Low-Temperature Fabrication of Plate-like α-Al2O3 with Less NH4F Additive
Abstract
:1. Introduction
2. Experimental Procedures
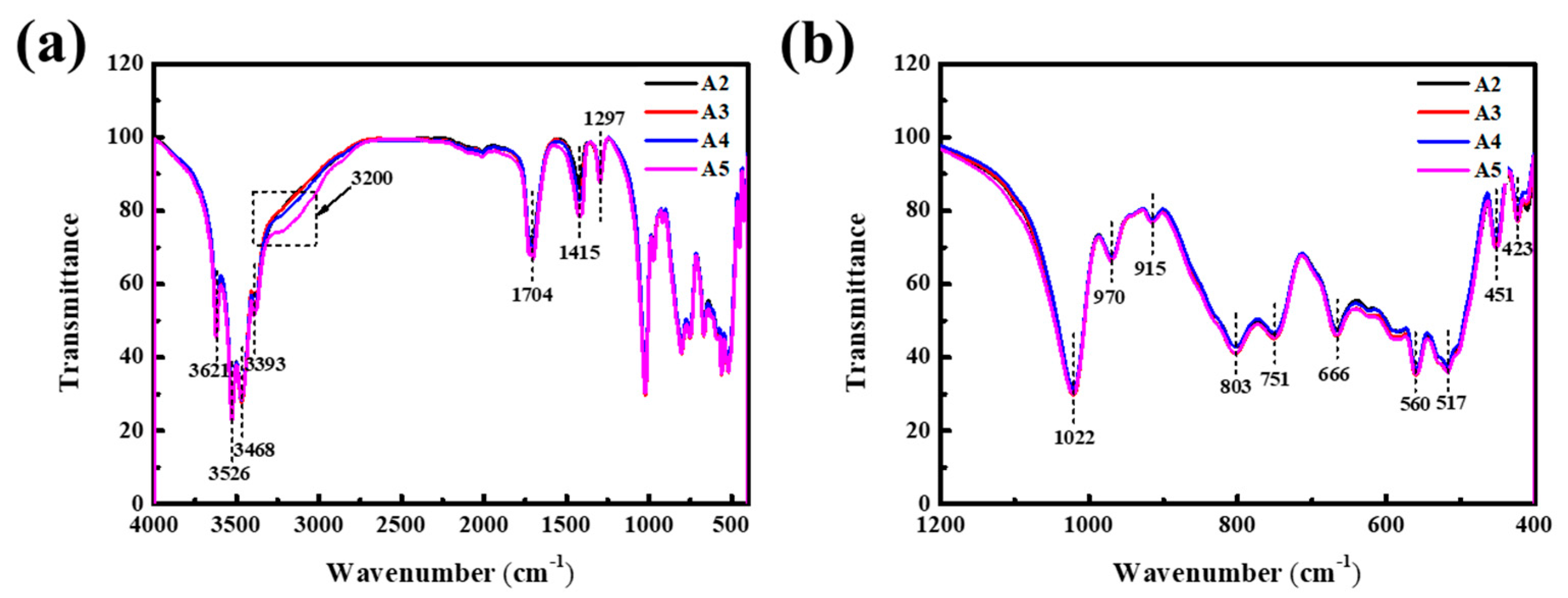
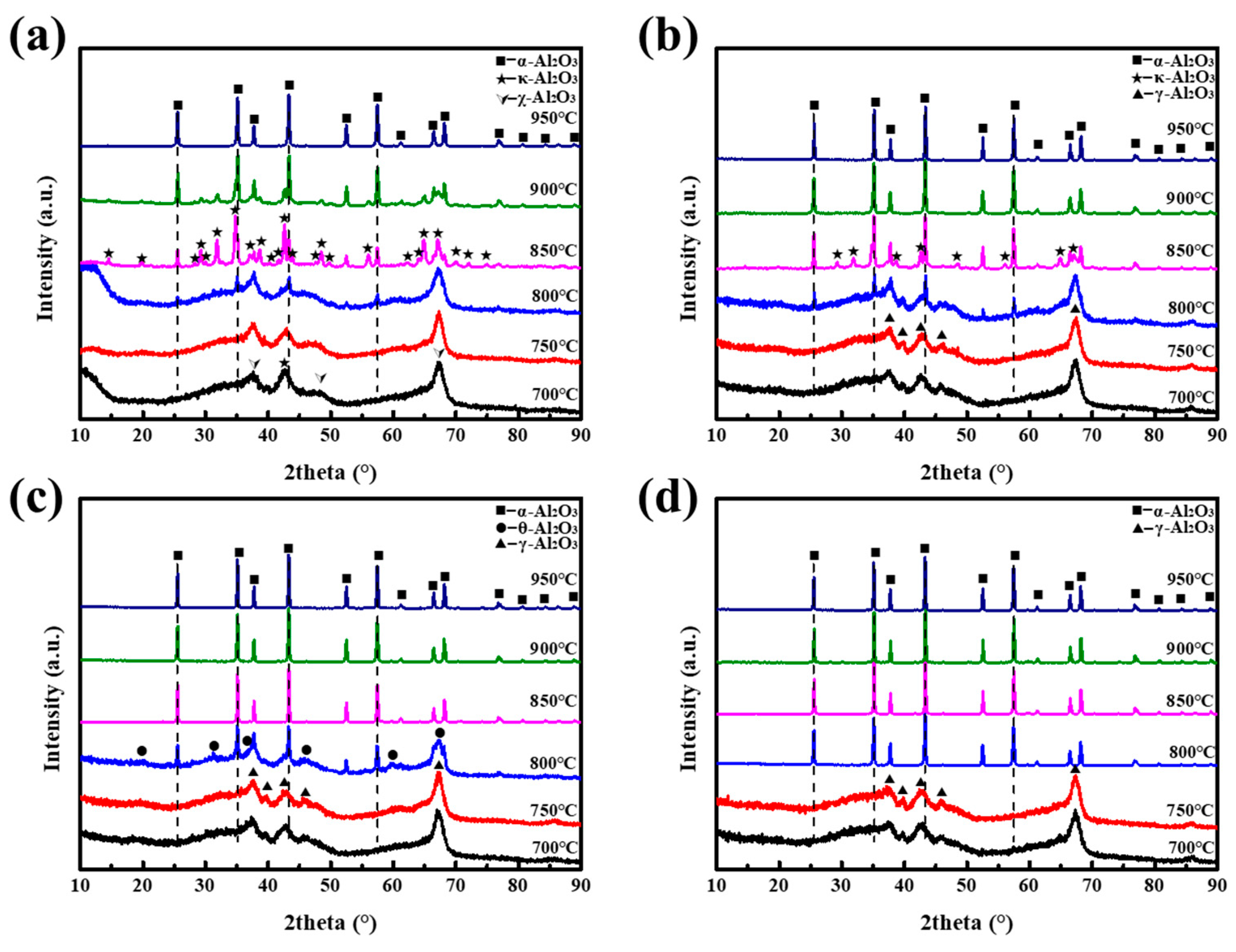
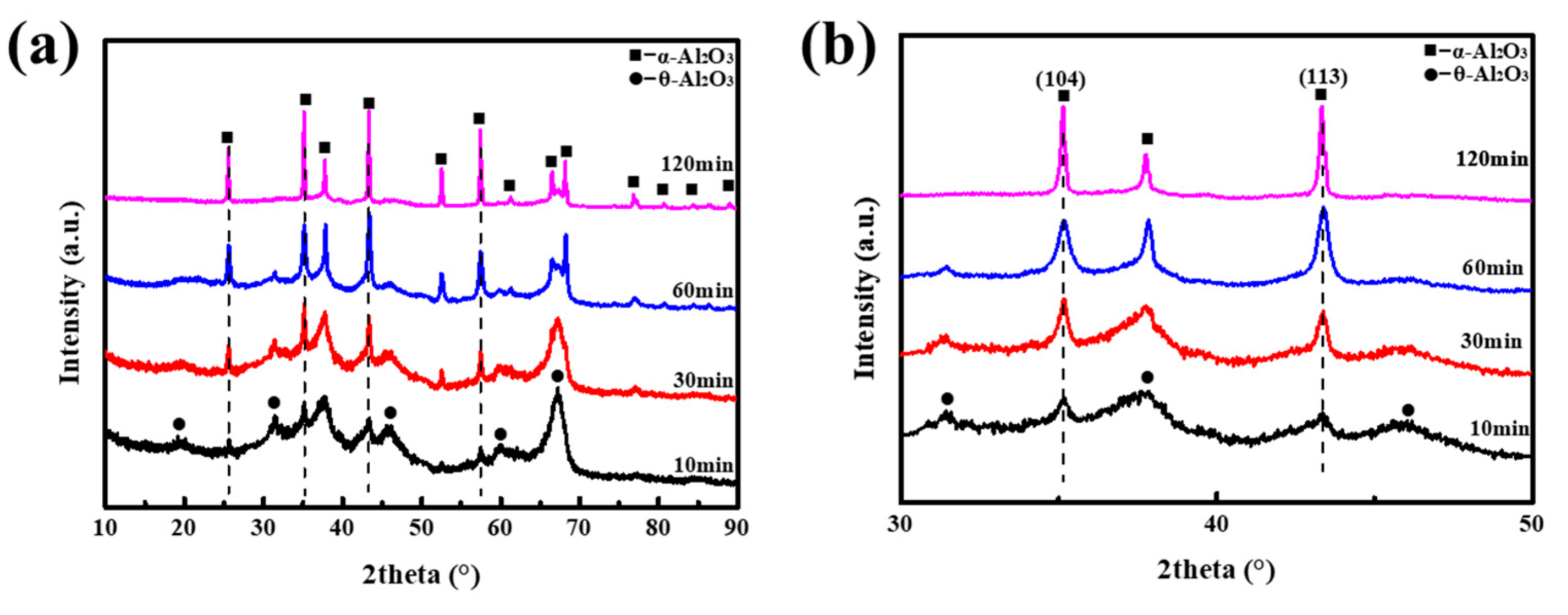
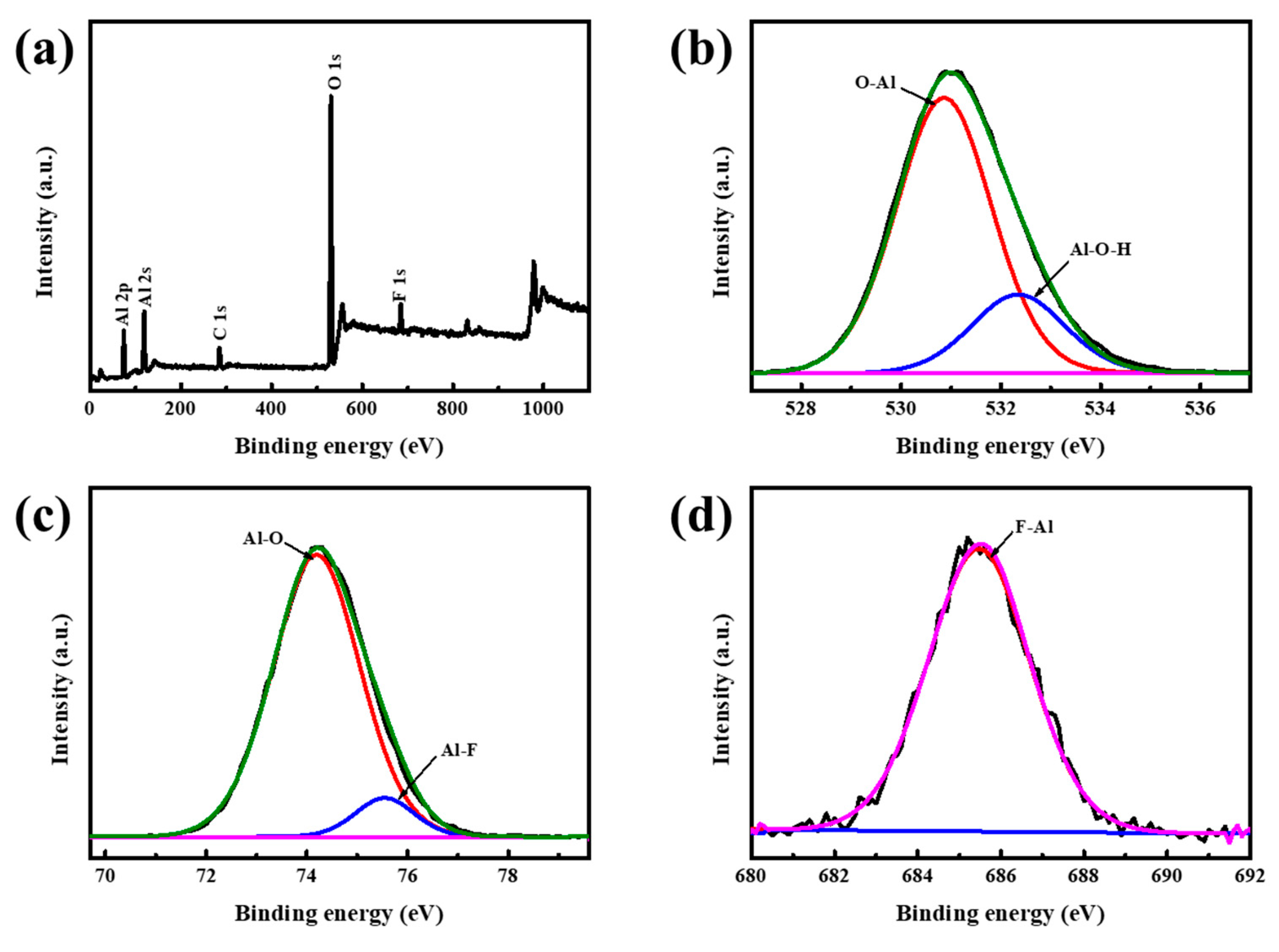
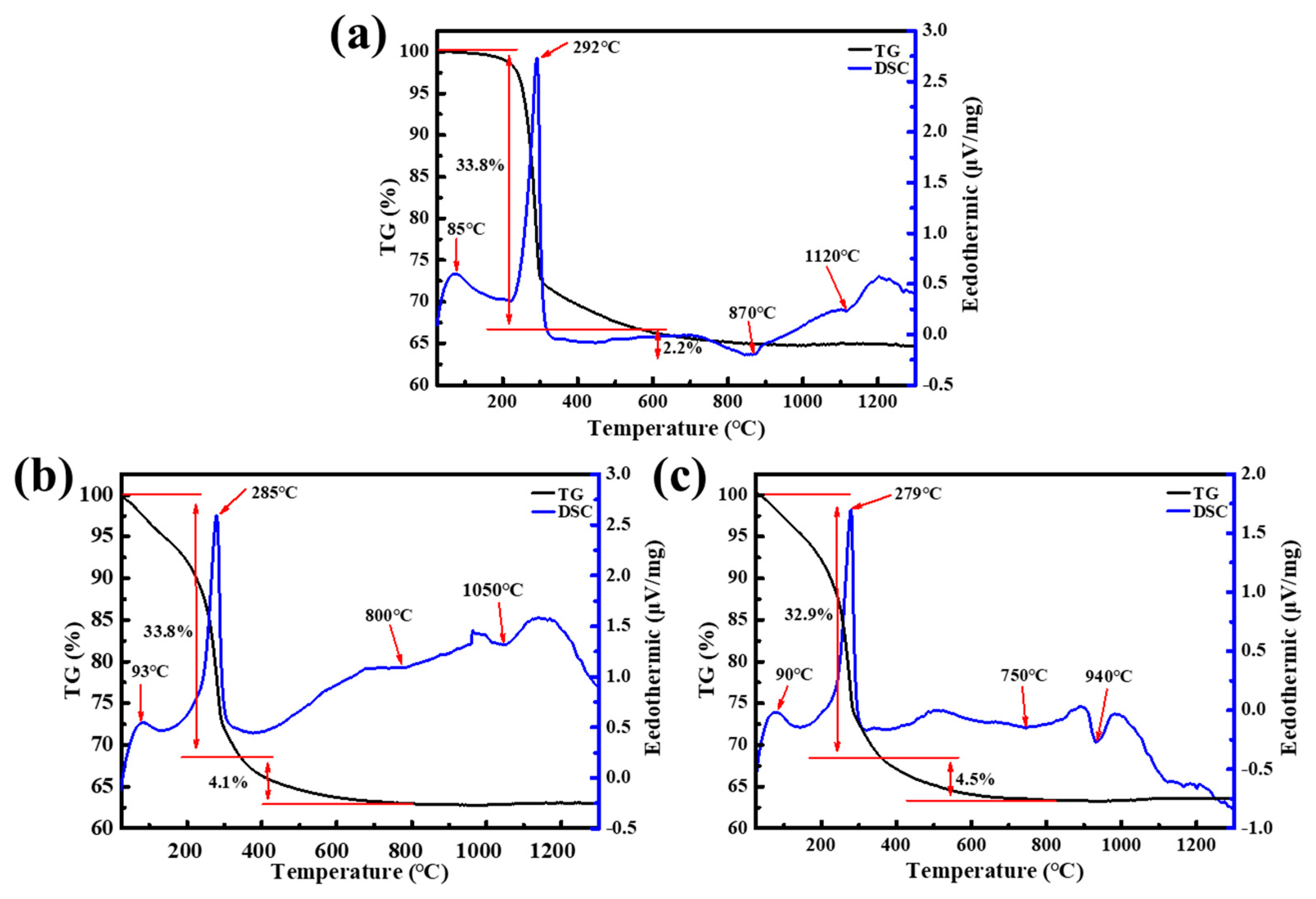
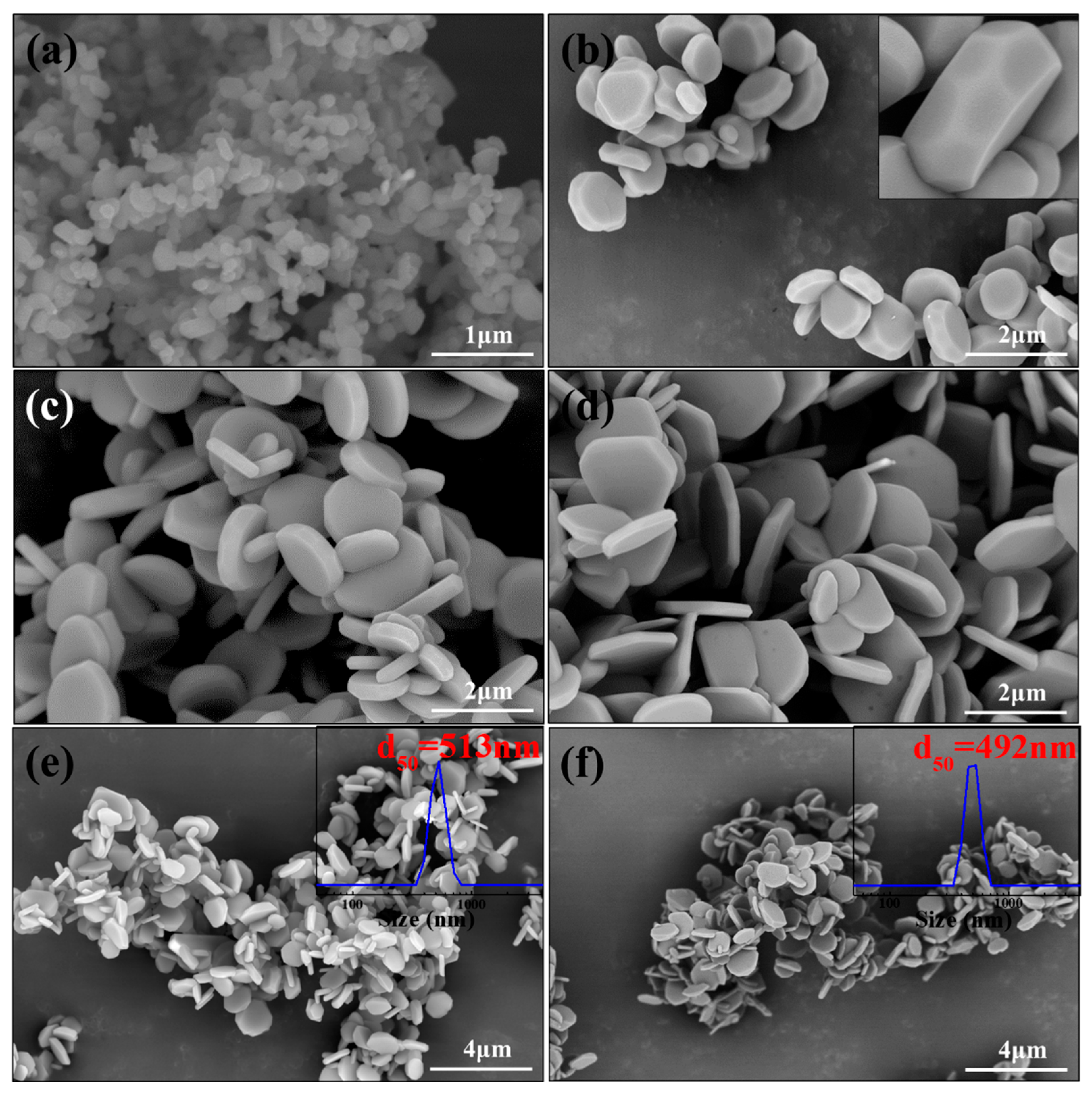
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; Mai, Z.; Wu, Y.; Wang, Y.; Cao, Z.; Li, M.; Fan, B.; Shao, G.; Wang, H.; Xu, H.; et al. Preparation of spherical α-Al2O3 nanoparticles by microwave hydrothermal synthesis and addition of nano-Al seeds. J. Am. Ceram. Soc. 2022, 105, 5585–5597. [Google Scholar]
- Panasyuk, G.P.; Belan, V.N.; Voroshilov, I.L.; Kozerozhets, V.I.; Luchkov, V.; Kondakov, D.F.; Demina, L.I. The study of hydrargillite and γ-alumina conversion process in boehmite in different hydrothermal media. Theor. Found. Chem. Eng. 2013, 47, 415–421. [Google Scholar]
- Xu, X.; Zhou, S.; Wu, J.; Zhang, Q.; Zhang, Y.; Zhu, G. Preparation of highly dispersive solid microspherical α-Al2O3 powder with a hydrophobic surface for stereolithography-based 3D printing technology. Ceram. Int. 2020, 46, 1895–1906. [Google Scholar]
- Kozerozhets, I.V.; Panasyuk, G.P.; Semenov, E.A.; Danchevskaya, M.N.; Azarova, L.A.; Simonenko, N.P. Transformations of nanosized boehmite and γ-Al2O3 upon heat treatment. Russ. J. Inorg. Chem. 2020, 65, 587–591. [Google Scholar]
- Zhu, L.; Liu, L.; Sun, C.; Zhang, X.; Zhang, L.; Gao, Z.; Ye, G.; Li, H. Low temperature synthesis of polyhedral α-Al2O3 nanoparticles through two different modes of planetary ball milling. Ceram. Int. 2020, 46, 28414–28421. [Google Scholar]
- Miyake, K.; Hirata, Y.; Shimonosono, T.; Sameshima, S. The Effect of particle shape on sintering behavior and compressive strength of porous alumina. Materials 2018, 11, 1137. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Zhu, L.; Liu, L.; Chen, L.; Li, H.; Sun, C. Influence of high-energy ball milling and additives on the formation of sphere-like α-Al2O3 powder by high-temperature calcination. Z. Naturforsch. B 2018, 73, 589–596. [Google Scholar]
- Tian, Q.; Zhang, Y.; Yang, X.; Dai, J.; Lv, Z. Influences of NH4F on transformation and morphology of high-pure α-alumina. Mater. Res. Express 2017, 4, 105904. [Google Scholar]
- Li, X.; Su, X.; Xiao, H.; Chen, L.; Li, S.; Tang, M. Continuous α-Al2O3 fibers grown by seeding with in-situ suspension. Ceram. Int. 2020, 46, 15638–15645. [Google Scholar]
- Hill, R.; Danzer, R.; Paine, R. Synthesis of aluminum oxide platelets. J. Am. Ceram. Soc. 2001, 84, 514–520. [Google Scholar]
- Zhou, J.; Laoui, T.; Biest, O. Toughening of X-sialon with Al2O3 platelets. J. Eur. Ceram. Soc. 1995, 15, 297–305. [Google Scholar]
- Karl-Heinz Heussner, N.C. Yttria- and ceria-stabilized tetragonal zirconia polycrystals (Y-TZP, Ce-TZP) reinforced with A12O3 platelets. J. Eur. Ceram. Soc. 1989, 5, 193–200. [Google Scholar]
- Jin, X.; Gao, L. Size control of α-A12O3 platelets synthesized in molten Na2SO4 flux. J. Am. Ceram. Soc. 2004, 87, 533–540. [Google Scholar]
- Liu, L.; Zhang, X.; Zhu, L.; Wei, Y.; Guo, C.; Li, H. Preparation of hexagonal micro-sized α-A12O3 platelets from a milled Al(OH)3 precursor with NH4F and NH4Cl additives. Z. Naturforsch. B 2019, 74, 579–583. [Google Scholar]
- Yin, X.; Zhang, Q.; Liu, J.; Zhong, X.; Chai, C. Polishing property of α-Al2O3 nanoflakes prepared by self-burning method. Acta Phys-Chim. Sin. 2009, 25, 1443–1448. [Google Scholar]
- Suchanek, W.; Garcés, J.; Fulvio, P.; Jaroniec, M. Hydrothermal synthesis and surface sharacteristics of novel alpha alumina nanosheets with controlled chemical composition. Chem. Mater. 2010, 22, 6564–6574. [Google Scholar]
- Zhu, L.; Tu, R.; Huang, Q. Molten salt synthesis of α-Al2O3 platelets using NaAlO2 as raw material. Ceram. Int. 2012, 38, 901–908. [Google Scholar]
- Wei, M.; Zhi, D.; Choy, K. Novel synthesis of α-alumina hexagonal nanoplatelets using electrostatic spray assisted chemical vapour deposition. Nanotechnology 2006, 17, 181–184. [Google Scholar]
- Amrute, A.; Lodziana, Z.; Schreyer, H.; Weidenthaler, C.; Schuth, F. High-surface-area corundum by mechanochemically induced phase transformation of boehmite. Science 2019, 366, 485–489. [Google Scholar]
- Deng, B.; Advincula, P.; Luong, D.; Zhou, J.; Zhang, B.; Wang, Z.; McHugh, E.; Chen, J.; Carter, R.; Kittrell, C.; et al. High-surface-area corundum nanoparticles by resistive hotspot-induced phase transformation. Nat. Commun. 2022, 13, 5027. [Google Scholar]
- Wang, W.; Bi, J.; Qi, Y.; Zhang, Z.; Xing, Z.; Zhu, H.; Guo, D.; Xu, J.; Bai, Y. Preparation of micrometer-sized α-Al2O3 platelets by thermal decomposition of AACH. Powder Technol. 2010, 201, 273–276. [Google Scholar]
- Fu, G.; Wang, J.; Kang, J. Influence of AlF3 and hydrothermal conditions on morphologies of α-Al2O3. T. Nonferr. Metal. Soc. 2008, 18, 743–748. [Google Scholar]
- Tian, Q. The influences of fluorides on the transformation of α-alumina crystals. Ceram.-Silik. 2017, 64, 357–366. [Google Scholar]
- Riello, D.; Zetterström, C.; Parr, C.; Braulio, M.; Moreira, M.; Gallo, J.; Pandolfelli, V. AlF3 reaction mechanism and its influence on α-Al2O3 mineralization. Ceram. Int. 2016, 42, 9804–9814. [Google Scholar]
- Kim, H.; Park, N.; Lee, T.; Kang, M. Effect of AlF3 seed concentrations and calcination temperatures on the crystal growth of hexagonally shaped α-alumina powders. Ceram. Int. 2014, 40, 3813–3818. [Google Scholar]
- Kim, H.; Kang, M. Rapid crystal phase transformation into hexagonally shaped α-alumina using AlF3 seeds. J. Sol-Gel Sci. Techn. 2013, 68, 110–120. [Google Scholar]
- Shimbo, M.; Yamamoto, O.; Hayashi, S.; Nakagawa, Z. Influence of addition of AlF3 on thermal decomposition of gibbsite and phase transition of the intermediate alumina to α-Al2O3. J. Ceram. Soc. Jpn. 2007, 115, 536–540. [Google Scholar]
- Wu, Y.; Pezzotti, G.; Guo, J. Influence of AlF3 and ZnF2 on the phase transformation of gamma to alpha alumina. Mater. Lett. 2002, 52, 366–369. [Google Scholar]
- Živkovic, Ž.; Štrbac, N.; Šesták, J. Influence of fluorides on polymorphous transformation of α-Al2O3 formation. Thermochim. Acta 1995, 266, 293–300. [Google Scholar]
- Zhu, L.; Sun, C.; Chen, L.; Lu, X.; Li, S.; Ye, G.; Liu, L. Influences of NH4F additive and calcination time on the morphological evolution of α-Al2O3 from a milled γ-Al2O3 precursor. Z. Naturforsch. B 2017, 72, 665–670. [Google Scholar]
- Kang, J.; Wang, J.; Zhang, W. Influence of AlF3 additive on microstructure of alumina powder. Light Met. 2008, 3, 13–16. [Google Scholar]
- Islam, M.; Patel, R. Thermal activation of basic oxygen furnace slag and evaluation of its fluoride removal efficiency. Chem. Eng. J. 2011, 169, 68–77. [Google Scholar]
- Chen, H.; Ren, B.; Chen, L.; Zhao, F.; Bai, Q.; Chen, J.; Li, B. Preparation of equiaxed α-Al2O3 by adding oxalic acid. Ceram. Int. 2021, 47, 31512–31517. [Google Scholar]
- Ibrahim, D.; Abu-Ayana, Y. Preparation and characterization of ultrafine alumina via sol–gel polymeric route. Mater. Chem. Phys. 2008, 111, 326–330. [Google Scholar]
- Meher, T.; Basu, A.; Ghatak, S. Physicochemical characteristics of alumina gel in hydroxyhydrogel and normal form. Ceram. Int. 2005, 31, 831–838. [Google Scholar]
- Wilson, S. Phase transformations and development of microstructure in boehmite-derived transition aluminas. Proc. Brit. Ceram. Soc. 1979, 28, 281–294. [Google Scholar]
- Igor Levin, D.B. Metastable alumina polymorphs crystal structures and transition sequences. J. Am. Ceram. Soc. 1998, 81, 1995–2012. [Google Scholar]
- Brindley, G.; Choe, J. The reaction series, gibbsite→chi alumina→kappa alumina→corundum. Am. Mineral. 1961, 46, 771–785. [Google Scholar]
- Yan, F.; Huang, H. Habit evolution of alumina crystallite during κ- to α-phase transformation. Chin. J. Process Eng. 2004, 4, 366–370. [Google Scholar]
- Chang, P.; Yen, F.; Cheng, K.; Wen, H. Examinations on the critical and primary crystallite sizes during θ- to α-phase transformation of ultrafine alumina powders. Nano Lett. 2001, 1, 253–261. [Google Scholar]
- Bagwell, R.; Messing, G. Effect of seeding and water vapor on the nucleation and growth of γ-Al2O3 from α-Al2O3. J. Am. Ceram. Soc. 1999, 82, 825–832. [Google Scholar]
- Arai, H.; Machida, M. Thermal stabilization of catalyst supports and their application to high-temperature catalytic. Appl. Catal. A-Gen. 1996, 138, 161–176. [Google Scholar]
- Barth, C.; Reichling, M. Imaging the atomic arrangements on the high-temperature reconstructed α-Al2O3 (0001) surface. Nature 2001, 414, 54–57. [Google Scholar]
- Mohri, M.; Uchida, Y.; Sawabe, Y.; Watanabe, H. α-Alumina. U.S. Patent 6165437, 26 December 2000. [Google Scholar]

| Morphology | Additive | Mixing Amount | Material | Temperature/Time | Ref. |
|---|---|---|---|---|---|
| Polyhedral | AlF3 | 1 wt.% | Al(OH)3 | 910 °C/3 h | [5] |
| Sphere-like | NH4BF4 | 5 wt.% | Al(OH)3 | 1450 °C/3 h | [7] |
| Plate-like | NH4F | 5 wt.% | AlOOH | 900 °C/2.5 h | [8] |
| Plate-like | NH4F+NH4F | 5 wt.% + 5 wt.% | Al(OH)3 | 1300 °C/3 h | [14] |
| Plate-like | AlF3 | 5 wt.% | AACH | 1200 °C/1 h | [21] |
| Plate-like | AlF3 | 2 wt.% | AIP | 900 °C/3 h | [22] |
| Plate-like | AlF3 NH4F | 2.8 wt.% of F | AlOOH | 1050 °C/1 h 950 °C/1 h | [23] |
| Plate-like | AlF3 | 0.6 wt.% | t-Al2O3Al(OH)3 | 1000 °C/0.5 h 950 °C/0.5 h | [24] |
| Plate-like | AlF3 | 1 mol% | Al(OH)3 | 750 °C/10 h | [25] |
| Plate-like | AlF3 | 2 mol% | AlOOH | 750 °C/10 h | [26] |
| Plate-like | ZnF2 AlF3 | - | Al(NO3)3 | 920 °C/10 h 900 °C/1 h | [28] |
| Square-like | NH4F | 2 wt.% | γ-Al2O3 | 1300 °C/2 h | [30] |
| Plate-like | Oxalic acid + NH4F | 0.16 mol/L + 1 wt.% | Al(OH)3 | 850 °C/3 h | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Li, B.; Liu, M.; Yang, X.; Liu, J.; Qin, T.; Xue, Z.; Xing, Y.; Chen, J. Low-Temperature Fabrication of Plate-like α-Al2O3 with Less NH4F Additive. Materials 2023, 16, 4415. https://doi.org/10.3390/ma16124415
Chen H, Li B, Liu M, Yang X, Liu J, Qin T, Xue Z, Xing Y, Chen J. Low-Temperature Fabrication of Plate-like α-Al2O3 with Less NH4F Additive. Materials. 2023; 16(12):4415. https://doi.org/10.3390/ma16124415
Chicago/Turabian StyleChen, Haiyang, Bin Li, Meng Liu, Xue Yang, Jie Liu, Tingwei Qin, Zejian Xue, Yun Xing, and Junhong Chen. 2023. "Low-Temperature Fabrication of Plate-like α-Al2O3 with Less NH4F Additive" Materials 16, no. 12: 4415. https://doi.org/10.3390/ma16124415






