A New Methodological Approach on the Characterization of Optimal Charging Rates at the Hydrogen Plasma Smelting Reduction Process Part 2: Results
Abstract
:1. Introduction
2. Equipment and Methods
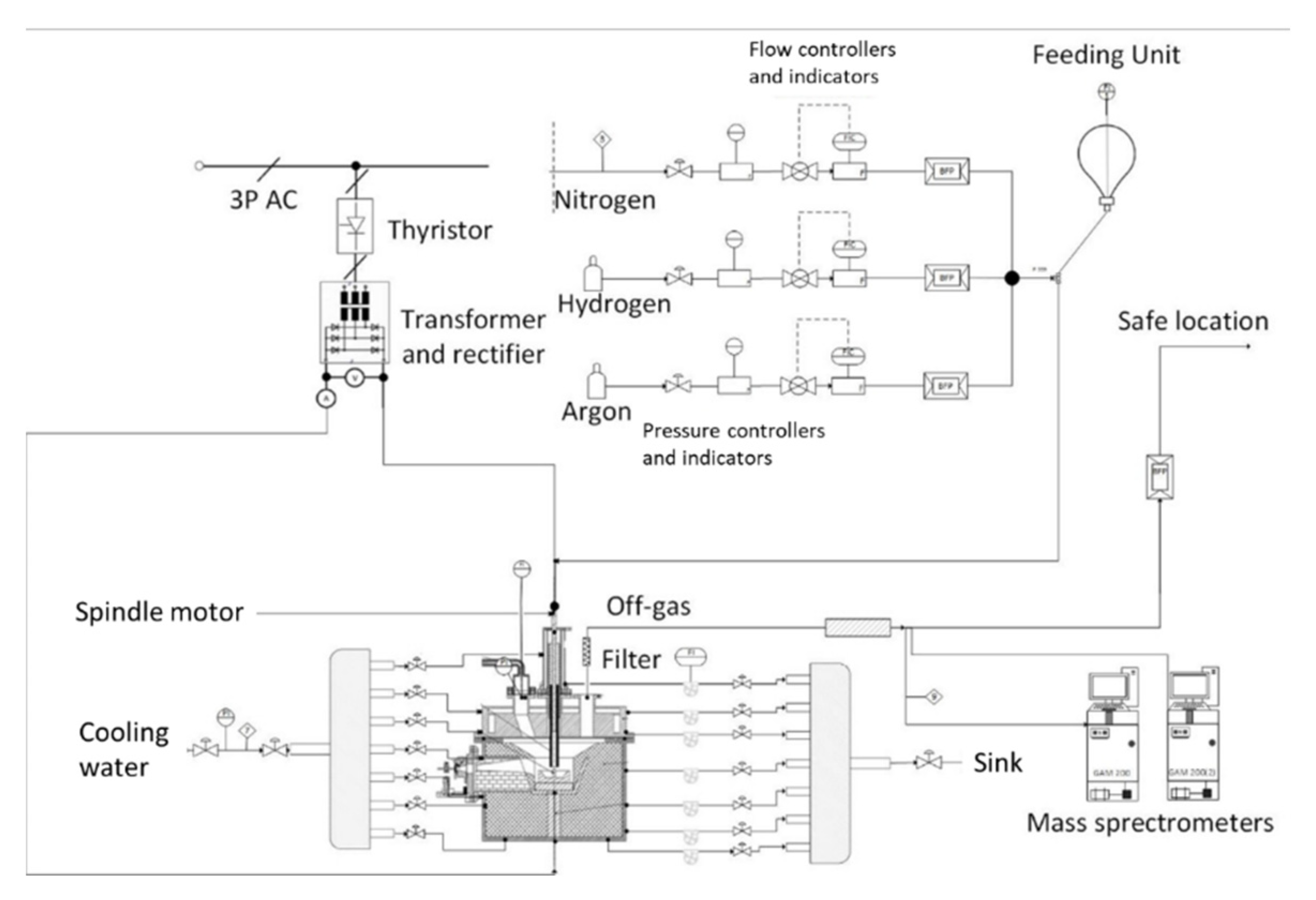
2.1. HPSR Laboratory Equipment
2.2. Procedure Description
- Pure argon is injected into the system to create a completely inert atmosphere. In this phase, no electrical energy was supplied.
- In the pre-melting step, the arc was lit between the HGE and the ignition pin in the steel crucible under pure argon to meltdown and reduce the batch-wise iron ore due to thermal decomposition.
- With the start of the pre-reduction phase, the plasma gas composition was changed according to the experimental plan. In the preliminary tests, this phase was performed for 5 min. The time depends on the desired degree of pre-reduction for the main trials and can vary in the range of 1–5 min.
- After the pre-reduction of the iron ore, the continuous charging of oxidic material through the HGE was carried out. For this purpose, the plasma gas composition and the charging rate were adjusted according to the trial program in Section 3.
- The post-reduction phase aims to reduce the remaining oxide melt to metallic iron. This phase ended when the exhaust gas composition at the mass spectrometer showed no further increase in hydrogen.
- The reactor was purged with argon to remove the remaining hydrogen as the last step.
2.3. Evaluation Methods
3. Experimental Program
4. Results and Discussion
4.1. Preliminary Tests and Parameter Definition for the Main Tests
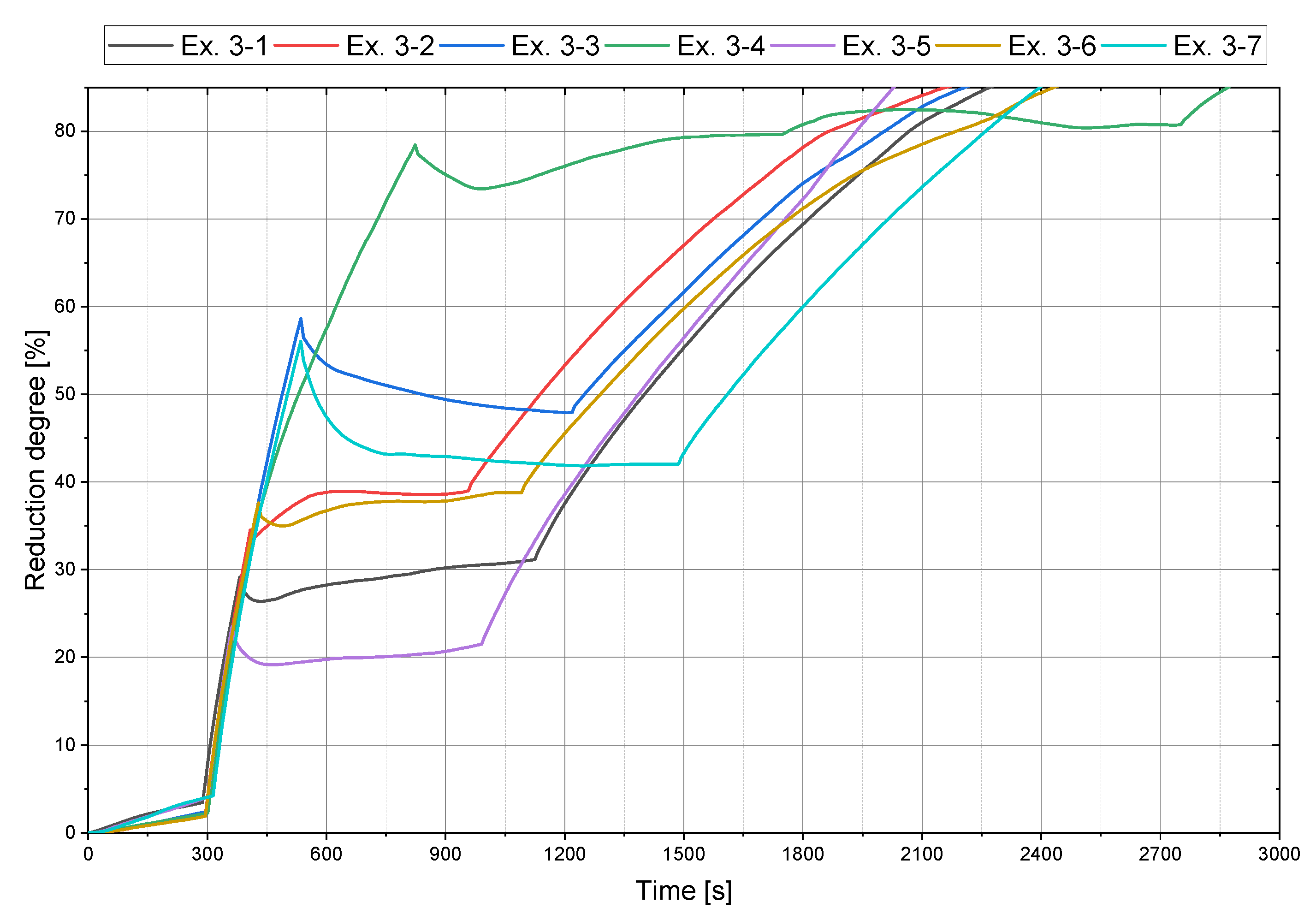
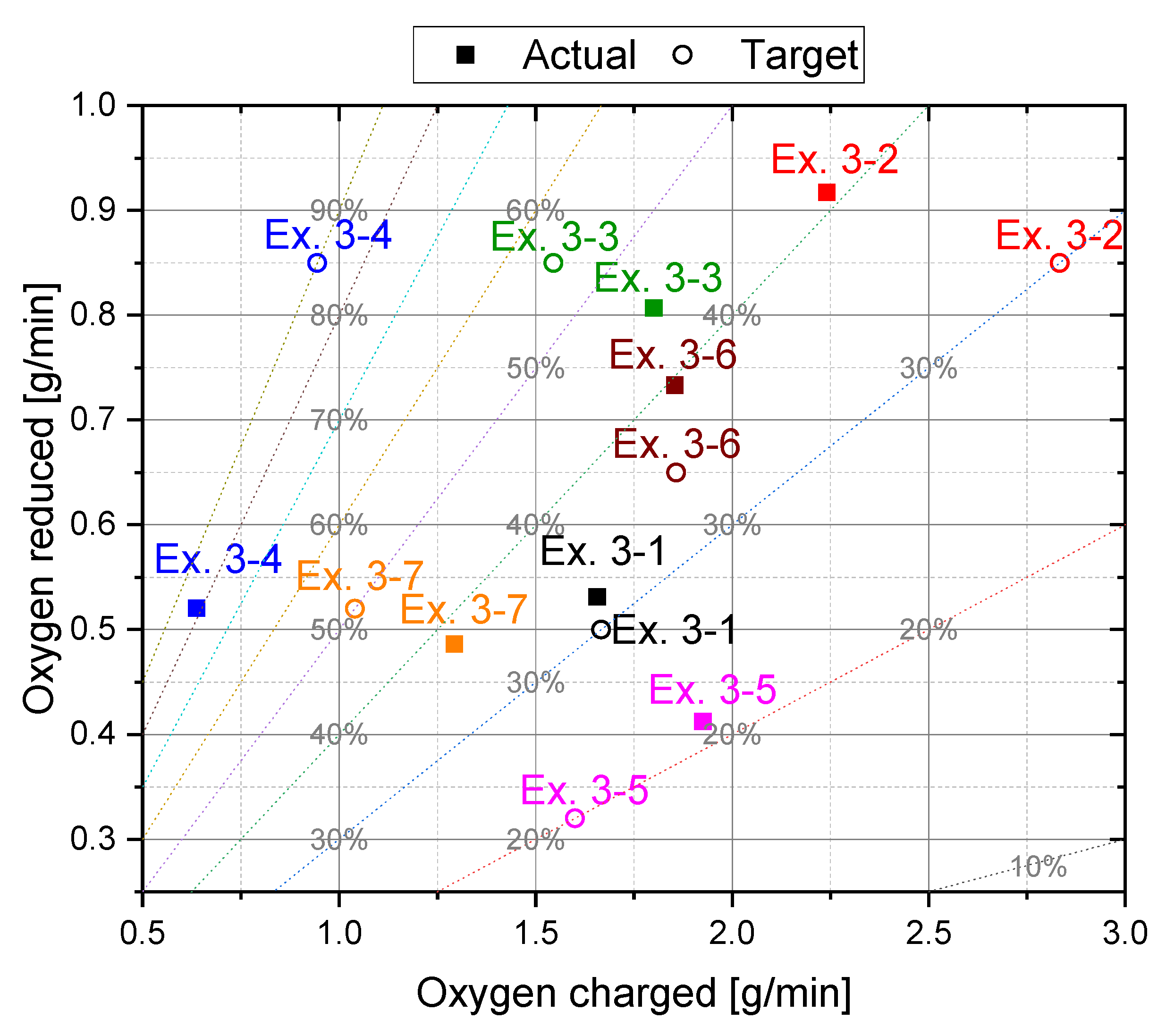
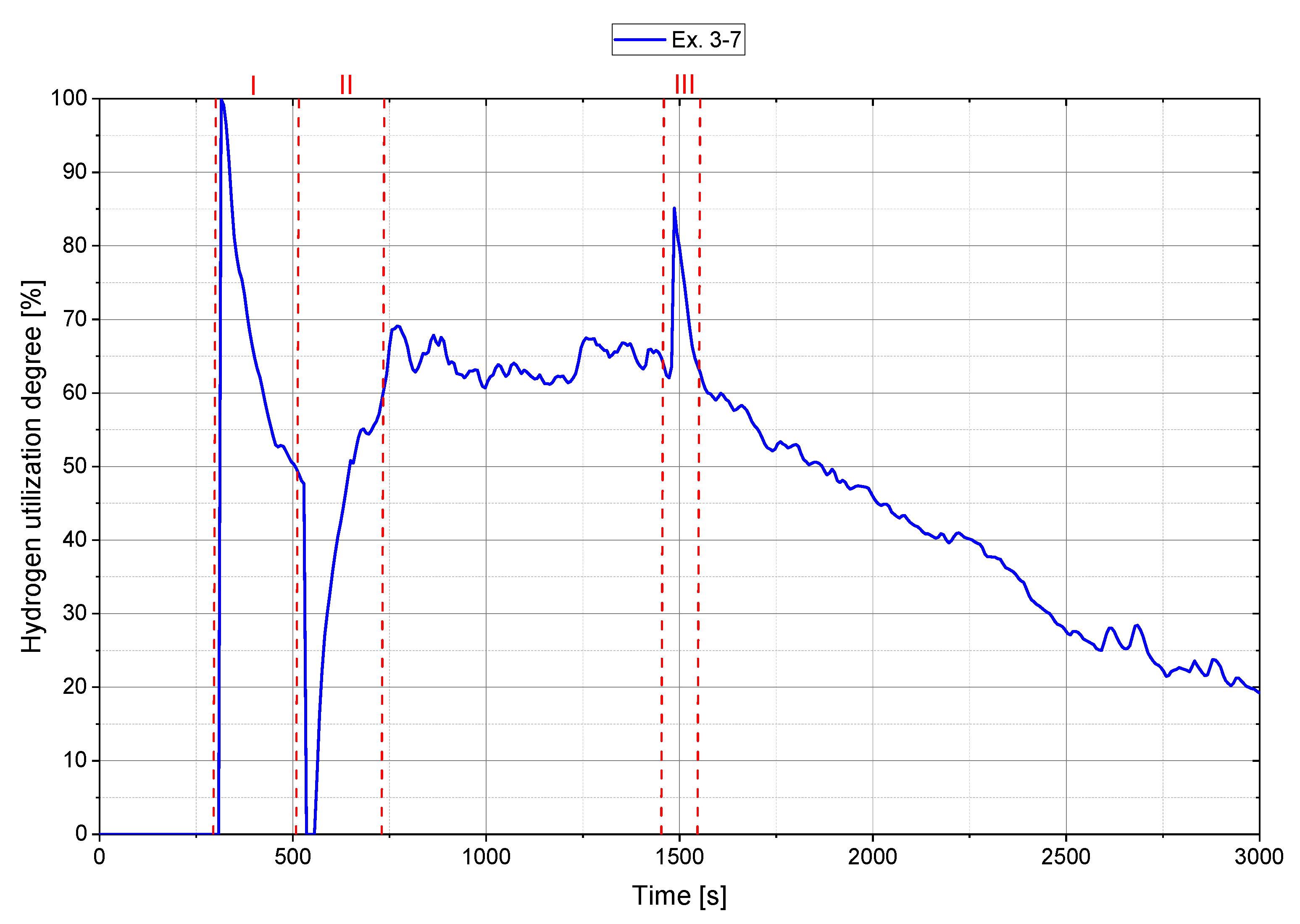
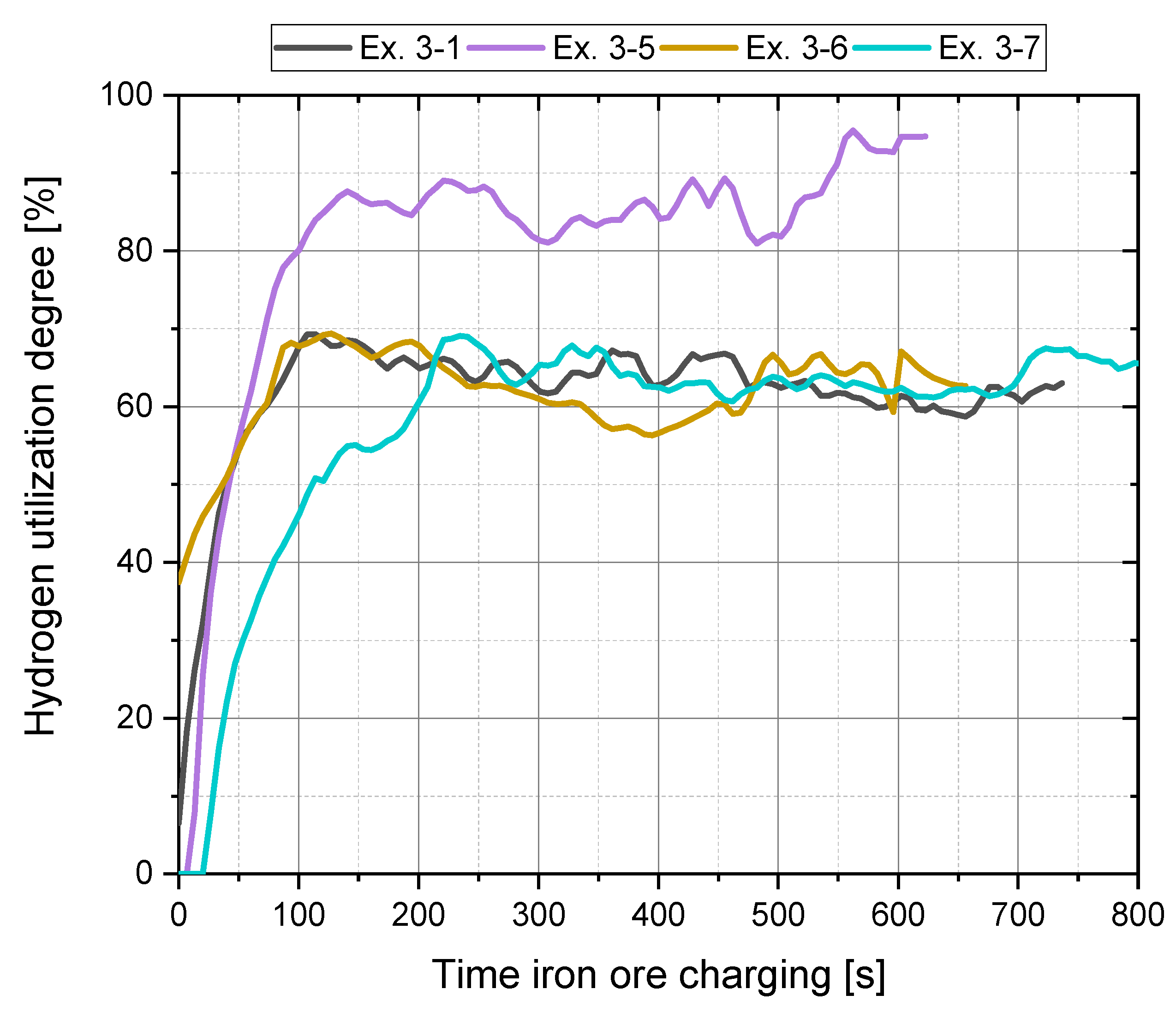
4.2. Main Experiments
4.3. Statistical Analysis
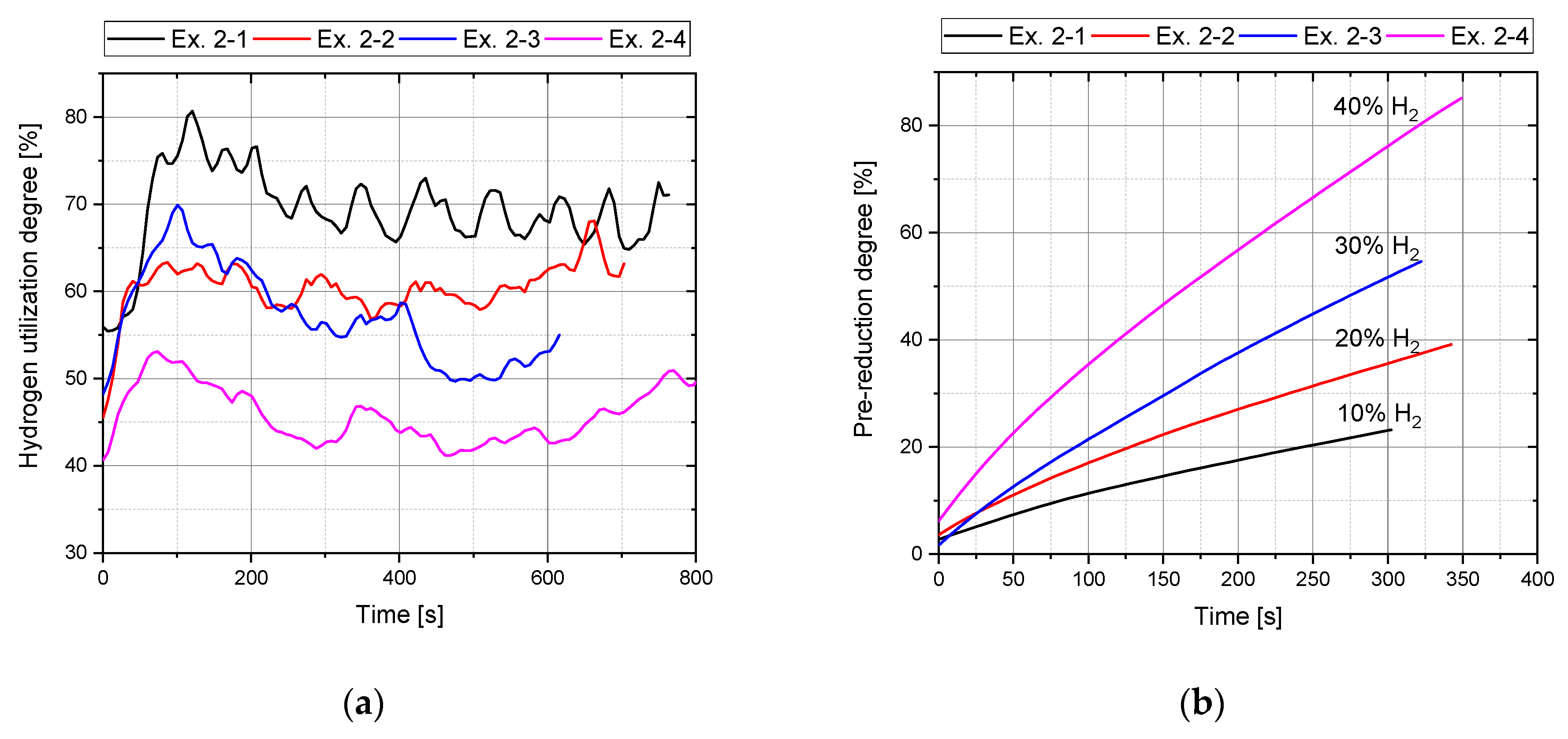
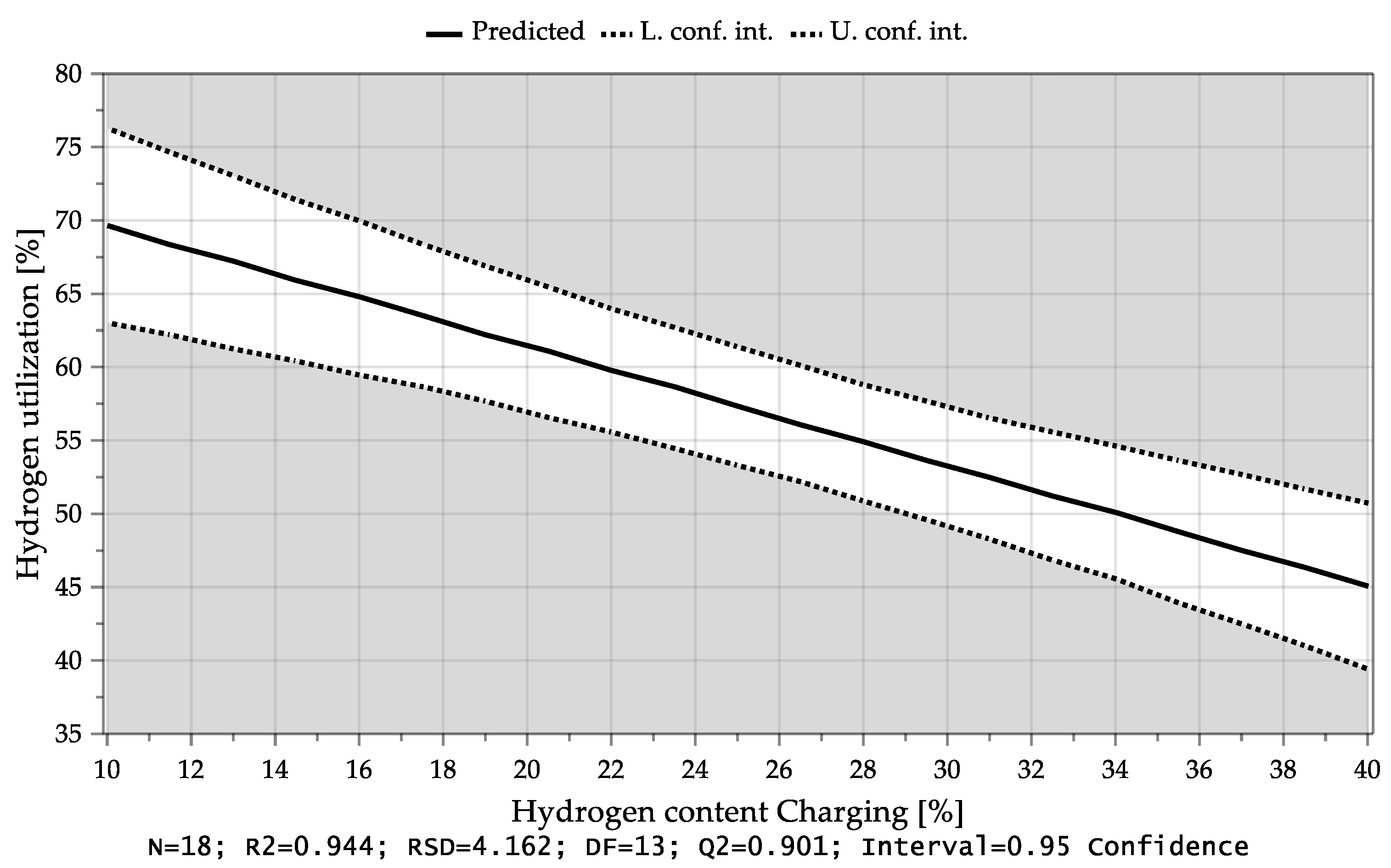
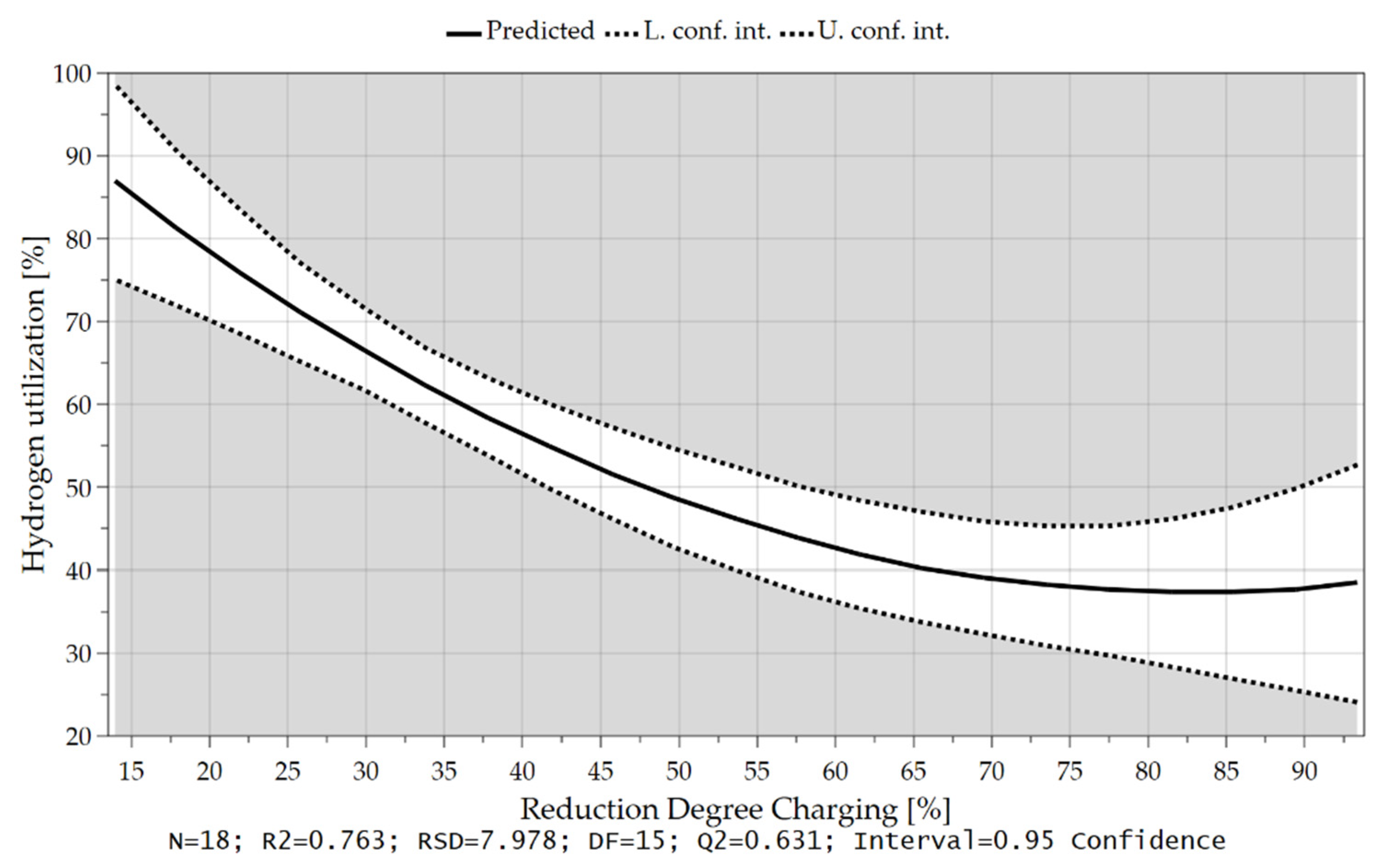
4.3.1. Hydrogen Utilization
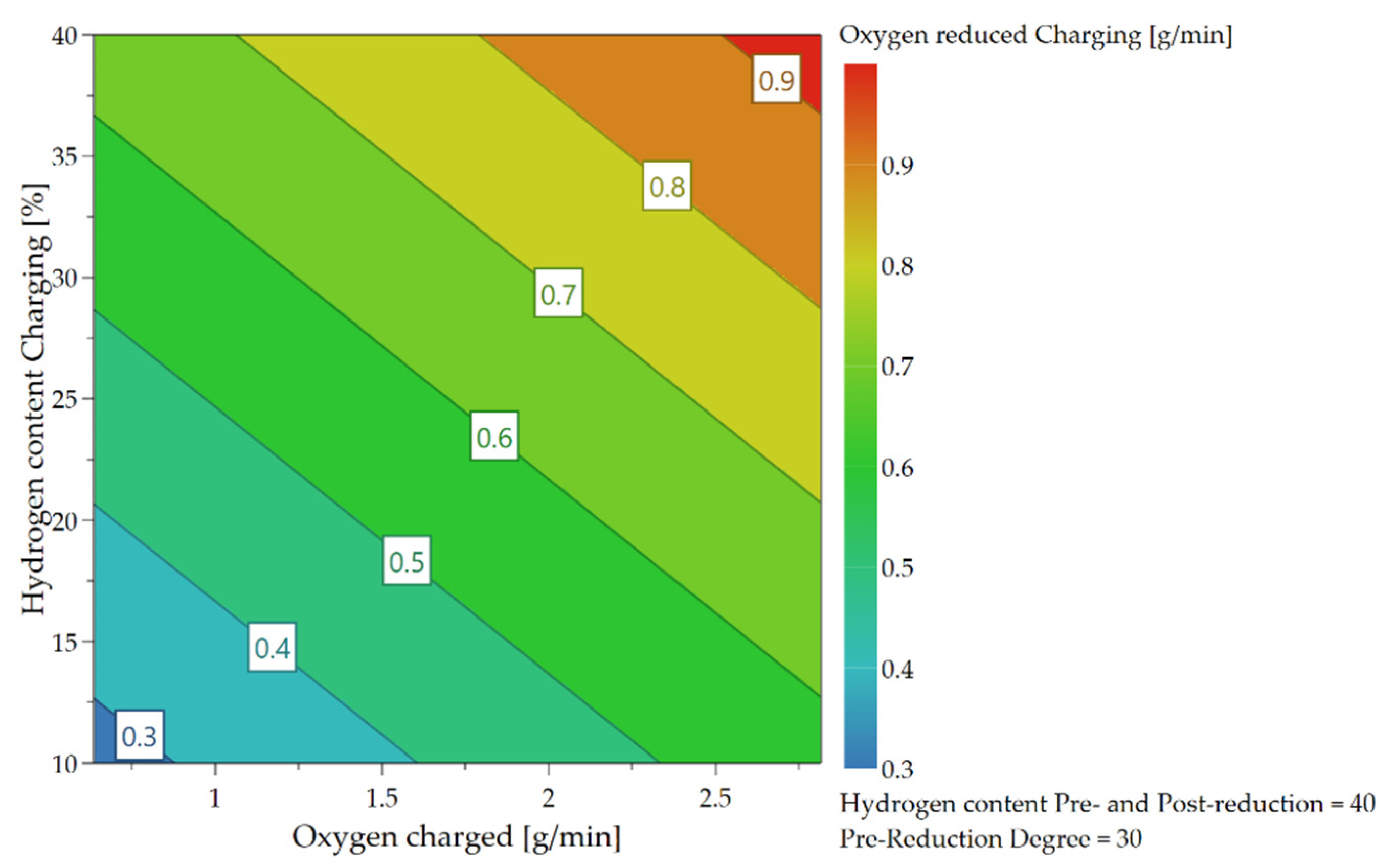
4.3.2. Oxygen Removal Rate during Continuous Charging
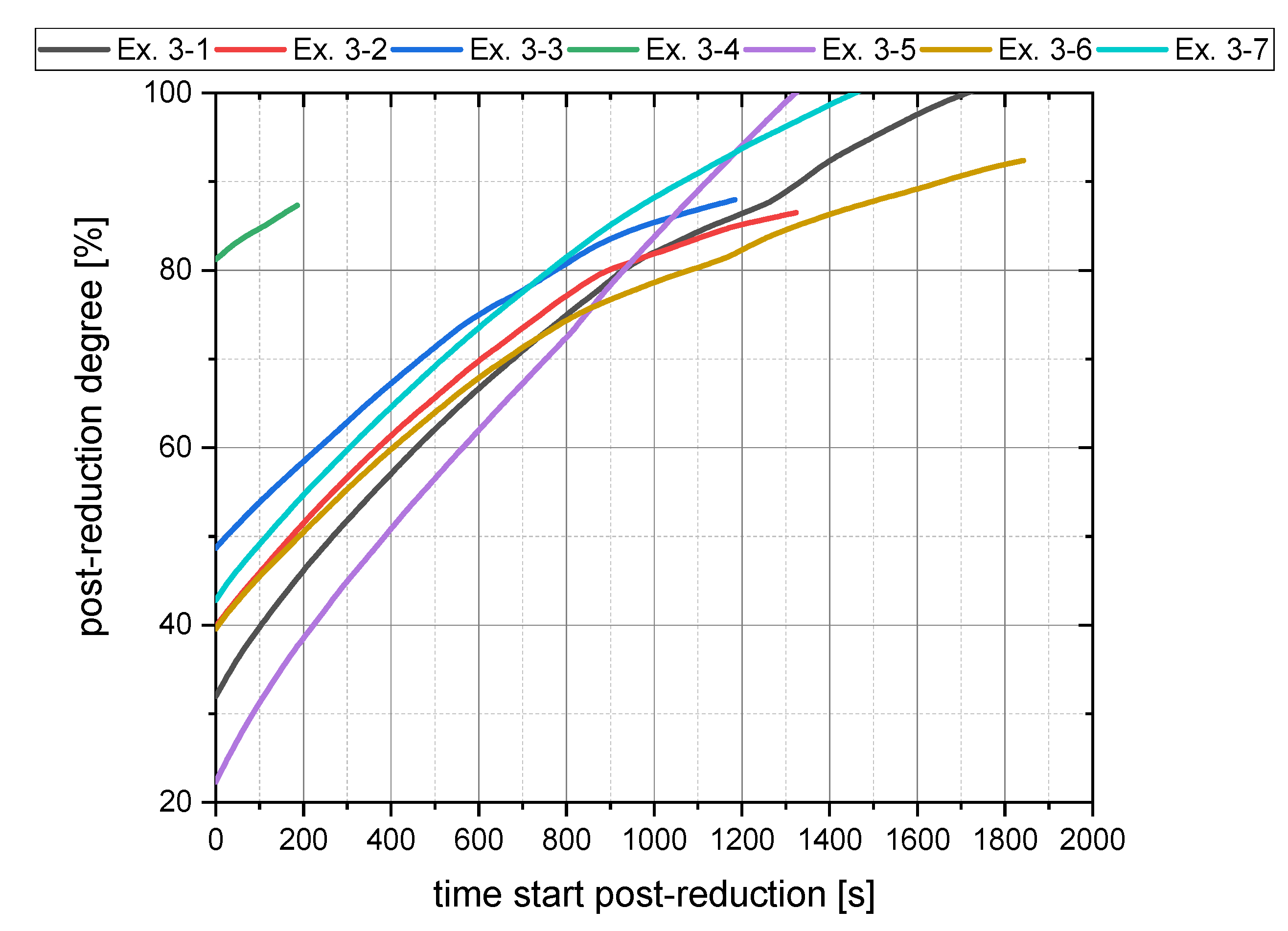
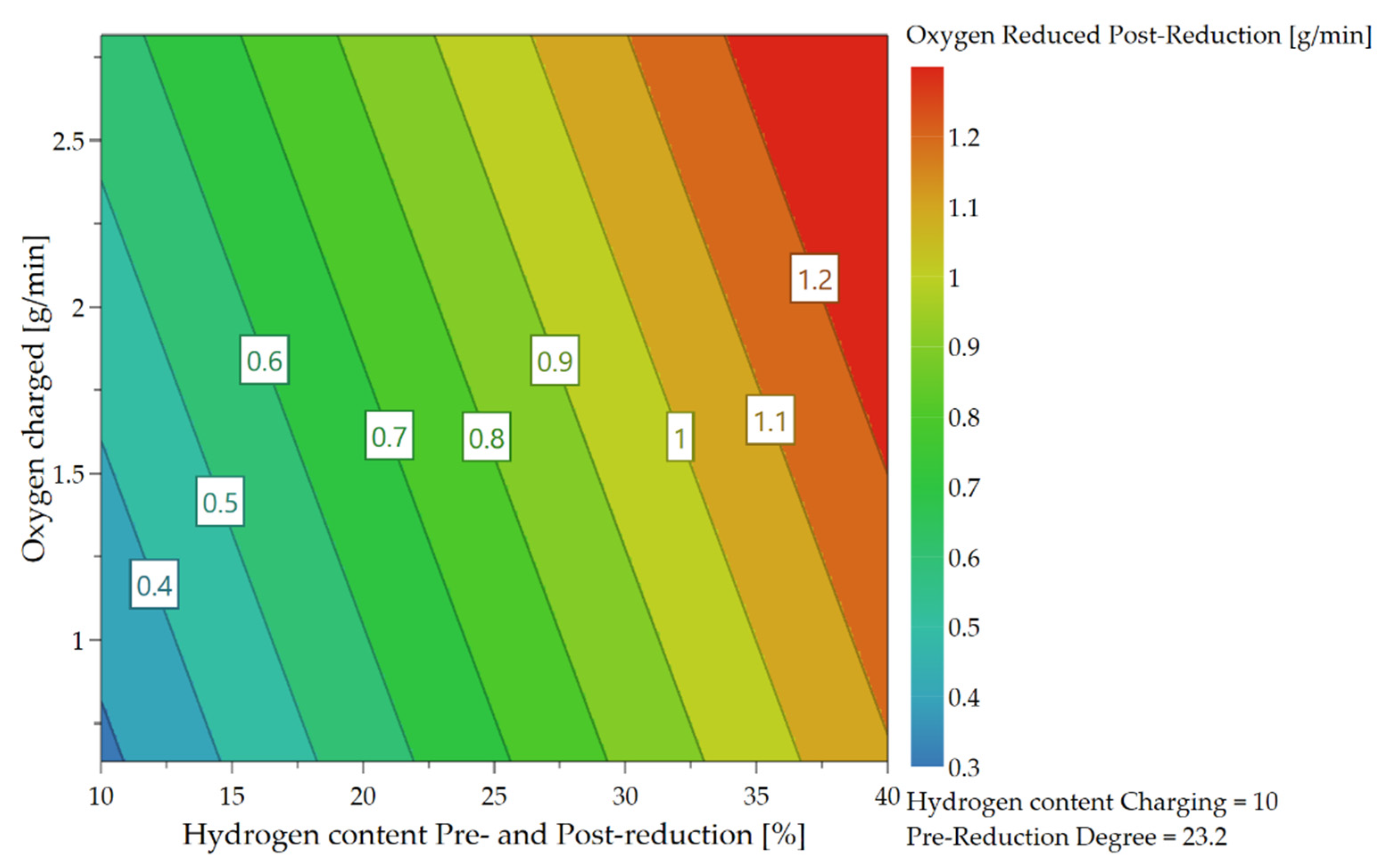
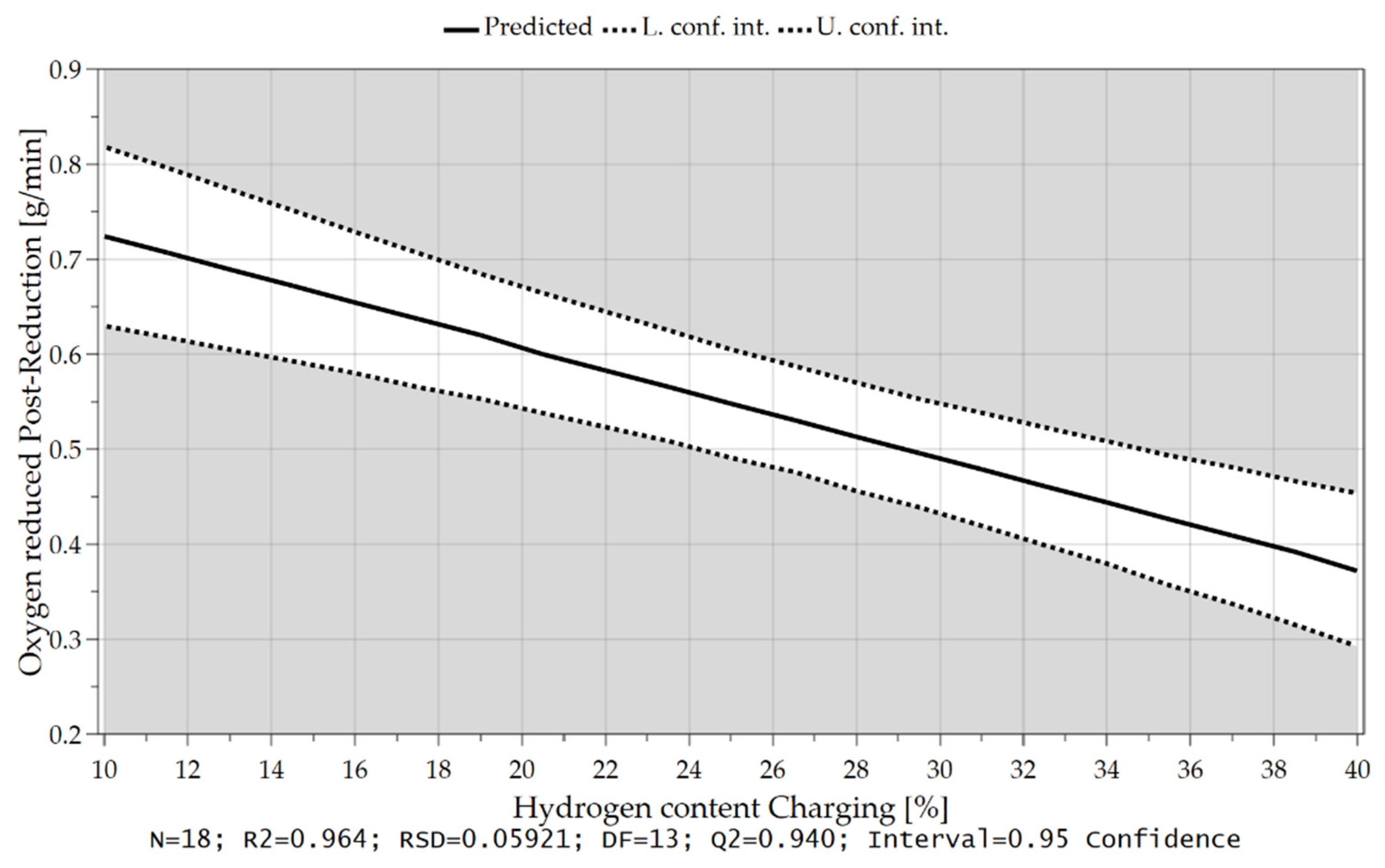
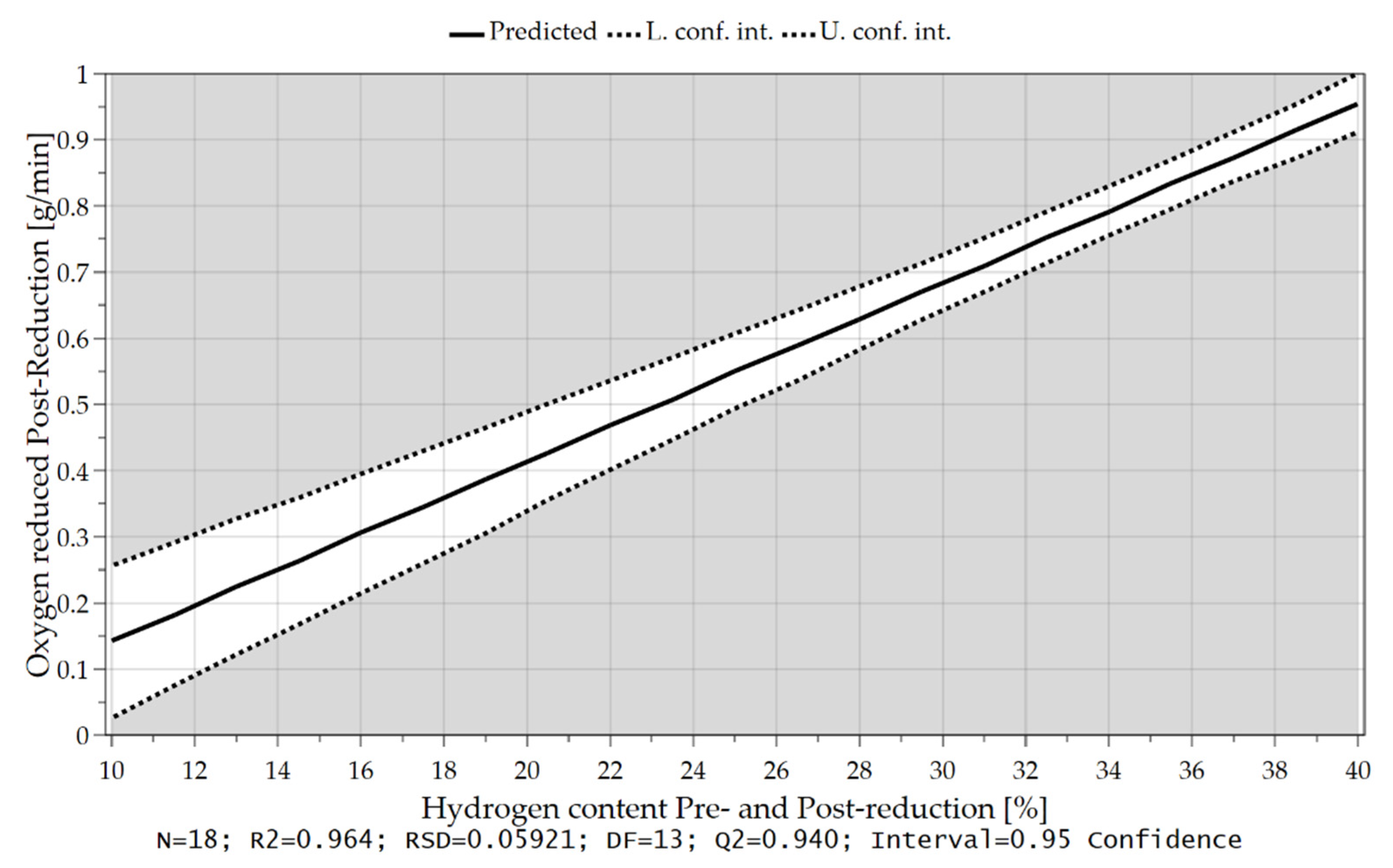
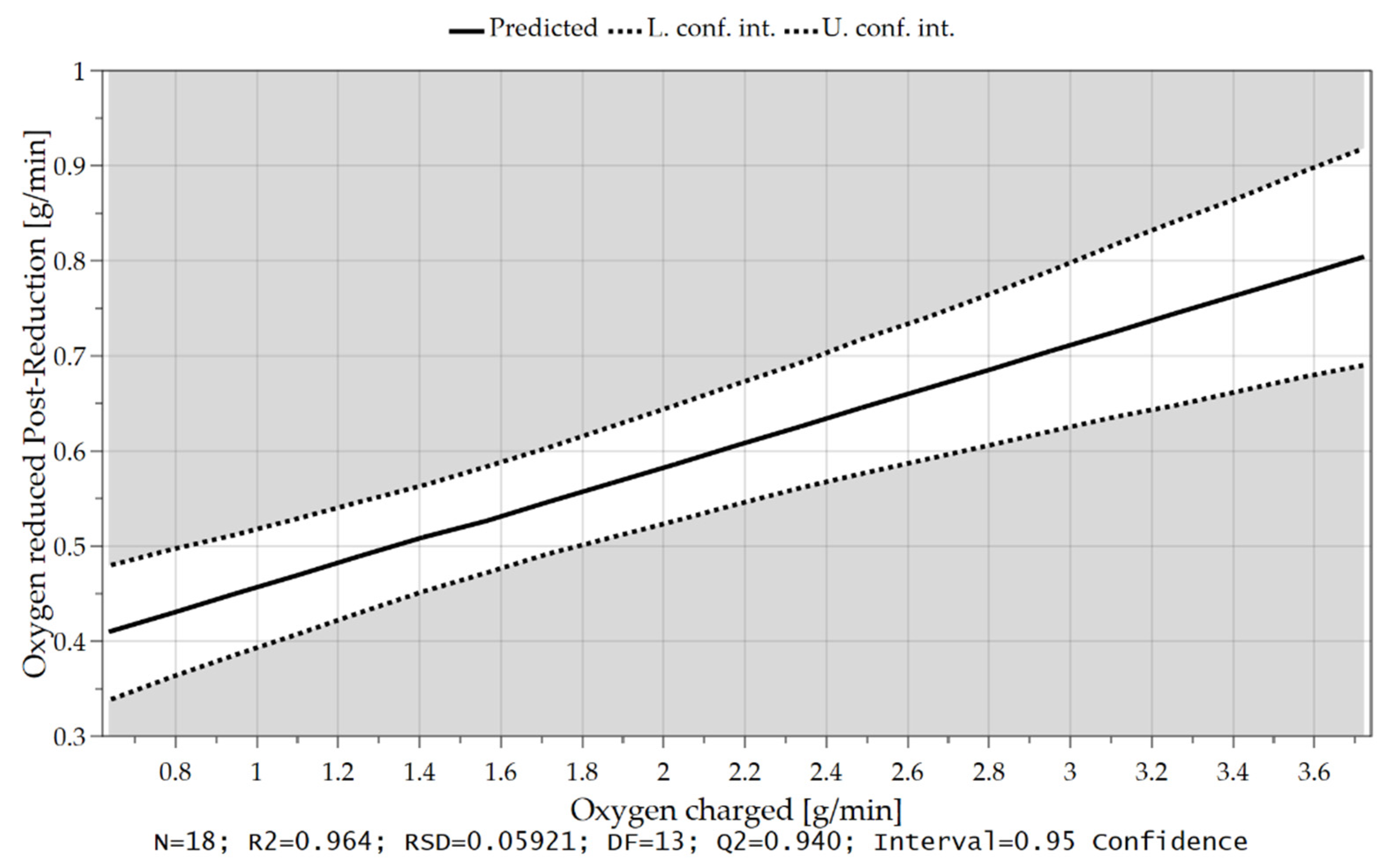
4.3.3. Oxygen Removal Rate during Post-Reduction
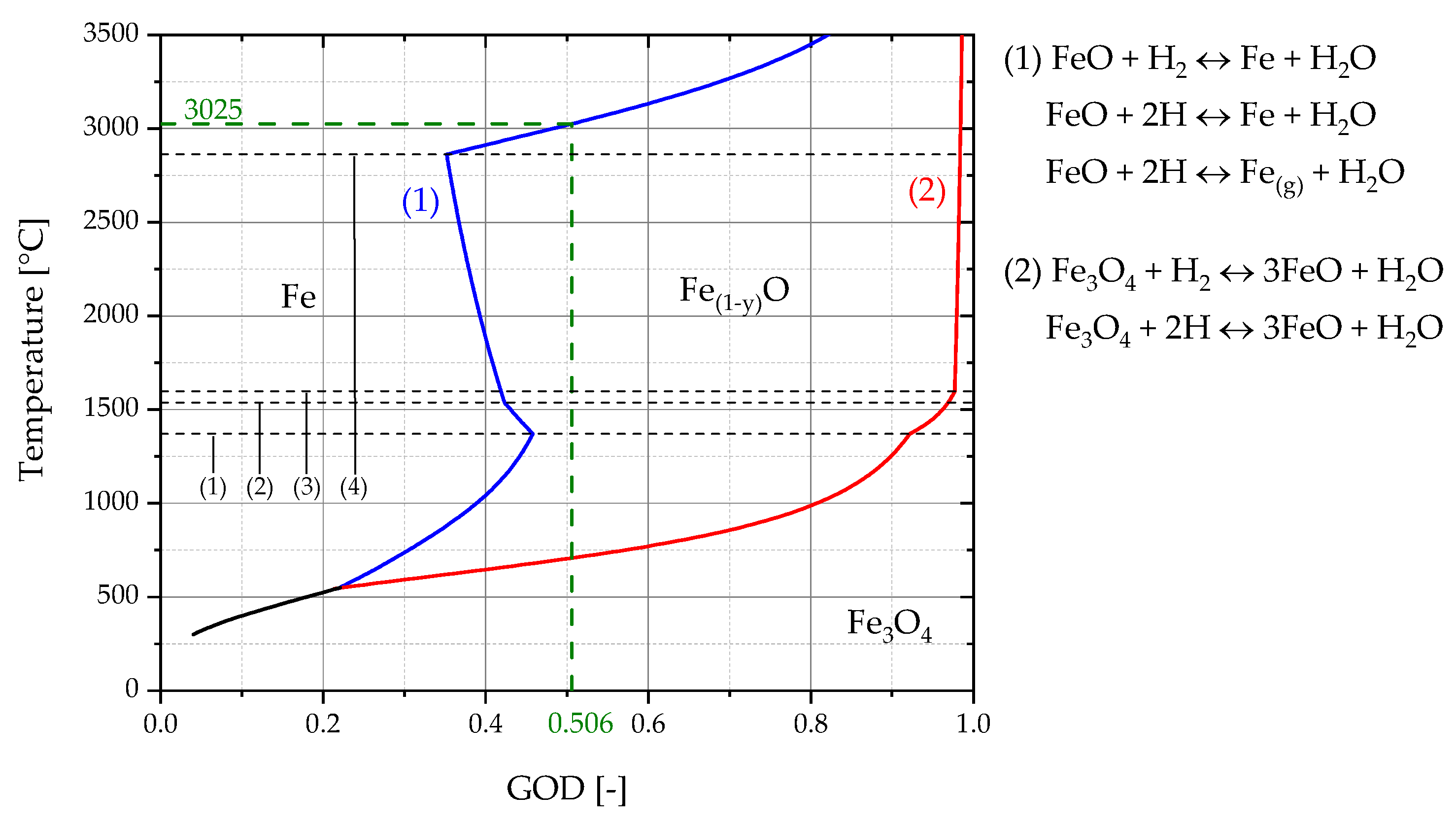
4.4. Reaction Rate Constant for Hydrogen Reduction
- (1)
- 1371 °C FeO(s) → FeO(l)
- (2)
- 1538 °C Fe(s) → Fe(l)
- (3)
- 1597 °C Fe3O4(s) → Fe3O4(l)
- (4)
- 2862 °C Fe(l) → Fe(g)
5. Conclusions and Outlook
5.1. Conclusions
5.1.1. Experimental Results
5.1.2. Comparison with the Results Reported in the Literature
5.1.3. Ecological Aspect
5.2. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Draxler, M.; Schenk, J.; Bürgler, T.; Sormann, A. The Steel Industry in the European Union on the Crossroad to Carbon Lean Production—Status, Initiatives and Challenges. BHM Berg-Und Hüttenmännische Mon. 2020, 165, 221–226. [Google Scholar] [CrossRef] [Green Version]
- Vogl, V.; Åhman, M.; Nilsson, L.J. The making of green steel in the EU: A policy evaluation for the early commercialization phase. Clim. Policy 2021, 21, 78–92. [Google Scholar] [CrossRef]
- Holappa, L. A General Vision for Reduction of Energy Consumption and CO2 Emissions from the Steel Industry. Metals 2020, 10, 1117. [Google Scholar] [CrossRef]
- Rieger, J.; Colla, V.; Matino, I.; Branca, T.A.; Stubbe, G.; Panizza, A.; Brondi, C.; Falsafi, M.; Hage, J.; Wang, X.; et al. Residue Valorization in the Iron and Steel Industries: Sustainable Solutions for a Cleaner and More Competitive Future Europe. Metals 2021, 11, 1202. [Google Scholar] [CrossRef]
- Eurofer, The European Steel Association. Low Carbon Roadmap: Pathways to a CO2-Neutral European Steel Industry [Online]; Eurofer, The European Steel Association: Brussels, Belgium, 2019; Available online: https://www.eurofer.eu/assets/Uploads/EUROFER-Low-Carbon-Roadmap-Pathways-to-a-CO2-neutral-European-Steel-Industry.pdf (accessed on 1 May 2022).
- Sormann, A. Untersuchung zur Schmelzreduktion von Eisenoxiden mit Wasserstoff als Reduktionsmittel. Ph.D. Thesis, Montanuniversität Leoben, Leoben, Austria, 1992. [Google Scholar]
- Bäck, E. Schmelzreduktion von Eisenoxiden mit Argon-Wasserstoff-Plasma. Ph.D. Thesis, Montanuniversität Leoben, Leoben, Austria, 1998. [Google Scholar]
- Plaul, J.F. Schmelzreduktion von hämatitischen Feinerzen im Wasserstoff-Argon-Plasma. Ph.D. Thesis, Montanuniversität Leoben, Leoben, Austria, 2005. [Google Scholar]
- Badr, K. Smelting of Iron Oxides Using Hydrogen Based Plasmas. Ph.D. Thesis, Montanuniversität Leoben, Leoben, Austria, 2007. [Google Scholar]
- Seftejani, M.N. Reduktion von Hämatit Mittels Wasserstoffplasma-Schmelzreduktion. Ph.D. Thesis, Montanuniversität Leoben, Leoben, Austria, 2020. [Google Scholar]
- Zarl, M.A. Ermittlung der Grundlagen für die Skalierung von Plasma-Schmelzreduktionsreaktoren für die Eisenherstellung. Ph.D. Thesis, Montanuniversität Leoben, Leoben, Austria, 2021. [Google Scholar]
- Zarl, M.A.; Ernst, D.; Cejka, J.; Schenk, J. A New Methodological Approach on the Characterization of Optimal Charging Rates at the Hydrogen Plasma Smelting Reduction Process: Part 1: Method. 2022. [Google Scholar]
- Zarl, M.A.; Farkas, M.A.; Schenk, J. A Study on the Stability Fields of Arc Plasma in the HPSR Process. Metals 2020, 10, 1394. [Google Scholar] [CrossRef]
- Cejka, J.F. Parameterevaluierung für den kontinuierlichen Chargiervorgang von Eisenerzen und Zuschlägen im Wasserstoff-Plasma-Schmelzreduktionsprozess. Master’s Thesis, Montanuniversitaet Leoben, Leoben, Austria, 2021. [Google Scholar]
- Nagasaka, T.; Hino, M.; Ban-Ya, S. Interfacial kinetics of hydrogen with liquid slag containing iron oxide. Met. Mater. Trans B 2000, 31, 945–955. [Google Scholar] [CrossRef]
- Nakamura, Y.; Ito, M.; Ishikawa, H. Reduction and dephosphorization of molten iron oxide with hy-drogen-argon plasma. Plasma Chem. Plasma Process. 1981, 1, 149–160. [Google Scholar] [CrossRef]
- Souza Filho, I.R.; Ma, Y.; Kulse, M.; Ponge, D.; Gault, B.; Springer, H.; Raabe, D. Sustainable steel through hydrogen plasma reduction of iron ore: Process, kinetics, microstructure, chemistry. Acta Mater. 2021, 213, 116971. [Google Scholar] [CrossRef]
- Kamiya, K.; Kitahara, N.; Morinaka, I.; Sakuraya, K.; Ozawa, M.; Tanaka, M. Reduction of Molten Iron Oxide and FeO Bearing Slags by H2-Ar Plasma. Trans. Iron Steel Inst. Jpn. 1984, 24, 7–16. [Google Scholar] [CrossRef]











| Experiment Designation | Speed of the Charging System (-) | Total Gas Flow (Nl/min) | Hydrogen Content (%) |
|---|---|---|---|
| Ex. 1–1 | 15 | 5 | 40 |
| Ex. 1–2 | 25 | 5 | 40 |
| Ex. 1–3 | 35 | 5 | 40 |
| Ex. 1–4 | 45 | 5 | 40 |
| Ex. 1–5 | 55 | 5 | 40 |
| Ex. 1–6 | 65 | 5 | 40 |
| Ex. 2–1 | 45 | 5 | 10 |
| Ex. 2–2 | 45 | 5 | 20 |
| Ex. 2–3 | 45 | 5 | 30 |
| Ex. 2–4 | 45 | 5 | 40 |
| Experiment Designation | Speed of the Charging System (-) | H2 Content Pre-/Post-Reduction (%) | H2 Content Charging (%) | Aimed Pre-Reduction Degree (%) |
|---|---|---|---|---|
| Ex. 3–1 | 45 | 40 | 20 | 30 |
| Ex. 3–2 | 65 | 40 | 40 | 30 |
| Ex. 3–3 | 45 | 40 | 40 | 55 |
| Ex. 3–4 | 15 | 40 | 40 | 90 |
| Ex. 3–5 | 45 | 40 | 10 | 20 |
| Ex. 3–6 | 45 | 40 | 30 | 35 |
| Ex. 3–7 | 25 | 40 | 20 | 50 |
| No | Element | (wt.%) |
|---|---|---|
| 1 | Fe2O3 1 | 92.83 |
| 2 | FeO | 1.07 |
| 3 | Total Fe | 65.81 |
| 4 | Silica | 1.694 |
| 5 | Aluminum oxide | 1.01 |
| 6 | Manganese | 0.17 |
| 7 | Phosphorus | 0.057 |
| 8 | Total sulfur | 0.014 |
| 9 | LOI 2 | 2.79 |
| Mesh Size (μm) | Fraction (wt.%) | Cum (wt.%) |
|---|---|---|
| 63–125 | 50 | 50 |
| 25–63 | 50 | 100 |
| Element | Unit | C | Si | Mn | P | S | Cr | Mo | Ni | Al | Cu |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Steel crucible | (wt.%) | 0.178 | 0.261 | 1.325 | 0.009 | 0.005 | 0.083 | 0.031 | 0.168 | 0.027 | 0.179 |
| Ignition pin | (wt.%) | 0.441 | 0.217 | 0.85 | 0.008 | 0.028 | 0.985 | 0.162 | 0.085 | 0.021 | 0.116 |
| Product Name | Purity (%) | O2 (ppm) | H2O (ppm) | N2 (ppm) |
|---|---|---|---|---|
| Hydrogen 5.0 | ≥99.999 | ≤2 | ≤5 | ≤3 |
| Argon 5.0 | ≥99.999 | ≤2 | ≤3 | ≤5 |
| Nitrogen 5.0 | ≥99.999 | ≤3 | ≤5 | - |
| Factors | Responses | |||||||
|---|---|---|---|---|---|---|---|---|
| Exp Name | Oxygen Charging Rate (g/min) | H2 Content Charging (%) | H2 Content Pre-/Post-Reduction (%) | Pre-Reduction Degree (%) | Time for Reduction (RD 85%) (s) | Average η H2 Charging (%) | Oxygen Reduction Charging (g/min) | Oxygen Reduction Post-Reduction (g/min) |
| Ex. 1–1 | 0.765 | 40 | 40 | 81.2 | 1245.4 | 41 | 0.714 | 0.602 |
| Ex. 1–2 | 1.111 | 40 | 40 | 78.9 | 2318.6 | 42.5 | 0.713 | 0.614 |
| Ex. 1–3 | 1.397 | 40 | 40 | 71.2 | 2524.8 | 50.1 | 0.784 | 0.701 |
| Ex. 1–4 | 1.566 | 40 | 40 | 64.8 | 2083.8 | 51.4 | 0.828 | 0.815 |
| Ex. 1–5 | 2.691 | 40 | 40 | 75.9 | 2672.3 | 53.7 | 0.87 | 0.768 |
| Ex. 1–6 | 2.816 | 40 | 40 | 73.4 | 2636.3 | 50.9 | 0.854 | 0.905 |
| Ex. 2–1 | 1.596 | 10 | 10 | 23.2 | 5871.5 | 69.3 | 0.311 | 0.396 |
| Ex. 2–2 | 1.708 | 20 | 20 | 39.2 | 5317.5 | 60.4 | 0.527 | 0.487 |
| Ex. 2–3 | 1.868 | 30 | 30 | 54.6 | 2758.2 | 57.1 | 0.68 | 0.713 |
| Ex. 2–4 | 1.445 | 40 | 40 | 85.1 | 2276.7 | 46 | 0.794 | 0.650 |
| Ex. 3–1 | 1.655 | 20 | 40 | 29.1 | 2276.9 | 63.79 | 0.532 | 1.129 |
| Ex. 3–2 | 2.239 | 40 | 40 | 34.5 | 2161.1 | 59.3 | 0.918 | 0.902 |
| Ex. 3–3 | 1.8 | 40 | 40 | 58.7 | 2208.4 | 49.6 | 0.807 | 0.767 |
| Ex. 3–4 | 0.636 | 40 | 40 | 78.4 | 2875.4 | 29.5 | 0.521 | 0.494 |
| Ex. 3–5 | 1.925 | 10 | 40 | 23.5 | 2028.8 | 86.3 | 0.413 | 1.303 |
| Ex. 3–6 | 1.853 | 30 | 40 | 37.6 | 2438.3 | 63.2 | 0.734 | 0.853 |
| Ex. 3–7 | 1.292 | 20 | 40 | 56 | 2397.4 | 63.2 | 0.487 | 0.914 |
| Ex. 3–8 | 3.724 | 10 | 40 | 37.3 | 1413.1 | 93.8 | 0.701 | 1.424 |
| Acronym | Meaning |
|---|---|
| N | Number of valid experiments responses |
| R2 | Coefficient of determination |
| RSD | Residual standard deviation |
| DF | Number of degrees of freedom |
| Q2 | Percent of the variation of the response predicted by the model |
| Interval | Confidence interval |
| Parameter | Ex. 1–4 | Ex. 1–6 | Ex. 3–3 |
|---|---|---|---|
| Focal Spot Area A1 (mm2) | 53.1 | 39.4 | 22.9 |
| Focal Spot Area A2 (mm2) | 30.3 | 19.3 | 25.1 |
| Focal Spot Area A3 (mm2) | 43.9 | 35.5 | 74.1 |
| Focal Spot Area A4 (mm2) | 59.6 | 52.3 | 23.5 |
| Focal Spot Area A5 (mm2) | 56.9 | 68.8 | 23.1 |
| Average Spot Area (mm2) | 48.8 | 43.0 | 33.7 |
| Oxygen removal rate (g/min) | 0.828 | 0.854 | 0.807 |
| Oxygen supply rate (g/min) | 1.566 | 2.816 | 1.800 |
| Reduction degree (%) | 52.8 | 30.3 | 44.8 |
| Hydrogen supply rate (L/min) | 2 | 2 | 2 |
| Specific hydrogen supply (l hydrogen/g oxygen bond to iron) | 1.28 | 0.71 | 1.11 |
| Partial pressure pH2 (kPa) | 40.52 | 40.52 | 40.52 |
| Partial pressure pH2O (kPa) | 10.98 | 10.92 | 10.75 |
| GOD/Hydrogen Utilization (-) | 0.514 | 0.509 | 0.496 |
| Equilibrium partial pressure ratio K’H (-) | 1.057 | 1.038 | 0.986 |
| Reaction rate r (kg O2/m2 s) | 0.28 | 0.33 | 0.40 |
| Reaction rate constant ka (kg O2/m2 s Pa) | 9.39 × 10−6 | 1.10 × 10−5 | 1.35 × 10−5 |
| O/Fe (-) | 0.71 | 1.05 | 0.83 |
| Estimated temperature (°C) | 3035 | 3025 | 3015 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ernst, D.; Zarl, M.A.; Cejka, J.; Schenk, J. A New Methodological Approach on the Characterization of Optimal Charging Rates at the Hydrogen Plasma Smelting Reduction Process Part 2: Results. Materials 2022, 15, 4065. https://doi.org/10.3390/ma15124065
Ernst D, Zarl MA, Cejka J, Schenk J. A New Methodological Approach on the Characterization of Optimal Charging Rates at the Hydrogen Plasma Smelting Reduction Process Part 2: Results. Materials. 2022; 15(12):4065. https://doi.org/10.3390/ma15124065
Chicago/Turabian StyleErnst, Daniel, Michael Andreas Zarl, Julian Cejka, and Johannes Schenk. 2022. "A New Methodological Approach on the Characterization of Optimal Charging Rates at the Hydrogen Plasma Smelting Reduction Process Part 2: Results" Materials 15, no. 12: 4065. https://doi.org/10.3390/ma15124065








