A New Methodological Approach to the Characterization of Optimal Charging Rates at the Hydrogen Plasma Smelting Reduction Process Part 1: Method
Abstract
:1. Introduction
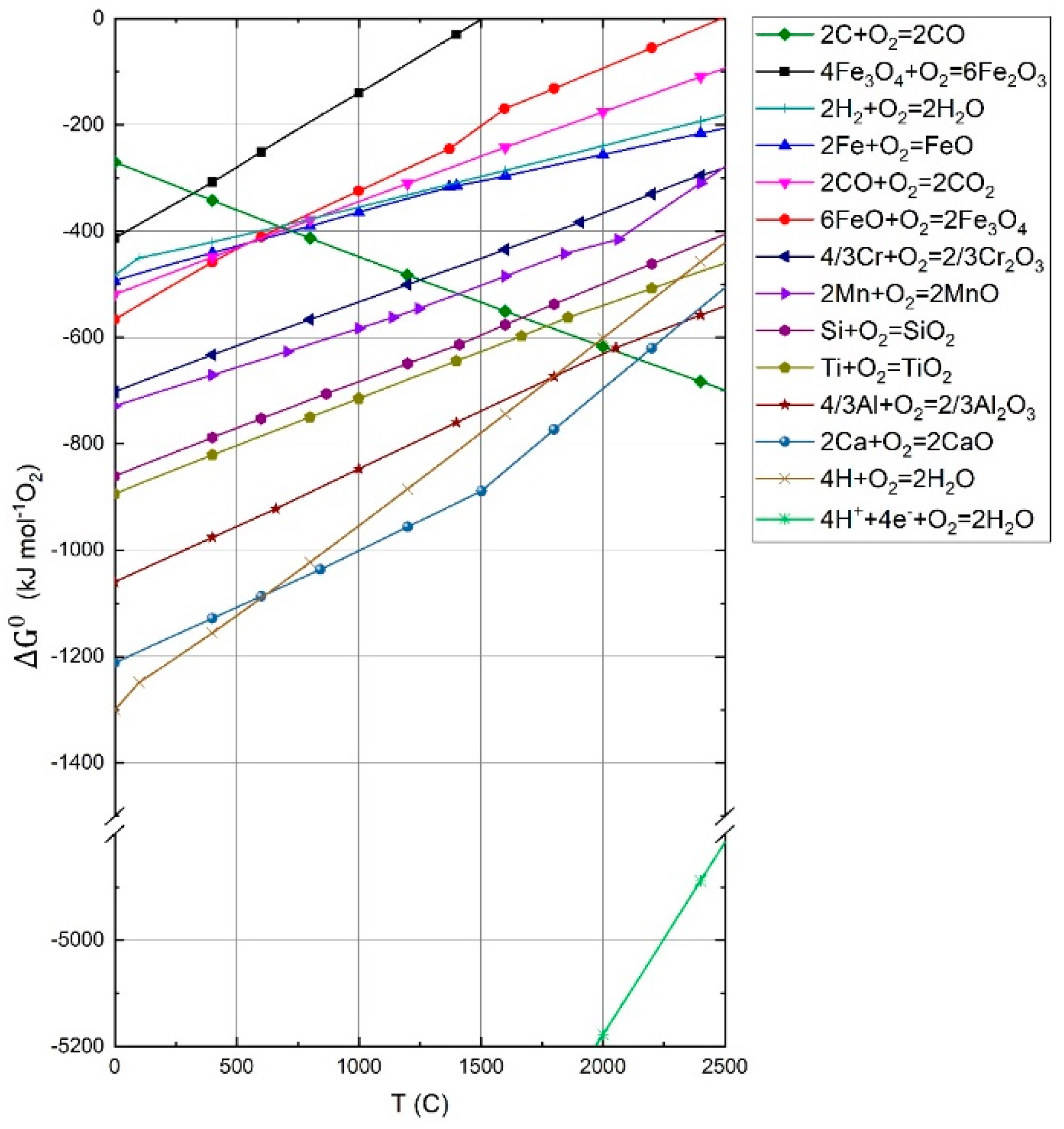
2. Arc Plasma Basics for the HPSR Process
2.1. HPSR Basics of Thermodynamics and Kinetics
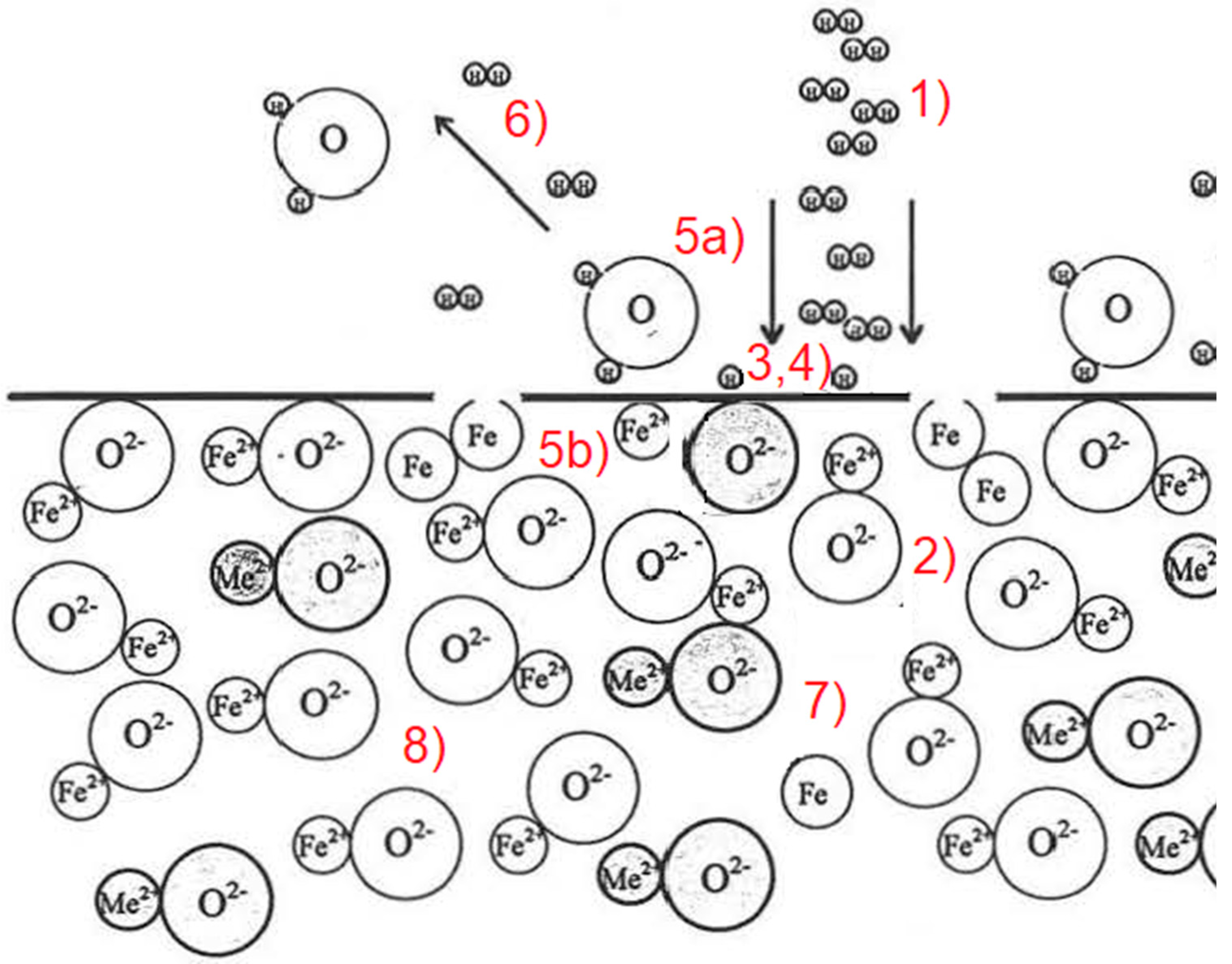
- Transport of hydrogen in a molecular, excited, or ionized state to the oxide melt through the boundary layer, consisting of reaction products and inert gases, to the phase interface between the plasma gas and slag phase.
- The transportation of the iron oxides of the slag to the phase boundary through the boundary layer may consist of accompanying oxides. Furthermore, solid precipitation due to an increased liquidus temperature or build-up of iron can have an inhibitory effect.
- Adsorption of molecular or atomic hydrogen and oxygen of the dissociated iron oxides at the phase boundary.
- The chemical reaction at the phase boundary.
- Desorption of water vapor (5a) and metallic iron (5b) from the phase boundary.
- Transport of water vapor through the boundary layer into the gas phase.
- The higher density usually drives the removal of the reduced iron compared to the oxide melt.
- The removal of the accompanying oxides, which often have a high melting point (CaO, SiO2, and Al2O3), and can therefore cause an increase in the liquidus temperature when the FeO content decreases, unless they are removed from the reaction site by the flow.
2.2. Aim of the Work Shown
3. Materials and Methods
3.1. HPSR Laboratory Equipment
3.2. Experimental Program and Materials
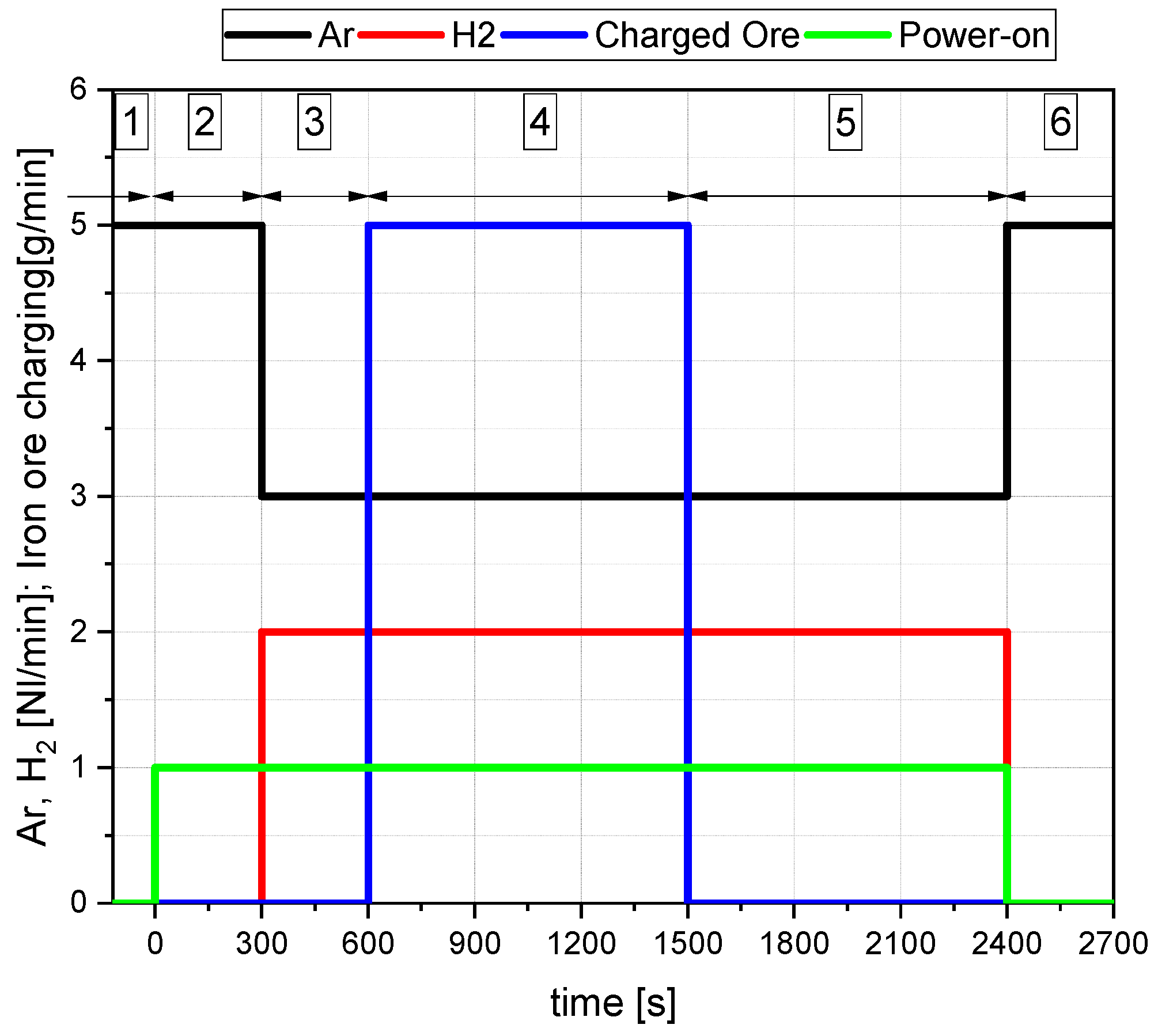
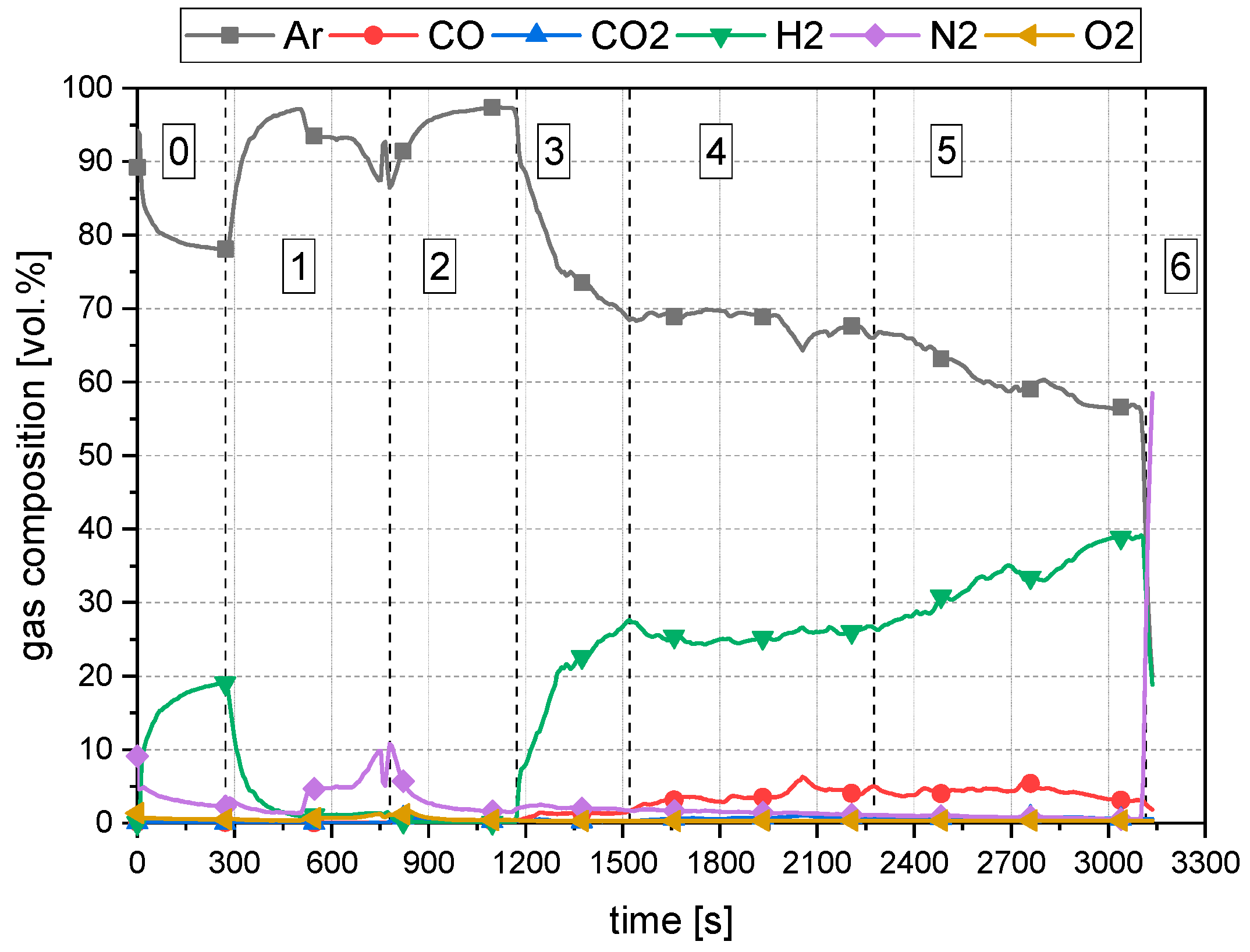
3.3. Description of the Operation
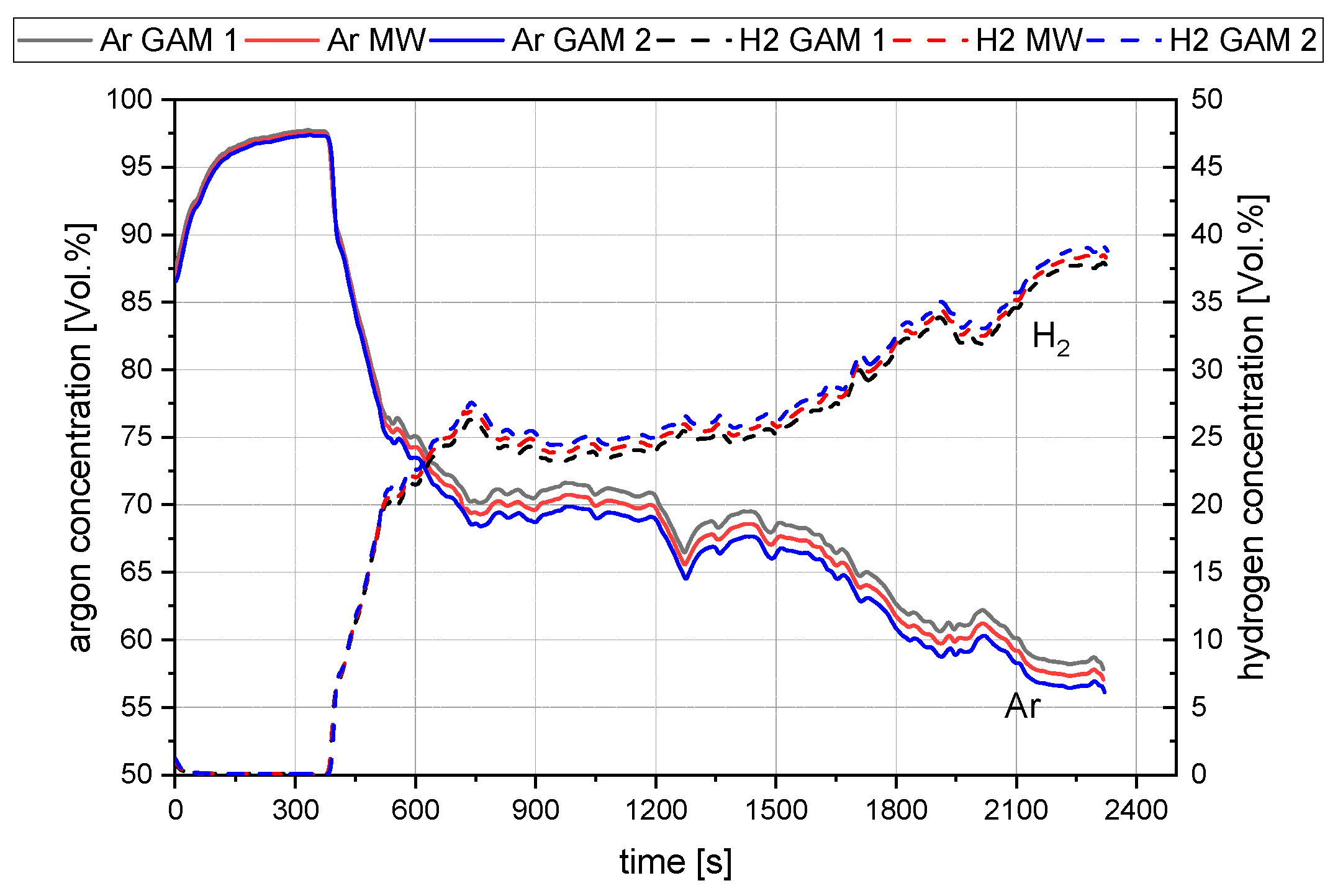
3.4. Methods for the Evaluation
4. Results and Discussion
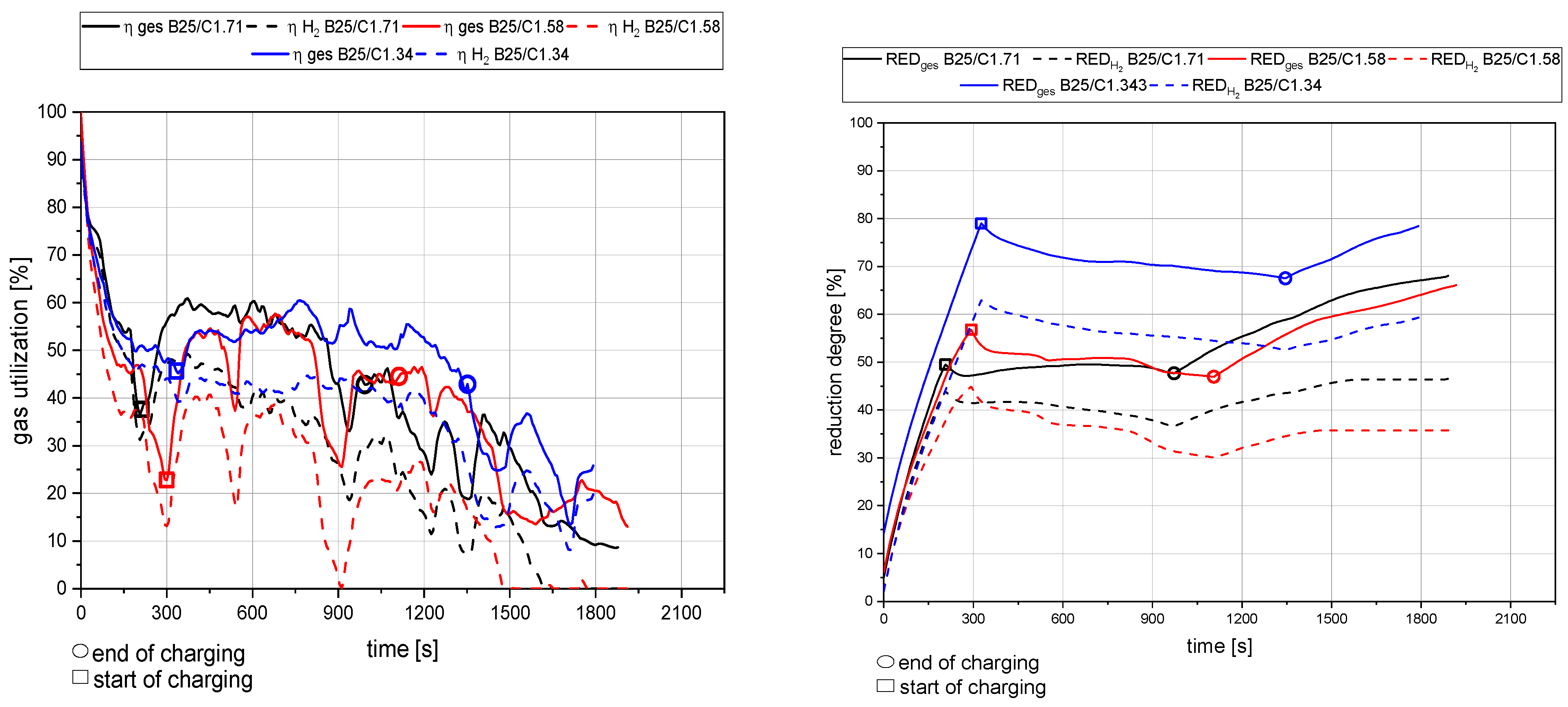
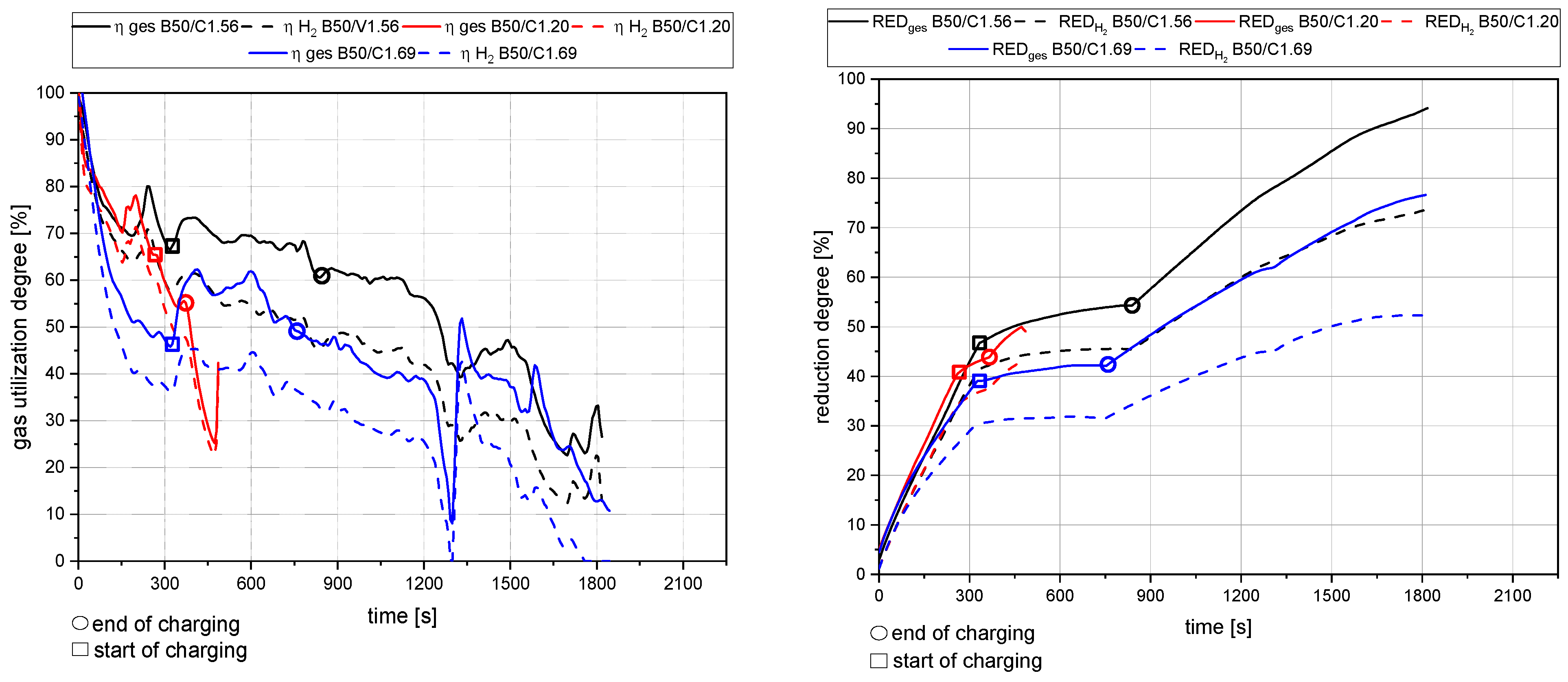
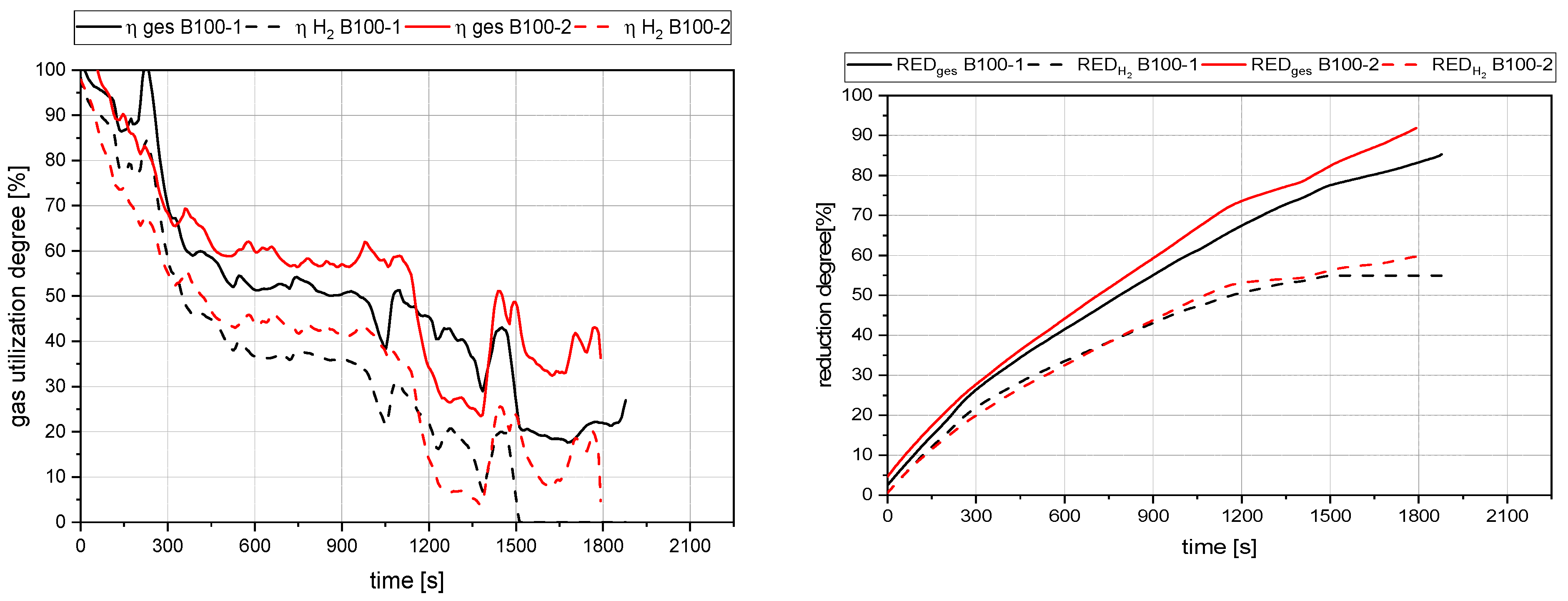
4.1. General Findings
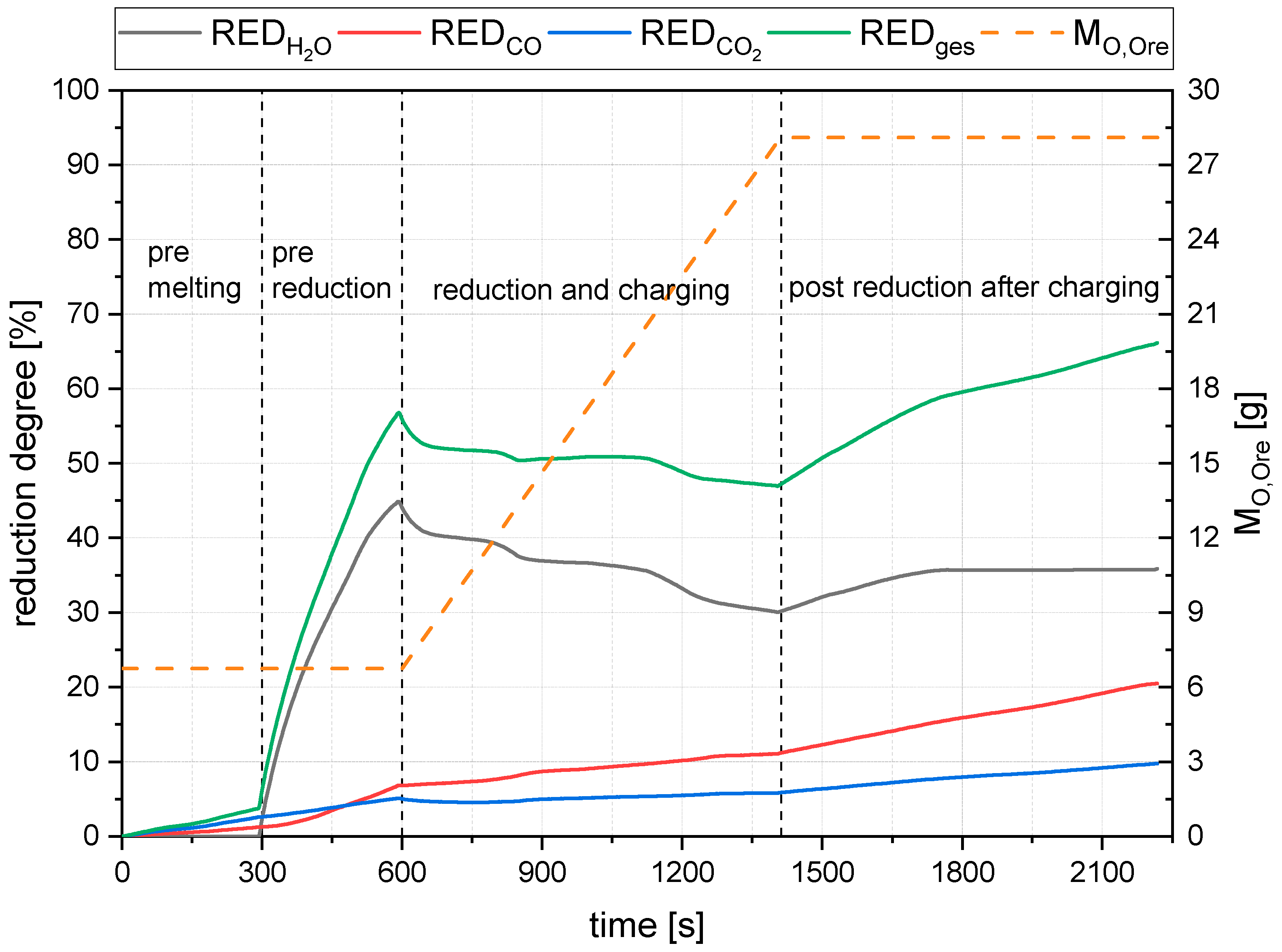
- The primary reduction of the batch material is indicated by a steep increase of the RD line (until the squared value);
- The start of charging, indicated by a less steep phase of the RD graph (between the square and the circle);
- The increase in the RD’s gradient due to the cessation of charging (after the circle).
4.2. Detailed Discussion of the Results for All Three Phases
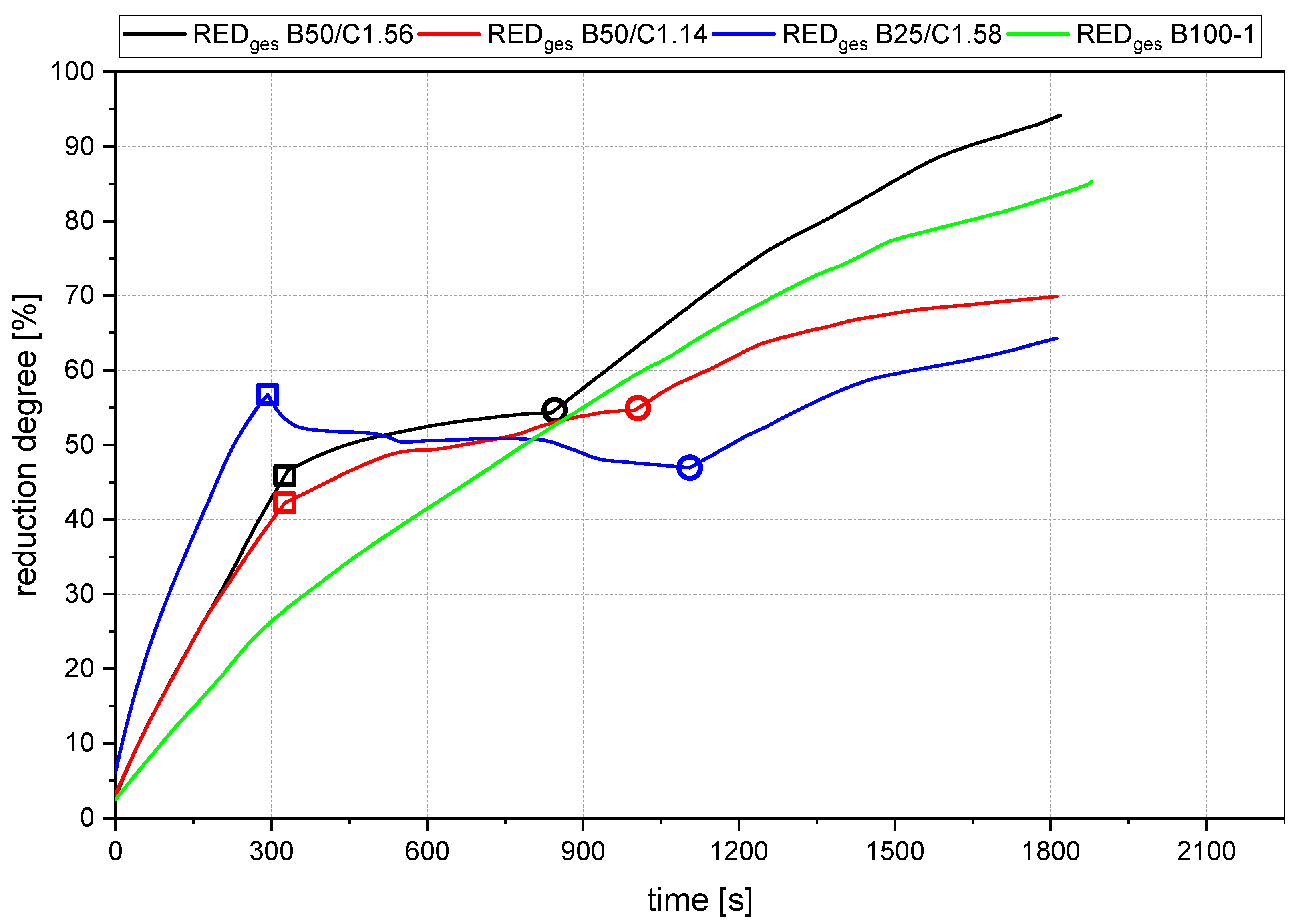
- The rate of absolute removed oxygen per time in phase one seems similar for all trials. Therefore, the slope of the reduction degree differs at different starting masses of ore in the crucible. For lower amounts of batch-wise charged ore, the reduction degree increases faster.
- The slope of the reduction degree is directly linked to the charging rate. A higher/lower amount of oxygen is introduced at different charging rates. Therefore, the reduction degree changes until the equilibrium between the introduced and removed oxygen is reached. For higher amounts of charged ore, lower equilibrium reduction degrees are observed.
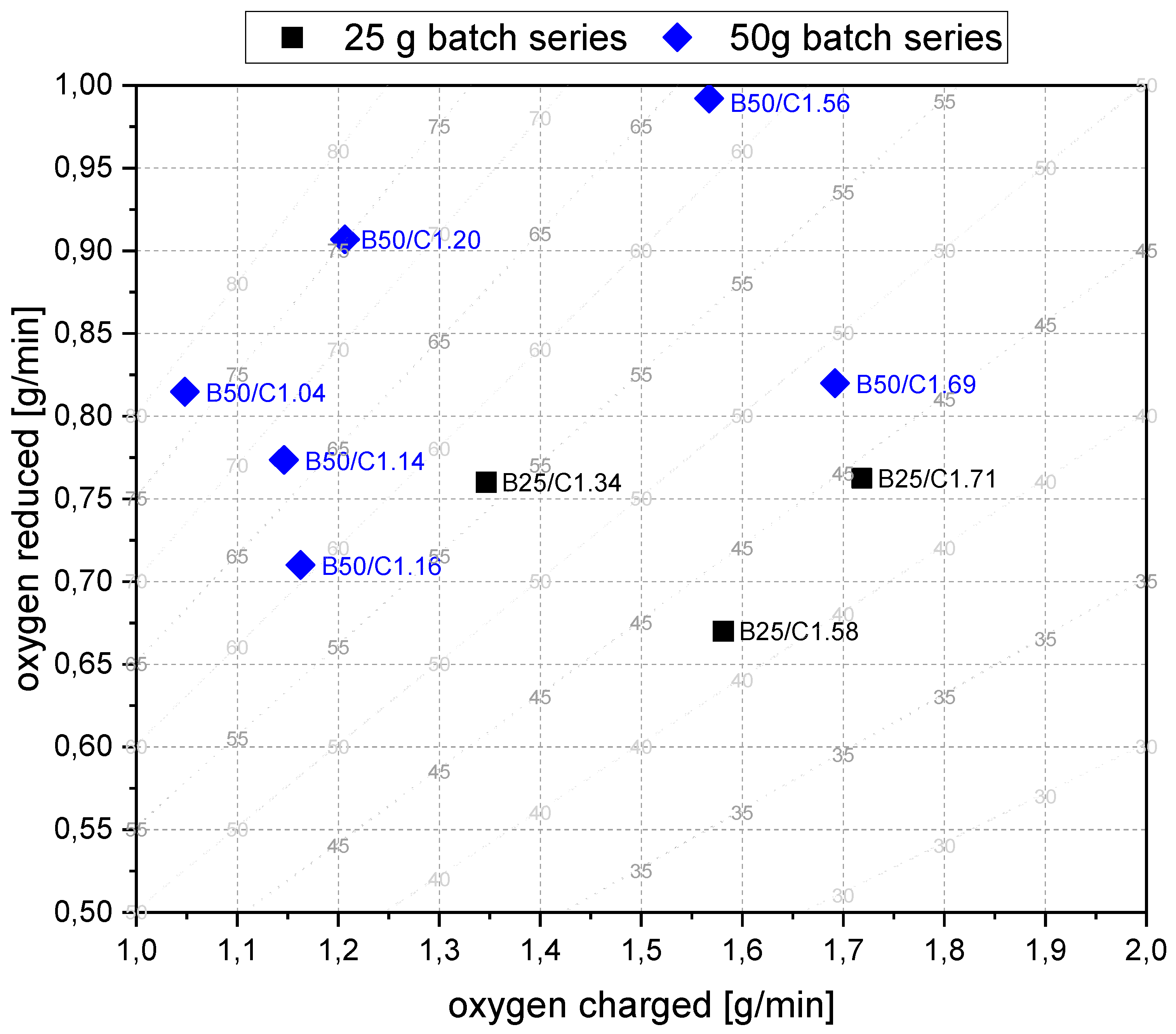
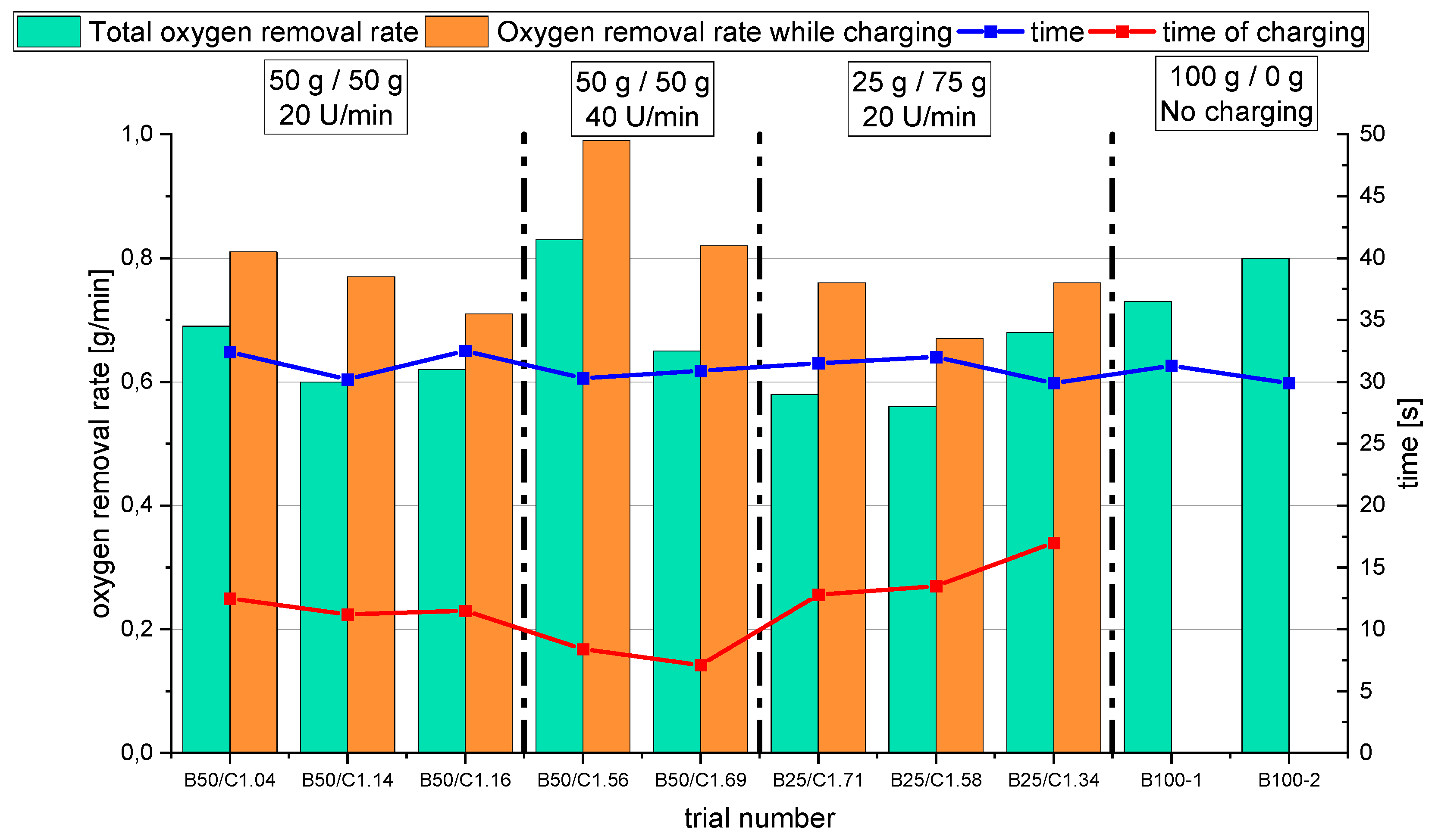
- For the B50 trials, an optimum reduction rate of 1 g/min could be evaluated at 1.56 g/min of introduced oxygen. At B25 tests, this optimum is not reached. A possible cause could be the higher reduction degree achieved in the first phase, which means the absolute amount of oxygen is lower; therefore, the kinetics are partially inhibited. This finding needs further investigation. Nevertheless, the demonstrated method seems to be able to compare and evaluate the optimal conditions for the reduction rate of the process.
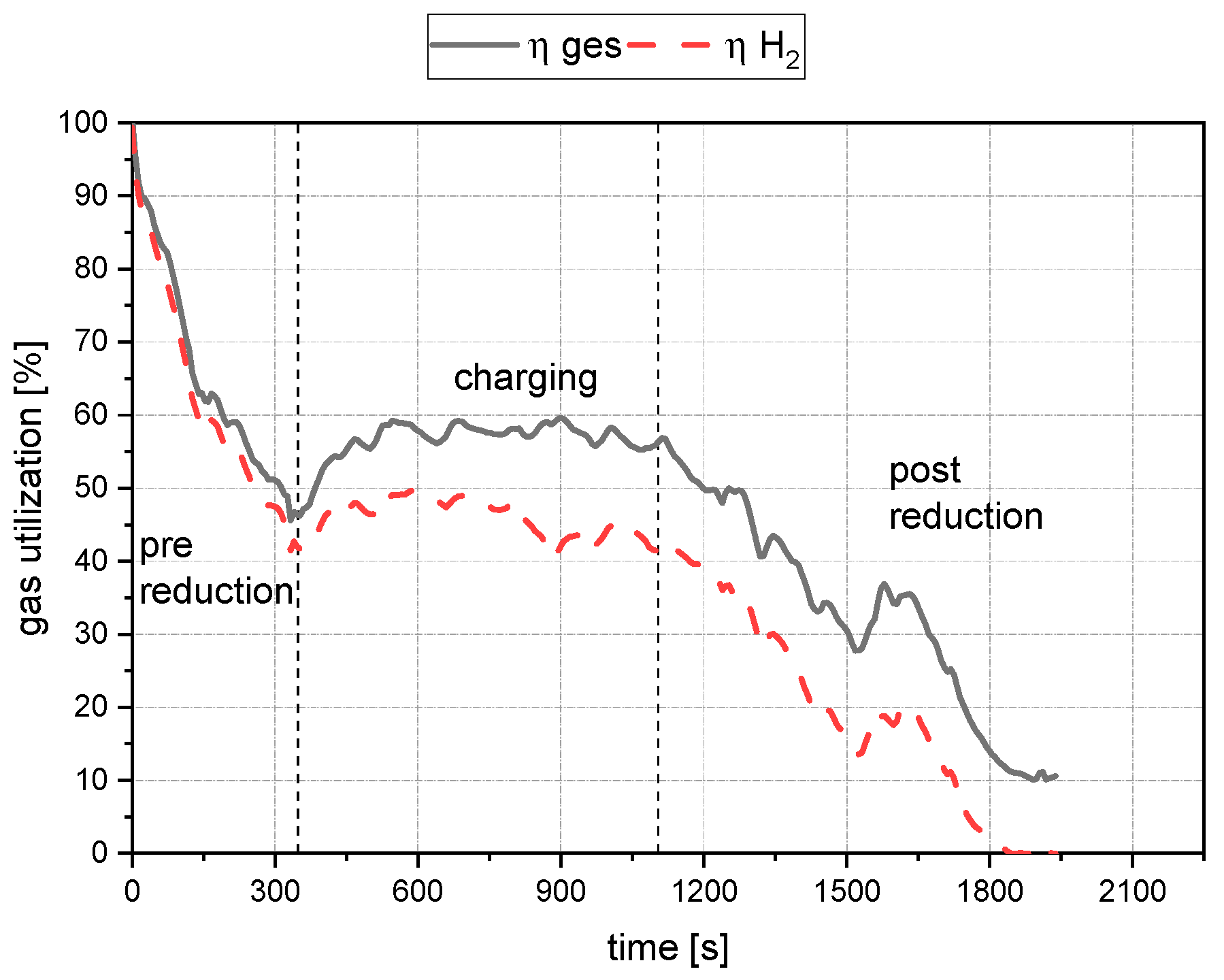
- Comparing the reduction rates of the total trial and the charging time in Figure 19, the oxygen removal while charging is higher than the compared total rate. This means the reduction happens faster while charging compared to the batch trial.
- The reduction rates and the accompanying reduction degrees vary for all trials. Therefore, pre-reduction and charging procedures can influence the final phase’s starting point and the reduction’s progression. A definitive statement is not possible due to the lack of data from systematic investigations. Further trials need to be carried out using a suitable experimental program
- In addition, the final reduction degree differs over a wide value range. The influencing factors of this phenomenon also require further investigations.
5. Conclusions and Outlook
Planned Investigations for Part 2
- The influence of the specific amount of reducing agent in the gas phase needs to be investigated for further trials. This means that the optimal hydrogen rate for the steepest slope at the reduction degree curve needs to be found. This will lead to an optimal hydrogen gas rate for the pre-reduction phase.
- In addition, the influence of the specific gas rate (lower charging rate or higher total gas flow) is of significant interest in the description of the most favorable reduction conditions.
- The final reduction degree after the pre-reduction step could be an influencing factor for the ongoing reduction while charging. This could be because the rate of FeO-rich slag to produce iron influences the melting point of the bath. Therefore, the total size of the pool decreases at higher reduction degrees, and the kinetics for the reduction are lowered. Therefore, additional investigations need to be conducted.
- The possible connection between the oxygen supplied and oxygen reduced needs to be investigated.
- A suitable program for an accurate statistical evaluation needs to be defined.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 3P | 3 Phases |
| AC | Alternating Current |
| BF | Blast Furnace |
| BFP | Backflow preventer |
| BG Diagram | Baur Glaessner Diagram |
| DC | Direct Current |
| FIC | Flow indicator and controller |
| FR | Full Reduction |
| GOD | Gas Oxidation Degree |
| HGE | Hollow Graphite Electrode |
| HPSR | Hydrogen Plasma Smelting Reduction |
| LOI | Loss of Ignition |
| MIG Welding | Metal Inert Gas Welding |
| MS | Mass spectrometry |
| MW | Mean Value (Mittelwert) |
| OF | Operational Field |
| PI | Pressure indicator |
| RD, RED | Reduction Degree |
| SCR | Silicon Controlled Rectifier |
| TDC | Thermal Decomposition |
| Formula Characters | |
| Volume flow of H2 in the gas supply [Nl/min] | |
| Volume flow of Ar [Nl/min] | |
| Volume of the species X (CO, CO2, H2O) [Nl] | |
| Activation energy [kJ/mol] | |
| Equilibrium constants of Formulas (26) to (28) | |
| Partial pressure ratio (pH2O)/(pH2) in equilibrium | |
| Molar mass of oxygen [g/mol] | |
| Reduction degree with H2, CO, and C [%] | |
| Total reduction degree as a sum of RED [%] | |
| Specific surface [m2/mol] | |
| Volume of the produced H2O in the measuring cycle [Nl] | |
| VAr | Volume of Ar for a measuring cycle [Nl] |
| , | Volume of CO, CO2, H2 in the exhaust gas for a measuring cycle [Nl] |
| Molar volume according to standard conditions [l/mol] | |
| Concentration of hydrogen/water in the gas [mol/m3] | |
| Gas concentration of CO, CO2, H2, and Ar in exhaust gas [vol.%] | |
| Pre-exponential factor [m/s] | |
| Mass of Oxygen removed in the previous periods [g] | |
| Oxygen mass in ore [g] | |
| Mass of Oxygen removed [g] | |
| Quantity of hematite/magnetite/wüstite [molh/m/w] | |
| Partial pressure of hydrogen, water, and Oxygen [Pa] | |
| Time of a measuring cycle [min] | |
| Gas quantity of CO2, H2O, CO, and H2 [mol] | |
| Gas utilization rate for hydrogen | |
| Gas utilization rate for all oxygen carriers | |
| Gibbs free energy change [J/mol] | |
| A | Area of the focal spot [m2] |
| ka[H2] | Reaction rate constant wüstite/H2 [kg oxygen/m2 s Pa] |
| kCh | rate constant of the chemical reaction [mol/s] |
| R | Ideal gas constant [kJ/mol * K] |
| R | Reaction rate [kg oxygen/m2s] |
| r’ | Oxygen removal rate [kg oxygen/s] |
| t | Time [s] |
| T | Reaction temperature [K] |
| β | mass transport coefficient [m/s] |
| Gas Oxidation Degree [-] |
References
- Sormann, A. Untersuchung zur Schmelzreduktion von Eisenoxiden mit Wasserstoff als Reduktionsmittel. Ph.D. Thesis, Montanuniversität, Leoben, Austria, 1992. [Google Scholar]
- Bäck, E. Schmelzreduktion von Eisenoxiden mit Argon-Wasserstoff-Plasma. Ph.D. Thesis, Montanuniversität, Leoben, Austria, 1998. [Google Scholar]
- Badr, K. Smelting of Iron Oxides Using Hydrogen Based Plasmas. Ph.D. Thesis, Montanuniversität, Leoben, Austria, 2007. [Google Scholar]
- Plaul, J.F. Schmelzreduktion von hämatitischen Feinerzen im Wasserstoff-Argon-Plasma. Ph.D. Thesis, Montanuniversität, Leoben, Austria, 2005. [Google Scholar]
- Seftejani, M.N. Reduktion von Hämatit mittels Wasserstoffplasma-Schmelzreduktion. Ph.D. Thesis, Montanuniversität, Leoben, Austria, 2020. [Google Scholar]
- Energy Transitions Commission. Reaching Zero Carbon Emissions from Steel. Available online: http://energy-transitions.org/sites/default/files/ETC_Consultation_Paper_-_Steel.pdf (accessed on 15 July 2020).
- BMWI. Nationales Reformprogramm 2020: Die Nationale Wasserstoffstrategie; Bundesministerium für Wirtschaft und Energie (BMWi): Berlin, Germany, 2020. [Google Scholar]
- de Beer, J.; Worrell, E.; Blok, K. Future Technologies for Energy-Efiicient Iron and Steel. Annu. Rev. Energy. Environ. 1998, 23, 123–205. [Google Scholar] [CrossRef]
- Eurofer European Steel association. Low Carbon Roadmap: Pathways to a CO2- neutral European Steel Industry. Final Report 2019, 1–18. Available online: https://www.eurofer.eu/publications/reports-or-studies/low-carbon-roadmap-pathways-to-a-co2-neutral-european-steel-industry/ (accessed on 23 December 2020).
- Europäische Kommission. CO2-Arme Wirtschaft Bis 2050: Die Europäische Wirtschaft Soll Umweltschonender Und Energieeffizienter Werden. Die Europäische Kommission Untersucht, Wie Dies Möglichst Kosteneffizient Erreicht Werden Kann. Available online: https://ec.europa.eu/clima/policies/strategies/2050_de (accessed on 26 January 2019).
- McKinsey. McKinsey on Climate Change. San Francisco. 2020. Available online: https://www.mckinsey.com/~/media/McKinsey/Business%20Functions/Sustainability/Our%20Insights/McKinsey%20on%20Climate%20Change/McKinsey-on-Climate-Change-Report-v2.pdf (accessed on 23 December 2020).
- Bürgler, T. Presentation, Symposioum CO2 freie Stahlherstellung. In Hydrogen from Renewable Energy; IRENA: Leoben, Austria, 2020. [Google Scholar]
- World Steel Association. Steel’s Contribution to a Low Carbon Future and Climate Resilient Societies: Worldsteel Position Paper, Canada. 2020. Available online: https://www.worldsteel.org/en/dam/jcr:7ec64bc1-c51c-439b-84b8-94496686b8c6/Position_paper_climate_2020_vfinal.pdf (accessed on 9 October 2020).
- Kordesch, M.; Bierbaumer, K. Diplomarbeit über die Prozessüberwachung eines Plasmaversuchsschmelzofens in Kooperation mit der Montanuniversität Leoben; Abschlussarbeit Mittelschule: Klagenfurt, Austria, 2020. [Google Scholar]
- Farkas, M. Vergleich und Evaluierung der Lichtbogenstabilitätsfelder im Bereich der Reduktion von Feinerzen mit Wasserstoff—Argon Plasma. Bachelor’s Thesis, Montanuniversität, Leoben, Austria, 2020. [Google Scholar]
- Farkas, M. Thermodynamische Simulation zur Systemauslegung von zukünftigen HPSR Anlagen. Master’s Thesis, Montanuniversität Leoben, Leoben, Austria.
- Cejka, J. Auswirkung der Zufuhrrate von Eisenerz auf die Reduktionsrate bei Verwendung von Wasserstoffplasmaschmelzreduktion. Bachelor’s Thesis, Montanuniversität, Leoben, Austria, 2020. [Google Scholar]
- Leitner, E.M. Methodenentwicklung zur Stabilisation des Elektrischen Lichtbogens im Wasserstoff-Argon Schmelzreduktionsplasma. Master’s Thesis, Montanuniversität, Leoben, Austria, 2021. [Google Scholar]
- Bäck, E.; Badr, K.; Plaul, J.F.; Sormann, A. Überblick über die Entwicklung der Wasserstoff-Schmelzreduktion am Lehrstuhl für Metallurgie. BHM Berg-Und Hüttenmännische Mon. 2009, 154, 6–9. [Google Scholar] [CrossRef]
- Fabing, C. Vergleich und Evaluierung der bisherigen Versuchsergebnisse im Bereich der Reduktion von Feinerzen mit Wasserstoff-Argon Plasmen; Montanuniversität: Leoben, Austria, 2019. [Google Scholar]
- Bäck, E.; Schenk, J.; Badr, K.; Sormann, A.; Plaul, J.F. Wasserstoff als Reduktionsmittel für die Eisen- und Rohstahlerzeugung—Ist-Situation, Potentiale und Herausforderungen. BHM Berg Und Hüttenmännische Mon. 2015, 160, 96–102. [Google Scholar] [CrossRef]
- Sammer, B. Analyse der Einflüsse von Prozessparametern auf das Verschleißverhalten von MA-Spinell im HPSR Prozess. Bachelor’s Thesis, Montanuniversität, Leoben, Austria, 2020. [Google Scholar]
- Zarl, M.A.; Farkas, M.A.; Schenk, J. A Study on the Stability Fields of Arc Plasma in the HPSR Process. Metals 2020, 10, 1394. [Google Scholar] [CrossRef]
- Naseri Seftejani, M.; Schenk, J. Reaction kinetics of molten iron oxides reduction using hydrogen. La Metallurgia Italiana 2018, 7/8, 5–14. [Google Scholar]
- Naseri Seftejani, M.; Schenk, J. Thermodynamic Aspects of Hydrogen Plasma Smelting Reduction of Iron Oxides, 8 th International Congress on Science and Technology of Ironmaking (ICSTI 2018) At: Vienna, Austria, 2018. Available online: https://www.researchgate.net/publication/328075326_THERMODYNAMIC_ASPECTS_OF_HYDROGEN_PLASMA_SMELTING_REDUCTION_OF_IRON_OXIDES/citations (accessed on 23 December 2020).
- Naseri Seftejani, M.; Schenk, J. Thermodynamic of Liquid Iron Ore Reduction by Hydrogen Thermal Plasma. Metals 2018, 8, 1051. [Google Scholar] [CrossRef] [Green Version]
- Naseri Seftejani, M.; Schenk, J. Transport Properties of HPSR Gases; CSST: Tokyo, Japan, 2018. [Google Scholar]
- Naseri Seftejani, M.; Schenk, J.; Zarl, M. Reduction of Haematite Using Hydrogen Thermal Plasma. Materials 2019, 12, 1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seftejani, M.N.; Schenk, J. Fundamentals of hydrogen plasma smelting reduction (HPSR) of iron oxides, a new generation of steelmaking processes. Equilibrium 2018, 24, 26. [Google Scholar]
- Zhang, Y.; Ding, W.Z.; Guo, S.; Xu, K.D. Reduction of metal oxide in nonequilibrium hydrogen plasma. Chin. J. Nonferrous Met. 2004, 14, 317–321. [Google Scholar]
- Sormann, A.; Schenk, J.; Seftejani, M.N.; Spreitzer, D.; Zarl, M. The Way to a Carbon Free Steelmaking; ADMET: Lviv, Ukraine, 2018. [Google Scholar]
- Seftejani, M.N.; Schenk, J.; Spreitzer, D.; Zarl, M. Slag Formation During Reduction of Iron Oxide Using Hydrogen Plasma Smelting Reduction. Materials 2020, 13, 935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamiya, K.; Kithrara, N.; Morinaka, I.; Sakuraya, K.; Ozawa, M.; Tanaka, M. Reduction of Molten Iron Oxide and FeO Bearing Slags by H2-Ar Plasma. Trans. Iron Steel Inst. Jpn. 1984, 24, 7–16. [Google Scholar] [CrossRef]
- Hou, B.; Zhang, H.; Li, H.; Zhu, Q. Study on Kinetics of Iron Oxide Reduction by Hydrogen. Chin. J. Chem. Eng. 2012, 20, 10–17. [Google Scholar] [CrossRef]
- Nagasaka, T.; Hino, M.; Ban-Ya, S. Interfacial kinetics of hydrogen with liquid slag containing iron oxide. Metall. Mater. Trans. B 2000, 31, 945–955. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical Reaction Engineering, 2nd ed.; Wiley: New York, NY, USA, 1972; ISBN 0471530190. [Google Scholar]

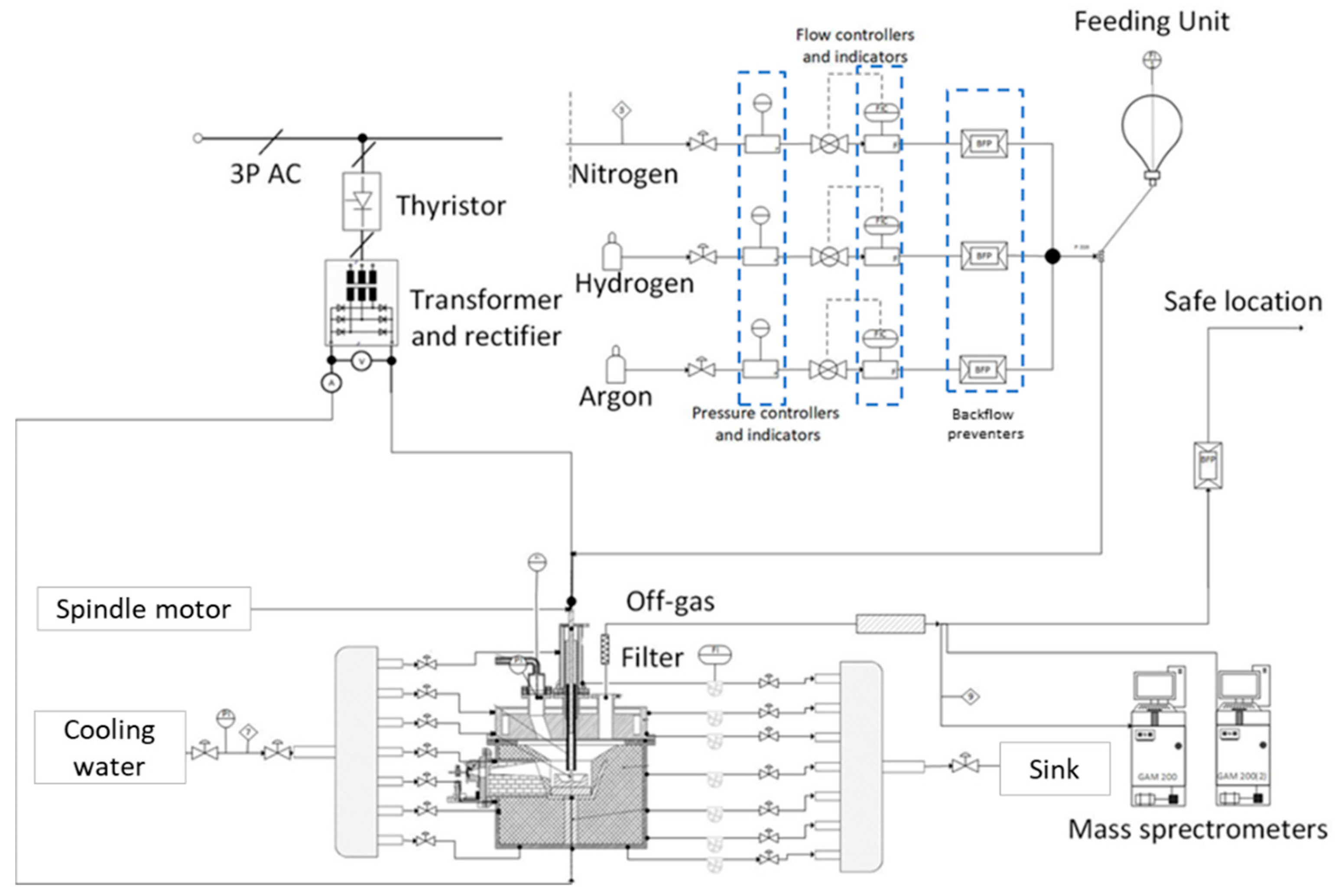




| Facility Manufacturer Info | Short Description |
|---|---|
| Power Supply inHouse, Leoben, Austria | The arc is a transferred DC arc with an average power input of ~5 kVA. |
| SCR Power controller inHouse, Leoben, Austria | Due to the implementation, the system can operate seamlessly from 1 to 16 kVA. |
| Reactor and subdivisions inHouse, Leoben, Austria | The hollow graphite electrode (HGE) has an inner diameter of 5 mm and a 24–26 mm outer diameter. |
| The ignition pin has a 10 mm diameter and a height of 20 mm and is MIG welded to the crucible in two spots in the intersection area. | |
| The crucible has an outer diameter of 100 mm, a height of 35 mm, and can carry up to 100 g of ore at the start of the trial if the trial is operated in batch mode. The diameter of the flat base of the crucible is 54 mm. | |
| A refractory ring protects the bottom disc in case of a meltdown. | |
| Feeding System LAMBDA Laboratory Instruments, Baar, Switzerland | Powder dosing system, using a powder distributor, which is coupled to a stepping motor. |
| 3x Process Gas Linde Gas, Stadl-Paura, Austria | Argon and hydrogen are injected through the HGE into the arc zone to create ionized species. Nitrogen is used as a purging gas. |
| 3x Flow Controller Bronkhorst High-tech B.V, AK Ruurlo, The Netherlands | Mass flow controllers type EL-FLOW PRESTIGE FG were utilized. |
| 2x Mass Spectrometry Pfeiffer Vacuum Technologies, Aßlar, Germany | For the analysis of the off-gas, two mass spectrometers (MS Type GAM200) were used. |
| Off Gas Cleaning System inHouse, Leoben, Austria | Dedusting and dehumidification were ensured through the system. |
| Camera System Pieper GMBH, Berlin, Germany | The camera system Axis-Q1775 was used. |
| Denomination of the Experiment [–] | Iron ore Distribution Crucible/Continuous Feeding [g] | Continuous Charged Iron Ore [g] | Mass Flow of Iron Ore [g/min] | Oxygen Charged during Continuous Ore Feeding [g/min] |
|---|---|---|---|---|
| B50/C0 | 50/50 | X | X | 0 |
| B50/C1.04 | 50/50 | 49.4 | 3.94 | 1.04 |
| B50/C1.14 | 50/50 | 46.1 | 4.11 | 1.14 |
| B50/C1.16 | 50/50 | 46.5 | 4.03 | 1.16 |
| B50/C1.20 | 50/50 | 8.8 | 4.96 | 1.20 |
| B50/C1.56, | 50/50 | 48.6 | 5.76 | 1.56 |
| B50/C1.69 | 50/50 | 46.5 | 6.55 | 1.69 |
| B25/C1.37 | 25/75 | 73.6 | 4.33 | 1.34 |
| B25/C1.58 | 25/75 | 75.1 | 5.54 | 1.58 |
| B25/C1.71 | 25/75 | 75 | 5.88 | 1.71 |
| B100-1 | 100/0 (Reference) | X | X | 0 |
| B100-2 | 100/0 (Reference) | X | X | 0 |
| No | Element | [wt.%] |
|---|---|---|
| 1 | Fe2O3 1 | 92.83 |
| 2 | FeO | 1.07 |
| 3 | Total Fe | 65.81 |
| 4 | SiO2 | 1.694 |
| 5 | Al2O3 | 1.01 |
| 6 | Manganese | 0.17 |
| 7 | Phosphorus | 0.057 |
| 8 | Total sulfur | 0.014 |
| 9 | LOI 2 | 2.79 |
| Mesh Size [μm] | Fraction [wt.%] | Cum [wt.%] |
|---|---|---|
| 63–125 | 50 | 50 |
| 25–63 | 50 | 100 |
| Element | Unit | C | Si | Mn | P | S | Cr | Mo | Ni | Al | Cu |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Steel crucible | (wt.%) | 0.178 | 0.261 | 1.325 | 0.009 | 0.005 | 0.083 | 0.031 | 0.168 | 0.027 | 0.179 |
| Ignition pin | (wt.%) | 0.441 | 0.217 | 0.85 | 0.008 | 0.028 | 0.985 | 0.162 | 0.085 | 0.021 | 0.116 |
| Gas | Purity | Remarks |
|---|---|---|
| H2 | 5.0 | - |
| Ar | 5.0 | O2, N2, H2O ≤ 5ppm |
| N2 | 5.0 | O2, H2O ≤ 5ppm |
| Parameter | Type of Evaluation | Description of the Method |
|---|---|---|
| r | measurement and calculation | The hydrogen content in the off-gas is measured, and according to Equation (40), the removed oxygen is calculated |
| PH2 | measurement | The mass spectrometry measures the value during the trial |
| PH2O | measurement and calculation | The mass spectrometry measures the hydrogen content, and the partial pressure of the water vapor is calculated according to the missing hydrogen of Equation (39) |
| K’H | set to a fixed value | The value is set to the gas utilization degree. Further explanations follow (Figure 12) |
| A | video, estimation, and measuring | The Area is measured via an image processing program from images of the reactor’s interior shown in Figure 11b |
| Trial [-] | rO,red while H2-Supply [g/min] | H2 Flow [min] | rO,red While Charging [g/min] | Charging [min] |
|---|---|---|---|---|
| B50/C0 | 0,40 | 29.4 | X | X |
| B50/C1.04 | 0.68 | 32.4 | 0.81 | 12.5 |
| B50/C1.14 | 0.60 | 30.2 | 0.77 | 11.2 |
| B50/C1.16 | 0.62 | 32.5 | 0.71 | 11.5 |
| B50/C1.20 | 0.84 | 8.1 | 0.91 | 1.8 |
| B50/C1.56 | 0.83 | 30.3 | 0.99 | 8.4 |
| B50/C1.69 | 0.65 | 30.9 | 0.82 | 7.1 |
| B25/C1.34 | 0.68 | 29.9 | 0.76 | 17.0 |
| B25/C1.58 | 0.56 | 32.0 | 0.67 | 13.5 |
| B25/C1.71 | 0.58 | 31.5 | 0.76 | 12.8 |
| B100-1 (ref) | 0.72 | 31.3 | X | X |
| B100-2 (ref) | 0.80 | 29.9 | X | X |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarl, M.A.; Ernst, D.; Cejka, J.; Schenk, J. A New Methodological Approach to the Characterization of Optimal Charging Rates at the Hydrogen Plasma Smelting Reduction Process Part 1: Method. Materials 2022, 15, 4767. https://doi.org/10.3390/ma15144767
Zarl MA, Ernst D, Cejka J, Schenk J. A New Methodological Approach to the Characterization of Optimal Charging Rates at the Hydrogen Plasma Smelting Reduction Process Part 1: Method. Materials. 2022; 15(14):4767. https://doi.org/10.3390/ma15144767
Chicago/Turabian StyleZarl, Michael Andreas, Daniel Ernst, Julian Cejka, and Johannes Schenk. 2022. "A New Methodological Approach to the Characterization of Optimal Charging Rates at the Hydrogen Plasma Smelting Reduction Process Part 1: Method" Materials 15, no. 14: 4767. https://doi.org/10.3390/ma15144767








