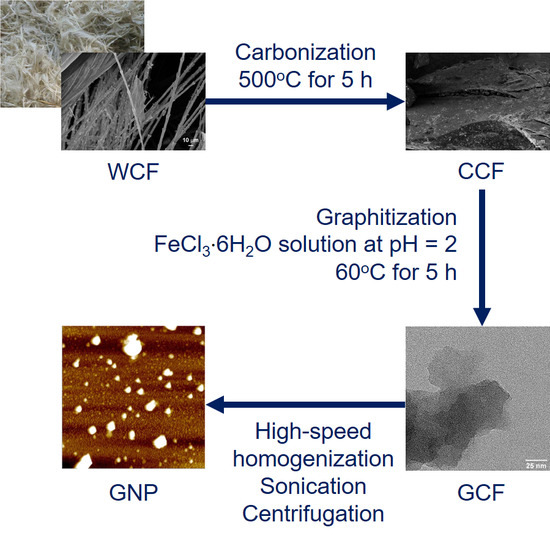
Graphite Nanoplatelets from Waste Chicken Feathers
Abstract
:1. Introduction
2. Materials and Methods
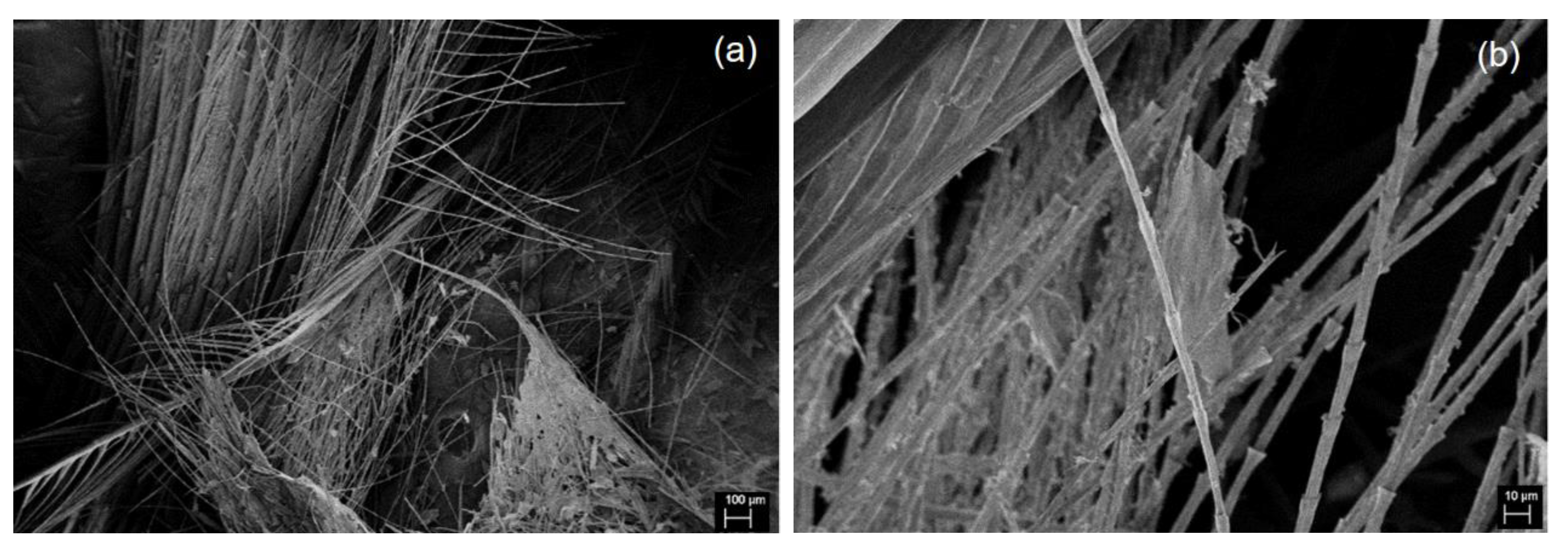
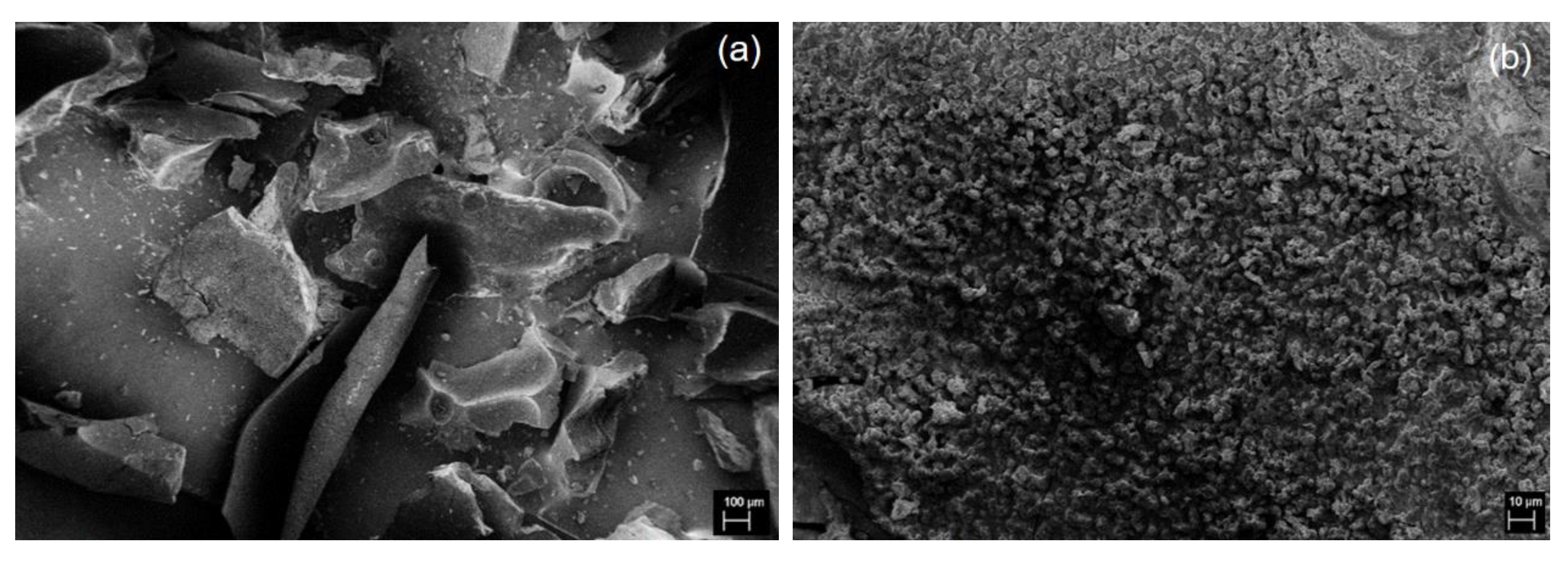
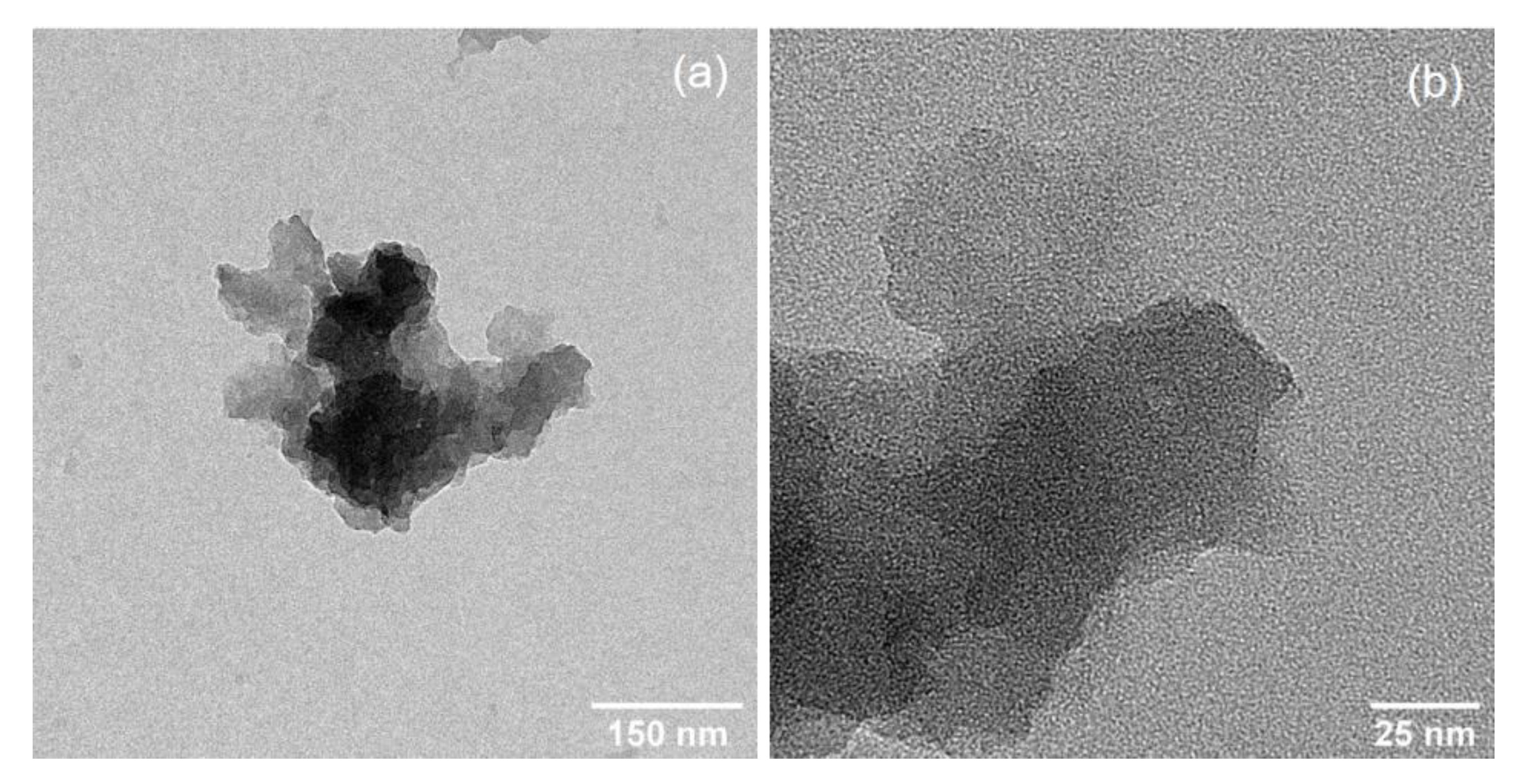
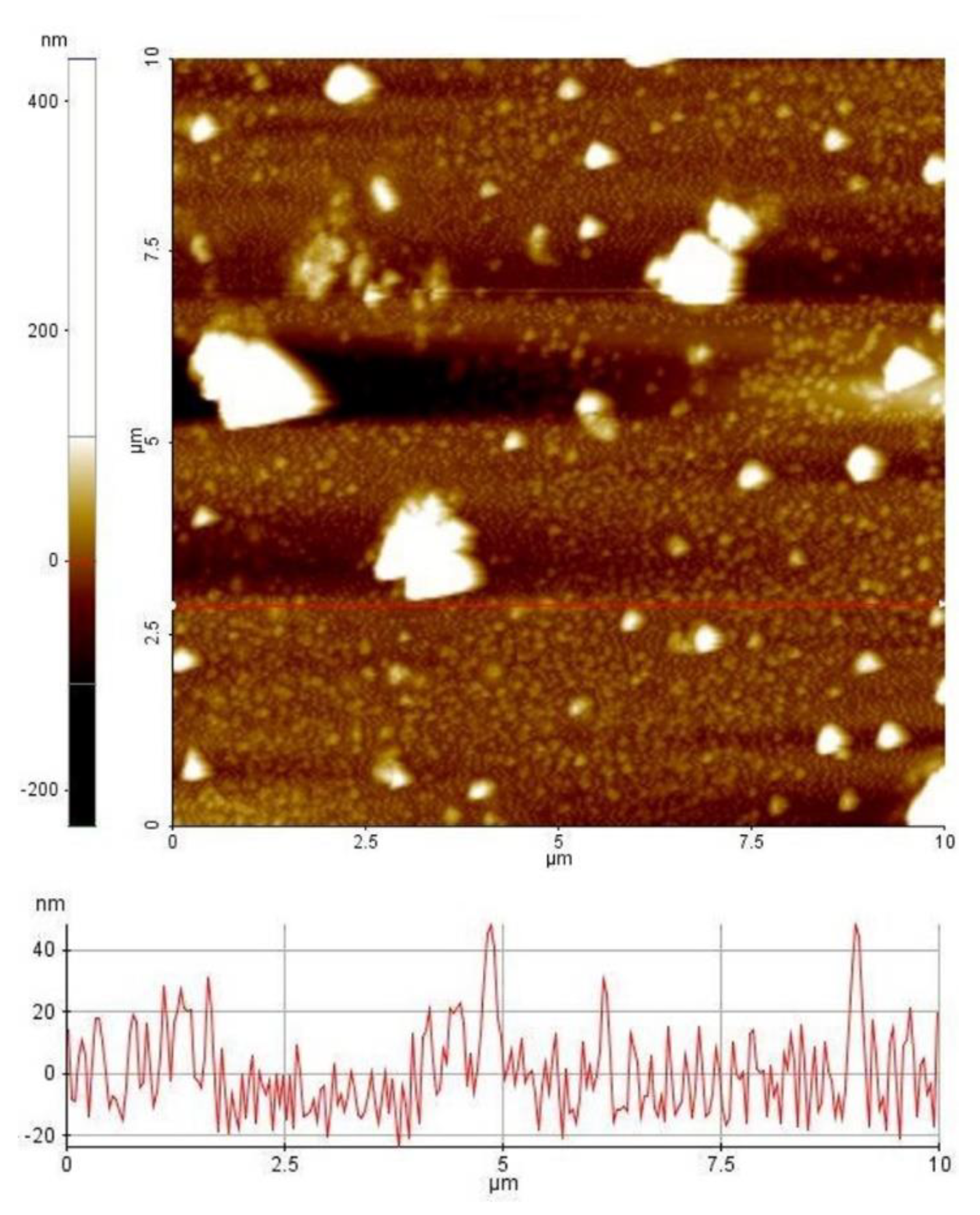
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Huang, X.; Zeng, L.; Li, R.; Tian, H.; Fu, X.; Wang, Y.; Zhong, W.H. A review of the electrical and mechanical properties of carbon nanofiller-reinforced polymer composites. J. Mater. Sci. 2019, 54, 1036–1076. [Google Scholar] [CrossRef]
- Afzal, A.; Kausar, A.; Siddiq, M. Perspectives of polystyrene composite with fullerene, carbon black, graphene, and carbon nanotube: A review. Polym. Plast. Technol. Eng. 2016, 55, 1988–2011. [Google Scholar] [CrossRef]
- Naz, A.; Kausar, A.; Siddiq, M.; Choudhary, M.A. Comparative review on structure, properties, fabrication techniques, and relevance of polymer nanocomposites reinforced with carbon nanotube and graphite fillers. Polym. Plast. Technol. Eng. 2016, 55, 171–198. [Google Scholar] [CrossRef]
- Chung, D.D. A review of exfoliated graphite. J. Mater. Sci. 2016, 51, 554–568. [Google Scholar] [CrossRef]
- Li, B.; Zhong, W.H. Review on polymer/graphite nanoplatelet nanocomposites. J. Mater. Sci. 2011, 46, 5595–5614. [Google Scholar] [CrossRef]
- Sengupta, R.; Bhattacharya, M.; Bandyopadhyay, S.; Bhowmick, A.K. A review on the mechanical and electrical properties of graphite and modified graphite reinforced polymer composites. Prog. Polym. Sci. 2011, 36, 638–670. [Google Scholar] [CrossRef]
- Zabihi, O.; Ahmadi, M.; Abdollahi, T.; Nikafshar, S.; Naebe, M. Collision-induced activation: Towards industrially scalable approach to graphite nanoplatelets functionalization for superior polymer nanocomposites. Sci. Rep. 2017, 7, 3560. [Google Scholar] [CrossRef] [Green Version]
- Van Thanh, D.; Van Thien, N.; Thang, B.H.; Van Chuc, N.; Hong, N.M.; Trang, B.T.; Dai Lam, T.; Huyen, D.T.; Hong, P.N.; Minh, P.N. A highly efficient and facile approach for fabricating graphite nanoplatelets. J. Electron. Mater. 2016, 45, 2522–2528. [Google Scholar] [CrossRef]
- Tian, X.; Itkis, M.E.; Bekyarova, E.B.; Haddon, R.C. Anisotropic thermal and electrical properties of thin thermal interface layers of graphite nanoplatelet-based composites. Sci. Rep. 2013, 3, 1710. [Google Scholar] [CrossRef] [Green Version]
- Xiang, J.; Drzal, L.T. Investigation of exfoliated graphite nanoplatelets (xGnP) in improving thermal conductivity of paraffin wax-based phase change material. Sol. Energy Mater. Sol. Cells 2011, 95, 1811–1818. [Google Scholar] [CrossRef]
- Geng, Y.; Wang, S.J.; Kim, J.K. Preparation of graphite nanoplatelets and graphene sheets. J. Colloid Interface Sci. 2009, 336, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Piner, R.D.; Chen, X.; Wu, N.; Nguyen, S.T.; Ruoff, R.S. Stable aqueous dispersions of graphitic nanoplatelets via the reduction of exfoliated graphite oxide in the presence of poly (sodium 4-styrenesulfonate). J. Mater. Chem. 2006, 16, 155–158. [Google Scholar] [CrossRef]
- Viculis, L.M.; Mack, J.J.; Mayer, O.M.; Hahn, H.T.; Kaner, B.B. Intercalation and exfoliation routes to graphite nanoplatelets. J. Mater. Chem. 2005, 15, 974–978. [Google Scholar] [CrossRef]
- Yu, A.; Ramesh, P.; Itkis, M.E.; Bekyarova, E.; Haddon, R.C. Graphite nanoplatelet− epoxy composite thermal interface materials. J. Phys. Chem. C 2007, 111, 7565–7569. [Google Scholar] [CrossRef]
- Jeddi, J.; Katbab, A.A.; Mehranvari, M. Investigation of microstructure, electrical behavior, and EMI shielding effectiveness of silicone rubber/carbon black/nanographite hybrid composites. Polym. Compos. 2019, 40, 4056–4066. [Google Scholar] [CrossRef]
- Raza, M.A.; Westwood, A.; Stirling, C. Graphite nanoplatelet/rubbery epoxy composites as adhesives and pads for thermal interface applications. J. Mater. Sci.—Mater. Electron. 2018, 29, 8822–8837. [Google Scholar] [CrossRef]
- Mhike, W.; Focke, W.W.; Asante, J.K. Rotomolded antistatic and flame-retarded graphite nanocomposites. J. Thermoplast. Compos. Mater. 2018, 31, 535–552. [Google Scholar] [CrossRef] [Green Version]
- Rosehr, A.; Griebe, D.; Luinstra, G.A. Graphite nanosheets-polypropylene composites from in toluene delaminated graphite using atactic polypropylene as dispersant. Compos. Sci. Technol. 2018, 156, 28–38. [Google Scholar] [CrossRef]
- Lin, S.; Anwer, M.A.; Zhou, Y.; Sinha, A.; Carson, L.; Naguib, H.E. Evaluation of the thermal, mechanical and dynamic mechanical characteristics of modified graphite nanoplatelets and graphene oxide high-density polyethylene composites. Compos. Part B 2018, 132, 61–68. [Google Scholar] [CrossRef]
- Sullivan, E.M.; Karimineghlani, P.; Naraghi, M.; Gerhardt, R.A.; Kalaitzidou, K. The effect of nanofiller geometry and compounding method on polylactic acid nanocomposite films. Eur. Polym. J. 2016, 77, 31–42. [Google Scholar] [CrossRef]
- Lv, Q.; Wu, D.; Qiu, Y.; Chen, J.; Yao, X.; Ding, K.; Wei, N. Crystallization of Poly (ϵ-caprolactone) composites with graphite nanoplatelets: Relations between nucleation and platelet thickness. Thermochim. Acta 2015, 612, 25–33. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, Y.; Chen, J.; Wu, D.; Qiu, Y.; Yao, X.; Zhou, Y.; Chen, C. Percolation networks and transient rheology of polylactide composites containing graphite nanosheets with various thicknesses. Polymer 2015, 67, 216–226. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, H.; Tang, M.; Tu, W.; Zhang, X. Improved thermal property of a multilayered graphite nanoplatelets filled silicone resin composite. J. Mater. Eng. Perform. 2015, 24, 920–929. [Google Scholar] [CrossRef]
- Plyushch, A.; Macutkevic, J.; Samulionis, V.; Banys, J.; Bychanok, D.; Kuzhir, P.; Mathieu, S.; Fierro, V.; Celzard, A. Synergetic effect of triglycine sulfate and graphite nanoplatelets on dielectric and piezoelectric properties of epoxy resin composites. Polym. Compos. 2019, 40, E1181–E1188. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, S.; Shariatpanahi, H.; Taromi, F.A.; Neshati, J. Electrochemical and anticorrosion behaviors of hybrid functionalized graphite nano-platelets/tripolyphosphate in epoxy-coated carbon steel. Mater. Res. Bull. 2016, 80, 7–22. [Google Scholar] [CrossRef]
- Xiang, J.; Drzal, L.T. Thermal conductivity of exfoliated graphite nanoplatelet paper. Carbon 2011, 49, 773–778. [Google Scholar] [CrossRef]
- Quan, H.; Zhang, B.Q.; Zhao, Q.; Yuen, R.K.; Li, R.K. Facile preparation and thermal degradation studies of graphite nanoplatelets (GNPs) filled thermoplastic polyurethane (TPU) nanocomposites. Compos. Part A 2009, 40, 1506–1513. [Google Scholar] [CrossRef]
- Li, J.; Vaisman, L.; Marom, G.; Kim, J.K. Br treated graphite nanoplatelets for improved electrical conductivity of polymer composites. Carbon 2007, 45, 744–750. [Google Scholar] [CrossRef] [Green Version]
- Cho, D.; Lee, S.; Yang, G.; Fukushima, H.; Drzal, L.T. Dynamic mechanical and thermal properties of phenylethynyl-terminated polyimide composites reinforced with expanded graphite nanoplatelets. Macromol. Mater. Eng. 2005, 290, 179–187. [Google Scholar] [CrossRef]
- Chen, G.; Weng, W.; Wu, D.; Wu, C.; Lu, J.; Wang, P.; Chen, X. Preparation and characterization of graphite nanosheets from ultrasonic powdering technique. Carbon 2004, 42, 753–759. [Google Scholar] [CrossRef]
- Jara, A.D.; Betemariam, A.; Woldetinsae, G.; Kim, J.Y. Purification, application and current market trend of natural graphite: A review. Int. J. Min. Sci. Technol. 2019, 29, 671–689. [Google Scholar] [CrossRef]
- Olson, D.W.; Virta, R.L.; Mahdavi, M.; Sangine, E.S.; Fortier, S.M. Natural graphite demand and supply—Implications for electric vehicle battery requirements. Geol. Soc. Am. Spec. Pap. 2016, 520, 67–77. [Google Scholar]
- Zhang, W.; Liu, Z.; Xia, J.; Li, F.; He, W.; Li, G.; Huang, J. Preparing graphene from anode graphite of spent lithium-ion batteries. Front. Environ. Sci. Eng. 2017, 11, 6. [Google Scholar] [CrossRef]
- Esparza, Y.; Ullah, A.; Wu, J. Preparation and characterization of graphite oxide nano-reinforced biocomposites from chicken feather keratin. J. Chem. Technol. Biot. 2017, 92, 2023–2031. [Google Scholar] [CrossRef]
- Gao, L.; Li, R.; Sui, X.; Li, R.; Chen, C.; Chen, Q. Conversion of chicken feather waste to N-doped carbon nanotubes for the catalytic reduction of 4-nitrophenol. Environ. Sci. Technol. 2014, 48, 10191–10197. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, D.; Wu, C.; Gu, S. State-of-the-art on the production and application of carbon nanomaterials from biomass. Green Chem. 2018, 20, 5031–5057. [Google Scholar] [CrossRef] [Green Version]
- Purkait, T.; Singh, G.; Singh, M.; Kumar, D.; Dey, R.S. Large area few-layer graphene with scalable preparation from waste biomass for high-performance supercapacitor. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.K.; Singh, D.P. Natural and waste hydrocarbon precursors for the synthesis of carbon based nanomaterials: Graphene and CNTs. Renew. Sust. Energ. Rev. 2016, 58, 976–1006. [Google Scholar] [CrossRef]
- Zhuo, C.; Alves, J.O.; Tenorio, J.A.; Levendis, Y.A. Synthesis of carbon nanomaterials through up-cycling agricultural and municipal solid wastes. Ind. Eng. Chem. Res. 2012, 51, 2922–2930. [Google Scholar] [CrossRef]
- Alves, J.O.; Zhuo, C.; Levendis, Y.A.; Tenório, J.A. Catalytic conversion of wastes from the bioethanol production into carbon nanomaterials. Appl. Catal. B-Environ. 2011, 106, 433–444. [Google Scholar] [CrossRef]
- Cataldi, P.; Condurache, O.; Spirito, D.; Krahne, R.; Bayer, I.S.; Athanassiou, A.; Perotto, G. Keratin-graphene nanocomposite: Transformation of waste wool in electronic devices. ACS Sustain. Chem. Eng. 2019, 7, 12544–12551. [Google Scholar] [CrossRef]
- Tesfaye, T.; Sithole, B.; Ramjugernath, D. Valorisation of chicken feathers: A review on recycling and recovery route—current status and future prospects. Clean Technol. Envir. 2017, 19, 2363–2378. [Google Scholar] [CrossRef]
- Gurav, R.G.; Jadhav, J.P. A novel source of biofertiliser from feather biomass for banana cultivation. Environ. Sci. Pollut. Res. 2013, 20, 4532–4539. [Google Scholar] [CrossRef] [PubMed]
- Ozdal, M.; Kurbanoglu, E.B. Citric acid production by Aspergillus niger from agro-industrial by-products: Molasses and chicken feather peptone. Waste Biomass Valori. 2019, 10, 631–640. [Google Scholar] [CrossRef]
- del Río, F.; Boado, M.G.; Rama, A.; Guitián, F. A comparative study on different aqueous-phase graphite exfoliation methods for few-layer graphene production and its application in alumina matrix composites. J. Eur. Ceram. Soc. 2017, 37, 3681–3693. [Google Scholar]
- Munuera, J.M.; Paredes, J.I.; Villar-Rodil, S.; Ayán-Varela, M.; Pagán, A.; Aznar-Cervantes, S.D.; Cenis, J.L.; Martínez-Alonso, A.; Tascón, J.M.D. High quality, low oxygen content and biocompatible graphene nanosheets obtained by anodic exfoliation of different graphite types. Carbon 2015, 94, 729–739. [Google Scholar] [CrossRef] [Green Version]
- Akhavan, O.; Bijanzad, K.; Mirsepah, A. Synthesis of graphene from natural and industrial carbonaceous wastes. RSC Adv. 2014, 4, 20441–20448. [Google Scholar] [CrossRef]
- Kun, P.; Wéber, F.; Balázsi, C. Preparation and examination of multilayer graphene nanosheets by exfoliation of graphite in high efficient attritor mill. Cent. Eur. J. Chem. 2011, 9, 47–51. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Structure and properties of chicken feather barbs as natural protein fibers. J. Polym. Environ. 2007, 15, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Wang, Y.; Li, M.; Yang, R. High performance N-doped porous activated carbon based on chicken feather for supercapacitors and CO2 capture. RSC Adv. 2015, 5, 34803–34811. [Google Scholar] [CrossRef]
- Thompson, E.; Danks, A.E.; Bourgeois, L.; Schnepp, Z. Iron-catalyzed graphitization of biomass. Green Chem. 2015, 17, 551–556. [Google Scholar] [CrossRef]
- Pajarito, B.B.; Cayabyab, C.A.; Costales, P.A.; Francisco, J.R. Exfoliated graphite/acrylic composite film as hydrophobic coating of 3D-printed polylactic acid surfaces. J. Coat. Technol. Res. 2019, 16, 1133–1140. [Google Scholar] [CrossRef]
- Li, J.; Östling, M. Prevention of graphene restacking for performance boost of supercapacitors—A review. Crystals 2013, 3, 163–190. [Google Scholar] [CrossRef]
- Shahriary, L.; Athawale, A.A. Graphene oxide synthesized by using modified Hummers approach. Int. J. Renew. Energy Environ. Eng. 2014, 2, 58–63. [Google Scholar]
- Ţucureanu, V.; Matei, A.; Avram, A.M. FTIR spectroscopy for carbon family study. Crit. Rev. Anal. Chem. 2016, 46, 502–520. [Google Scholar] [CrossRef]
- Tesfaye, T.; Sithole, B.; Ramjugernath, D.; Chunilall, V. Valorisation of chicken feathers: Characterisation of chemical properties. Waste Manag. 2017, 68, 626–635. [Google Scholar] [CrossRef]
- Ma, B.; Qiao, X.; Hou, X.; Yang, Y. Pure keratin membrane and fibers from chicken feather. Int. J. Biol. Macromol. 2016, 89, 614–621. [Google Scholar] [CrossRef] [Green Version]
- Tuna, A.; Okumuş, Y.; Celebi, H.; Seyhan, A.T. Thermochemical conversion of poultry chicken feather fibers of different colors into microporous fibers. J. Anal. Appl. Pyrolysis 2015, 115, 112–124. [Google Scholar] [CrossRef]
- Yi, M.; Shen, Z.; Ma, S.; Zhang, X. A mixed-solvent strategy for facile and green preparation of graphene by liquid-phase exfoliation of graphite. J. Nanopart. Res. 2012, 14, 1003. [Google Scholar] [CrossRef]
- Huh, S.H. Thermal reduction of graphene oxide. In Physics and Applications of Graphene—Experiments; Mikhailov, S., Ed.; InTech: Rijeka, Croatia, 2011; pp. 73–90. [Google Scholar]
- Huang, H.H.; De Silva, K.K.; Kumara, G.R.; Yoshimura, M. Structural evolution of hydrothermally derived reduced graphene oxide. Sci. Rep. 2018, 8, 6849. [Google Scholar] [CrossRef]
- Sole, C.; Drewett, N.E.; Liu, F.; Abdelkader, A.M.; Kinloch, I.A.; Hardwick, L.J. The role of re-aggregation on the performance of electrochemically exfoliated many-layer graphene for Li-ion batteries. J. Electroanal. Chem. 2015, 753, 35–41. [Google Scholar] [CrossRef]
- Blanton, T.N.; Barnes, C.L. Quantitative analysis of calcium oxide desiccant conversion to calcium hydroxide using X-ray diffraction. Int. Cent. Differ. Data. 2005, 48, 45–51. [Google Scholar] [CrossRef]
- Kamhi, S.R. On the structure of vaterite CaCO3. Acta Crystallogr. 1963, 16, 770–772. [Google Scholar] [CrossRef]
- Showell, M.S. Handbook of Detergents Part D: Formulation; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Hoekstra, J.; Beale, A.M.; Soulimani, F.; Versluijs-Helder, M.; Geus, J.W.; Jenneskens, L.W. Base metal catalyzed graphitization of cellulose: A combined Raman spectroscopy, temperature-dependent X-ray diffraction and high-resolution transmission electron microscopy study. J. Phys. Chem. C 2015, 119, 10653–10661. [Google Scholar] [CrossRef]
- Rhim, Y.R.; Zhang, D.; Fairbrother, D.H.; Wepasnick, K.A.; Livi, K.J.; Bodnar, R.J.; Nagle, D.C. Changes in electrical and microstructural properties of microcrystalline cellulose as function of carbonization temperature. Carbon 2010, 48, 1012–1024. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Jawhari, T.; Roid, A.; Casado, J. Raman spectroscopic characterization of some commercially available carbon black materials. Carbon 1995, 33, 1561–1565. [Google Scholar] [CrossRef]
- Cuesta, A.; Dhamelincourt, P.; Laureyns, J.; Martinez-Alonso, A.; Tascón, J.D. Raman microprobe studies on carbon materials. Carbon 1994, 32, 1523–1532. [Google Scholar] [CrossRef]
- Muzyka, R.; Drewniak, S.; Pustelny, T.; Chrubasik, M.; Gryglewicz, G. Characterization of graphite oxide and reduced graphene oxide obtained from different graphite precursors and oxidized by different methods using Raman spectroscopy. Materials 2018, 11, 1050. [Google Scholar] [CrossRef] [Green Version]
- Yi, M.; Shen, Z. Kitchen blender for producing high-quality few-layer graphene. Carbon 2014, 78, 622–626. [Google Scholar] [CrossRef]
- Xu, Y.; Cao, H.; Xue, Y.; Li, B.; Cai, W. Liquid-phase exfoliation of graphene: An overview on exfoliation media, techniques, and challenges. Nanomaterials 2018, 8, 942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gayathri, S.; Jayabal, P.; Kottaisamy, M.; Ramakrishnan, V. Synthesis of few layer graphene by direct exfoliation of graphite and a Raman spectroscopic study. AIP Adv. 2014, 4, 027116. [Google Scholar] [CrossRef]





| Sample | Peak (cm−1) | FWHM (cm−1) | I/ITotal (%) |
|---|---|---|---|
| GNP | 1560.1 | 167.7 | 49.7 |
| 1431.1 | 84.3 | 8.1 | |
| 1340.6 | 128.0 | 27.8 | |
| 1224.4 | 108.1 | 9.2 | |
| 1114.0 | 71.6 | 1.6 | |
| GCF | 1560.3 | 154.0 | 46.2 |
| 1435.8 | 96.5 | 10.4 | |
| 1337.6 | 138.6 | 34.8 | |
| 1222.0 | 94.9 | 6.8 | |
| 1137.6 | 38.6 | 0.2 | |
| CCF | 1560.1 | 165.1 | 44.2 |
| 1432.9 | 84.4 | 7.1 | |
| 1337.8 | 140.4 | 31.2 | |
| 1225.5 | 86.7 | 5.2 | |
| 1140.1 | 191.6 | 9.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pajarito, B.; Belarmino, A.J.; Calimbas, R.M.; Gonzales, J.R. Graphite Nanoplatelets from Waste Chicken Feathers. Materials 2020, 13, 2109. https://doi.org/10.3390/ma13092109
Pajarito B, Belarmino AJ, Calimbas RM, Gonzales JR. Graphite Nanoplatelets from Waste Chicken Feathers. Materials. 2020; 13(9):2109. https://doi.org/10.3390/ma13092109
Chicago/Turabian StylePajarito, Bryan, Amelia Jane Belarmino, Rizza Mae Calimbas, and Jillian Rae Gonzales. 2020. "Graphite Nanoplatelets from Waste Chicken Feathers" Materials 13, no. 9: 2109. https://doi.org/10.3390/ma13092109