Formation of ZrC–SiC Composites from the Molecular Scale through the Synthesis of Multielement Polymers
Abstract
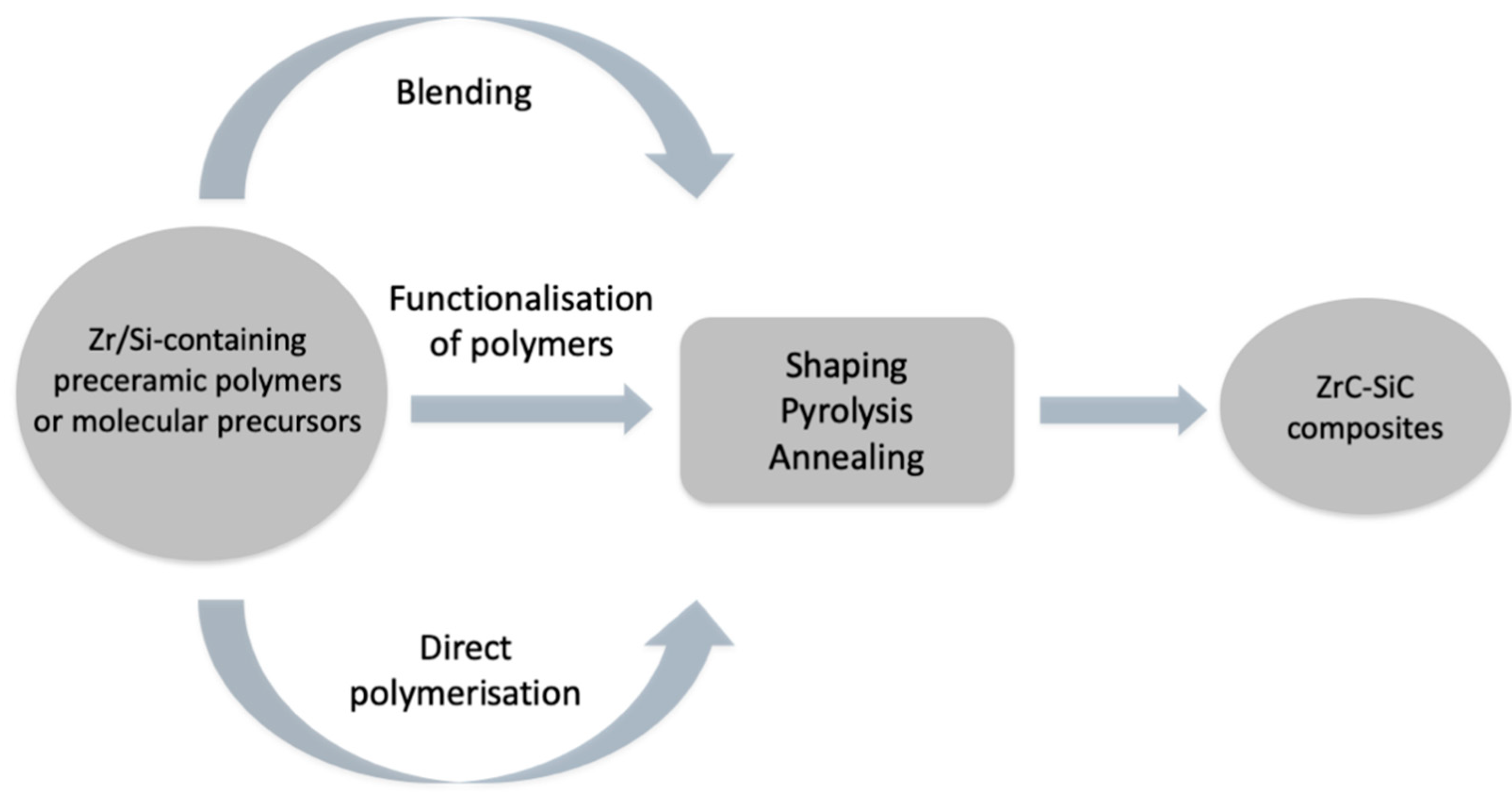
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterisations
2.3. Synthesis
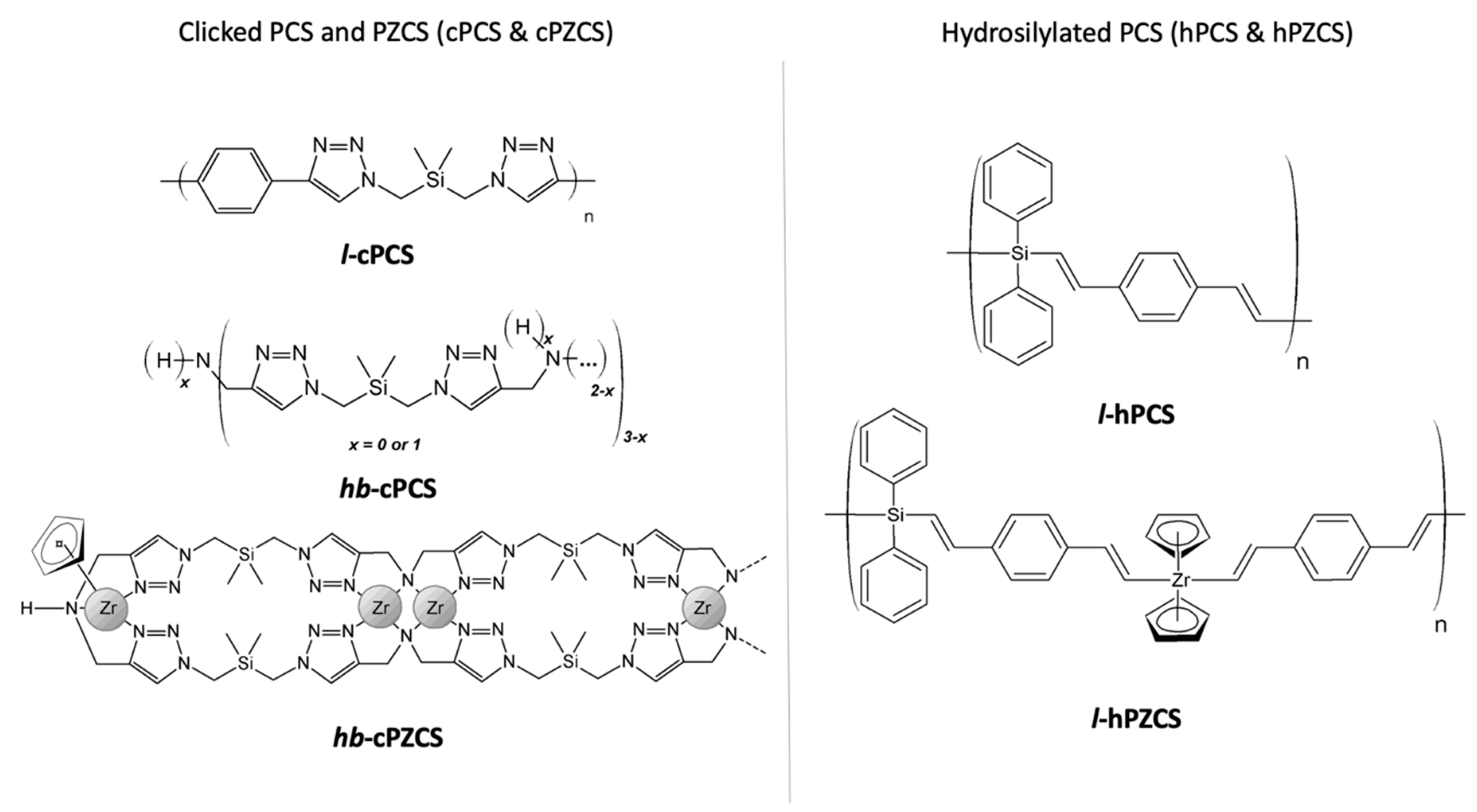
2.3.1. Polycycloaddition (CuAAC)
Linear polycarbosilane (l-cPCS, as linear-clicked PCS)
Hyperbranched polycarbosilane (hb-cPCS, as hyperbranched-clicked PCS)
Zr-modified hyperbranched polycarbosilane (hb-cPZCS, as hyperbranched-clicked Zr-containing PCS)
2.3.2. Hydrosilylation
Linear polycarbosilane obtained by hydrosilylation (l-hPCS, as linear-hydrosilylated PCS)
Polyzirconocarbosilane obtained by hydrosilylation (l-hPZCS, as linear-hydrosilylated Zr-containing PCS)
2.4. Pyrolysis
3. Results and Discussion
3.1. Characterisation of the Polymeric Precursors
3.1.1. Polycarbosilanes (l-cPCS, hb-cPCS, and l-hPCS)
3.1.2. Zr-Containing Polycarbosilanes (hb-cPZCS, l-hPZCS)
| Polymer | Reaction | Aspect | Solubility in Toluene * | Polymer Yield (%) |
|---|---|---|---|---|
| l-cPCS | Click-chemistry (CuAAC) | Brown solid | - | 84 |
| hb-cPCS | Click-chemistry (CuAAC) | Orange gel | - | 92 |
| hb-cPZCS | Click-chemistry (CuAAC) | Black solid | - | 66 |
| l-hPCS | Hydrosilylation | Orange solid | ++ | 93 |
| l-hPZCS | Hydrosilylation & Hydrozirconation | Brown solid | -- | 87 |
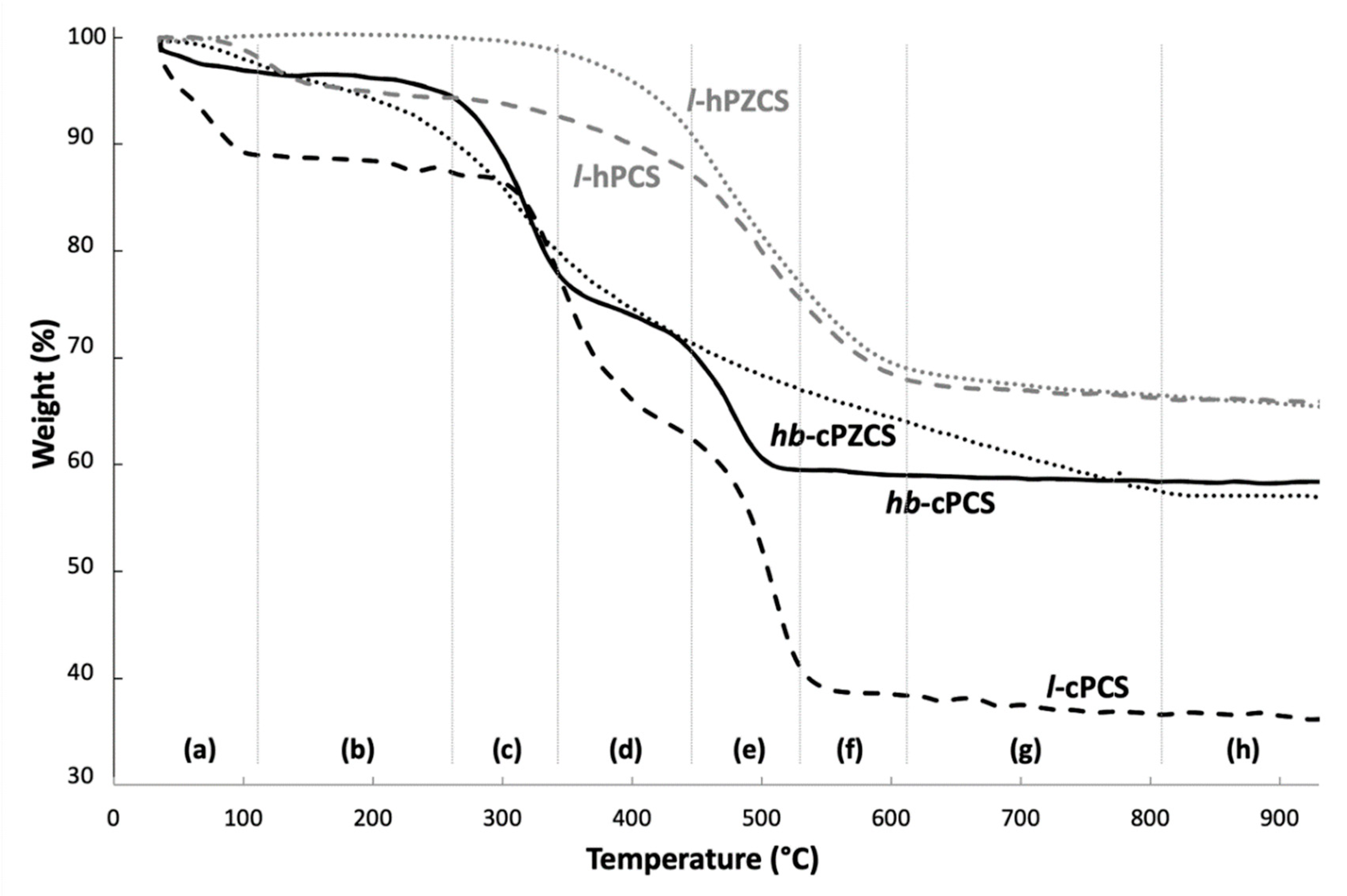
3.2. Thermal Behaviour of the Preceramic Polymers
3.2.1. Polycarbosilanes
PCS Obtained through CuAAC: l-cPCS and hb-cPCS
Two Linear Preceramic Precursors: l-cPCS and l-hPCS
3.2.2. Zr-Containing Polycarbosilanes (PZCS)
PCS and PZCS Obtained through Click-Chemistry: hb-cPCS and hb-cPZCS
PCS and PZCS Obtained through Hydrosilylation: l-hPCS and l-hPZCS
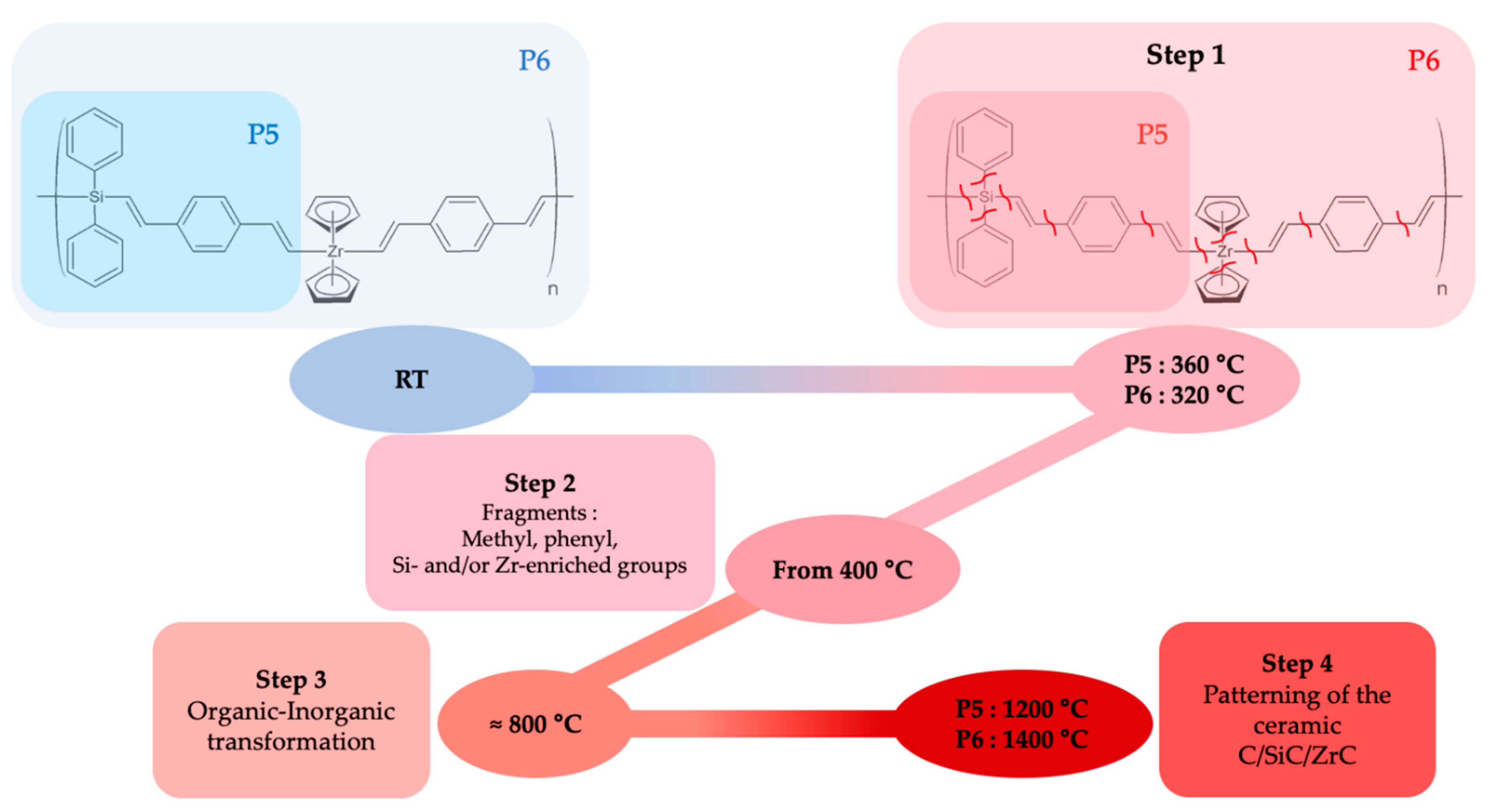
Ceramisation Mechanisms
3.3. Ceramics Issued from l-hPCS and l-hPZCS
3.4. Overview of the Thermal Behaviour of the Different Preceramic Polymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balasubramanian, K.; Sultan, M.T.H.; Rajeswari, N. Manufacturing techniques of composites for aerospace applications. In Sustainable Composites for Aerospace Applications; Jawaid, M., Thariq, M., Eds.; Woodhead Publishing Series in Composites Science and Engineering; Woodhead Publishing: Royston Rd, UK; Duxford, UK, 2018; pp. 55–67. [Google Scholar]
- Rana, S.; Fangueiro, R. (Eds.) Advanced composites in aerospace engineering. In Advanced Composites in Aerospace Engineering; Woodhead Publishing Series in Composites Science and Engineering; Woodhead Publishing: Royston Rd, UK; Duxford, UK, 2016; pp. 1–15. [Google Scholar]
- Levine, S.R.; Opila, E.J.; Halbig, M.C.; Kiser, J.D.; Singh, M.; Salem, J.A. Evaluation of ultra-high temperature ceramics for aeropropulsion use. J. Eur. Ceram. Soc. 2002, 22, 2757–2767. [Google Scholar] [CrossRef]
- Ionescu, E.; Bernard, S.; Lucas, R.; Kroll, P.; Ushakov, S.; Navrotsky, A.; Riedel, R. Polymer-derived ultra-high temperature ceramics (UHTCs) and related materials. Adv. Eng. Mater. 2019, 21, 1900269. [Google Scholar] [CrossRef] [Green Version]
- Opeka, M.M.; Talmy, I.G.; Wuchina, E.J.; Zaykoski, J.A.; Causey, S.J. Mechanical, thermal, and oxidation properties of refractory hafnium and zirconium compounds. J. Eur. Ceram. Soc. 1999, 19, 2405–2414. [Google Scholar] [CrossRef] [Green Version]
- Balat, M.J.H. Determination of the active-to-passive transition in the oxidation of silicon carbide in standard and microwave-excited air. J. Eur. Ceram. Soc. 1996, 16, 55. [Google Scholar] [CrossRef]
- Columbo, P.; Mera, G.; Riedel, R.; Sorarù, G.D. Polymer-derived ceramics: 40 years of research and innovation in advanced ceramics. J. Am. Ceram. Soc. 2010, 93, 1805–1837. [Google Scholar] [CrossRef]
- Yajima, S.; Okamura, K.; Hayashi, J.; Omori, M. Synthesis of continuous sic fibers with high tensile strength. J. Am. Ceram. Soc. 1976, 59, 324–327. [Google Scholar] [CrossRef]
- Greil, P. Polymer derived engineering ceramics. Adv. Eng. Mater. 2000, 2, 339–348. [Google Scholar] [CrossRef]
- Riedel, R.; Mera, G.; Hauser, R.; Klonczynski, A. Silicon-based Polymer-derived ceramics: Synthesis properties and applications-a review. J. Ceram. Soc. Jpn. 2006, 114, 425–444. [Google Scholar] [CrossRef] [Green Version]
- Bouzat, F.; Darsy, G.; Foucaud, S.; Lucas, R. Group 4 metal-containing polymers: An overview. Polym. Rev. 2016, 56, 187–224. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L.; Cheng, L.; Wang, Y.; Yu, Z.; Huang, M.; Tu, H.; Xia, H. Effect of the polycarbosilane structure on its final ceramic yield. J. Eur. Ceram. Soc. 2008, 28, 887–891. [Google Scholar] [CrossRef]
- Yu, Z.; Min, H.; Yang, L.; Feng, Y.; Zhang, P.; Riedel, R. Influence of the architecture of dendritic-like polycarbosilanes on the ceramic yield. J. Eur. Ceram. Soc. 2014, 35, 1161–1171. [Google Scholar] [CrossRef]
- Wen, Q.; Xu, Y.; Xu, B.; Fasel, C.; Guillon, O.; Buntkowsky, G.; Yu, Z.; Riedel, R.; Ionescu, E. Single-source-precursor synthesis of dense SiC/HfCxN1-x-based ultrahigh-temperature ceramic nanocomposites. Nanoscale 2014, 6, 13678–13689. [Google Scholar] [CrossRef] [PubMed]
- Tornoe, C.W.; Meldal, M. Peptidotriazoles: Copper(I)-catalysed 1,3-dipolar cycloadditions on solid-phase. In Peptides:the wave of the future, Proceedings of the Second International and the Seventeenth American Peptide Symposium, San Diego, CA, USA, 9–14 June 2001; Lebl, M., Houghten, R.A., Eds.; Springer: Dordrecht, Netherlands, 2001; pp. 263–264. [Google Scholar]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise huisgen cycloaddition process: Copper(I)-catalyzed regioselective “Ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Meldal, M. Polymer “clicking” by CuAAC reactions. Macromol. Rapid Commun. 2008, 29, 1016–1051. [Google Scholar] [CrossRef]
- Drohmann, C.; Möller, M.; Gorbatsevich, O.B.; Muzafarov, A.M. Hyperbranched polyalkenylsilanes by hydrosilylation with platinum catalysts. I. Polymerization. J. Polym. Sci. Pol. Chem. 2000, 38, 741–751. [Google Scholar] [CrossRef]
- Sanchez, J.C.; Trogler, W.C. Hydrosilylation of dyines as a route to functional polymers delocalized through silicon. Macromol. Chem. Phys. 2008, 209, 1527–1540. [Google Scholar] [CrossRef]
- Wang, Y.; Xang, D.; Han, J.; Feng, S. Facile synthesis of σ–π conjugated organosilicon polymers via click polymerization. J. Organomet. Chem. 2011, 696, 1874–1878. [Google Scholar] [CrossRef]
- Nomura, N.; Ishii, R.; Yamamoto, Y.; Kondo, T. Stereoselective ring-opening polymerization of a racemic lactide by using achiral salen– and homosalen–aluminum complexes. Chem. Eur. J. 2007, 13, 4433–4451. [Google Scholar] [CrossRef]
- Bouzat, F.; Foucaud, S.; Leconte, Y.; Maître, A.; Lucas, R. From click to ceramic: An efficient way to generate multielement Si/Zr/C clicked-polymer-derived ceramics (cPDC). Mater. Lett. 2013, 106, 337–340. [Google Scholar] [CrossRef]
- Schunack, M.; Gragert, M.; Döhler, D.; Michael, P.; Binder, W.H. Low-temperature Cu(I)-Catalyzed “Click” reactions for self-healing polymers. Macromol. Chem. Phys. 2012, 213, 205–214. [Google Scholar] [CrossRef]
- Schwartz, J.; Labinger, J.A. Hydrozirconation: A new transition metal reagent for organic synthesis. Angew. Chem. Int. Ed. 1976, 15, 333–340. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L.; Cheng, L.; Yu, Z.; Huang, M.; Tu, H.; Xia, H. Effect of curing and pyrolysis processing on the ceramic yield of a highly branched polycarbosilane. J. Mater. Sci. 2008, 44, 721–725. [Google Scholar] [CrossRef] [Green Version]
- Dasan, A.; Lucas, R.; Laborde, E.; Piriou, C.; Foucaud, S. Towards a surface functionalisation and grafting of a polycarbosilane onto zirconium carbide particles for the development of hybrid core-shell structures. Appl. Surf. Sci. 2019, 495, 143409. [Google Scholar] [CrossRef]
- Mocacer, D.; Pailler, R.; Naslain, R.; Richard, C.; Pillot, J.P.; Dunogues, J.; Gerardin, C.; Taulette, F. Si-C-N ceramics with a high microstructural stability elaborated from the pyrolysis of new polycarbosilazane precursors. J. Mater. Sci. 1993, 28, 2615–2631. [Google Scholar] [CrossRef]
- Qiao, W.M.; Lim, S.Y.; Yoon, S.H.; Mochida, I.; Ling, L.C.; Yang, J.H. Synthesis of crystalline SiC nanofiber through the pyrolysis of polycarbomethylsilane coated platelet carbon nanofiber. Appl. Surf. Sci. 2007, 253, 4467–4471. [Google Scholar] [CrossRef]
- Bouillon, E.; Langlais, F.; Pailler, R.; Naslain, R.; Cruege, F.; Huong, P.V.; Sarthou, J.C.; Delpuech, A.; Laffon, C.; Lagarde, P.; et al. Conversion mechanisms of a polycarbosilane precursor into a SiC-based ceramic material. J. Mater. Sci. 1991, 26, 1333–1345. [Google Scholar] [CrossRef]
- Bouzat, F.; Graff, A.R.; Lucas, R.; Foucaud, S. Preparation of C/SiC ceramics using a preceramic polycarbosilane synthesized via hydrosilylation. J. Eur. Ceram. Soc. 2016, 36, 2913–2921. [Google Scholar] [CrossRef]
- Pizon, D.; Lucas, R.; Foucaud, S.; Maître, A. ZrC-SiC materials from the polymer-derived ceramics. Route. Adv. Eng. Mater. 2011, 13, 599–603. [Google Scholar] [CrossRef]

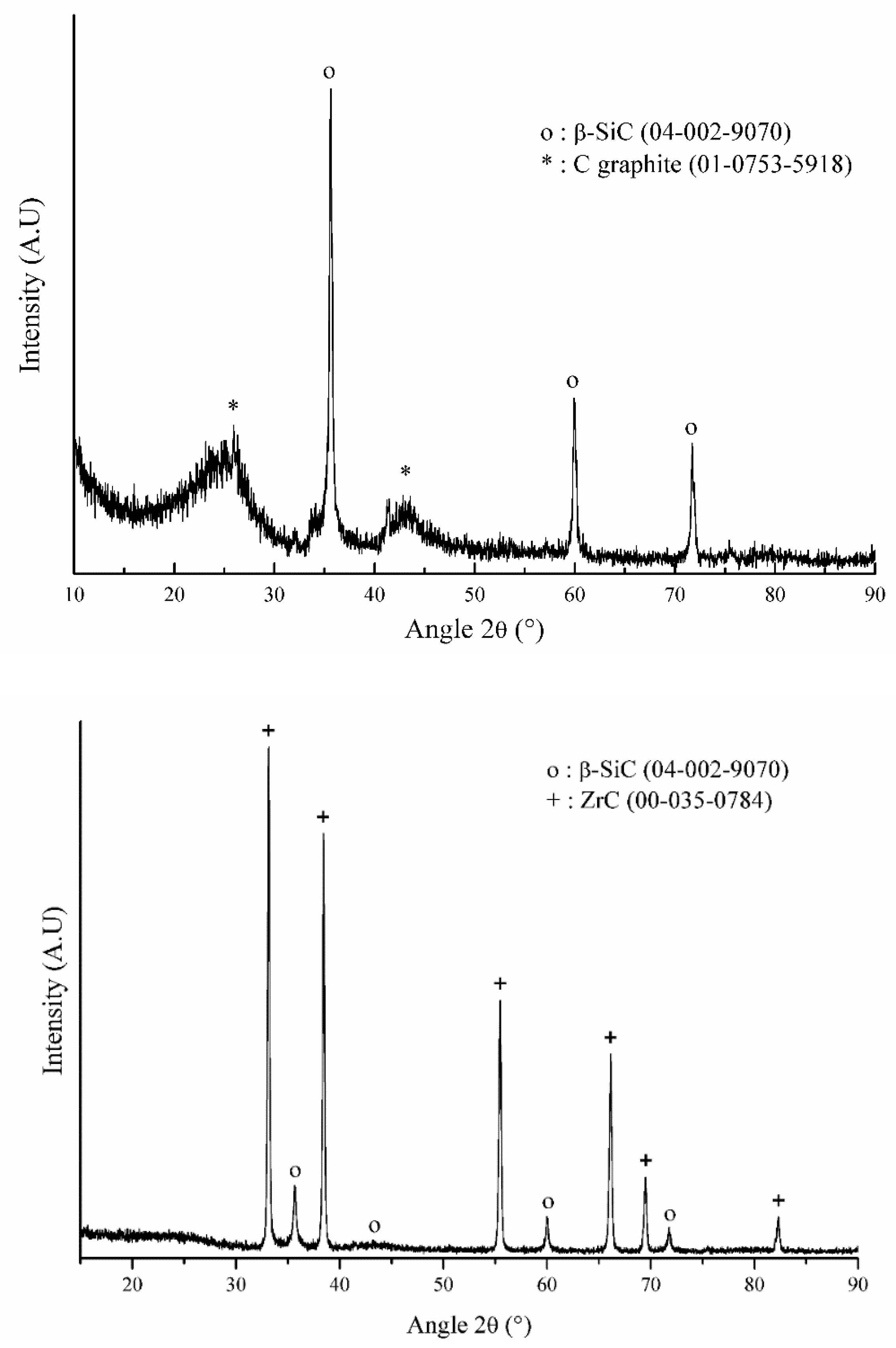
| Polymer | Synthetic Process | Ceramic Yield (wt.%) | Organic-to-Inorganic Transition Range of Temperature (°C) | Ceramic Composition |
|---|---|---|---|---|
| l-cPCS | Click-chemistry (CuAAC) | 36 | 511 | SiC + Cgraphite |
| hb-cPCS | Click-chemistry (CuAAC) | 58 | 489 | SiC + Cgraphite |
| hb-cPZCS | Click-chemistry (CuAAC) | 54 | 407 | SiC + ZrC |
| l-hPCS | Hydrosilylation | 62 | 780 | SiC + Camorphous |
| l-hPZCS | Hydrosilylation and Hydrozirconation | 62 | 770 | SiC + ZrC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouzat, F.; Lucas, R.; Leconte, Y.; Foucaud, S.; Champavier, Y.; Coelho Diogo, C.; Babonneau, F. Formation of ZrC–SiC Composites from the Molecular Scale through the Synthesis of Multielement Polymers. Materials 2021, 14, 3901. https://doi.org/10.3390/ma14143901
Bouzat F, Lucas R, Leconte Y, Foucaud S, Champavier Y, Coelho Diogo C, Babonneau F. Formation of ZrC–SiC Composites from the Molecular Scale through the Synthesis of Multielement Polymers. Materials. 2021; 14(14):3901. https://doi.org/10.3390/ma14143901
Chicago/Turabian StyleBouzat, Fabien, Romain Lucas, Yann Leconte, Sylvie Foucaud, Yves Champavier, Cristina Coelho Diogo, and Florence Babonneau. 2021. "Formation of ZrC–SiC Composites from the Molecular Scale through the Synthesis of Multielement Polymers" Materials 14, no. 14: 3901. https://doi.org/10.3390/ma14143901







