Effect of Zr2Al4C5 Content on the Mechanical Properties and Oxidation Behavior of ZrB2-SiC-Zr2Al4C5 Ceramics
Abstract
:1. Introduction
2. Experimental Details
2.1. Preparation
2.2. Characterization and Measurement
3. Results and Discussion
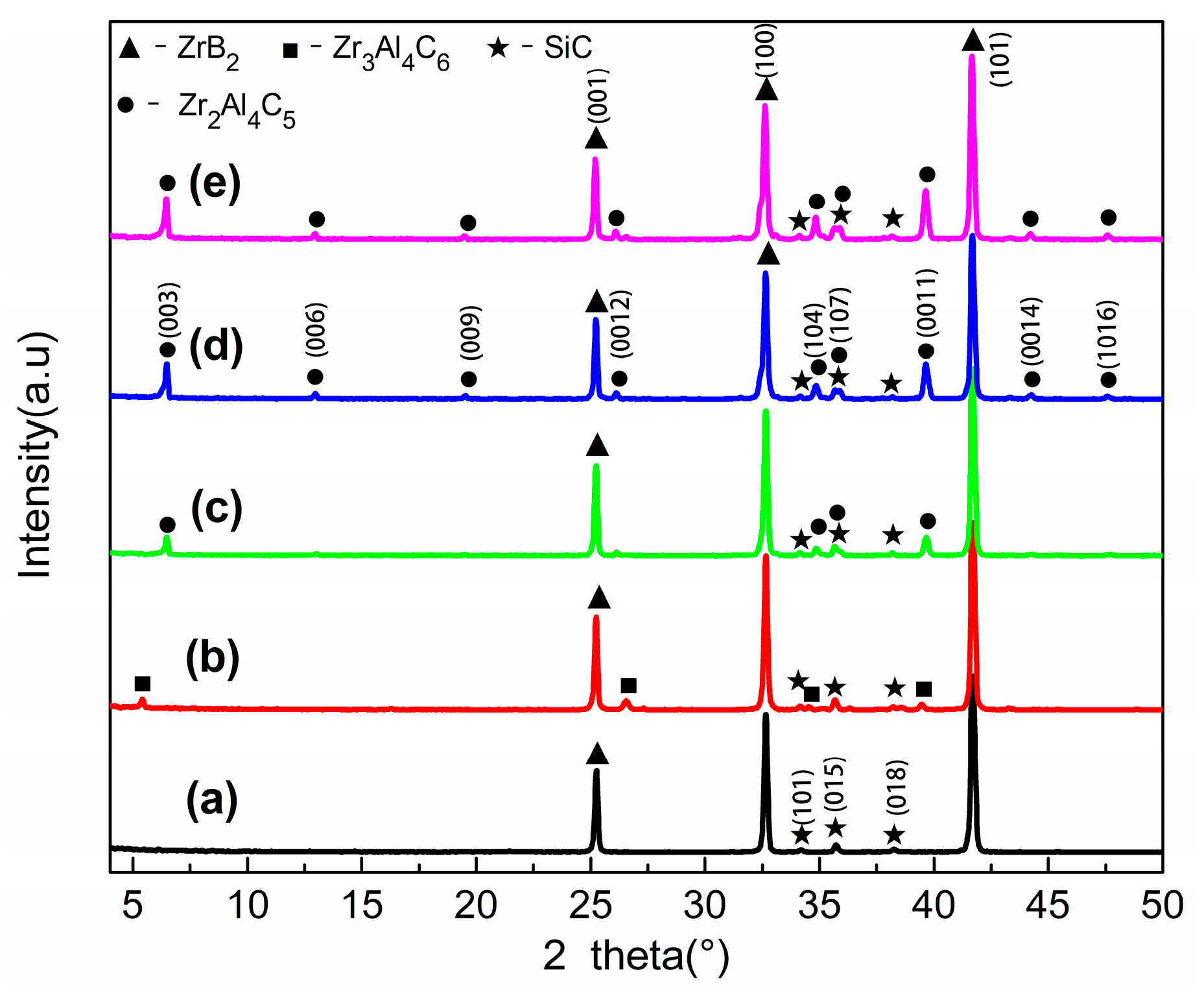
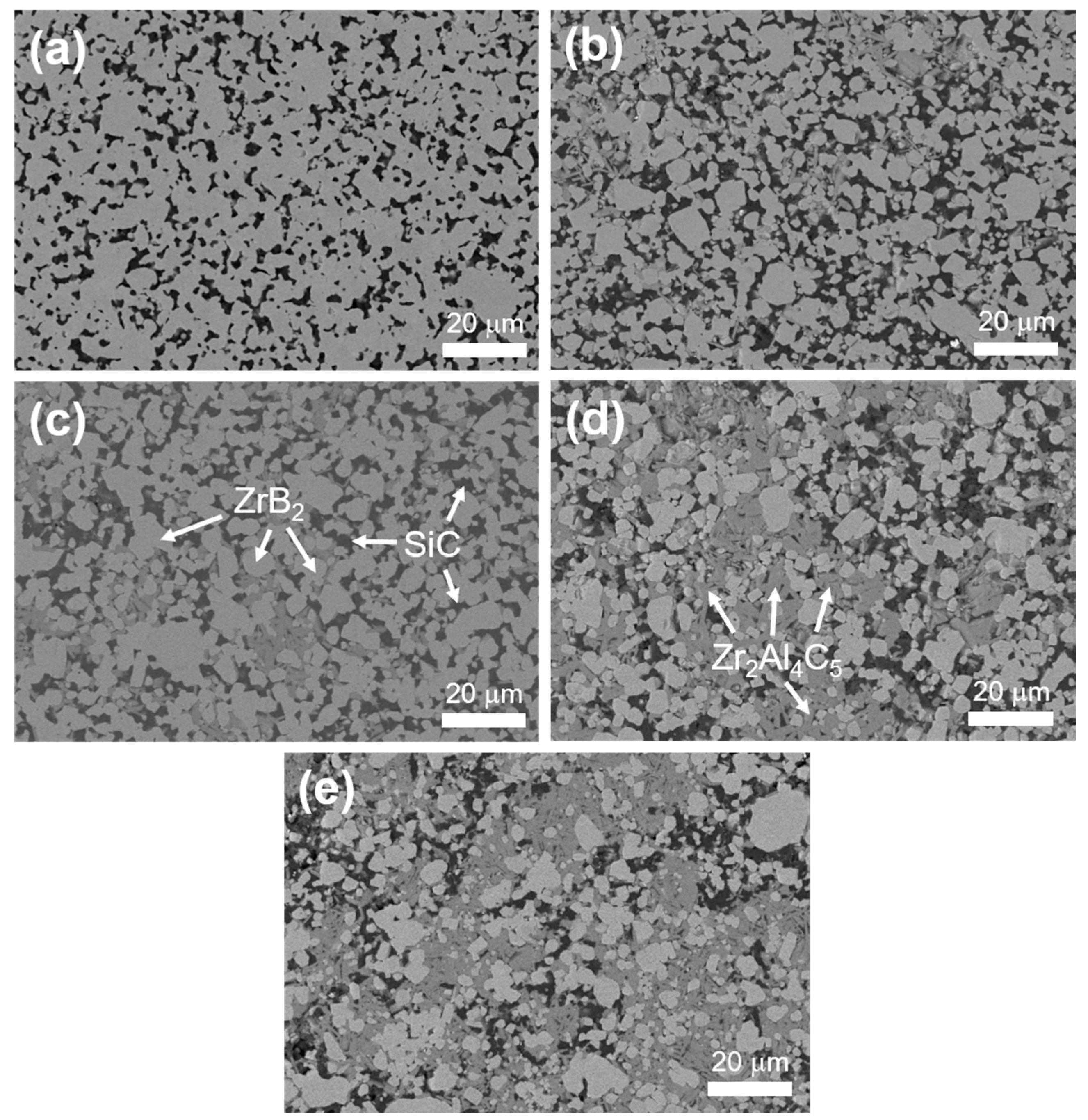
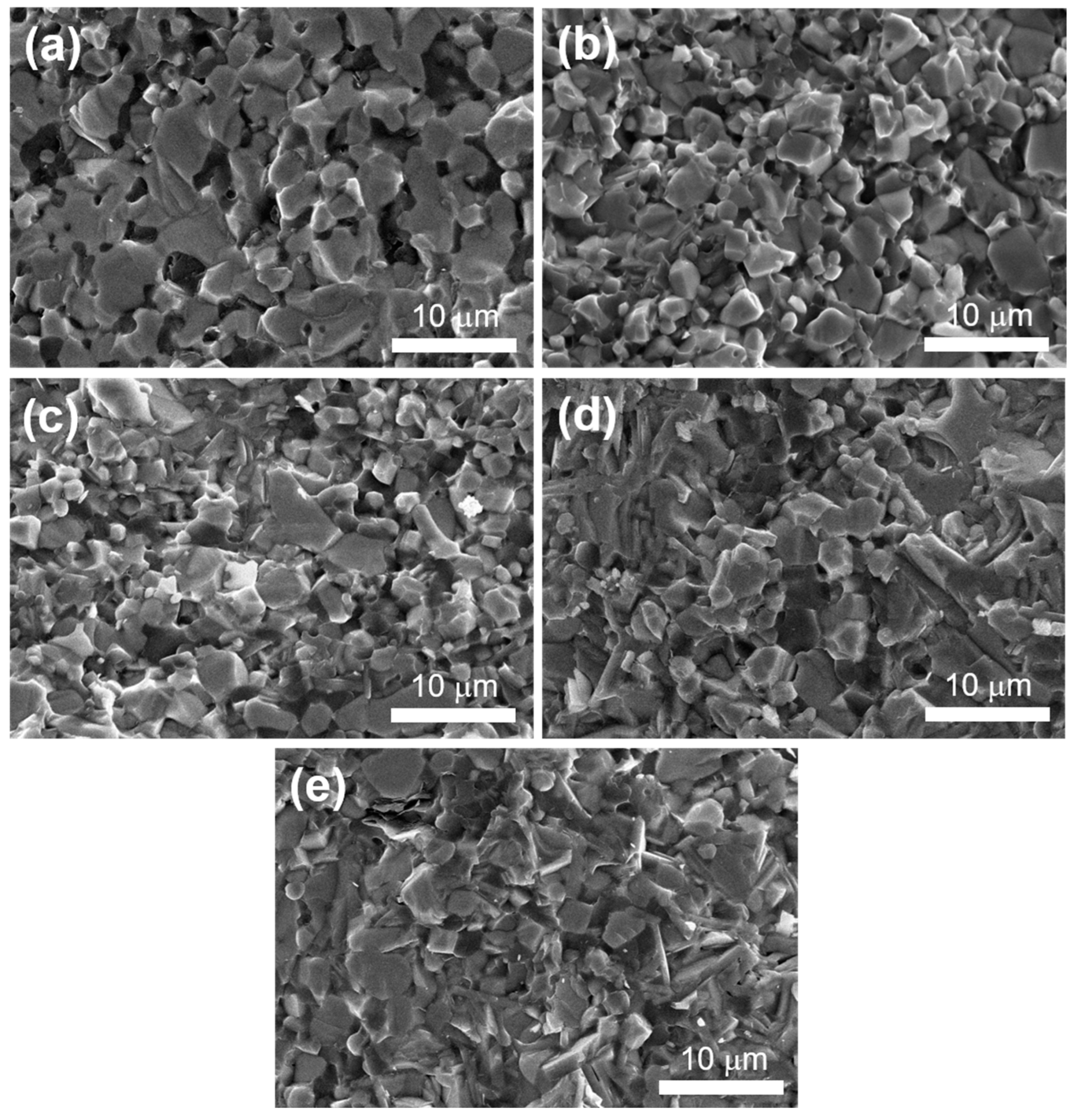
3.1. Phase Composition and Microstructure
3.2. Mechanical Properties
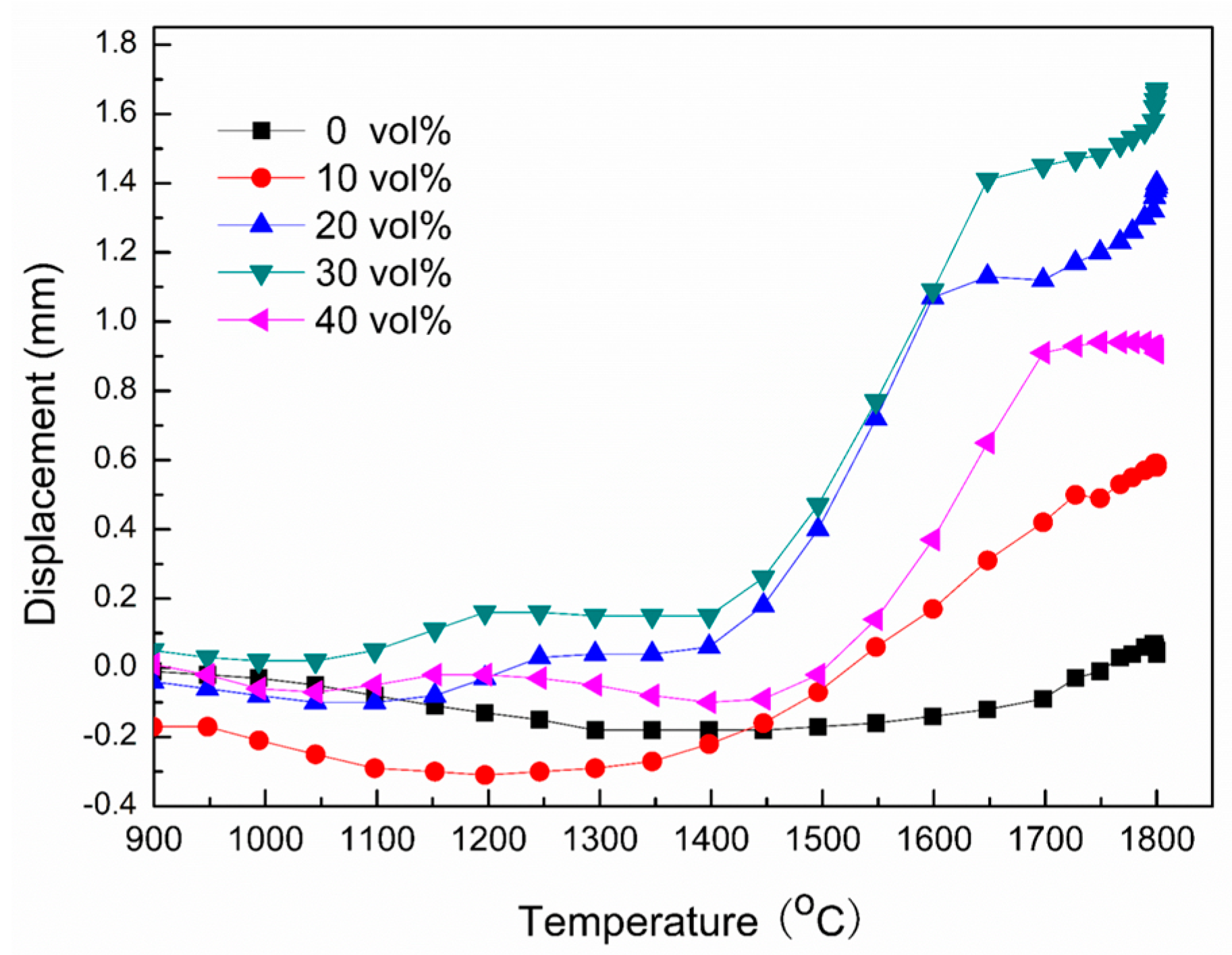
3.3. Oxidation Resistance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aguirre, T.G.; Lamm, B.W.; Cramer, C.L.; Mitchell, D.J. Zirconium-diboride silicon-carbide composites: A review. Ceram. Int. 2022, 48, 7344–7361. [Google Scholar] [CrossRef]
- Mohammadzadeh, B.; Jung, S.; Lee, T.H.; Le, Q.V.; Cha, J.H.; Jang, H.W. Manufacturing ZrB2-SiC–TaC Composite: Potential Application for Aircraft Wing Assessed by Frequency Analysis through Finite Element Model. Materials 2020, 13, 2213. [Google Scholar] [CrossRef]
- Fahrenholtz, W.G.; Hilmas, G.E.; Talmy, I.G.; Zaykoski, J.A. Refractory diborides of zirconium and hafnium. J. Am. Ceram. Soc. 2007, 90, 1347–1364. [Google Scholar] [CrossRef]
- Guo, S.Q. Densincation of ZrB2-based composites and their mechanical and physical properties: A review. J. Eur. Ceram. Soc. 2009, 29, 995–1011. [Google Scholar] [CrossRef]
- Asl, M.S.; Nayebi, B.; Ahmadi, Z.; Zamharir, M.J.; Shokouhimehr, M. Effects of carbon additives on the properties of ZrB2-based composites: A review. Ceram. Int. 2018, 44, 7334–7348. [Google Scholar] [CrossRef]
- Zhang, X.H.; Hu, P.; Han, J.C.; Meng, S.H. Ablation behavior of ZrB2-SiC ultra high temperature ceramics under simulated atmospheric re-entry conditions. Compos. Sci. Technol. 2008, 68, 1718–1726. [Google Scholar] [CrossRef]
- Guicciardi, S.; Silvestroni, L.; Nygren, M.; Sciti, D. Microstructure and Toughening Mechanisms in Spark Plasma-sintered ZrB2 Ceramics Reinforced by SiC Whiskers or SiC-chopped Fibers. J. Am. Ceram. Soc. 2010, 93, 2384–2391. [Google Scholar] [CrossRef]
- Asl, M.S.; Zamharir, M.J.; Ahmadi, Z.; Parvizi, S. Effects of nano-graphite content on the characteristics of spark plasma sintered ZrB2-SiC composites. Mater. Sci. Eng. A 2018, 716, 99–106. [Google Scholar]
- Zhang, X.H.; An, Y.M.; Han, J.C.; Han, W.B.; Zhao, G.D.; Jin, X.X. Graphene nanosheet reinforced ZrB2-SiC ceramic composite by thermal reduction of graphene oxide. Rsc. Adv. 2015, 5, 47060–47065. [Google Scholar] [CrossRef]
- Yue, C.G.; Liu, W.W.; Zhang, L.; Zhang, T.H.; Chen, Y. Fracture toughness and toughening mechanisms in a (ZrB2-SiC) composite reinforced with boron nitride nanotubes and boron nitride nanoplatelets. Scr. Mater. 2013, 68, 579–582. [Google Scholar] [CrossRef] [Green Version]
- Asl, M.S.; Farahbakhsh, I.; Nayebi, B. Characteristics of multi-walled carbon nanotube toughened ZrB2-SiC ceramic composite prepared by hot pressing. Ceram. Int. 2016, 42, 1950–1958. [Google Scholar]
- Lin, J.; Zhang, X.H.; Wang, Z.; Han, W.B. Microstructure and mechanical properties of ZrB2-SiC-ZrO2f ceramic. Scr. Mater. 2011, 64, 872–875. [Google Scholar]
- Sha, J.J.; Li, J.; Lv, Z.Z.; Wang, S.H.; Zhang, Z.F.; Zu, Y.F.; Flauder, S.; Krenkel, W. ZrB2-based composites toughened by as-received and heat-treated short carbon fibers. J. Eur. Ceram. Soc. 2017, 37, 549–558. [Google Scholar] [CrossRef]
- Fukuda, K.; Hisamura, M.; Kawamoto, Y.; Iwata, T. Synthesis, Crystal structure and thermoelectric properties of a new layered carbide (ZrC)3[Al3.56Si0.44]C3. J. Solid. State. Chem. 2007, 180, 1809–1815. [Google Scholar] [CrossRef]
- Zhou, Y.C.; He, L.F.; Lin, Z.J.; Wang, J.Y. Synthesis and structure-property relation ships of a new family of layered carbides in Zr-Al(Si)-C and Hf-Al(Si)-C systems. J. Eur. Ceram. Soc. 2013, 33, 2831–2865. [Google Scholar] [CrossRef]
- Guo, Q.L.; Cao, W.Z.; Gan, J.Z.; Pei, J.J.; Li, J.G.; Zhang, L.M. SPS Fabrication of ZrB2-SiC Ceramics Toughened by Zr2Al4C5 Compounds and Evaluation of their Properties. Raer. Metal. Mat. Eng. 2015, 44, 187–191. [Google Scholar]
- Guo, Q.L.; Pei, J.J.; Wang, J.; Li, J.G.; Zhang, L.M. Effect of the Content of SiC on Properties of ZrB2-SiC-Zr2Al4C5 Composite. Raer. Metal. Mat. Eng. 2018, 44, 282–287. [Google Scholar]
- Zhao, Y.; Wang, L.J.; Zhang, G.J.; Jiang, W.; Chen, L.D. Preparation and Microstructure of a ZrB2-SiC Composite Fabricated by the Spark Plasma Sintering-Reactive Synthesis (SPS-RS) Method. J. Am. Ceram. Soc. 2007, 90, 4040–4042. [Google Scholar] [CrossRef]
- Xu, J.Y.; Ma, P.F.; Zou, B.L. Reaction mechanism of ZrB2-ZrC formation in Ni-Zr-B4C system analyzed by differential scanning calorimetry. Materials 2021, 14, 6467. [Google Scholar] [CrossRef]
- Rangaraj, L.; Divakar, C.; Jayaram, V. Fabrication and mechanisms of densification of ZrB2-based ultra high temperature ceramics by reactive hot pressing. J. Eur. Ceram. Soc. 2010, 30, 129–138. [Google Scholar] [CrossRef]
- Fahrenholtz, W.G. Thermodynamic analysis of ZrB2-SiC oxidation: Formation of a SiC-depleted region. J. Am. Ceram. Soc. 2007, 90, 143–148. [Google Scholar] [CrossRef]
- Inoue, R.; Arai, Y.; Kubota, Y.; Kogo, Y.; Goto, K. Oxidation of ZrB2 and its composites: A review. J. Mater. Sci. 2018, 53, 14885–14906. [Google Scholar] [CrossRef]
- Talmy, I.G.; Zaykoski, J.A.; Opeka, M.M. High-temperature chemistry and oxidation of ZrB2 ceramics containing SiC, Si3N4, Ta5Si3, and TaSi2. J. Am. Ceram. Soc. 2008, 91, 2250–2257. [Google Scholar] [CrossRef]
- Opila, E.; Levine, S.; Lorincz, J. Oxidation of ZrB2- and HfB2-based ultra-high temperature ceramics: Effect of Ta additions. J. Mater. Sci. 2004, 39, 5969–5977. [Google Scholar] [CrossRef]
- Peng, F.; Speyer, R.F. Oxidation resistance of fully dense ZrB2 with SiC, TaB2, and TaSi2 additives. J. Am. Ceram. Soc. 2008, 91, 1489–1494. [Google Scholar] [CrossRef]
- Silvestroni, L.; Meriggi, G.; Sciti, D. Oxidation behavior of ZrB2 composites doped with various transition metal silicides. Corros. Sci. 2014, 83, 281–291. [Google Scholar] [CrossRef]
- Ouyang, G.Y.; Ray, P.K.; Kramer, M.J.; Akinc, M. Effect of AlN substitutions on the oxidation behavior of ZrB2-SiC composites at 1600 °C. J. Am. Ceram. Soc. 2016, 99, 3389–3397. [Google Scholar] [CrossRef]
- Sugiura, K.; Iwata, T.; Yoshida, H.; Hashimoto, S.; Fukuda, K. Syntheses, crystal structures and Si solubilities of new layered carbides Zr2Al4C5 and Zr3Al4C6. J. Solid State Chem. 2008, 181, 2864–2868. [Google Scholar] [CrossRef]
- Miyazaki, H.; Yoshizawa, Y. A reinvestigation of the validity of the indentation fracture (IF) method as applied to ceramics. J. Eur. Ceram. Soc. 2017, 37, 4437–4441. [Google Scholar] [CrossRef]
- Sktani, Z.D.I.; Rejab, N.A.; Ratnam, M.M.; Ahmad, Z.A. Fabrication of tougher ZTA ceramics with sustainable high hardness through (RSM) optimisation. Int. J. Refract. Met. Hard Mater. 2018, 74, 78–86. [Google Scholar] [CrossRef]
- Arab, A.; Sktani, Z.D.I.; Zhou, Q.; Ahmad, Z.A.; Chen, P.W. Effect of MgO Addition on the Mechanical and Dynamic Properties of Zirconia Toughened Alumina (ZTA) Ceramics. Materials 2019, 12, 2440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Q.L.; Wang, X.Q.; Wang, J. Study on Sintering Processing and Mechanical Properties of In-Situ Reaction-Synthesized Zr2Al4C5-Toughened ZrB2-SiC Composite Ceramics. Mat. Rep. 2021, 35, 06065–06070. [Google Scholar]
- Yang, C.; Yao, P.Y.; Huan, Y.; Fu, Z.Q.; Chen, F.; Lavernia, E.J. Tough TiB2 -based ceramic composites using metallic glass powder as the sintering aid. Adv. Eng. Mater. 2016, 18, 1936–1943. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.Q. Effects of VC additives on densification and elastic and mechanical properties of hot-pressed ZrB2-SiC composites. J. Mater. Sci. 2018, 53, 4010–4021. [Google Scholar] [CrossRef]
- Guo, Q.L.; Da Silva, C.V.J.; Lin, M.; Luo, S.J.; Pei, J.J.; Wang, X.Q.; Li, J.G.; Zhang, L.M. Influence of composition on microstructure, mechanical properties and oxidation behavior of ZrB2/ZrAlC composite ceramics. J. Ceram. Soc. Jap. 2019, 127, 878–886. [Google Scholar] [CrossRef]
- Yu, L.; Liu, H.; Fu, Y.H.; Hu, W.J.; Wang, Z.F.; Liu, Q.; Wei, B.; Yang, J.; Qiu, T. Design and preparation of an ultra-high temperature ceramic by in-situ introduction of Zr2[Al(Si)]4C5 into ZrB2-SiC: Investigation on the mechanical properties and oxidation behavior. J. Adv. Ceram. 2021, 10, 1082–1094. [Google Scholar] [CrossRef]
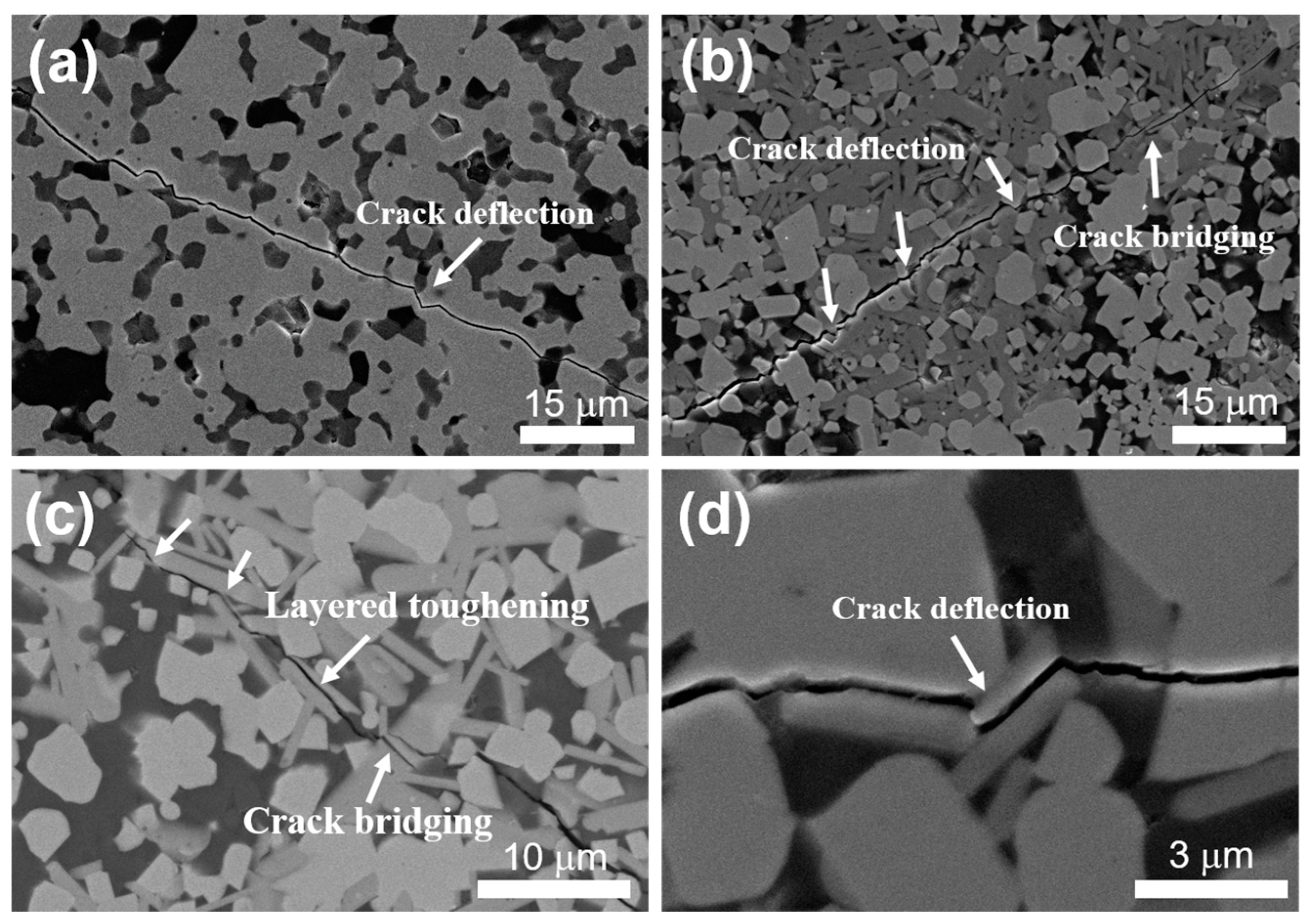


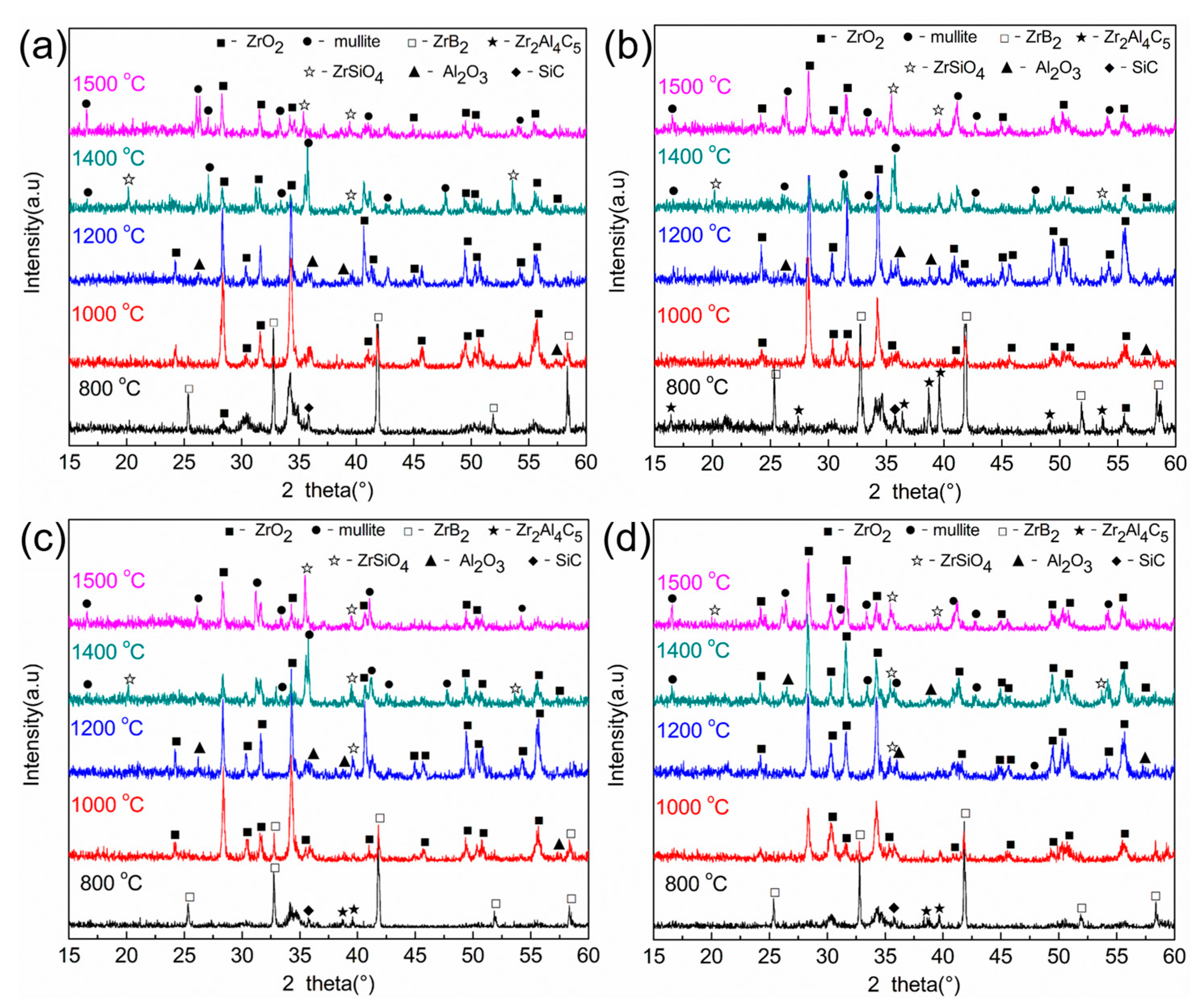

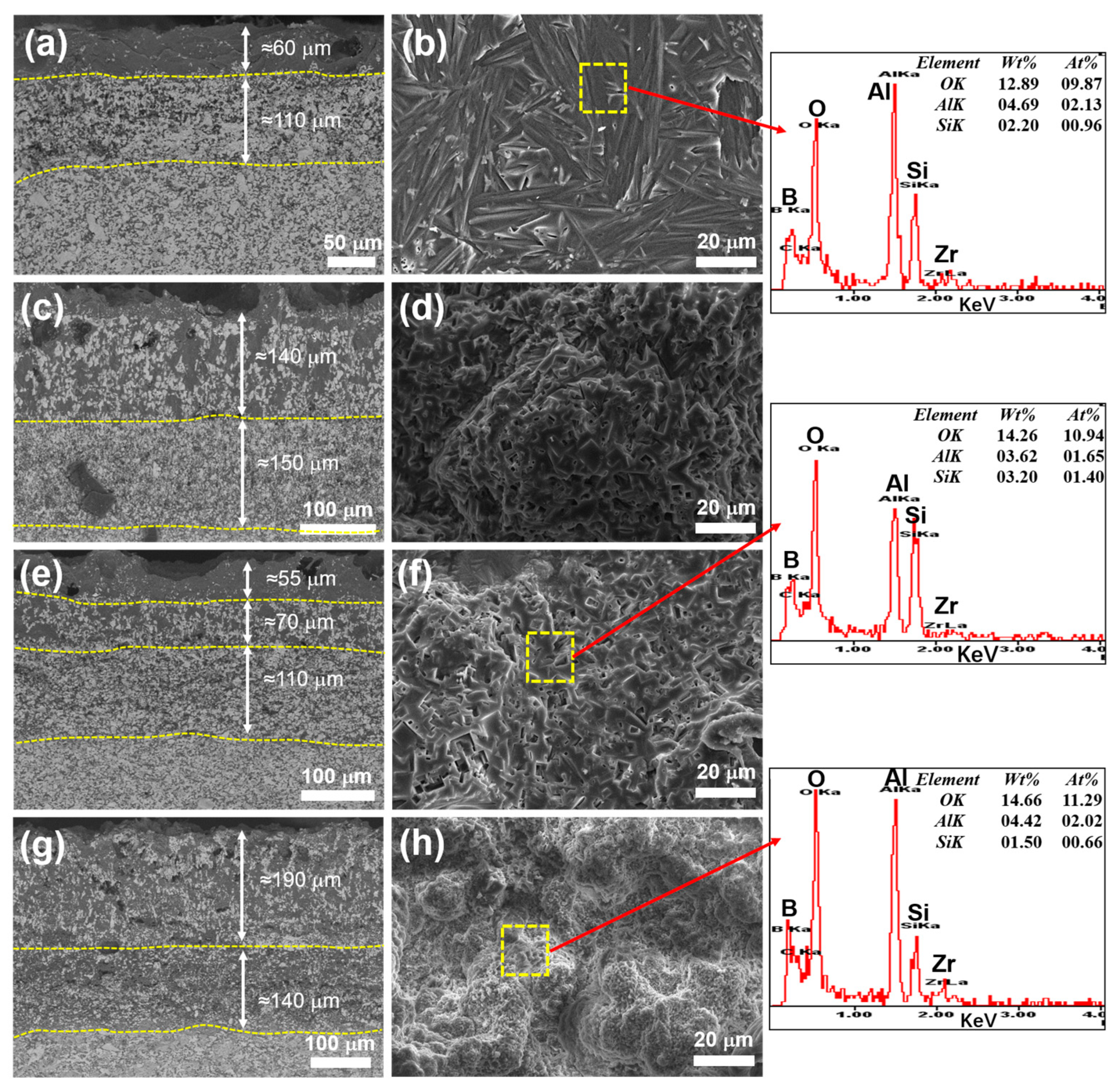


| Sample | Density (g/cm3) | Open Porosity (%) | ZrB2 Grain Size (μm) | Vickers Hardness (GPa) | Young’s Modulus (GPa) | Fracture Toughness (MPa·m1/2) |
|---|---|---|---|---|---|---|
| ZAC0 | 5.36 | 0.65 | 4.7 ± 0.7 | 17.9 ± 0.2 | 462 ± 15 | 4.02 ± 0.30 |
| ZAC10 | 5.07 | 0.45 | 4.2 ± 0.6 | 17.1 ± 0.2 | 435 ± 20 | 4.61 ± 0.33 |
| ZAC20 | 4.96 | 0.29 | 3.9 ± 0.6 | 16.8 ± 0.2 | 407 ± 15 | 5.12 ± 0.29 |
| ZAC30 | 4.83 | 0.22 | 3.3 ± 1.0 | 16.6 ± 0.4 | 392 ± 15 | 5.26 ± 0.32 |
| ZAC40 | 4.63 | 0.41 | 3.7 ± 1.1 | 15.8 ± 0.4 | 376 ± 15 | 4.53 ± 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Q.; Hua, L.; Ying, H.; Liu, R.; Lin, M.; Li, L.; Wang, J. Effect of Zr2Al4C5 Content on the Mechanical Properties and Oxidation Behavior of ZrB2-SiC-Zr2Al4C5 Ceramics. Materials 2023, 16, 4495. https://doi.org/10.3390/ma16124495
Guo Q, Hua L, Ying H, Liu R, Lin M, Li L, Wang J. Effect of Zr2Al4C5 Content on the Mechanical Properties and Oxidation Behavior of ZrB2-SiC-Zr2Al4C5 Ceramics. Materials. 2023; 16(12):4495. https://doi.org/10.3390/ma16124495
Chicago/Turabian StyleGuo, Qilong, Liang Hua, Hao Ying, Ronghao Liu, Mei Lin, Leilei Li, and Jing Wang. 2023. "Effect of Zr2Al4C5 Content on the Mechanical Properties and Oxidation Behavior of ZrB2-SiC-Zr2Al4C5 Ceramics" Materials 16, no. 12: 4495. https://doi.org/10.3390/ma16124495




