A Simple Method of Evaluating the Thermal Properties of Metallurgical Cokes under High Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Solution Reaction Tests and Characterization
3. Results and Discussion
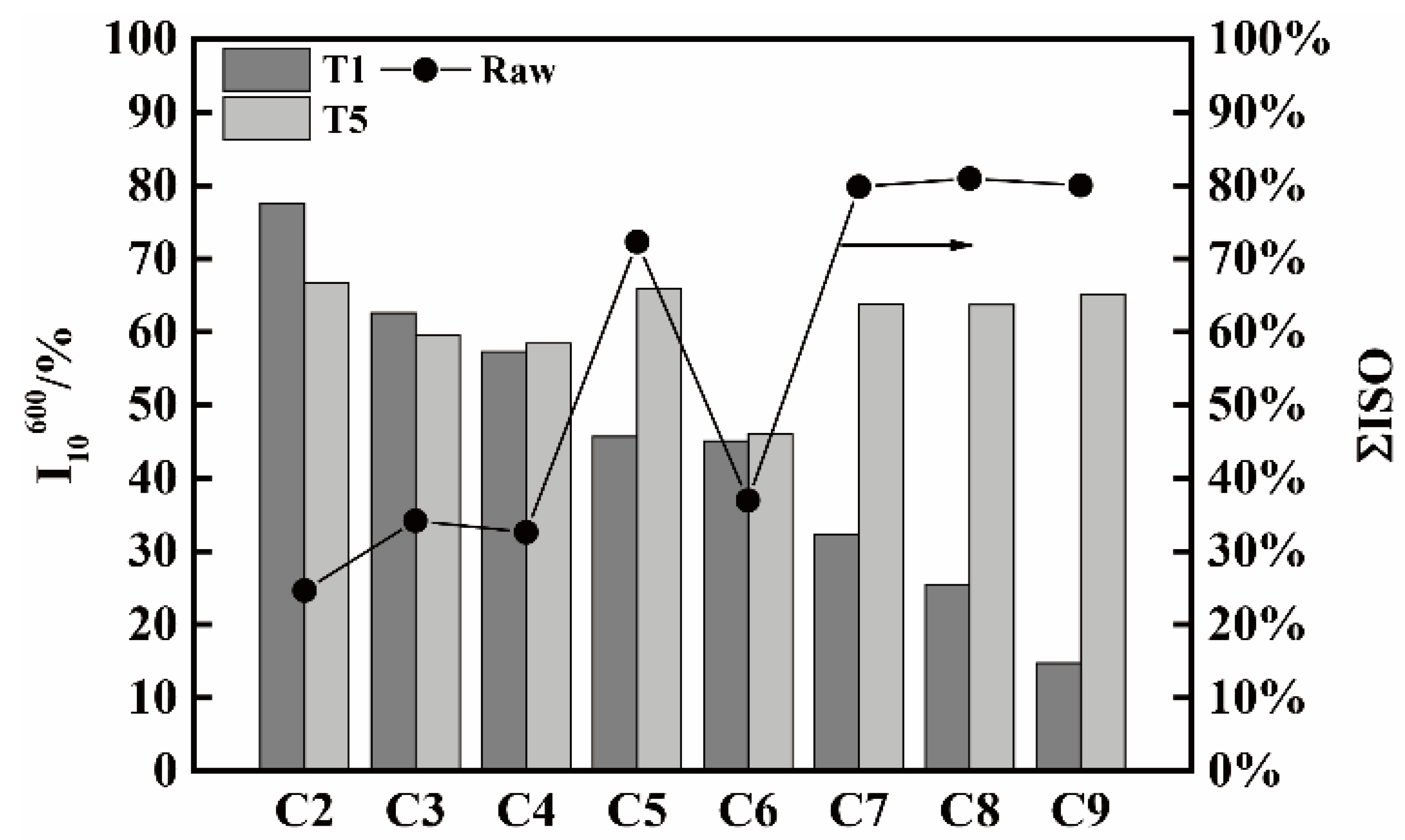
3.1. RI/I10600 of Metallurgical Coke under Different Reaction Conditions
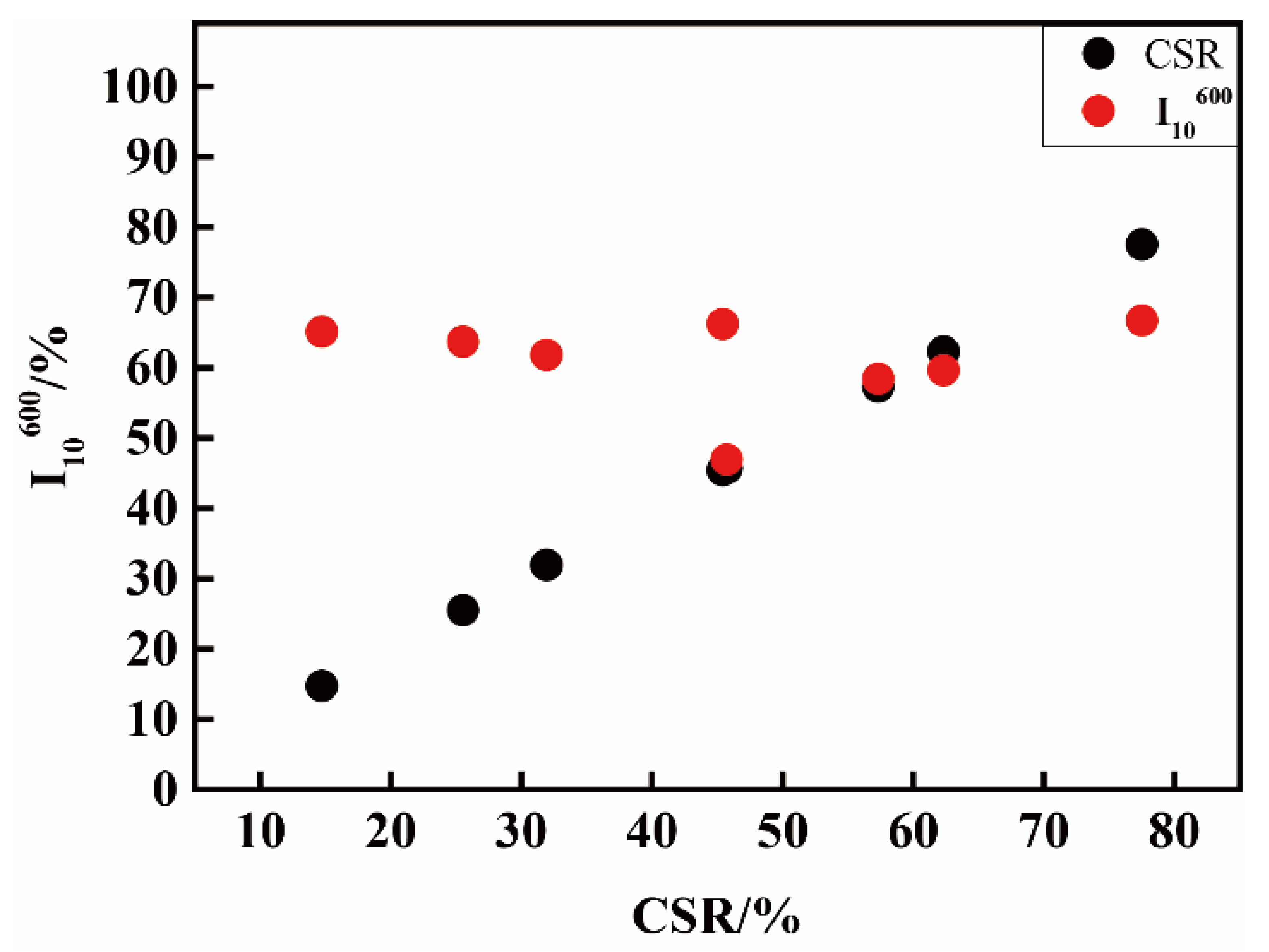
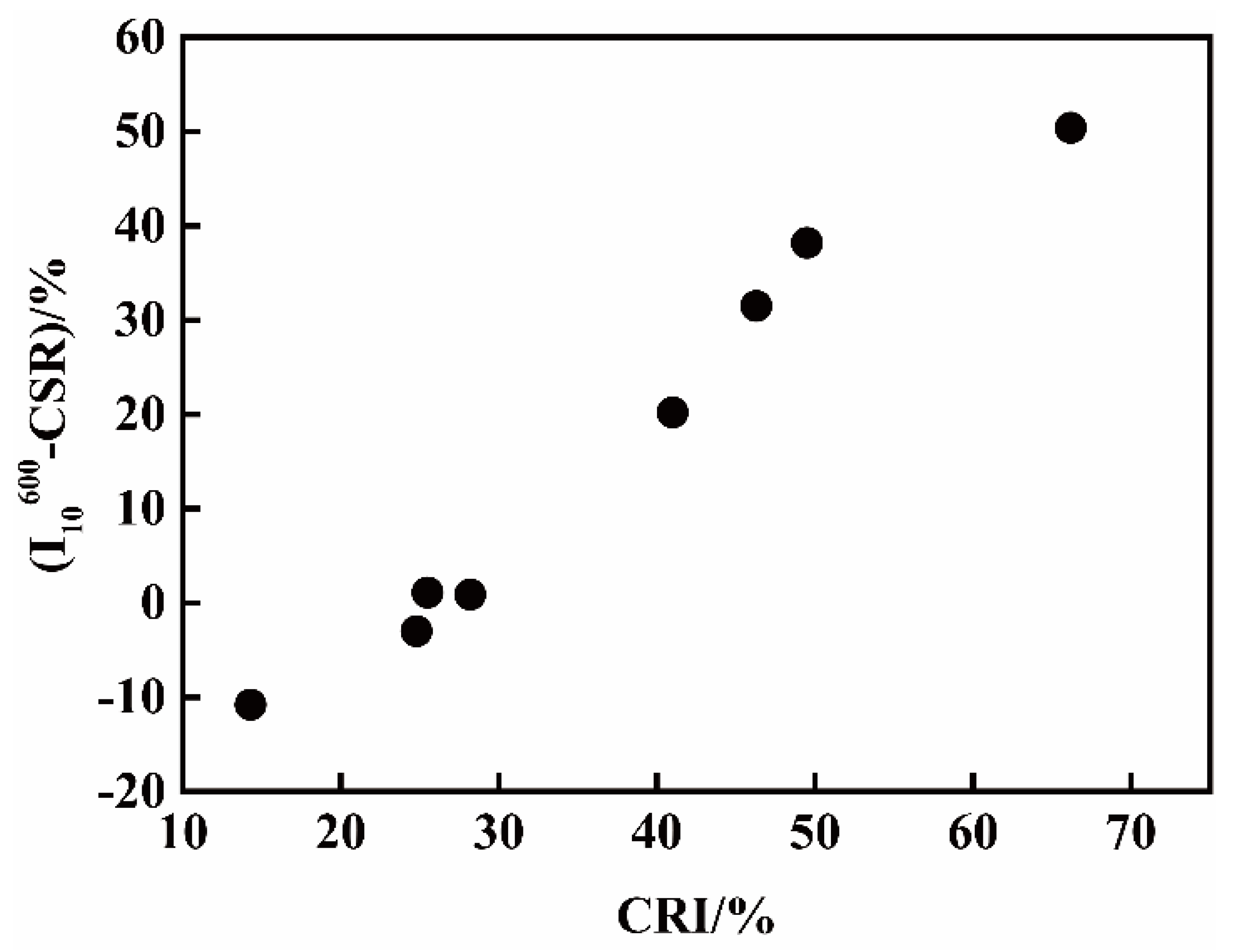
3.2. RI/I10600 of Pilot Cokes from Single Coals under High Temperature and Standard Conditions
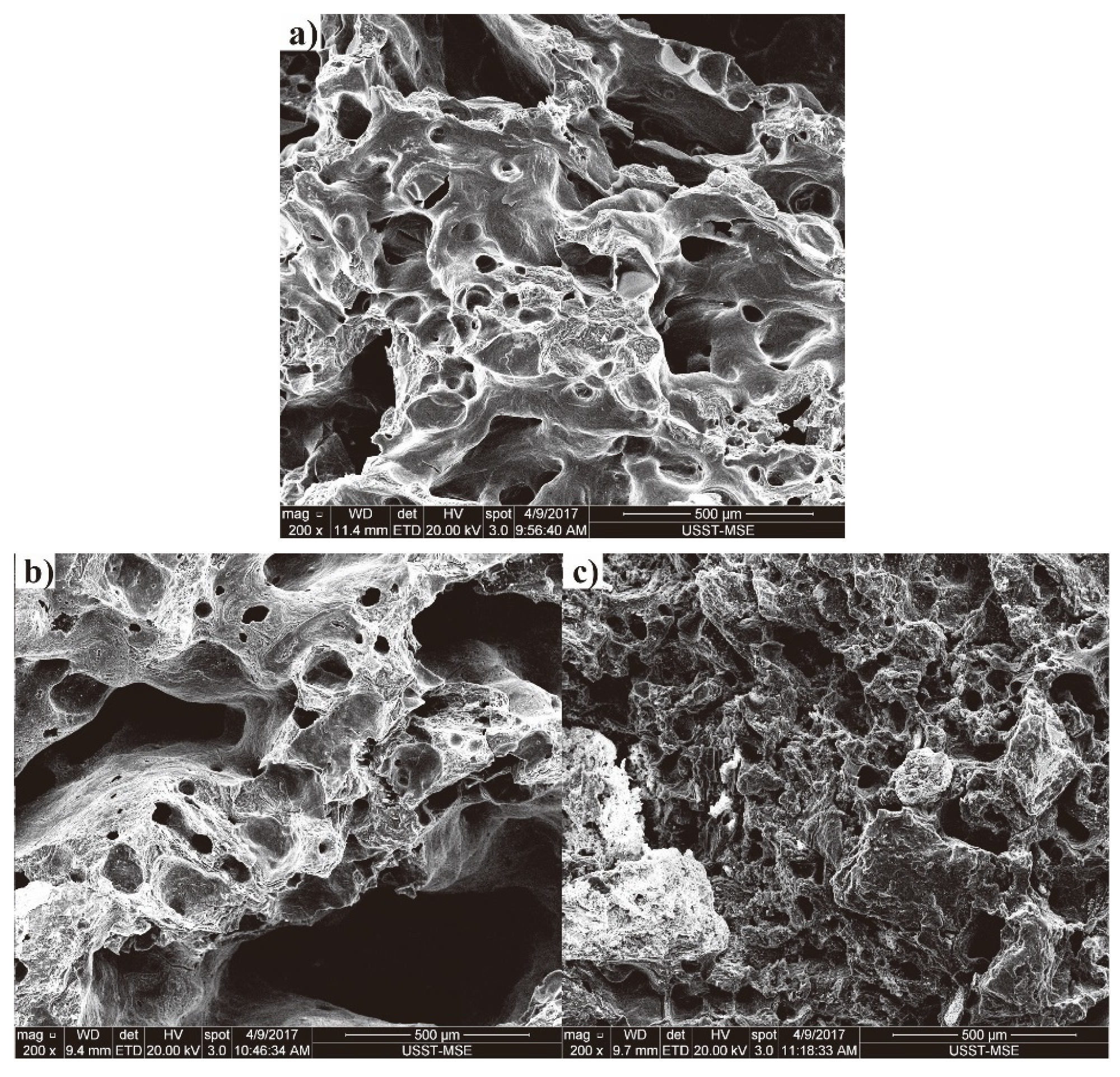
3.3. Morphology
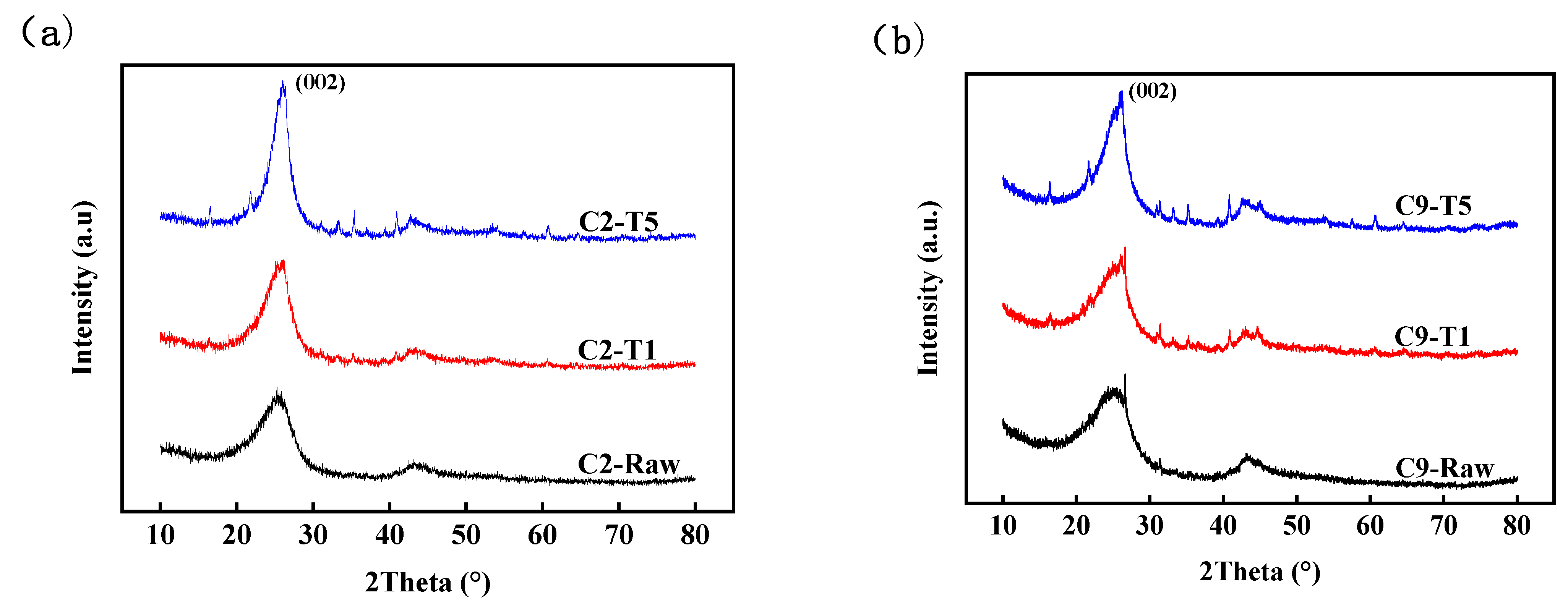
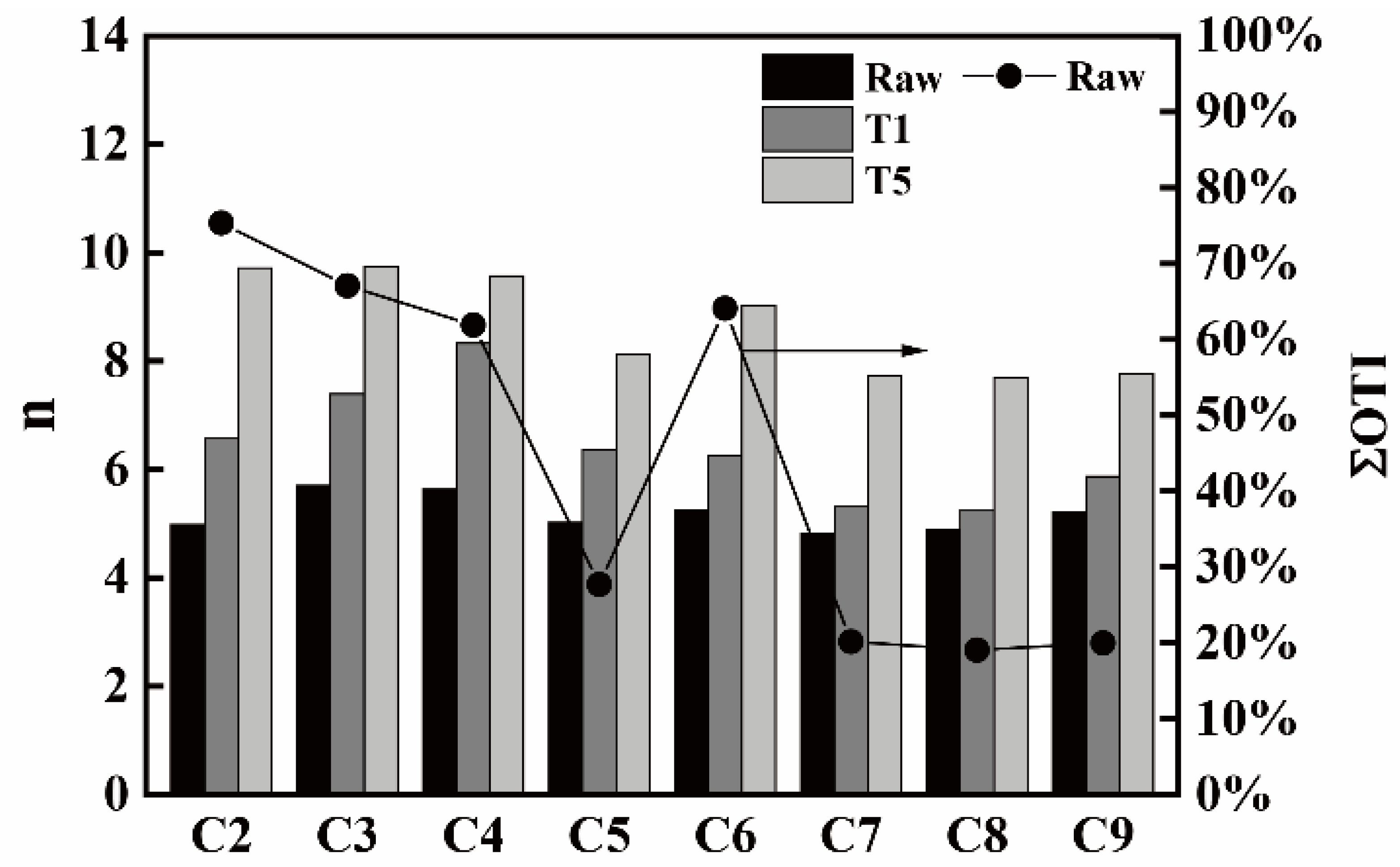
3.4. Carbon Structure
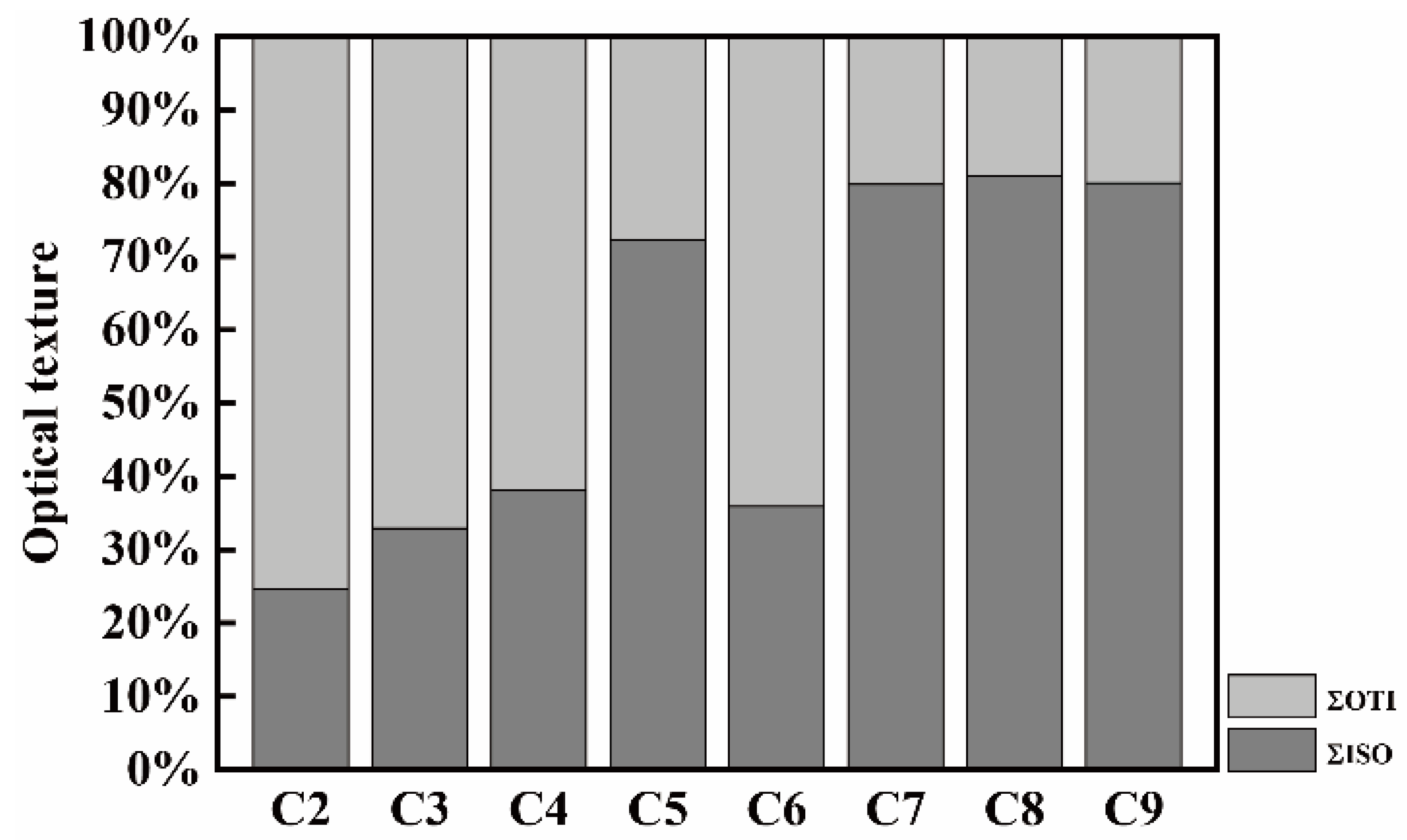
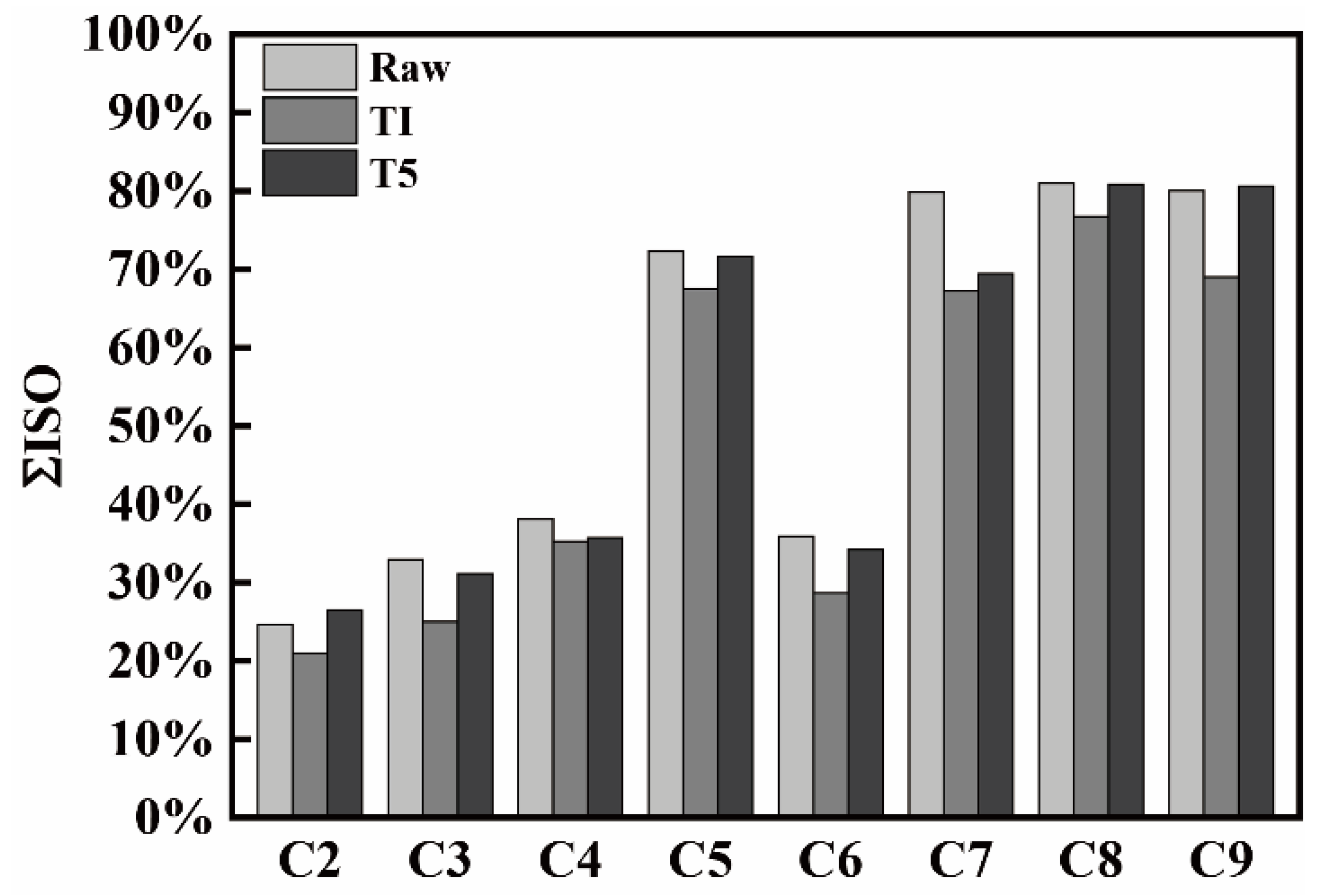
3.5. Optical Texture
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grigore, M.; Sakurovs, R.; French, D.; Sahajwalla, V. Mineral reactions during coke gasification with carbon dioxide. Int. J. Coal Geol. 2008, 75, 213–224. [Google Scholar] [CrossRef]
- Pusz, S.; Buszko, R. Reflectance parameters of cokes in relation to their reactivity index (CRI) and the strength after reaction (CSR), from coals of the Upper Silesian Coal Basin, Poland. Int. J. Coal Geol. 2012, 90–91, 43–49. [Google Scholar] [CrossRef]
- Donskoi, E.; Poliakov, A.; Mahoney, M.R.; Scholes, O. Novel optical image analysis coke characterisation and its application to study of the relationships between coke structure, coke strength and parent coal composition. Fuel 2017, 208, 281–295. [Google Scholar] [CrossRef]
- Flores, B.D.; Borrego, A.G.; Diez, M.A.; da Silva, G.L.; Zymla, V.; Vilela, A.C.; Osório, E. How coke optical texture became a relevant tool for understanding coal blending and coke quality. Fuel Process. Technol. 2017, 164, 13–23. [Google Scholar] [CrossRef]
- Haapakangas, J.; Suopajärvi, H.; Iljana, M.; Kemppainen, A.; Mattila, O.; Heikkinen, E.P.; Samuelsson, C.; Fabritius, T. Coke reactivity in simulated blast furnace shaft conditions. Metall. Mater. Trans. B 2016, 47, 2357–2370. [Google Scholar] [CrossRef]
- An, J.Y.; Seo, J.B.; Choi, J.H.; Lee, J.H.; Kim, H. Evaluation of characteristics of coke degradation after reaction in different conditions. ISIJ Int. 2016, 56, 226–232. [Google Scholar] [CrossRef] [Green Version]
- Nomura, S.; Naito, M.; Yamaguchi, K. Post-reaction strength of catalyst-added highly reactive coke. ISIJ Int. 2007, 47, 831–839. [Google Scholar] [CrossRef] [Green Version]
- Álvarez, R.; Díez, M.A.; Barriocanal, C.; Diaz-Faes, E.; Cimadevilla, J.L.G. An approach to blast furnace coke quality prediction. Fuel 2007, 86, 2159–2166. [Google Scholar] [CrossRef]
- Gupta, S.; Dubikova, M.; French, D.; Sahajwalla, V. Effect of CO2 gasification on the transformations of coke minerals. Energy Fuels 2007, 21, 1052–1061. [Google Scholar] [CrossRef]
- Sakurovs, R.; Burke, L. Influence of gas composition on the reactivity of cokes. Fuel Process. Technol. 2011, 92, 1220–1224. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, R.; Zhao, X.-F.; Sun, J.-F.; Zhang, S.; Liu, W.-Z. A new testing and evaluating method of cokes with greatly varied CRI and CSR. Fuel 2016, 182, 879–885. [Google Scholar] [CrossRef]
- Cheng, H.; Liang, Y.; Guo, R.; Sun, Z.; Wang, Q.; Wang, J. Effects of solution loss degree, reaction temperature, and high temperature heating on the thermal properties of metallurgical cokes. Fuel 2021, 283, 118936. [Google Scholar] [CrossRef]
- Lundgren, M.; Sundqvist Ökvist, L.; Björkman, B. Coke reactivity under blast furnace conditions and in the CSR/CRI test. Steel Res. Int. 2009, 80, 396–401. [Google Scholar] [CrossRef]
- Hilding, T.; Gupta, S.; Sahajwalla, V.; Björkman, B.; Wikström, J.O. Degradation behavior of a high CSR coke in an experimental blast furnace: Effect of carbon structure and alkali reactions. ISIJ Int. 2005, 45, 1041–1050. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Shi, T.; Zhang, Q.; Cao, Y.; Qian, H.; Wu, X.; Li, J.; Zhang, Q.; Yang, G.; Yang, J. Coke texture, reactivity and tumbler strength after reaction under simulated blast furnace conditions. Fuel 2019, 251, 218–223. [Google Scholar] [CrossRef]
- Li, K.; Khanna, R.; Zhang, J.; Liu, Z.; Sahajwalla, V.; Yang, T.; Kong, D. The evolution of structural order, microstructure and mineral matter of metallurgical coke in a blast furnace: A review. Fuel 2014, 133, 194–215. [Google Scholar] [CrossRef]
- Gornostayev, S.S.; Heino, J.J.; Kokkonen, T.M.; Makkonen, H.T.; Huttunen, S.M.; Fabritius, T.M. Textural changes in metallurgical coke prepared with polyethylene. Int. J. Miner. Metall. Mater. 2014, 21, 969–973. [Google Scholar] [CrossRef]
- Grigore, M.; Sakurovs, R.; French, D.; Sahajwalla, V. Properties and CO2 reactivity of the inert and reactive maceral-derived components in cokes. Int. J. Coal Geol. 2012, 98, 1–9. [Google Scholar] [CrossRef]
- Piechaczek, M.; Mianowski, A.; Sobolewski, A. Reprint of “The original concept of description of the coke optical texture”. Int. J. Coal Geol. 2015, 139, 184–190. [Google Scholar] [CrossRef]
- Yang, M.; Cui, P. Coal influenced to microstructure during reaction of coke gasification. Coal Sci. Technol. 2005, 33, 42–44. [Google Scholar]
- Geng, J.; Shen, H.; Liu, J. Current research situation and outlook of coke reactive capability. Yunnan Metall. 2009, 38, 59–63. [Google Scholar]
- Wang, W.; Wang, J.; Xu, R.; Yu, Y.; Jin, Y.; Xue, Z. Influence mechanism of zinc on the solution loss reaction of coke used in blast furnace. Fuel Process. Technol. 2017, 159, 118–127. [Google Scholar] [CrossRef]
- Zhou, S.; Zhao, J. Properties of Coking Coal and Quality of Coke for the Blast Furnace; Metallurgical Industry Press: Beijing, China, 2005. [Google Scholar]
- Cancino-Trejo, F.; Sáenz Padilla, M.; López-Honorato, E.; Carvajal-Nunez, U.; Boshoven, J.; Somers, J. The effect of heat treatment on the microstructure and diffusion of silver in pyrolytic carbon coatings. Carbon 2016, 109, 542–551. [Google Scholar] [CrossRef]

| Items | C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 | C9 |
|---|---|---|---|---|---|---|---|---|---|
| Moisture, % | 8.68 | 9.78 | 9.90 | 9.20 | 11.05 | 9.60 | 7.30 | 9.72 | 8.20 |
| Fixed carbon, % | 64.63 | 69.94 | 68.96 | 61.42 | 65.14 | 69.72 | 57.20 | 57.01 | 57.55 |
| Ash, % | 8.96 | 9.98 | 10.03 | 9.13 | 9.94 | 9.45 | 7.93 | 8.06 | 7.58 |
| Volatile, % | 26.41 | 20.08 | 21.01 | 29.45 | 24.93 | 20.81 | 34.86 | 34.93 | 34.87 |
| G | 84.00 | 90.75 | 86.00 | 90.00 | 90.50 | 75.43 | 80.60 | 79.80 | 78.70 |
| Y, mm | 14.00 | 14.50 | 11.00 | 23.00 | 23.75 | 10.14 | 11.40 | 13.00 | 13.33 |
| A + B, % | 43.50 | 81.25 | 58.00 | 176.00 | 113.50 | 11.71 | 29.80 | 39.00 | 122.50 |
| Test | Test Conditions | C1 | ||||||
|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Time (h) | CO2 (%) | Total Amount of CO2 Fed (L) | RI (%) | I10600 (%) | Reaction Amount of CO2 (L) | Reaction Ratio of CO2 (%) | |
| T1 a | 1100 | 2 | 100% | 600 | 24.7 | 68.6 | 92.2 | 15.4 |
| T2 | 1100 | 2 | 50% | 300 | 19.3 | 72.7 | 72.0 | 24.0 |
| T3 | 1100 | 2 | 25% | 150 | 12.9 | 78.4 | 48.2 | 32.2 |
| T4 | 1200 | 1 | 50% | 150 | 18.1 | 71.0 | 67.6 | 45.0 |
| T5 | 1300 | 0.5 | 100% | 150 | 23.3 | 68.1 | 87.0 | 58.0 |
| Test | C2 | C3 | C4 | C5 | C6 | C7 | C8 | C9 |
|---|---|---|---|---|---|---|---|---|
| T1 a (CRI/CSR) | 14.3/77.5 | 24.8/62.6 | 25.5/57.3 | 41.0/45.7 | 28.2/45.1 | 46.3/32.3 | 49.5/25.5 | 66.2/14.7 |
| T5 (RI/I10600) | 19.3/66.7 | 26.0/59.6 | 28.6/58.4 | 29.7/65.9 | 28.4/46.0 | 31.4/63.8 | 34.4/63.7 | 34.3/65.1 |
| (I10600-CSR) | −10.8% | −3% | 1.1% | 20.2% | 0.9% | 31.5% | 38.2% | 50.4% |
| Coke. | β(002) (°) | d(002) (nm) | Lc (nm) | La (nm) | n (Lc/d(002)) | (nT5 − nRaw)/nRaw (%) | |
|---|---|---|---|---|---|---|---|
| C2 | Raw | 4.694 | 0.3534 | 1.756 | 5.644 | 4.98 | 95.18 |
| T1 | 3.658 | 0.3438 | 2.256 | 6.054 | 6.57 | ||
| T5 | 2.482 | 0.3423 | 3.326 | 7.610 | 9.72 | ||
| C3 | Raw | 4.282 | 0.3387 | 1.929 | 8.611 | 5.70 | 70.88 |
| T1 | 3.316 | 0.3373 | 2.491 | 7.886 | 7.39 | ||
| T5 | 2.519 | 0.3371 | 3.280 | 9.401 | 9.74 | ||
| C4 | Raw | 4.382 | 0.3354 | 1.886 | 5.959 | 5.63 | 78.54 |
| T1 | 2.953 | 0.3362 | 2.799 | 7.994 | 8.33 | ||
| T5 | 2.508 | 0.3439 | 3.290 | 8.228 | 9.57 | ||
| C5 | Raw | 4.661 | 0.3521 | 1.769 | 6.041 | 5.02 | 61.75 |
| T1 | 3.798 | 0.3423 | 2.174 | 6.875 | 6.36 | ||
| T5 | 2.958 | 0.3437 | 2.790 | 8.244 | 8.12 | ||
| C6 | Raw | 4.482 | 0.3497 | 1.840 | 5.954 | 5.25 | 71.81 |
| T1 | 3.792 | 0.3469 | 2.175 | 9.086 | 6.26 | ||
| T5 | 2.663 | 0.3441 | 3.099 | 9.402 | 9.02 | ||
| C7 | Raw | 4.892 | 0.3496 | 1.686 | 8.146 | 4.82 | 60.63 |
| T1 | 3.440 | 0.3424 | 2.400 | 5.265 | 5.31 | ||
| T5 | 2.783 | 0.3396 | 2.628 | 5.185 | 7.74 | ||
| C8 | Raw | 5.052 | 0.3354 | 1.636 | 5.545 | 4.88 | 57.79 |
| T1 | 4.595 | 0.3407 | 1.797 | 4.898 | 5.26 | ||
| T5 | 2.479 | 0.3396 | 2.620 | 5.000 | 7.70 | ||
| C9 | Raw | 4.730 | 0.3362 | 1.747 | 3.698 | 5.20 | 49.23 |
| T1 | 4.002 | 0.3507 | 2.060 | 4.939 | 5.87 | ||
| T5 | 3.133 | 0.3395 | 2.636 | 5.268 | 7.76 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Wang, X.; Shi, T.; Wu, X.; Xue, Y. A Simple Method of Evaluating the Thermal Properties of Metallurgical Cokes under High Temperature. Materials 2021, 14, 5767. https://doi.org/10.3390/ma14195767
Yang G, Wang X, Shi T, Wu X, Xue Y. A Simple Method of Evaluating the Thermal Properties of Metallurgical Cokes under High Temperature. Materials. 2021; 14(19):5767. https://doi.org/10.3390/ma14195767
Chicago/Turabian StyleYang, Guangzhi, Xiaoqiang Wang, Ting Shi, Xinci Wu, and Yuhua Xue. 2021. "A Simple Method of Evaluating the Thermal Properties of Metallurgical Cokes under High Temperature" Materials 14, no. 19: 5767. https://doi.org/10.3390/ma14195767






