514–2518. [Google Scholar] [CrossRef]
- Yamazaki, T.; Kojima, K.; Sode, K. Extended-Range Glucose Sensor Employing Engineered Glucose Dehydrogenases. Anal. Chem. 2000, 72, 4689–4693. [Google Scholar] [CrossRef]
- Pal, R.K.; Farghaly, A.A.; Wang, C.; Collinson, M.M.; Kundu, S.C.; Yadavalli, V.K. Conducting Polymer-Silk Biocomposites for Flexible and Biodegradable Electrochemical Sensors. Biosens. Bioelectron. 2016, 81, 294–302. [Google Scholar] [CrossRef]
- Barsan, M.M.; Pifferi, V.; Falciola, L.; Brett, C.M.A. New CNT/Poly(Brilliant Green) and CNT/Poly(3,4-Ethylenedioxythiophene) Based Electrochemical Enzyme Biosensors. Anal. Chim. Acta 2016, 927, 35–45. [Google Scholar] [CrossRef]
- Chen, H.; Choo, T.K.; Huang, J.; Wang, Y.; Liu, Y.; Platt, M.; Palaniappan, A.; Liedberg, B.; Tok, A.I.Y. Label-Free Electronic Detection of Interleukin-6 Using Horizontally Aligned Carbon Nanotubes. Mater. Des. 2016, 90, 852–857. [Google Scholar] [CrossRef]
- Sun, X.; Hui, N.; Luo, X. Reagentless and Label-Free Voltammetric Immunosensor for Carcinoembryonic Antigen Based on Polyaniline Nanowires Grown on Porous Conducting Polymer Composite. Microchim. Acta 2017, 184, 889–896. [Google Scholar] [CrossRef]
- Taleat, Z.; Cristea, C.; Marrazza, G.; Mazloum-Ardakani, M.; Sǎndulescu, R. Electrochemical Immunoassay Based on Aptamer-Protein Interaction and Functionalized Polymer for Cancer Biomarker Detection. J. Electroanal. Chem. 2014, 717–718, 119–124. [Google Scholar] [CrossRef]
- Aydın, E.B.; Aydın, M.; Sezgintürk, M.K. A Highly Sensitive Immunosensor Based on ITO Thin Films Covered by a New Semi-Conductive Conjugated Polymer for the Determination of TNFα in Human Saliva and Serum Samples. Biosens. Bioelectron. 2017, 97, 169–176. [Google Scholar] [CrossRef]
- Pruna, R.; Baraket, A.; Bonhommé, A.; Zine, N.; Errachid, A.; López, M. Novel Nanostructured Indium Tin Oxide Electrode for Electrochemical Immunosensors: Suitability for the Detection of TNF-α. Electrochim. Acta 2018, 283, 1632–1639. [Google Scholar] [CrossRef]
- Shin, D.S.; Matharu, Z.; You, J.; Siltanen, C.; Vu, T.; Raghunathan, V.K.; Stybayeva, G.; Hill, A.E.; Revzin, A. Sensing Conductive Hydrogels for Rapid Detection of Cytokines in Blood. Adv. Healthc. Mater. 2016, 5, 659–664. [Google Scholar] [CrossRef]
- Aydın, E.B.; Aydın, M.; Sezgintürk, M.K. Highly Sensitive Electrochemical Immunosensor Based on Polythiophene Polymer with Densely Populated Carboxyl Groups as Immobilization Matrix for Detection of Interleukin 1Β in Human Serum and Saliva. Sens. Actuators B Chem. 2018, 270, 18–27. [Google Scholar] [CrossRef]
- Wang, H.; Ma, Z. A Cascade Reaction Signal-Amplified Amperometric Immunosensor Platform for Ultrasensitive Detection of Tumour Marker. Sens. Actuators B Chem. 2018, 254, 642–647. [Google Scholar] [CrossRef]
- Zhao, L.; Ma, Z. Facile Synthesis of Polyaniline-Polythionine Redox Hydrogel: Conductive, Antifouling and Enzyme-Linked Material for Ultrasensitive Label-Free Amperometric Immunosensor toward Carcinoma Antigen-125. Anal. Chim. Acta 2018, 997, 60–66. [Google Scholar] [CrossRef]
- Maeng, J.; Lee, B.-C.; Ko, Y.-J.; Ahn, Y.; Cho, N.-G.; Lee, S.-H.; Hwang, S.Y. A Novel Microfluidic Biosensor Based on an Electrical Detection System for Alpha-Fetoprotein. Biosens. Bioelectron. 2008, 23, 1319–1325. [Google Scholar] [CrossRef]
- Liu, S.; Ma, Y.; Cui, M.; Luo, X. Enhanced Electrochemical Biosensing of Alpha-Fetoprotein Based on Three-Dimensional Macroporous Conducting Polymer Polyaniline. Sens. Actuators B Chem. 2018, 255, 2568–2574. [Google Scholar] [CrossRef]
- Zhu, Q.; Liu, G.; Kai, M. DNA Aptamers in the Diagnosis and Treatment of Human Diseases. Molecules 2015, 20, 20979–20997. [Google Scholar] [CrossRef]
- Germer, K.; Leonard, M.; Zhang, X. Review Article RNA Aptamers and Their Therapeutic and Diagnostic Applications. Int. J. Biochem. Mol. Biol. 2013, 4, 27–40. [Google Scholar]
- Paleček, E. From Polarography of DNA to Microanalysis with Nucleic Acid-Modified Electrodes. Electroanalysis 1996, 8, 7–14. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, L.; Soeller, C.; Travas-Sejdic, J. Conducting Polymers for Electrochemical DNA Sensing. Biomaterials 2009, 30, 2132–2148. [Google Scholar] [CrossRef]
- Dutta, S.; Dutta Chowdhury, A.; Biswas, S.; Park, E.Y.; Agnihotri, N.; De, A.; De, S. Development of an Effective Electrochemical Platform for Highly Sensitive DNA Detection Using MoS 2—Polyaniline Nanocomposites. Biochem. Eng. J. 2018, 140, 130–139. [Google Scholar] [CrossRef]
- Rasheed, P.A.; Sandhyarani, N. Graphene-DNA Electrochemical Sensor for the Sensitive Detection of BRCA1 Gene. Sens. Actuators B Chem. 2014, 204, 777–782. [Google Scholar] [CrossRef]
- Shoaie, N.; Forouzandeh, M.; Omidfar, K. Highly Sensitive Electrochemical Biosensor Based on Polyaniline and Gold Nanoparticles for DNA Detection. IEEE Sens. J. 2018, 18, 1835–1843. [Google Scholar] [CrossRef]
- Soni, A.; Pandey, C.M.; Solanki, S.; Kotnala, R.K.; Sumana, G. Electrochemical Genosensor Based on Template Assisted Synthesized Polyaniline Nanotubes for Chronic Myelogenous Leukemia Detection. Talanta 2018, 187, 379–389. [Google Scholar] [CrossRef]
- Zhu, Q.; Gao, F.; Yang, Y.; Zhang, B.; Wang, W.; Hu, Z.; Wang, Q. Electrochemical Preparation of Polyaniline Capped Bi2S3 Nanocomposite and Its Application in Impedimetric DNA Biosensor. Sens. Actuators B Chem. 2015, 207, 819–826. [Google Scholar] [CrossRef]
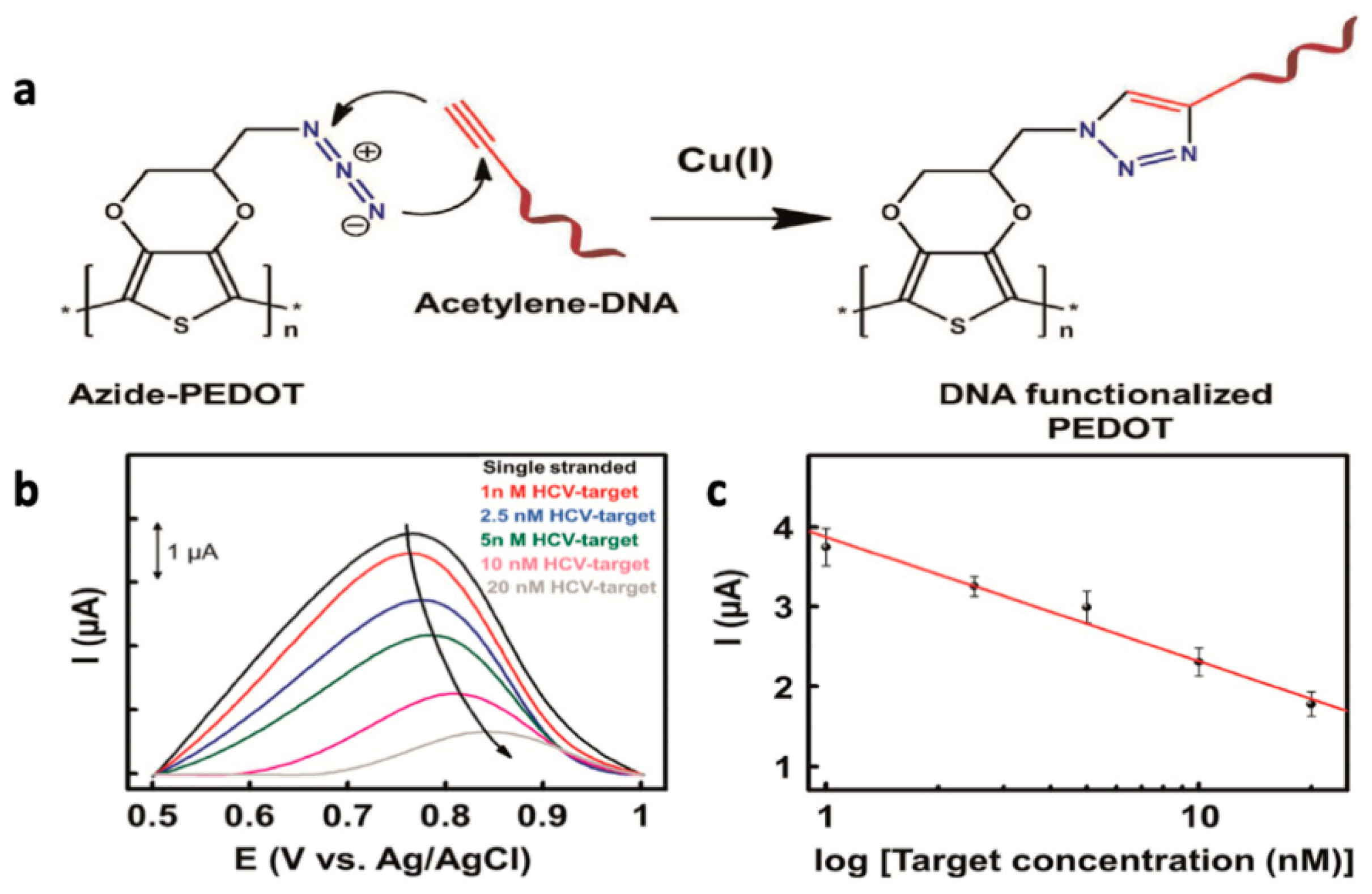
- Galán, T.; Prieto-Simón, B.; Alvira, M.; Eritja, R.; Götz, G.; Bäuerle, P.; Samitier, J. Label-Free Electrochemical DNA Sensor Using “Click”-Functionalized PEDOT Electrodes. Biosens. Bioelectron. 2015, 74, 751–756. [Google Scholar] [CrossRef]
- Aydemir, N.; Chan, E.; Baek, P.; Barker, D.; Williams, D.E.; Travas-Sejdic, J. New Immobilisation Method for Oligonucleotides on Electrodes Enables Highly-Sensitive, Electrochemical Label-Free Gene Sensing. Biosens. Bioelectron. 2017, 97, 128–135. [Google Scholar] [CrossRef]
- Aydemir, N.; Malmstrom, J.; Travas-Sejdic, J. Conducting Polymer Based Electrochemical Biosensors. Adv. Electrode Mater. 2016, 18, 8264–8277. [Google Scholar] [CrossRef]
- Cardoso, A.R.; Moreira, F.T.C.; Fernandes, R.; Sales, M.G.F. Novel and Simple Electrochemical Biosensor Monitoring Attomolar Levels of MiRNA-155 in Breast Cancer. Biosens. Bioelectron. 2016, 80, 621–630. [Google Scholar] [CrossRef]
- Song, K.M.; Lee, S.; Ban, C. Aptamers and Their Biological Applications. Sensors 2012, 12, 612–631. [Google Scholar] [CrossRef] [Green Version]
- Tuerk, C.; Gold, L. Systematic Evolution of Ligands by Exponential Enrichment: RNA Ligands to Bacteriophage T4 DNA Polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef]
- Maldarelli, C.; Jain, R.K.; Ivanov, I.B.; Ruckenstein, E. Stability of Symmetric and Unsymmetric Thin Liquid Films to Short and Long Wavelength Perturbations. J. Colloid Interface Sci. 1980, 78, 118–143. [Google Scholar] [CrossRef]
- Xu, D.; Xu, D.; Yu, X.; Liu, Z.; He, W.; Ma, Z. Label-Free Electrochemical Detection for Aptamer-Based Array Electrodes. Anal. Chem. 2005, 77, 5107–5113. [Google Scholar] [CrossRef]
- Huang, R.; Xi, Z.; Deng, Y.; He, N. Fluorescence Based Aptasensors for the Determination of Hepatitis B Virus e Antigen. Sci. Rep. 2016, 6, 31103. [Google Scholar] [CrossRef]
- Alsager, O.A.; Alotaib, K.M.; Alswieleh, A.M.; Alyamani, B.J. Colorimetric Aptasensor of Vitamin D3: A Novel Approach to Eliminate Residual Adhesion between Aptamers and Gold Nanoparticles. Sci. Rep. 2018, 8, 12947. [Google Scholar] [CrossRef]
- Jin, C.; Qiu, L.; Li, J.; Fu, T.; Zhang, X.; Tan, W. Cancer Biomarker Discovery Using DNA Aptamers. Analyst 2016, 141, 461–466. [Google Scholar] [CrossRef]
- Shen, G.; Zhang, H.; Yang, C.; Yang, Q.; Tang, Y. Thrombin Ultrasensitive Detection Based on Chiral Supramolecular Assembly Signal-Ampli Fi Ed Strategy Induced by Thrombin-Binding Aptamer. Anal. Chem. 2017, 89, 548–551. [Google Scholar] [CrossRef]
- Yoon, H.; Kim, J.; Lee, N.; Kim, B.; Jang, J. A Novel Sensor Platform Based on Aptamer-Conjugated Polypyrrole Nanotubes for Label-Free Electrochemical Protein Detection. ChemBioChem 2008, 9, 634–641. [Google Scholar] [CrossRef]
- Alpat, Ş.; Özdemir, K.; Kilinç Alpat, S. Voltammetric Determination of Epinephrine in Pharmaceutical Sample with a Tyrosinase Nanobiosensor. J. Sens. 2016, 2016, 5653975. [Google Scholar] [CrossRef]
- Moon, J.M.; Thapliyal, N.; Hussain, K.K.; Goyal, R.N.; Shim, Y.B. Conducting Polymer-Based Electrochemical Biosensors for Neurotransmitters: A Review. Biosens. Bioelectron. 2018, 102, 540–552. [Google Scholar] [CrossRef]
- Shahrokhian, S.; Saberi, R.S. Electrochemical Preparation of Over-Oxidized Polypyrrole/Multi-Walled Carbon Nanotube Composite on Glassy Carbon Electrode and Its Application in Epinephrine Determination. Electrochim. Acta 2011, 57, 132–138. [Google Scholar] [CrossRef]
- Johnson, T.A.; Jinnah, H.A.; Kamatani, N.; Stathis, C.G. Shortage of Cellular ATP as a Cause of Diseases and Strategies to Enhance ATP. Front. Pharm. 2019, 10, 98. [Google Scholar] [CrossRef]
- Li, Z.; Yin, J.; Gao, C.; Sheng, L.; Meng, A. A Glassy Carbon Electrode Modified with Graphene Oxide, Poly(3,4-Ethylenedioxythiophene), an Antifouling Peptide and an Aptamer for Ultrasensitive Detection of Adenosine Triphosphate. Microchim. Acta 2019, 186. [Google Scholar] [CrossRef]
- Xie, H.; Chai, Y.; Yuan, Y.; Yuan, R. Highly Effective Molecule Converting Strategy Based on Enzyme-Free Dual Recycling Amplification for Ultrasensitive Electrochemical Detection of ATP. Chem. Commun. 2017, 53, 8368–8371. [Google Scholar] [CrossRef]
- Daprà, J.; Lauridsen, L.H.; Nielsen, A.T.; Rozlosnik, N. Comparative Study on Aptamers as Recognition Elements for Antibiotics in a Label-Free All-Polymer Biosensor. Biosens. Bioelectron. 2013, 43, 315–320. [Google Scholar] [CrossRef]
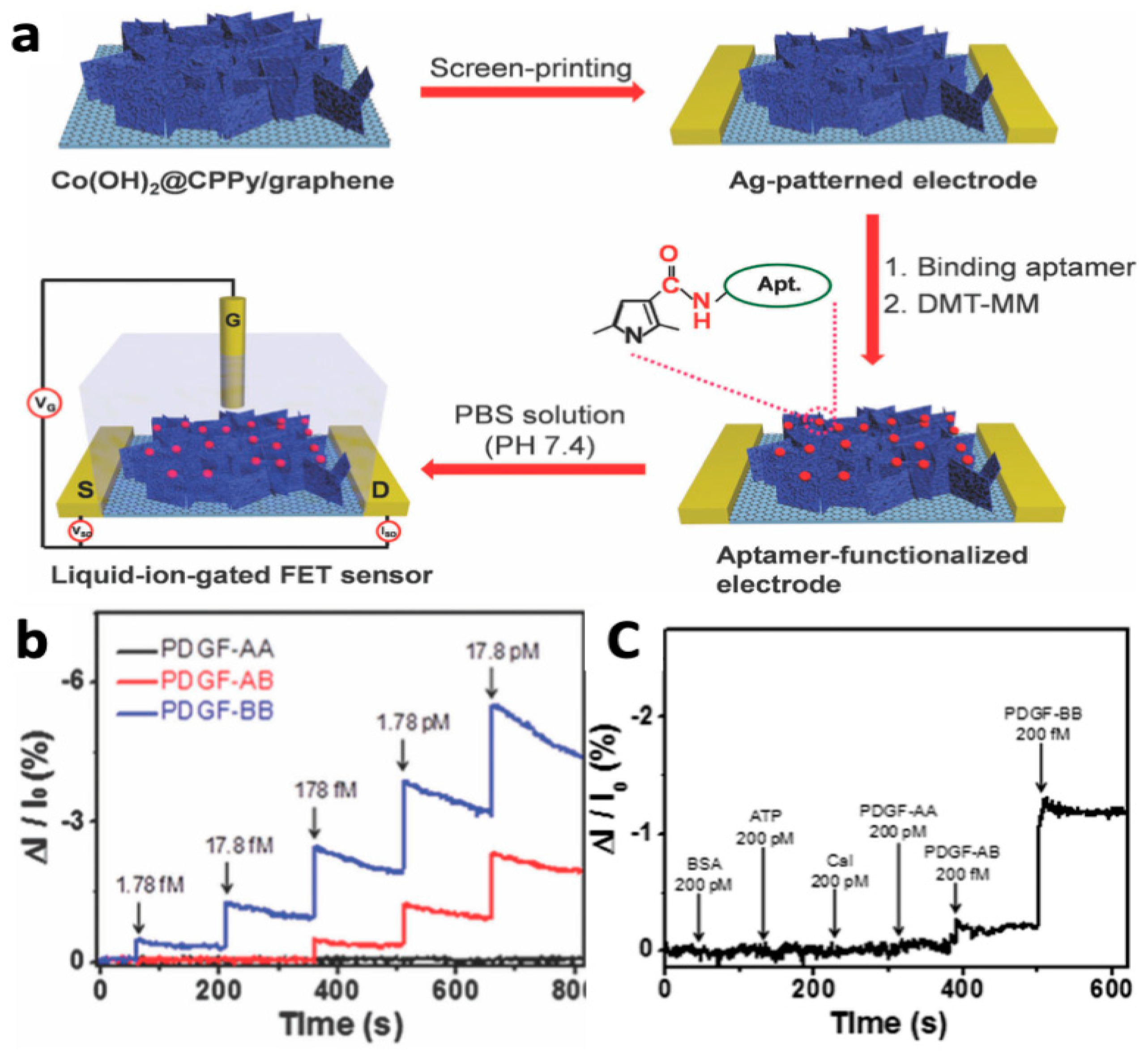
- Lee, J.S.; Kim, W.; Cho, S.; Jun, J.; Cho, K.H.; Jang, J. Multidimensional Hybrid Conductive Nanoplate-Based Aptasensor for Platelet-Derived Growth Factor Detection. J. Mater. Chem. B 2016, 4, 4447–4454. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Shadjou, N.; de la Guardia, M. Current Advancement in Electrochemical Analysis of Neurotransmitters in Biological Fluids. Trac Trends Anal. Chem. 2017, 86, 107–121. [Google Scholar] [CrossRef]
- Xu, G.; Jarjes, Z.A.; Desprez, V.; Kilmartin, P.A.; Travas-Sejdic, J. Sensitive, Selective, Disposable Electrochemical Dopamine Sensor Based on PEDOT-Modified Laser Scribed Graphene. Biosens. Bioelectron. 2018, 107, 184–191. [Google Scholar] [CrossRef]
- Taylor, I.M.; Robbins, E.M.; Catt, K.A.; Cody, P.A.; Happe, C.L.; Cui, X.T. Enhanced Dopamine Detection Sensitivity by PEDOT/Graphene Oxide Coating on in Vivo Carbon Fiber Electrodes. Biosens. Bioelectron. 2017, 89, 400–410. [Google Scholar] [CrossRef]
- Li, M.; Wang, W.; Chen, Z.; Song, Z.; Luo, X. Electrochemical Determination of Paracetamol Based on Au@graphene Core-Shell Nanoparticles Doped Conducting Polymer PEDOT Nanocomposite. Sens. Actuators B Chem. 2018, 260, 778–785. [Google Scholar] [CrossRef]
- Mandal, N.; Bhattacharjee, M.; Chattopadhyay, A.; Bandyopadhyay, D. Point-of-Care-Testing of α-Amylase Activity in Human Blood Serum. Biosens. Bioelectron. 2019, 124–125, 75–81. [Google Scholar] [CrossRef]
- Nater, U.M.; Rohleder, N.; Gaab, J.; Berger, S.; Jud, A.; Kirschbaum, C.; Ehlert, U. Human Salivary Alpha-Amylase Reactivity in a Psychosocial Stress Paradigm. Int. J. Psychophysiol. 2005, 55, 333–342. [Google Scholar] [CrossRef]
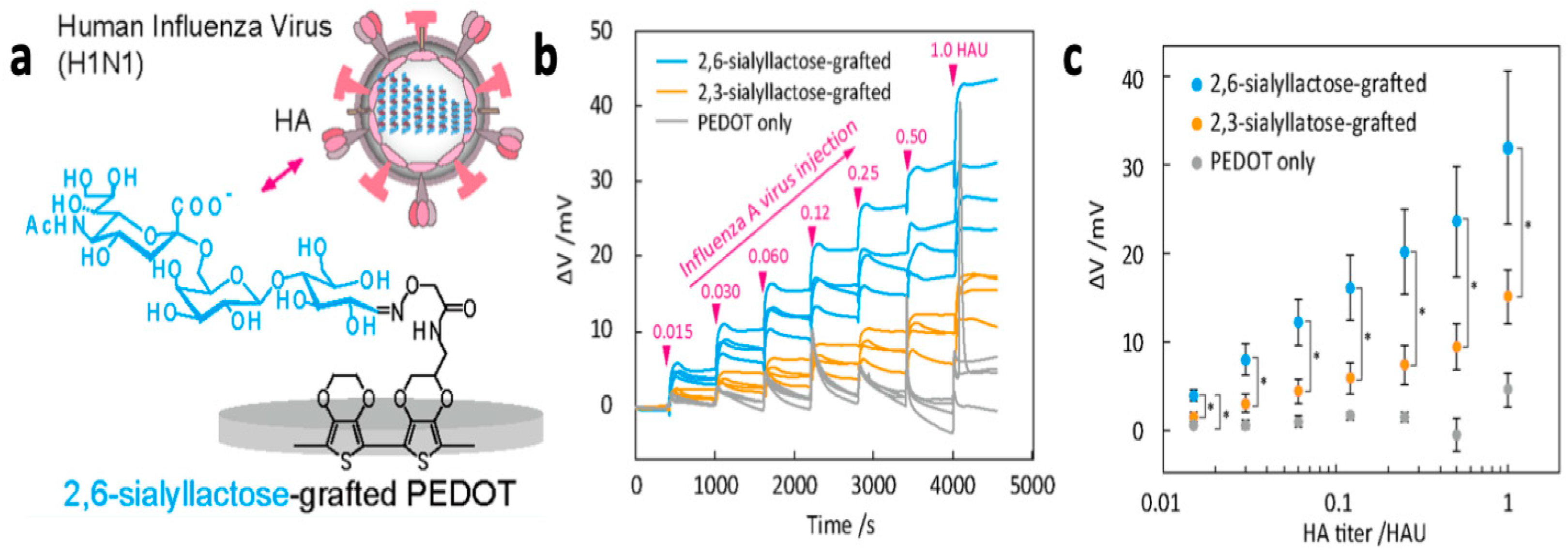
- Hai, W.; Goda, T.; Takeuchi, H.; Yamaoka, S.; Horiguchi, Y.; Matsumoto, A.; Miyahara, Y. Specific Recognition of Human Influenza Virus with PEDOT Bearing Sialic Acid-Terminated Trisaccharides. ACS Appl. Mater. Interfaces 2017, 9, 14162–14170. [Google Scholar] [CrossRef]
- Silva, B.V.M.; Rodríguez, B.A.G.; Sales, G.F.; Sotomayor, M.D.P.T.; Dutra, R.F. An Ultrasensitive Human Cardiac Troponin T Graphene Screen-Printed Electrode Based on Electropolymerized-Molecularly Imprinted Conducting Polymer. Biosens. Bioelectron. 2016, 77, 978–985. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive Polymers: Towards a Smart Biomaterial for Tissue Engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef]
- Tomczykowa, M.; Plonska-Brzezinska, M.E. Conducting Polymers, Hydrogels and Their Composites: Preparation, Properties and Bioapplications. Polymer 2019, 11, 350. [Google Scholar] [CrossRef]













© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Runsewe, D.; Betancourt, T.; Irvin, J.A. Biomedical Application of Electroactive Polymers in Electrochemical Sensors: A Review. Materials 2019, 12, 2629. https://doi.org/10.3390/ma12162629
Runsewe D, Betancourt T, Irvin JA. Biomedical Application of Electroactive Polymers in Electrochemical Sensors: A Review. Materials. 2019; 12(16):2629. https://doi.org/10.3390/ma12162629
Chicago/Turabian StyleRunsewe, Damilola, Tania Betancourt, and Jennifer A. Irvin. 2019. "Biomedical Application of Electroactive Polymers in Electrochemical Sensors: A Review" Materials 12, no. 16: 2629. https://doi.org/10.3390/ma12162629





