A Review on the Kinetics of Iron Ore Reduction by Hydrogen
Abstract
:1. Introduction
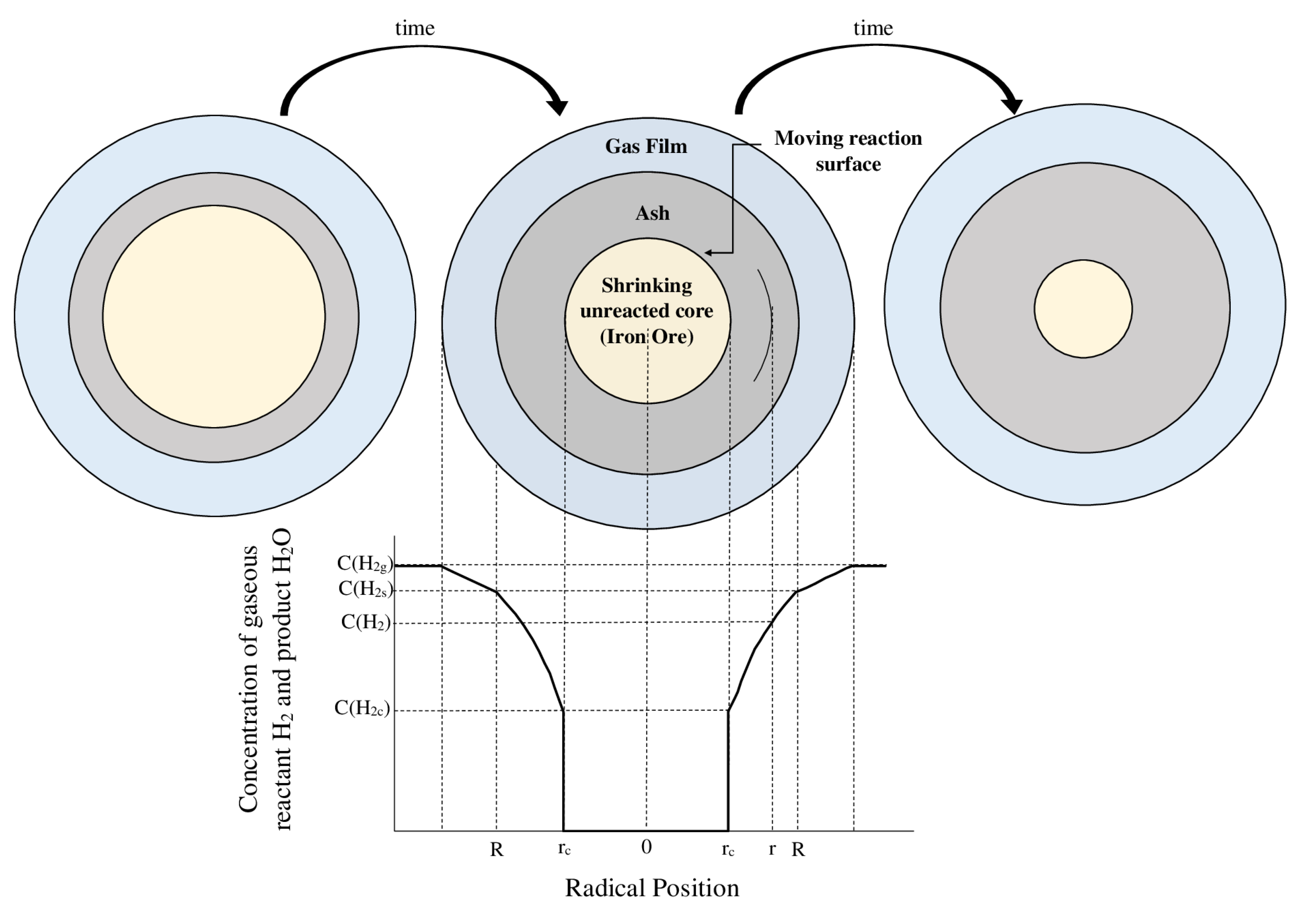
- Diffusion of hydrogen through the film surrounding the iron ore particle.
- Diffusion of hydrogen through the blanket of ash (consisting of the final product, i.e., iron, and gangue such as silica, alumina, etc.) to the surface of the unreacted iron ore.
- Chemical reaction of hydrogen with iron ore at this reaction surface.
- Diffusion of the gaseous product (H2O) through the ash back to the exterior surface of the particle.
- Control by diffusion through the gas film
- Control by diffusion through the ash layer
- Chemical reaction control
2. Effect of Different Parameters on the Kinetics of Reduction
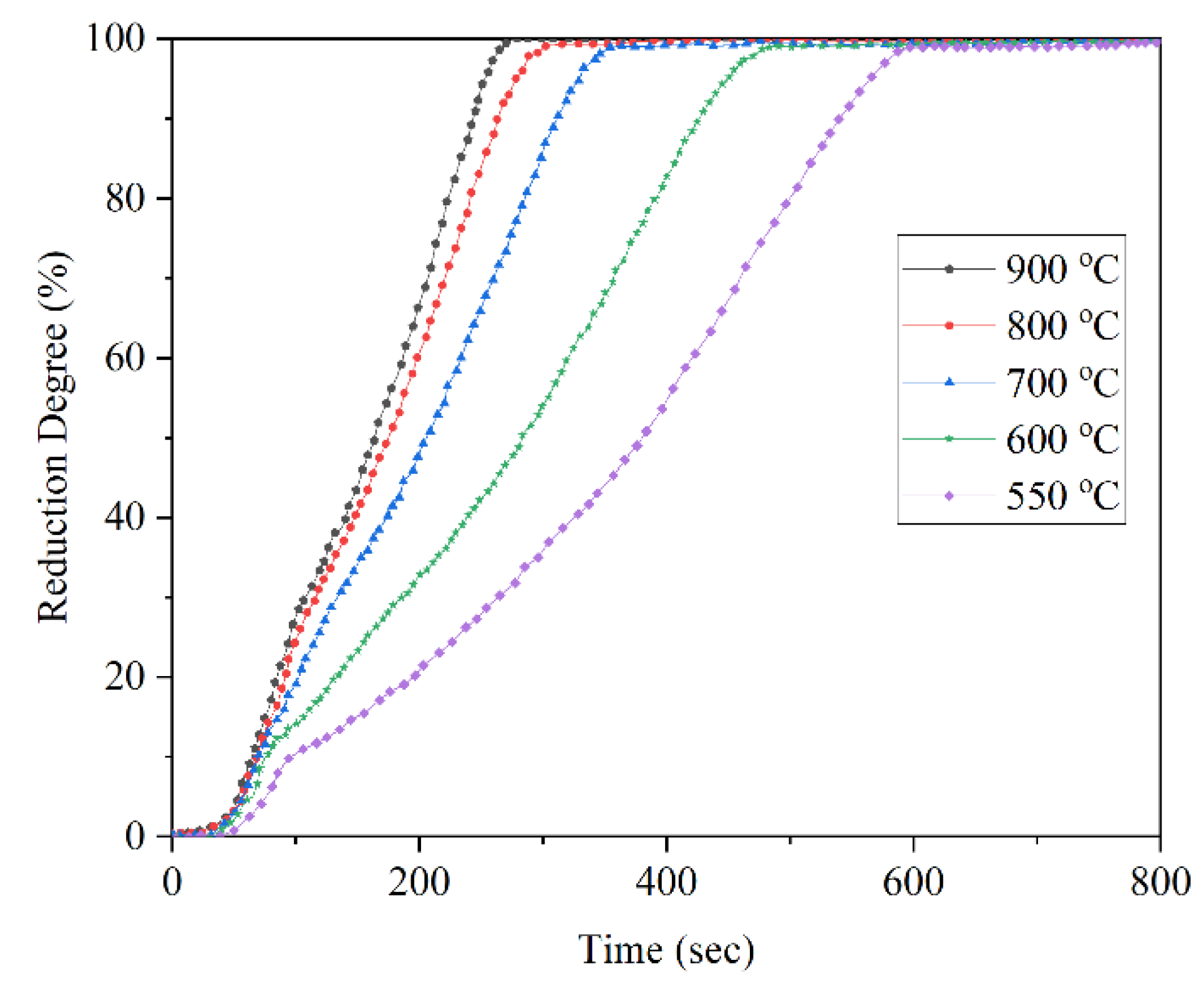
2.1. Effect of Temperature
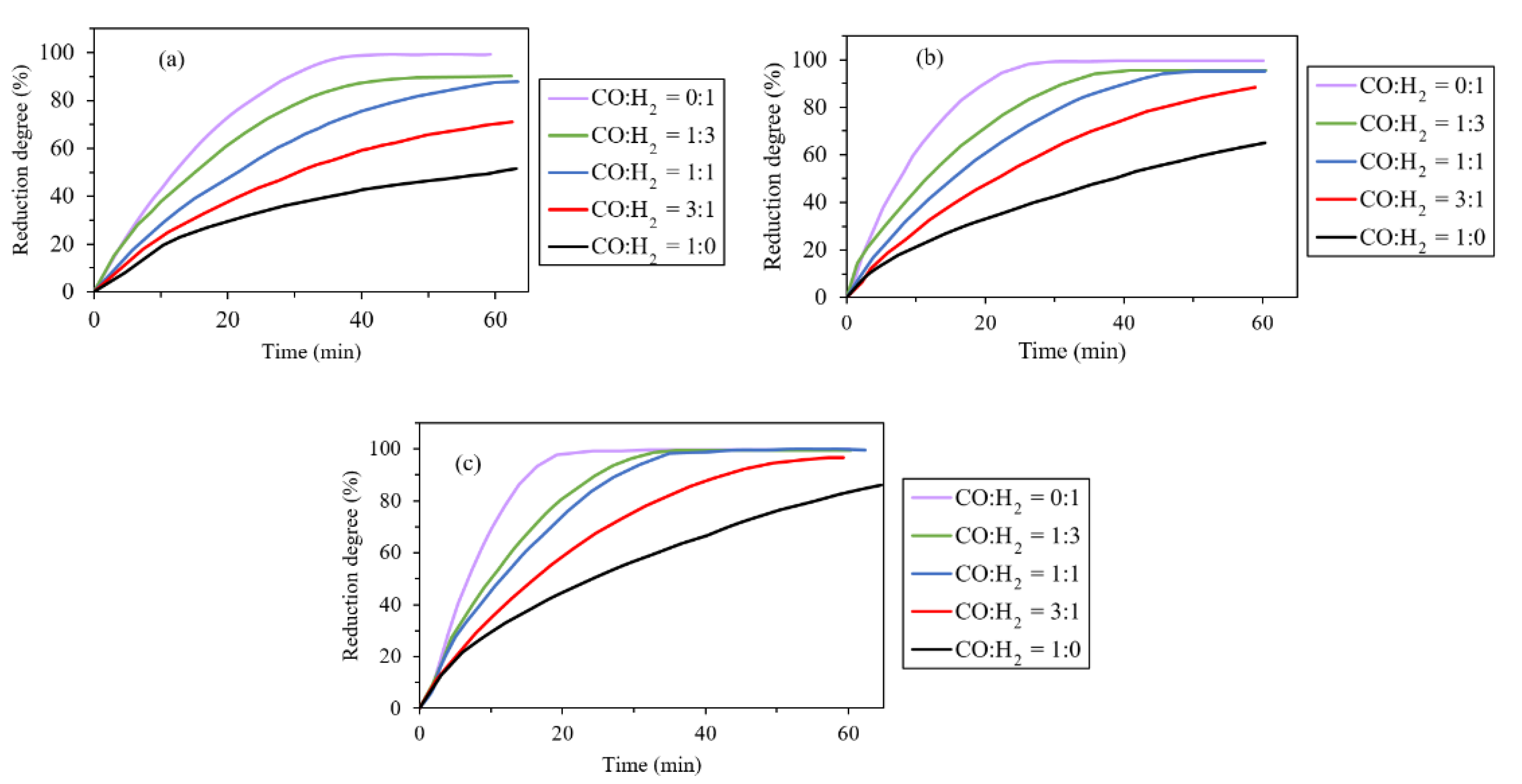
2.2. Effect of H2/CO Ratio
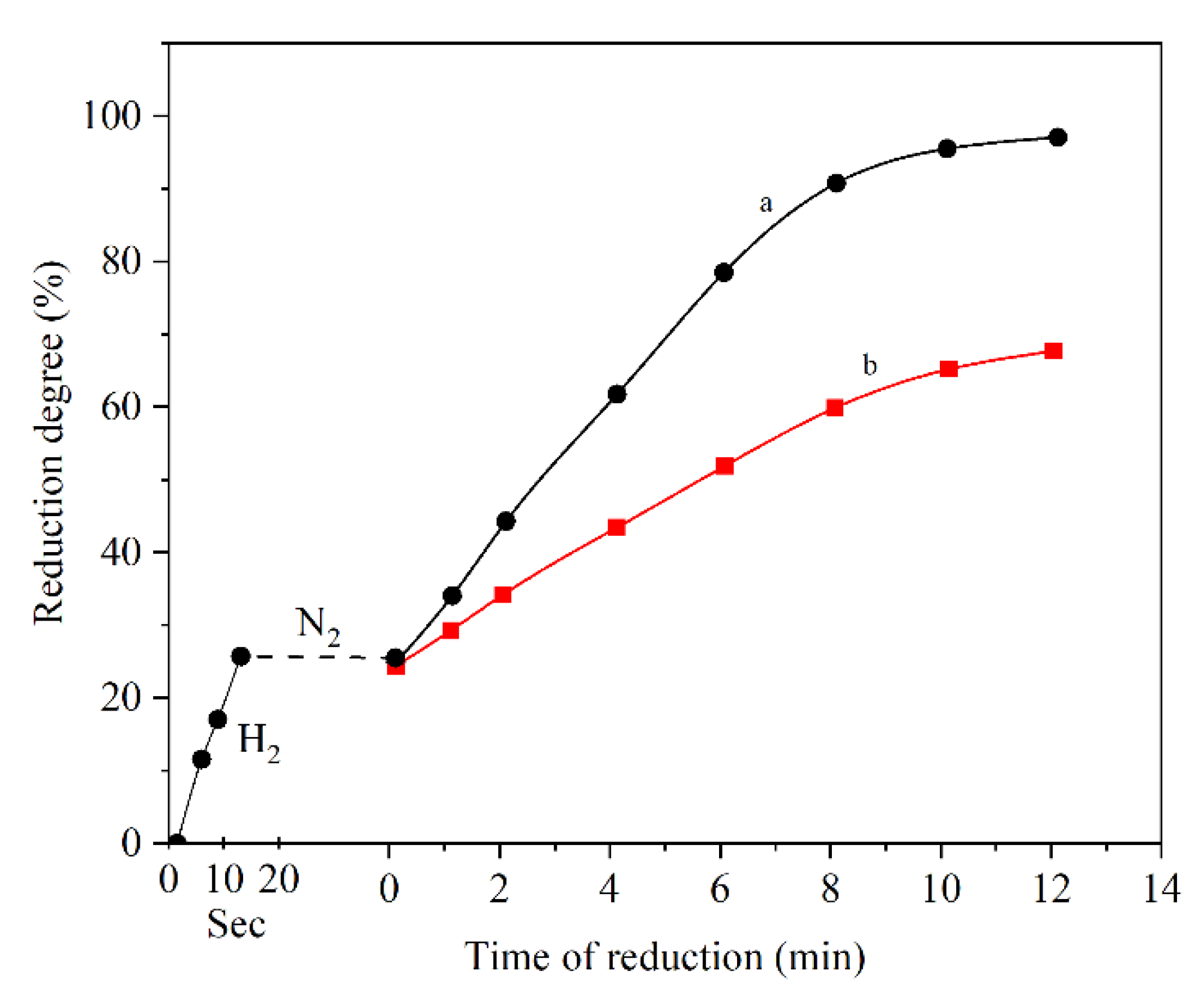
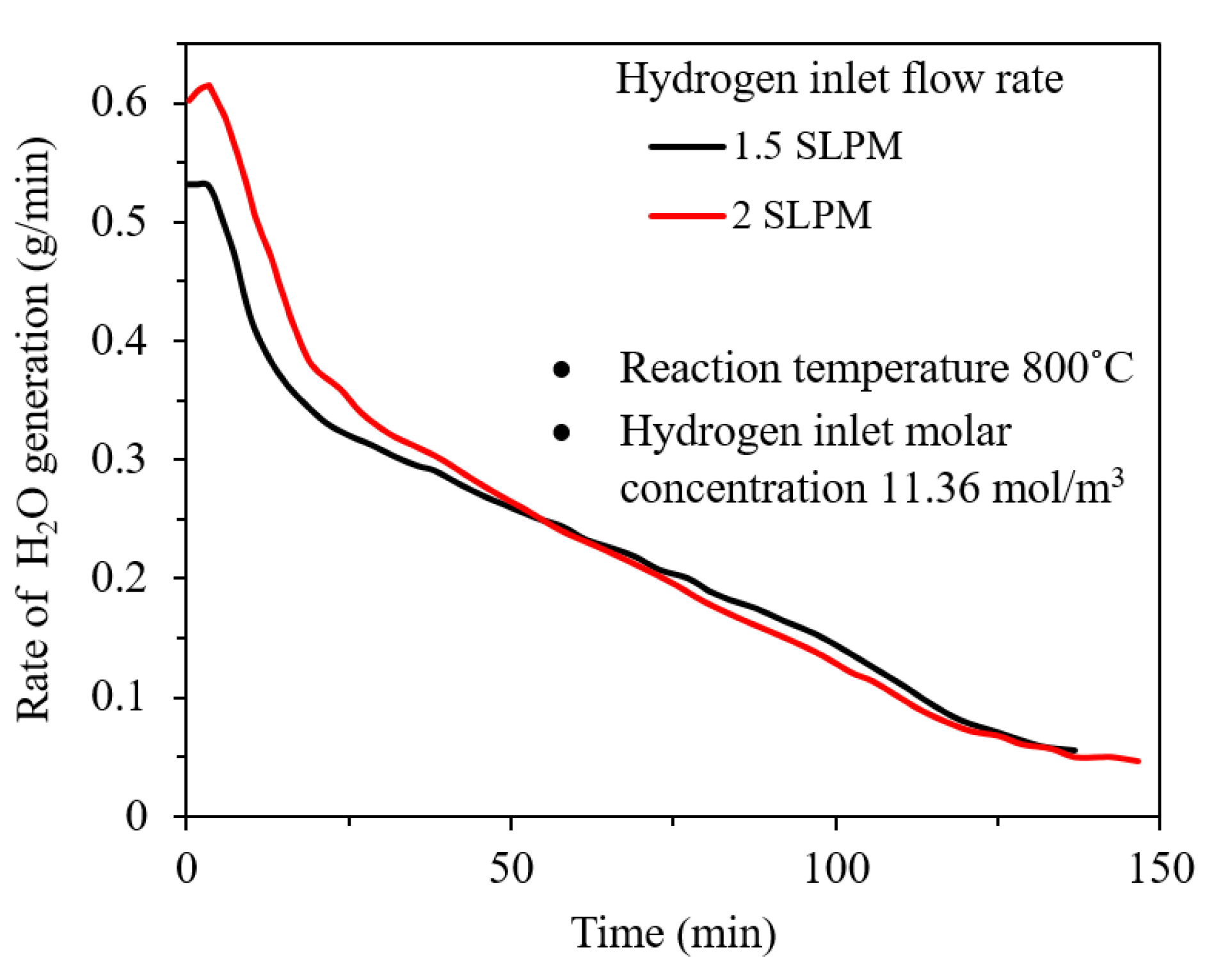
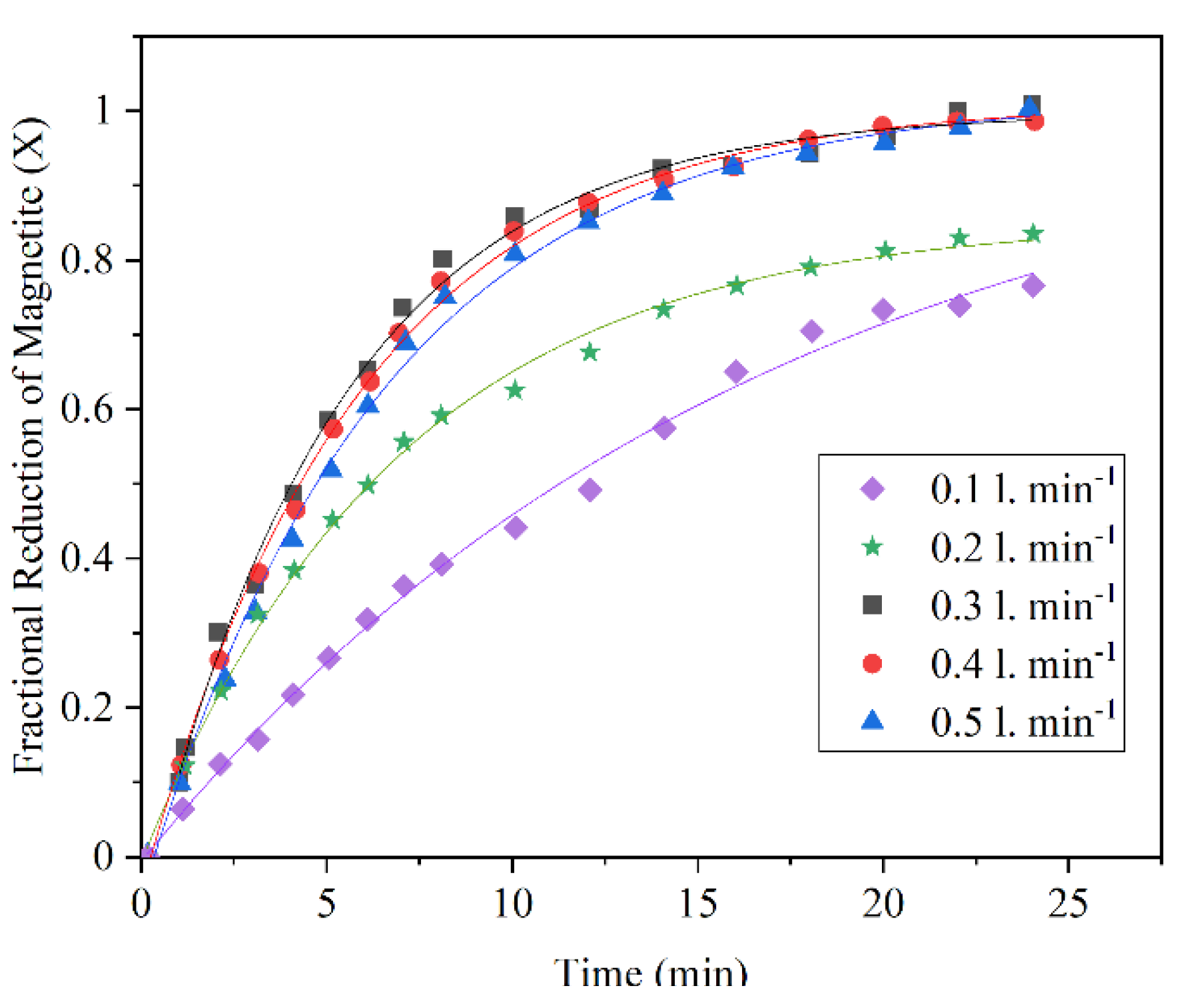
2.3. Effect of Hydrogen Flow Rate
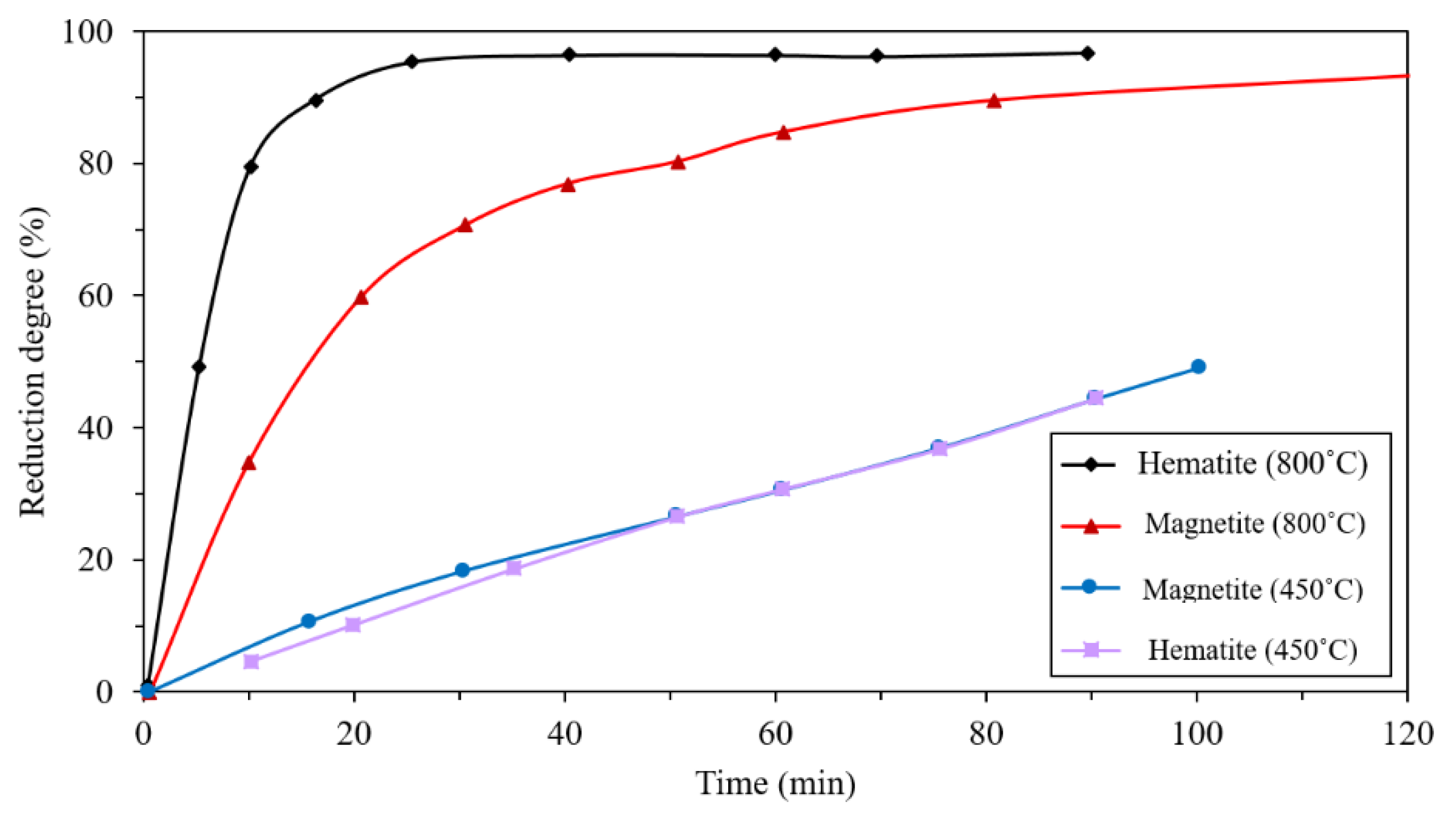
2.4. Effect of Mineralogy
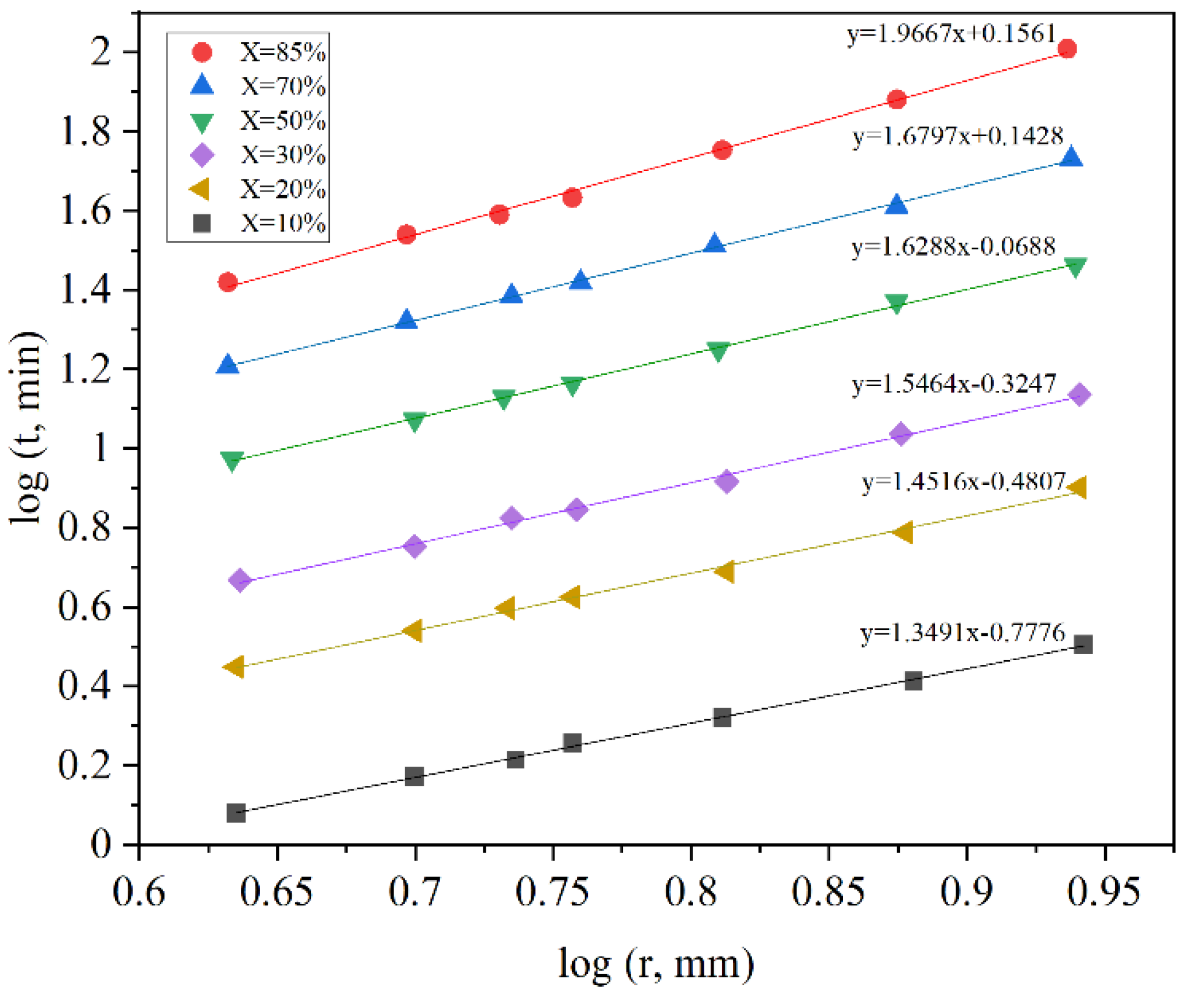
2.5. Effect of Particle Size
2.6. Effect of Impurities
2.7. Apparent Activation Energy
2.8. Kinetics Controlling Models
3. Conclusions
- Effect of Temperature: Due to the Arrhenius equation, by increasing the temperature, the rate of reduction will increase exponentially. At temperatures above 590 °C, the effect of temperature on the reduction of Fe2O3 to Fe3O4 and reduction of FeO to Fe is negligible, but for the reduction of Fe3O4 to FeO it is considerable.
- Effect of H2/CO ratio: The reaction rate would increase with the higher hydrogen content at temperatures above 1000 °C. Additionally, H2/CO proportion has the most beneficial effect on the reduction rate when being 1.6, and the higher ratios effect is negligible.
- Effect of hydrogen flow rate: Higher inlet flow rate causes higher steam generation at the early stages of the reaction and at the later stages, the effect is minor. Additionally, there is a critical gas velocity below which gas flow rate controls the rate of the reaction.
- Effect of iron ore mineralogy: Because of the hard and dense shell of magnetite in comparison with hematite, magnetite has lower diffusion. Thus, the reduction of hematite by hydrogen is faster than the reduction of magnetite, especially at higher temperatures.
- Effect of particle size: As the size of the particle decreases the specific area increases, therefore the reduction rate enlarges because the reaction starts from the surface. Furthermore, the smaller particle size leads to a shorter distance that gas has to pass to reach inner layers. However, as the particle size shrinks, the chance of agglomeration will increase and as a result, the specific area decreases.
- Effect of impurities: The effect of impurities can be assumed as reduction by H2 and CO. Impurities such as CaO, SiO2, and MgO and alumina forms can lead to the formation of micro-cracks that promote the reduction of wüstite. Contrarily, some impurities such as Al2O3 and MgO decrease the rate of magnetite reduction.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holappa, L. A general vision for reduction of energy consumption and CO2 emissions from the steel industry. Metals 2020, 10, 1117. [Google Scholar] [CrossRef]
- IEA. Iron and Steel; IEA: Paris, France, 2020. [Google Scholar]
- Bitsianes, G.; Joseph, T.L. Topochemical Aspects of Iron Ore Reduction. JOM 1955, 7, 639–645. [Google Scholar] [CrossRef]
- Tsay, Q.; Ray, W.; Szekley, J. The modelling of hematite reduction with hydrogen plus carbon monoxide mixtures: Part I. behavior of single pellets. AlChE J. 1976, 22, 1064–1072. [Google Scholar] [CrossRef]
- Spreitzer, D.; Schenk, J. Reduction of Iron Oxides with Hydrogen—A Review. Steel Res. Int. 2019, 90, 1900108. [Google Scholar] [CrossRef] [Green Version]
- Rechberger, K.; Spanlang, A.; Sasiain Conde, A.; Wolfmeir, H.; Harris, C. Green Hydrogen-Based Direct Reduction for Low-Carbon Steelmaking. Steel Res. Int. 2020, 91, 1–10. [Google Scholar] [CrossRef]
- Cuéllar-Franca, R.M.; Azapagic, A. Carbon capture, storage and utilisation technologies: A critical analysis and comparison of their life cycle environmental impacts. J. CO2 Util. 2015, 9, 82–102. [Google Scholar] [CrossRef]
- Birat, J.-P. 16—Carbon dioxide (CO2) capture and storage technology in the iron and steel industry. In Developments and Innovation in Carbon Dioxide (CO2) Capture and Storage Technology; Woodhead Publishing Series in Energy; Maroto-Valer, M., Ed.; Woodhead Publishing: Sawston, UK, 2010; Volume 1, pp. 492–521. ISBN 978–1–84569-533-0. [Google Scholar]
- Sjoberg Elf, J.; Espinosa, K.W. Carbon Capture and Utilisation in the Steel Industry. A Study Exploring the Integration of Carbon Capture Technology and High-Temperature CO-Electrolysis of CO2 and H2O to Produce Synthetic Gas; Kth Royal Institute of Technology: Stockolm, Sweden, 2017. [Google Scholar]
- Pérez-Fortes, M.; Moya, J.A.; Vatopoulos, K.; Tzimas, E. CO2 capture and utilization in cement and iron and steel industries. Energy Procedia 2014, 63, 6534–6543. [Google Scholar] [CrossRef]
- Suopajärvi, H.; Pongrácz, E.; Fabritius, T. The potential of using biomass-based reducing agents in the blast furnace: A review of thermochemical conversion technologies and assessments related to sustainability. Renew. Sustain. Energy Rev. 2013, 25, 511–528. [Google Scholar] [CrossRef]
- Hammerschmid, M.; Müller, S.; Fuchs, J.; Hofbauer, H. Evaluation of biomass-based production of below zero emission reducing gas for the iron and steel industry. Biomass Convers. Biorefinery 2021, 11, 169–187. [Google Scholar] [CrossRef]
- Gao, Y.M.; Wang, B.; Wang, S.B.; Peng, S. Study on electrolytic reduction with controlled oxygen flow for iron from molten oxide slag containing FeO. J. Min. Metall. Sect. B Metall. 2013, 49, 49–55. [Google Scholar] [CrossRef]
- Allanore, A.; Lavelaine, H.; Valentin, G.; Birat, J.P.; Lapicque, F. Iron Metal Production by Bulk Electrolysis of Iron Ore Particles in Aqueous Media. J. Electrochem. Soc. 2008, 155, E125. [Google Scholar] [CrossRef]
- Cavaliere, P. Electrolysis of Iron Ores: Most Efficient Technologies for Greenhouse Emissions Abatement. In Clean Ironmaking and Steelmaking Processes: Efficient Technologies for Greenhouse Emissions Abatement; Springer International Publishing: Cham, Switzerland, 2019; pp. 555–576. ISBN 978–3–030–21209–4. [Google Scholar]
- Lepage, T.; Kammoun, M.; Schmetz, Q.; Richel, A. Biomass-to-hydrogen: A review of main routes production, processes evaluation and techno-economical assessment. Biomass Bioenergy 2021, 144, 105920. [Google Scholar] [CrossRef]
- Patisson, F.; Mirgaux, O. Hydrogen ironmaking: How it works. Metals 2020, 10, 922. [Google Scholar] [CrossRef]
- Bessières, J.; Bessières, A.; Heizmann, J.J. Iron oxide reduction kinetics by hydrogen. Int. J. Hydrogen Energy 1980, 5, 585–595. [Google Scholar] [CrossRef]
- Hou, B.; Zhang, H.; Li, H.; Zhu, Q. Study on kinetics of iron oxide reduction by hydrogen. Chin. J. Chem. Eng. 2012, 20, 10–17. [Google Scholar] [CrossRef]
- McKewan, W.M. Kinetics of iron oxide reduction. Trans. Am. Inst. Min. Metall. Eng. 1960, 218, 2–6. [Google Scholar]
- Levenspiel, O. Chemical Reaction Engineering, 3rd ed.; Wiley: Hoboken, NJ, USA, 1999; ISBN 9780471530169. [Google Scholar]
- Von Bogdandy, L.; Engell, H.J. The Reduction of Iron Ores: Scientific Basis and Technology, 1st ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 1971; ISBN 9783662104002. [Google Scholar]
- Barde, A.A.; Klausner, J.F.; Mei, R. Solid state reaction kinetics of iron oxide reduction using hydrogen as a reducing agent. Int. J. Hydrogen Energy 2016, 41, 10103–10119. [Google Scholar] [CrossRef] [Green Version]
- Valipour, M.S.; Motamed Hashemi, M.Y.; Saboohi, Y. Mathematical modeling of the reaction in an iron ore pellet using a mixture of hydrogen, water vapor, carbon monoxide and carbon dioxide: An isothermal study. Adv. Powder Technol. 2006, 17, 277–295. [Google Scholar] [CrossRef]
- Tsay, Q.T.; Ray, W.H.; Szekely, J. The modeling of hematite reduction with hydrogen plus carbon monoxide mixtures: Part II. The direct reduction process in a shaft furnace arrangement. AIChE J. 1976, 22, 1072–1079. [Google Scholar] [CrossRef]
- Wagner, D.; Devisme, O.; Patisson, F.; Ablitzer, D. A Laboratory Study of the Reduction of Iron Oxides by Hydrogen. In Proceedings of the 2006 TMS Fall Extraction and Processing Division: Sohn International Symposium, San Diego, CA, USA, 27–31 August 2006; Volume 2, pp. 111–120. [Google Scholar]
- Choi, M.E.; Sohn, H.Y. Development of green suspension ironmaking technology based on hydrogen reduction of iron oxide concentrate: Rate measurements. Ironmak. Steelmak. 2010, 37, 81–88. [Google Scholar] [CrossRef]
- Fruehan, R.J.; Li, Y.; Brabie, L.; Kim, E.J. Final stage of reduction of iron ores by hydrogen. Scand. J. Metall. 2005, 34, 205–212. [Google Scholar] [CrossRef]
- Steffen, R.; Tacke, K.H. Hydrogen for the reduction of iron ores—State of the art and future aspects. Stahl Eisen 2004, 124, 45–52. [Google Scholar]
- Yang, Y.; Raipala, K.; Holappa, L. Ironmaking; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; Volume 3, ISBN 9780080969886. [Google Scholar]
- Kemppainen, A.; Mattila, O.; Heikkinen, E.P.; Paananen, T.; Fabritius, T. Effect of H2-H2O on the reduction of olivine pellets in CO-CO2 gas. ISIJ Int. 2012, 52, 1973–1978. [Google Scholar] [CrossRef]
- Zuo, H.B.; Wang, C.; Dong, J.J.; Jiao, K.X.; Xu, R.S. Reduction kinetics of iron oxide pellets with H2 and CO mixtures. Int. J. Miner. Metall. Mater. 2015, 22, 688–696. [Google Scholar] [CrossRef]
- Yi, L.; Huang, Z.; Peng, H.; Jiang, T. Action rules of H2 and CO in gas-based direct reduction of iron ore pellets. J. Cent. South. Univ. 2012, 19, 2291–2296. [Google Scholar] [CrossRef]
- Abdelrahim, A.; Iljana, M.; Omran, M.; Vuolio, T.; Bartusch, H.; Fabritius, T. Influence of H2–H2O content on the reduction of acid iron ore pellets in a CO–CO2–N2 reducing atmosphere. ISIJ Int. 2020, 60, 2206–2217. [Google Scholar] [CrossRef]
- Abu Tahari, M.N.; Salleh, F.; Tengku Saharuddin, T.S.; Samsuri, A.; Samidin, S.; Yarmo, M.A. Influence of hydrogen and carbon monoxide on reduction behavior of iron oxide at high temperature: Effect on reduction gas concentrations. Int. J. Hydrogen Energy 2021, 46, 24791–24805. [Google Scholar] [CrossRef]
- El-Geassy, A.H.A. Rate Controlling Step in the Reduction of Iron Oxides; Kinetics and Mechanism of Wüstite-Iron Step in H2, CO and H2/CO Gas Mixtures. IOP Conf. Ser. Mater. Sci. Eng. 2017, 229, 1–10. [Google Scholar] [CrossRef]
- Kawasaki, E.; Sanscrainte, J.; Walsh, T.J. Kinetics of reduction of iron oxide with carbon monoxide and hydrogen. AIChE J. 1962, 8, 48–52. [Google Scholar] [CrossRef]
- Kuila, S.K.; Chatterjee, R.; Ghosh, D. Kinetics of hydrogen reduction of magnetite ore fines. Int. J. Hydrogen Energy 2016, 41, 9256–9266. [Google Scholar] [CrossRef]
- Ohmi, M.; Usui, T. Improved Theory on the Rate of Reduction of Single Particles and Fixed Bed of Iron Oxide Pellets With Hydrogen. Trans. Iron Steel Inst. Jpn. 1982, 22, 66–74. [Google Scholar] [CrossRef]
- Ohmi, M.; Usui, T.; Naito, M.; Minamide, Y. Experimental Study of the Resistance duo to the Rate of Gas Flow on the Hydrogen Reduction of an Iron Oxide Pellet. Tetsu-to-Hangane 1981, 67, 1943–1951. [Google Scholar] [CrossRef]
- Edstrom, J.O. The mechanism of reduction of iron oxides. J. Iron Steel Inst. 1953, 175, 289. [Google Scholar]
- Rau, M.F.; Rieck, D.; Evans, J.W. Investigation of iron oxide reduction by TEM. Metall. Trans. B 1987, 18, 257–278. [Google Scholar] [CrossRef]
- Bogdandy, V.L.; Schulz, H.P.; Wiirzner, B.; Stranski, I.N.; von Bogdandy, L.; Schulz, H.P.; Würzner, B.; Stranski, I.N. Der Mechanismus der Reduktion von porigen Eisenerzen durch Wasserstoff. Arch. Eisenhüttenwes. 1963, 34, 401–409. [Google Scholar] [CrossRef]
- Heikkilä, A.; Iljana, M.; Bartusch, H.; Fabritius, T. Reduction of Iron Ore Pellets, Sinter, and Lump Ore under Simulated Blast Furnace Conditions. Steel Res. Int. 2020, 91, 2000047. [Google Scholar] [CrossRef] [Green Version]
- Jozwiak, W.K.; Kaczmarek, E.; Maniecki, T.P.; Ignaczak, W.; Maniukiewicz, W. Reduction behavior of iron oxides in hydrogen and carbon monoxide atmospheres. Appl. Catal. A Gen. 2007, 326, 17–27. [Google Scholar] [CrossRef]
- Zhang, A.; Monaghan, B.J.; Longbottom, R.J.; Nusheh, M.; Bumby, C.W. Reduction Kinetics of Oxidized New Zealand Ironsand Pellets in H2 at Temperatures up to 1443 K. Metall. Mater. Trans. B Process. Metall. Mater. Process. Sci. 2020, 51, 492–504. [Google Scholar] [CrossRef]
- Teplov, O.A. Kinetics of the low-temperature hydrogen reduction of magnetite concentrates. Russ. Metall. 2012, 2012, 8–21. [Google Scholar] [CrossRef]
- Chen, F.; Mohassab, Y.; Jiang, T.; Sohn, H.Y. Hydrogen Reduction Kinetics of Hematite Concentrate Particles Relevant to a Novel Flash Ironmaking Process. Metall. Mater. Trans. B 2015, 46, 1133–1145. [Google Scholar] [CrossRef]
- Turkdogan, E.T.; Vinters, J.V. Gaseous reduction of iron oxides: Part, I. Reduction of hematite in hydrogen. Metall. Mater. Trans. B 1971, 2, 3175–3188. [Google Scholar] [CrossRef]
- Corbari, R.; Fruehan, R.J. Reduction of iron oxide fines to wustite with CO/CO2 gas of low reducing potential. Metall. Mater. Trans. B 2010, 41, 318–329. [Google Scholar] [CrossRef]
- Qie, Y.; Lyu, Q.; Liu, X.; Li, J.; Lan, C.; Liu, X. Effect of hydrogen addition on reduction behavior of iron oxides in gas-injection blast furnace. ISIJ Int. 2017, 57, 404–417. [Google Scholar] [CrossRef] [Green Version]
- Kapelyushin, Y.; Xing, X.; Zhang, J.; Jeong, S.; Sasaki, Y.; Ostrovski, O. Effect of Alumina on the Gaseous Reduction of Magnetite in CO/CO2 Gas Mixtures. Metall. Mater. Trans. B Process. Metall. Mater. Process. Sci. 2015, 46, 1175–1185. [Google Scholar] [CrossRef]
- Paananen, T.; Kinnunen, K. EFfect of TiO2-content on Reduction of Iron Ore Agglomerates. Steel Res. Int. 2009, 80, 408–414. [Google Scholar] [CrossRef]
- Espenson, J.H. Chemical Kinetics and Reaction Mechanisms, 2nd ed.; Advanced Chemistry Series; McGraw-Hill: New York, NY, USA, 1995; ISBN 9780070202603. [Google Scholar]
- Piotrowski, K.; Mondal, K.; Lorethova, H.; Stonawski, L.; Szymański, T.; Wiltowski, T. Effect of gas composition on the kinetics of iron oxide reduction in a hydrogen production process. Int. J. Hydrogen Energy 2005, 30, 1543–1554. [Google Scholar] [CrossRef]
- Guo, D.; Hu, M.; Pu, C.; Xiao, B.; Hu, Z.; Liu, S.; Wang, X.; Zhu, X. Kinetics and mechanisms of direct reduction of iron ore-biomass composite pellets with hydrogen gas. Int. J. Hydrogen Energy 2015, 40, 4733–4740. [Google Scholar] [CrossRef]
- El-Geassy, A.A.; Shehata, K.A.; Ezz, S.Y. Mechanism of Iron Oxide Reduction With Hydrogen/Carbon Monoxide Mixtures. Trans. Iron Steel Inst. Jpn. 1977, 17, 629–635. [Google Scholar] [CrossRef] [Green Version]
- Pineau, A.; Kanari, N.; Gaballah, I. Kinetics of reduction of iron oxides by H2. Part I: Low temperature reduction of hematite. Thermochim. Acta 2006, 447, 89–100. [Google Scholar] [CrossRef]
- Manchili, S.K.; Wendel, J.; Hryha, E.; Nyborg, L. Analysis of iron oxide reduction kinetics in the nanometric scale using hydrogen. Nanomaterials 2020, 10, 1276. [Google Scholar] [CrossRef]
- Lin, H.Y.; Chen, Y.W.; Li, C. The mechanism of reduction of iron oxide by hydrogen. Thermochim. Acta 2003, 400, 61–67. [Google Scholar] [CrossRef]
- Kissinger, H.E. Variation of Peak Temperature With Heating Rate in Differential Thermal Analysis. J. Res. Natl. Bur. Stand. 1956, 57, 218–221. [Google Scholar] [CrossRef]
- Sastri, M.V.C.; Viswanath, R.P.; Viswanathan, B. Studies on the reduction of iron oxide with hydrogen. Int. J. Hydrogen Energy 1982, 7, 951–955. [Google Scholar] [CrossRef]
- Gray, N.B.; Henderson, J. Hydrogen reduction of dense hematites. AIME Met. Soc. Trans. 1966, 236, 1213–1217. [Google Scholar]
- Jung, S.S.; Lee, J.S. In-situ kinetic study of hydrogen reduction of Fe2O3 for the production of Fe nanopowder. Mater. Trans. 2009, 50, 2270–2276. [Google Scholar] [CrossRef] [Green Version]
- Sohn, I.; Jung, S.M. Effect of metal additions to the reduction of iron oxide composite pellets with hydrogen at moderate temperatures. Steel Res. Int. 2011, 82, 1345–1354. [Google Scholar] [CrossRef]
- Pineau, A.; Kanari, N.; Gaballah, I. Kinetics of reduction of iron oxides by H2. Part II. Low temperature reduction of magnetite. Thermochim. Acta 2007, 456, 75–88. [Google Scholar] [CrossRef]
- Tiernan, M.J.; Barnes, P.A.; Parkes, G.M.B. Reduction of iron oxide catalysts: The investigation of kinetic parameters using rate perturbation and linear heating thermoanalytical techniques. J. Phys. Chem. B 2001, 105, 220–228. [Google Scholar] [CrossRef]
- Kim, S.H.; Zhang, X.; Ma, Y.; Souza Filho, I.R.; Schweinar, K.; Angenendt, K.; Vogel, D.; Stephenson, L.T.; El-Zoka, A.A.; Mianroodi, J.R.; et al. Influence of microstructure and atomic-scale chemistry on the direct reduction of iron ore with hydrogen at 700 °C. Acta Mater. 2021, 212, 116933. [Google Scholar] [CrossRef]
- Bonalde, A.; Henriquez, A.; Manrique, M. Kinetic analysis of the iron oxide reduction using hydrogen-carbon monoxide mixtures as reducing agent. ISIJ Int. 2005, 45, 1255–1260. [Google Scholar] [CrossRef] [Green Version]
- Sohn, I.; Fruehan, R.J. The reduction of iron oxides by volatiles in a rotary hearth furnace process: Part, I. The role and kinetics of volatile reduction. Metall. Mater. Trans. B 2005, 36, 605–612. [Google Scholar] [CrossRef]
- Gaballah, N.M.; Zikry, A.F.; Khalifa, M.G.; Farag, A.B.; El-Hussiny, N.A.; Shalabi, M.E.H. Production of Iron from Mill Scale Industrial Waste via Hydrogen. Open, J. Inorg. Non Met. Mater. 2013, 3, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Kazemi, M.; Pour, M.S.; Sichen, D. Experimental and Modeling Study on Reduction of Hematite Pellets by Hydrogen Gas. Metall. Mater. Trans. B Process. Metall. Mater. Process. Sci. 2017, 48, 1114–1122. [Google Scholar] [CrossRef] [Green Version]

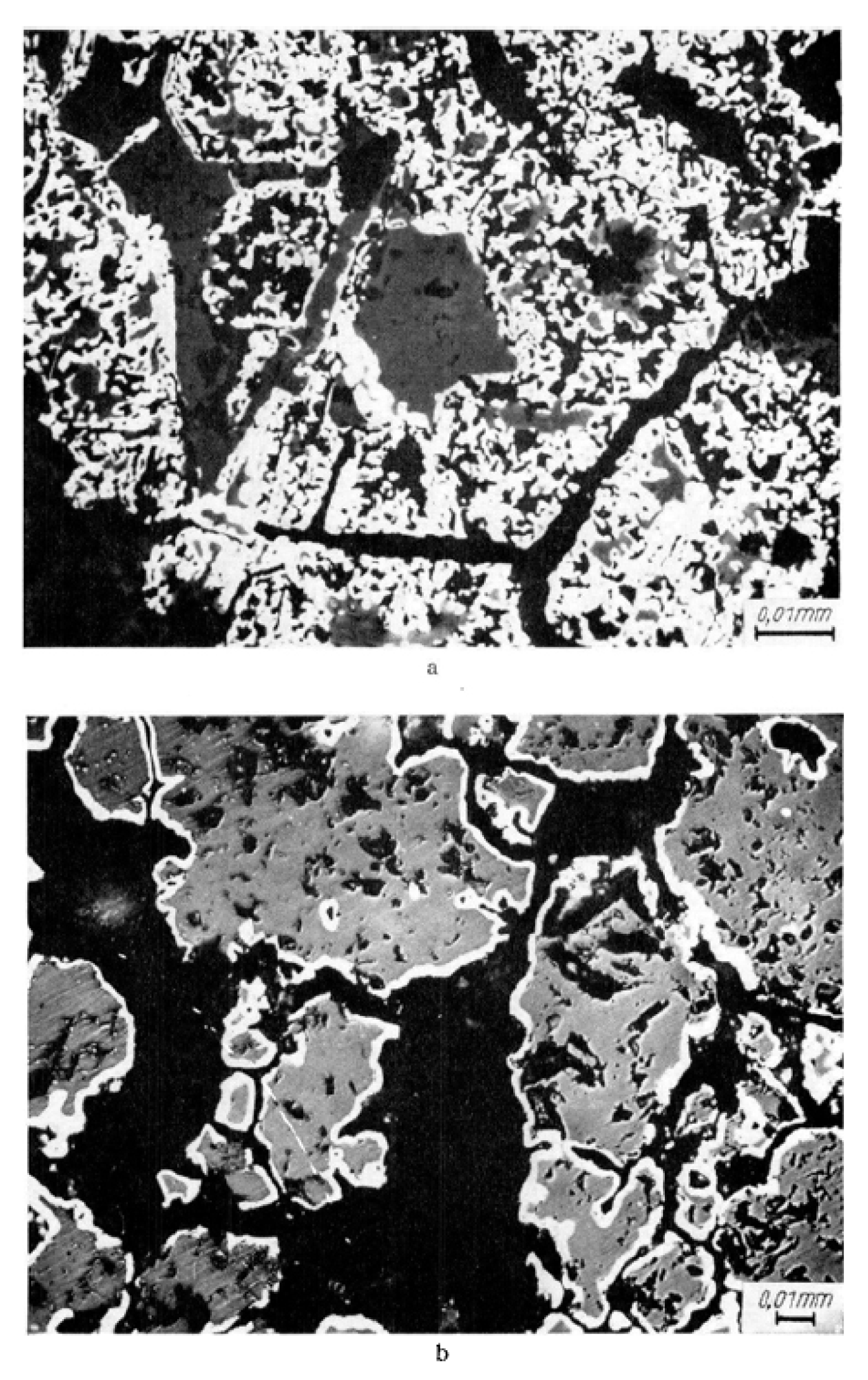
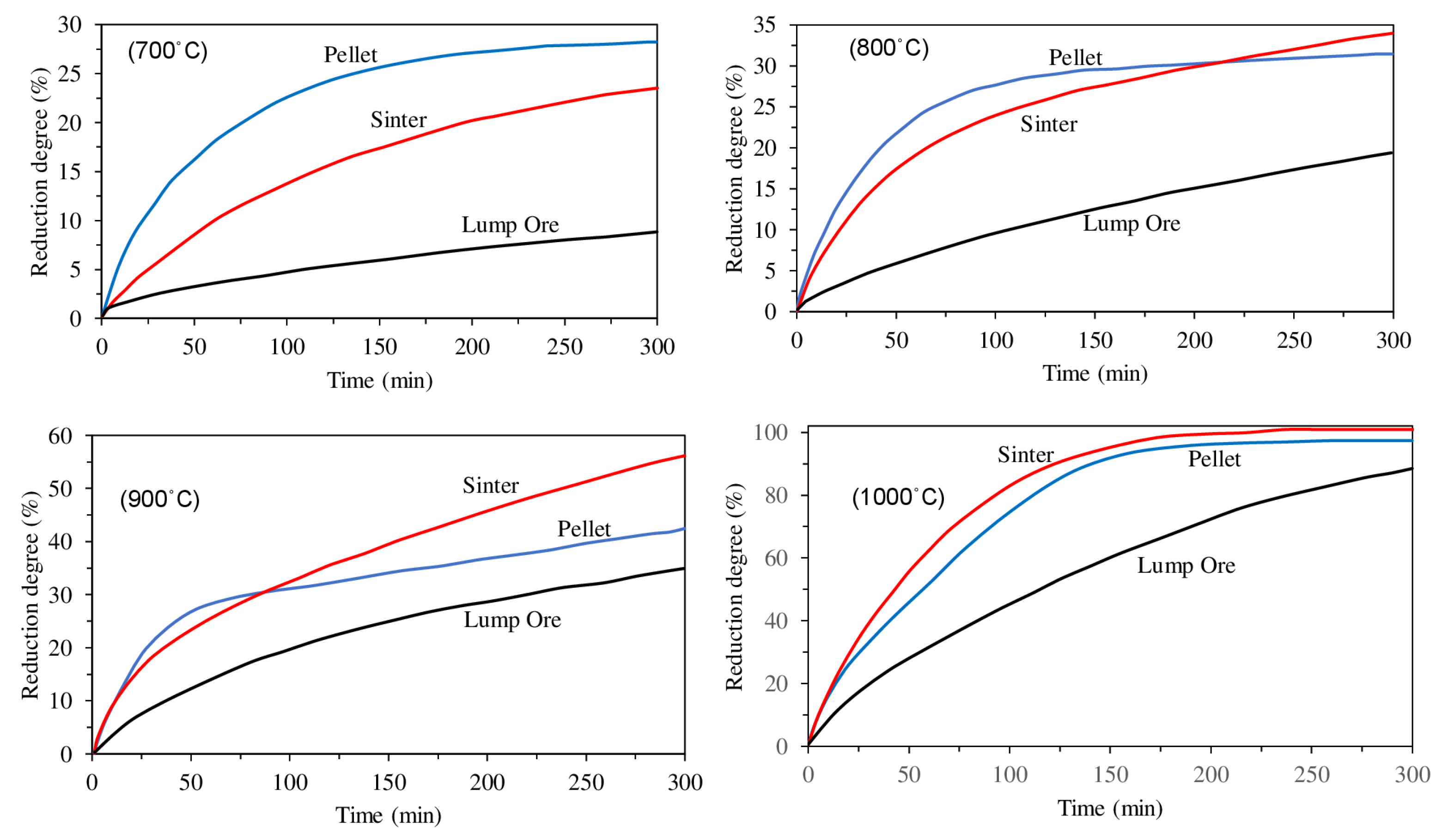


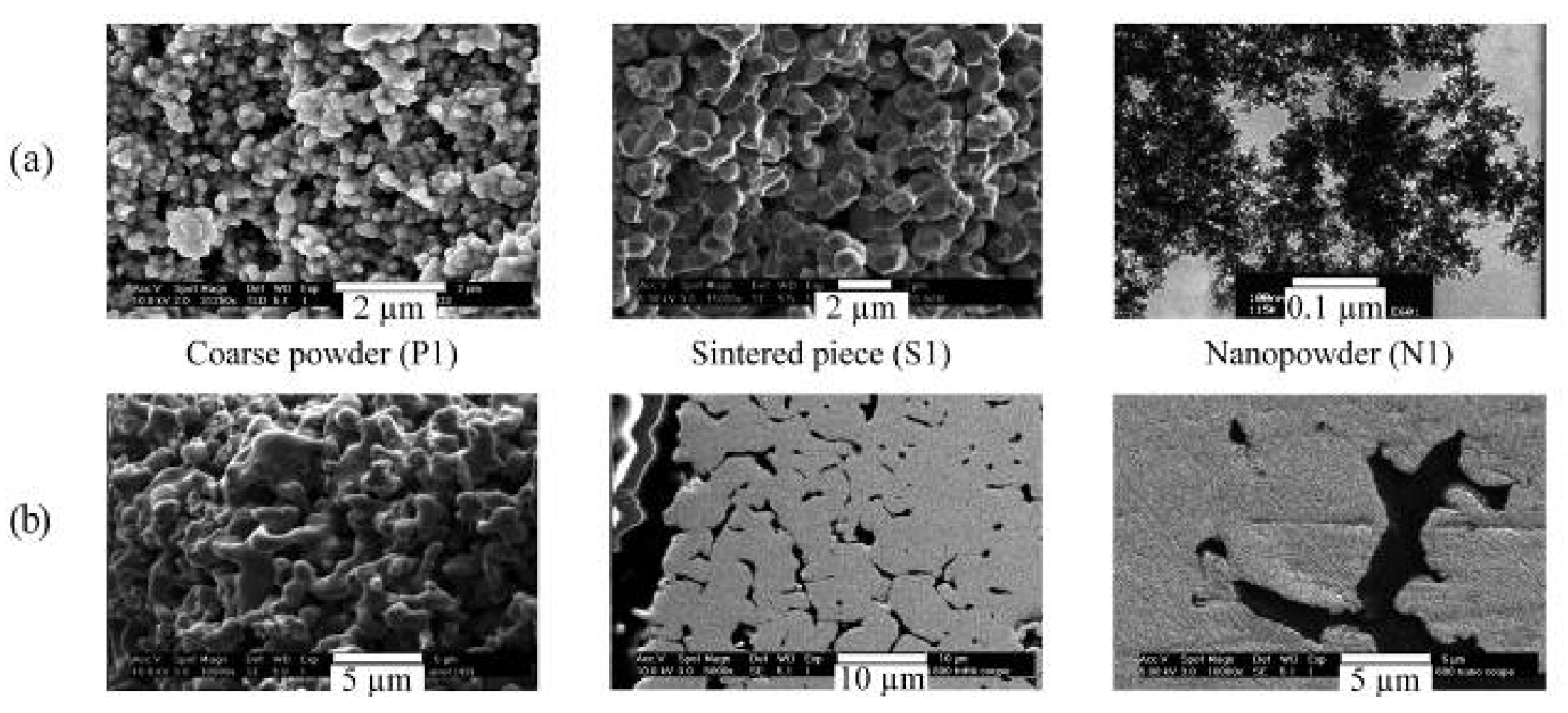


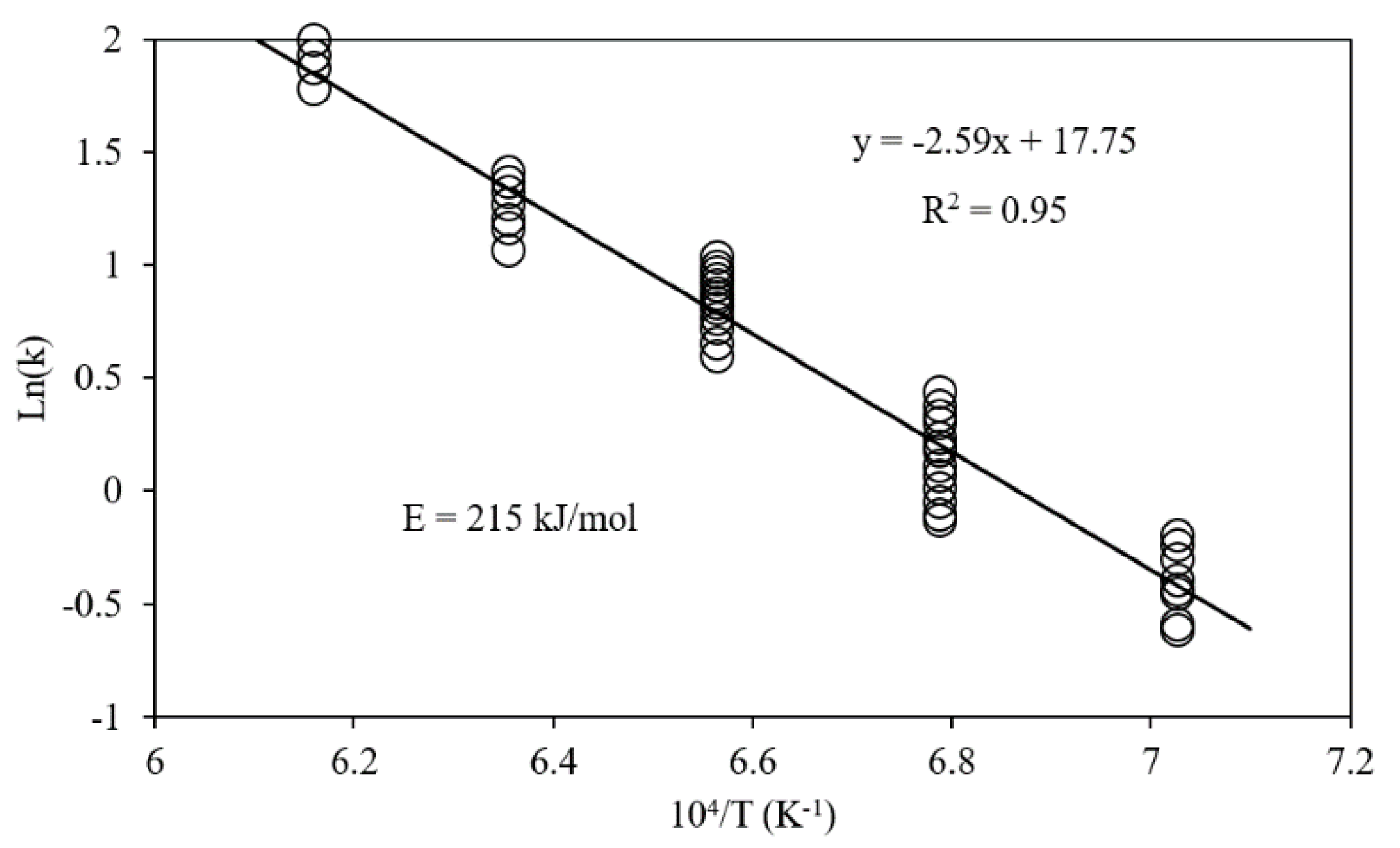
| Reference | Reduction Reaction/Step | Ea (kJ/mol) | Relevant Operating Conditions |
|---|---|---|---|
| [62] | Fe2O3 → Fe | 57.1 | Pure Fe2O3 |
| Fe2O3 → Fe | 110.5 | Fe2O3 mixed with MgO | |
| Fe2O3 → Fe | 108.4 | Fe2O3 mixed with Al2O3 | |
| Fe2O3 → Fe | 108.4 | Fe2O3 mixed with In2O3 | |
| Fe2O3 → Fe | 108.4 | Fe2O3 mixed with Li2O | |
| Fe2O3 → Fe | 130.0 | Fe2O3 mixed with TiO2 | |
| Fe2O3 → Fe | 89.9 | Hematite ore | |
| [60] | Fe2O3 → Fe3O4 | 89.1 | 5% H2 + 95% N2 |
| Fe3O4 → Fe | 70.4 | 5% H2 + 95% N2 | |
| [63] | Fe2O3 → Fe | 51.0 | Hematite ore |
| Fe2O3 → Fe | 96.1 | Natural single crystals | |
| [64] | Fe2O3 → Fe | 20–46 | Fe2O3 nanopowder |
| [65] | Fe2O3 → Fe | 15–20 | Fe2O3/metal Pellets |
| [58] | Fe2O3 → Fe3O4 | 75.9 | |
| Fe2O3 → Fe3O4 | 94.8 | 10% H2 + 90% N2 | |
| Fe3O4 → Fe | 88.0 | ||
| Fe3O4 → Fe | 103.0 | 10% H2 + 90% N2 | |
| [55] | Fe2O3 → Fe | 28.1 | 10% H2 + 90% N2 |
| Fe2O3 → Fe | 93.7 | 5.7% CO + 4.3% H2 + 90% N2 | |
| [56] | Fe2O3 → Fe | 111 | Hematite pellet with biomass |
| Fe2O3 → Fe | 122 | Hematite pellet without biomass | |
| [23] | Fe3O4 → FeO | 47 | |
| FeO → Fe | 30 | ||
| [66] | Fe3O4 → Fe | 200 | 227 °C < T < 250 °C |
| Fe3O4 → Fe | 71 | 250 °C < T < 390 °C | |
| Fe3O4 → Fe | 44 | T > 390 °C | |
| [67] | Fe3O4 → Fe (step) | 59–69 | 5% H2 + 95% He |
| Fe3O4 → Fe | 61–75 | 5% H2 + 95% He | |
| [19] | Fe3O4 → FeO | 13.5 | 5% H2 + 95% Ar |
| [57] | Fe2O3 → Fe | 37.4 | 25% H2 + 75% CO |
| Fe2O3 → Fe | 40.1 | 50% H2 + 50% CO | |
| Fe2O3 → Fe | 54.3 | 75% H2 + 25% CO | |
| Fe2O3 → Fe | 53.5 | 100% H2 | |
| [51] | Fe2O3 → Fe | 50.9 | 5% H2 + 30% CO + 65% N2 |
| Fe2O3 → Fe | 36.3 | 10% H2 + 30% CO + 60% N2 | |
| Fe2O3 → Fe | 35.8 | 15% H2 + 30% CO + 55% N2 | |
| Fe2O3 → Fe | 30.4 | 20% H2 + 30% CO + 50% N2 | |
| [25] | Fe2O3 → Fe3O4 | 92.0 | |
| Fe3O4 → FeO | 71.1 | ||
| FeO → Fe | 63.6 | ||
| [48] | Fe2O3 → Fe | 215 | |
| [36] | FeO → Fe | 53.7 | 100% H2 |
| FeO → Fe | 60.6 | 75% H2 + 25% CO | |
| FeO → Fe | 64.8 | 50% H2 + 50% CO | |
| [59] | Fe2O3 → Fe3O4 | 105–120 | Fe2O3 nanopowder |
| Fe3O4 → Fe | 55–45 | Fe2O3 nanopowder |
| Reference | Kinetics Controller | Condition/Description |
|---|---|---|
| [62] | topo chemical reaction | Pure Fe2O3 |
| [60] | two-dimensional nucleation | reduction of hematite to magnetite |
| [49] | diffusion through ash | |
| chemical reaction | ||
| [46] | chemical reaction | reduction of magnetite |
| [58] | Two- and three-dimensional nucleation | T < 420 °C |
| chemical reaction | T > 420 °C | |
| [55] | Two-dimensional nucleation and chemical reaction | initial stage |
| diffusion through ash | end of reaction | |
| [56] | chemical reaction | reduction of wüstite |
| [23] | chemical reaction | |
| [66] | diffusion | reduction of magnetite at low temperature |
| [57] | chemical reaction | |
| diffusion through ash | ||
| [51] | chemical reaction | |
| diffusion through ash | ||
| [65] | chemical reaction | reduction of hematite to magnetite |
| diffusion through ash | reduction of magnetite to wüstite | |
| [36] | chemical reaction | reduction of wüstite to iron |
| [59] | nucleation | reduction of hematite to magnetite |
| [68] | nucleation | reduction of wüstite to iron |
| [33] | chemical reaction | reduction of hematite to magnetite |
| chemical reaction | reduction of magnetite to wüstite | |
| diffusion through ash | reduction of wüstite to iron | |
| [69] | chemical reaction | |
| diffusion through ash | ||
| [37] | diffusion through film | |
| diffusion through ash | ||
| [25] | diffusion through ash | |
| [70] | nucleation | initial stage |
| chemical reaction and diffusion through ash | end of reaction | |
| [48] | nucleation | initial stage |
| [50] | diffusion through film | reduction of hematite to magnetite |
| chemical reaction | reduction of magnetite to wüstite | |
| [28] | diffusion through ash | reduction of wüstite to iron |
| [71] | diffusion through ash | |
| [72] | chemical reaction | |
| diffusion through ash |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heidari, A.; Niknahad, N.; Iljana, M.; Fabritius, T. A Review on the Kinetics of Iron Ore Reduction by Hydrogen. Materials 2021, 14, 7540. https://doi.org/10.3390/ma14247540
Heidari A, Niknahad N, Iljana M, Fabritius T. A Review on the Kinetics of Iron Ore Reduction by Hydrogen. Materials. 2021; 14(24):7540. https://doi.org/10.3390/ma14247540
Chicago/Turabian StyleHeidari, Aidin, Niusha Niknahad, Mikko Iljana, and Timo Fabritius. 2021. "A Review on the Kinetics of Iron Ore Reduction by Hydrogen" Materials 14, no. 24: 7540. https://doi.org/10.3390/ma14247540






