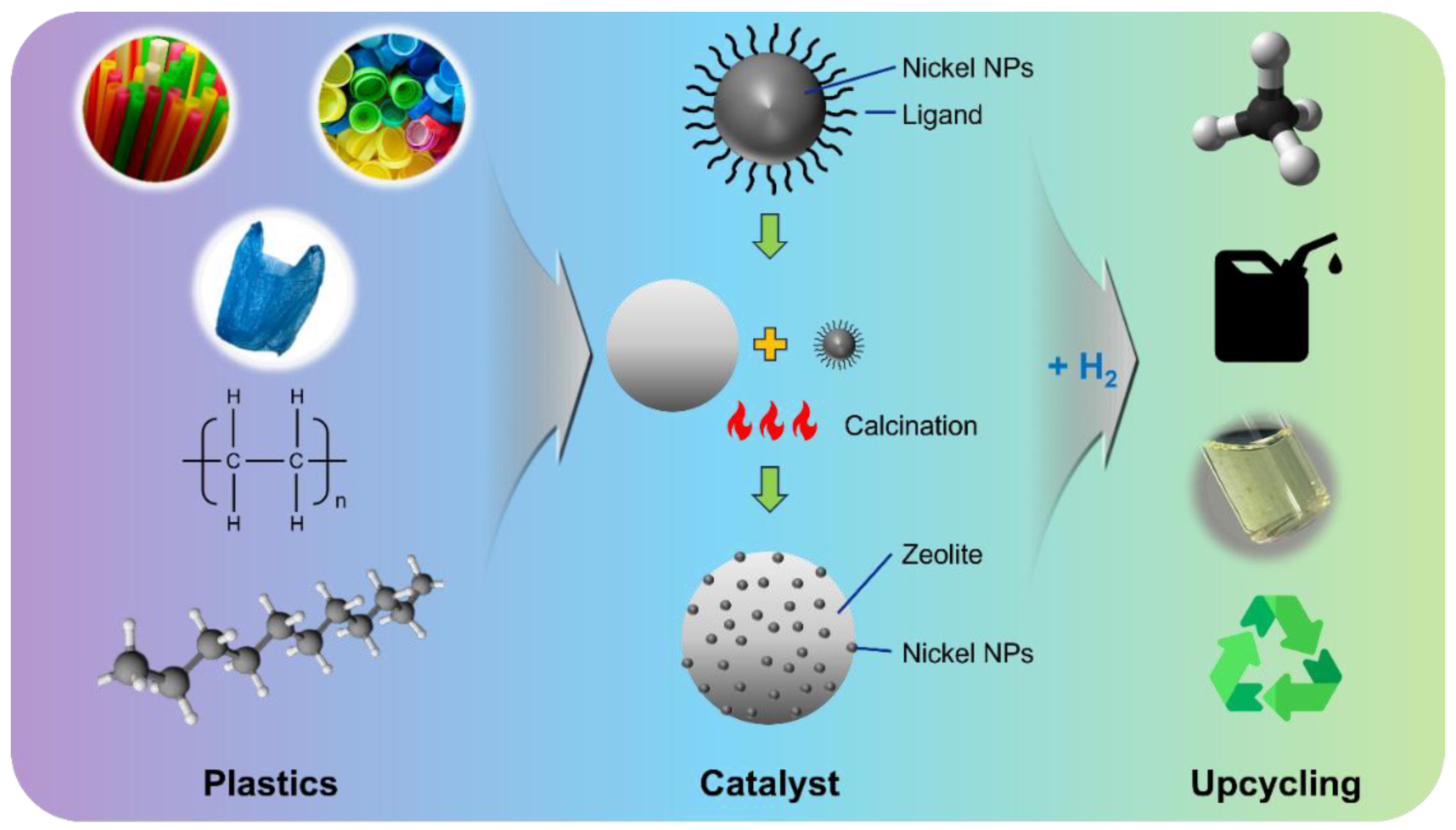
Conversion of Polyethylene to Low-Molecular-Weight Oil Products at Moderate Temperatures Using Nickel/Zeolite Nanocatalysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
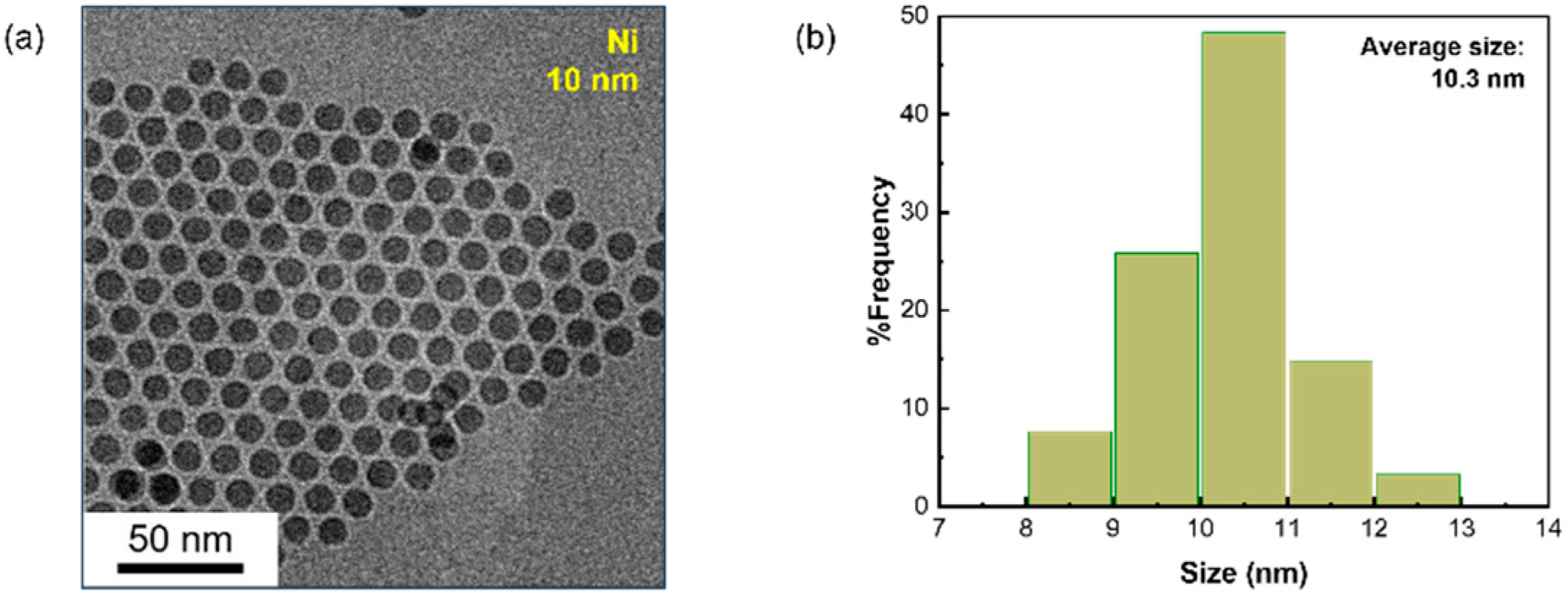
2.2.1. Synthesis of 10 nm Nickel Nanoparticles
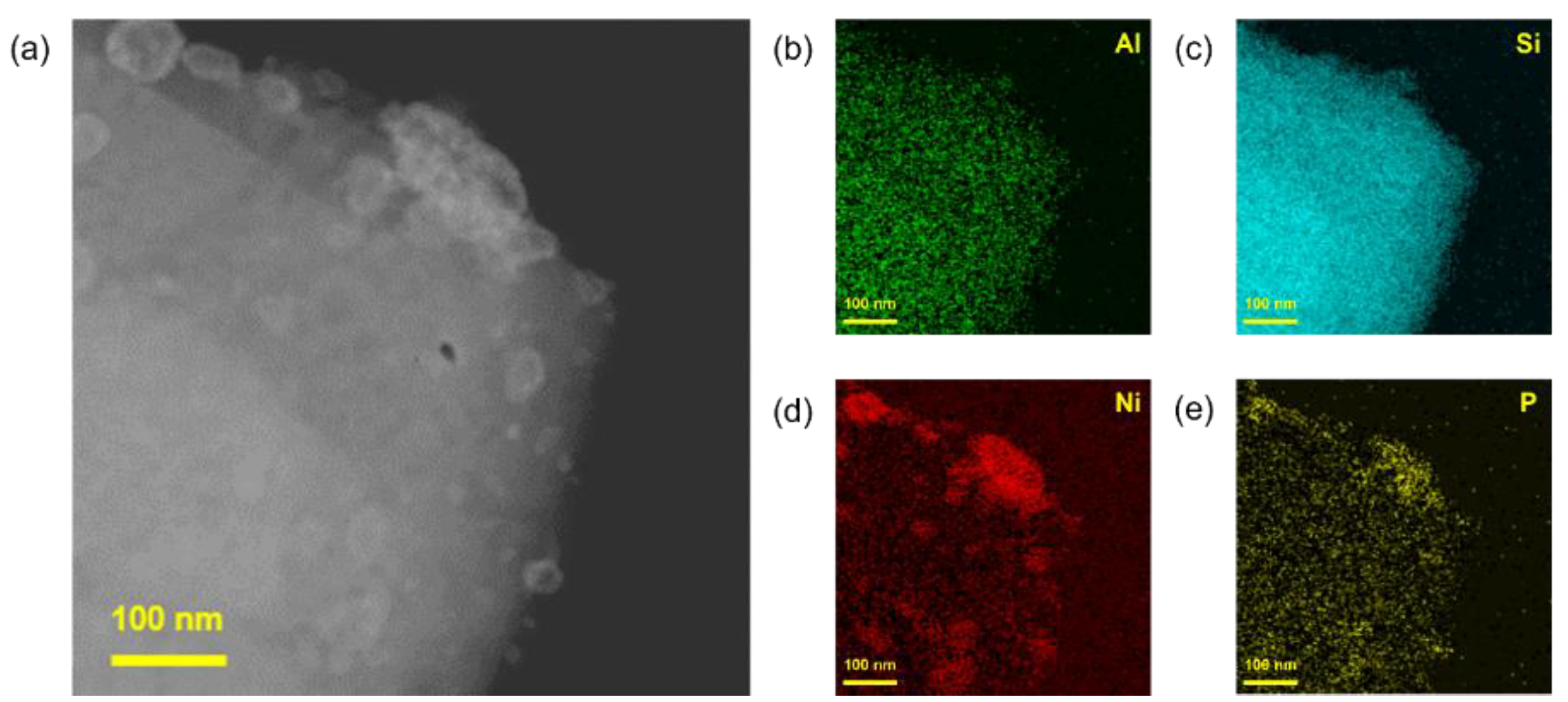
2.2.2. Preparation of Polyethylene Degradation Catalyst
2.2.3. Polyethylene Upcycling
2.2.4. Characterization of Materials
3. Results and Discussion
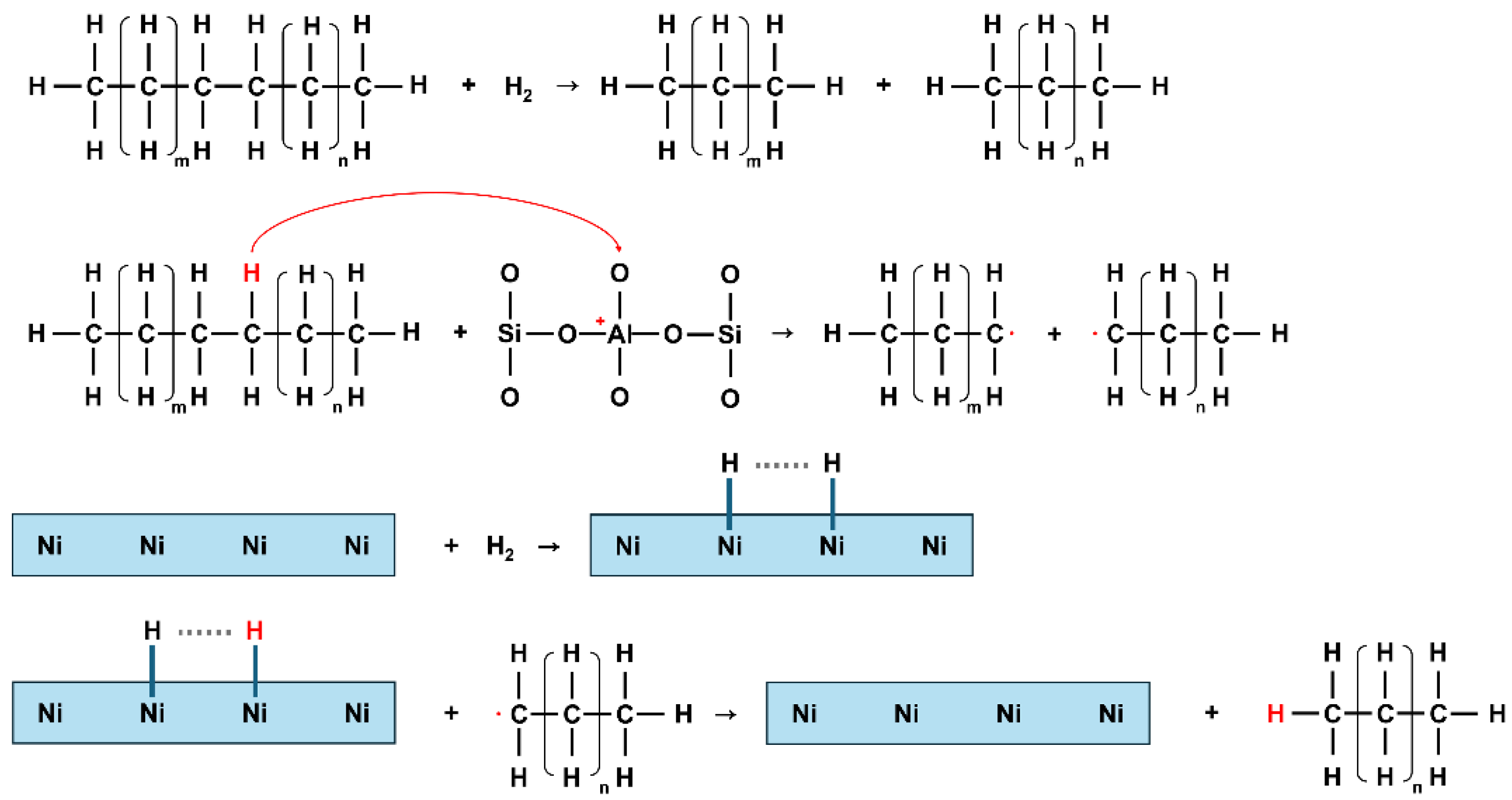
3.1. Analysis of Nanoparticles and Catalysts
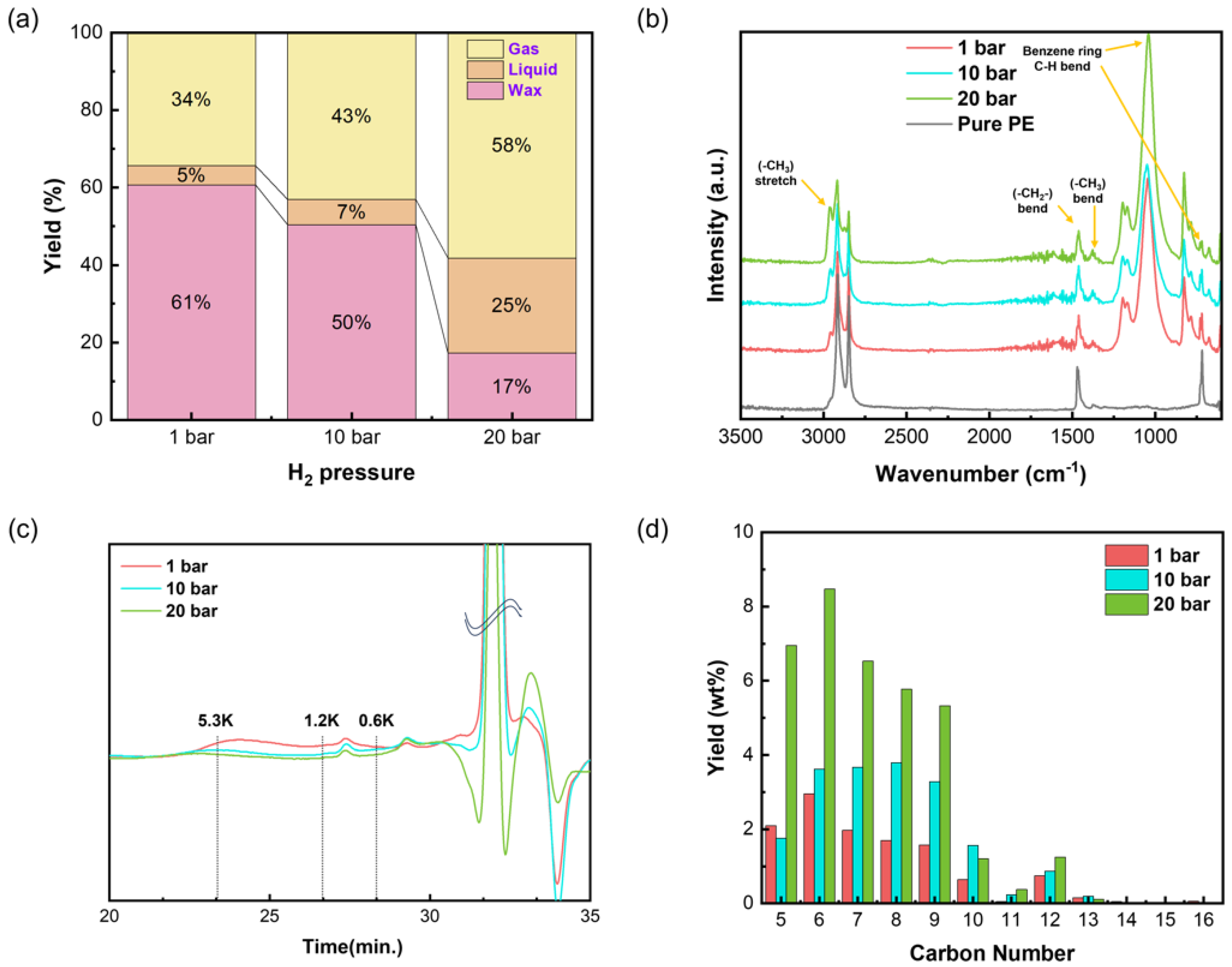
3.2. Analysis of PE Upcycling
3.2.1. Effect of Hydrogen Pressure on PE Upcycling
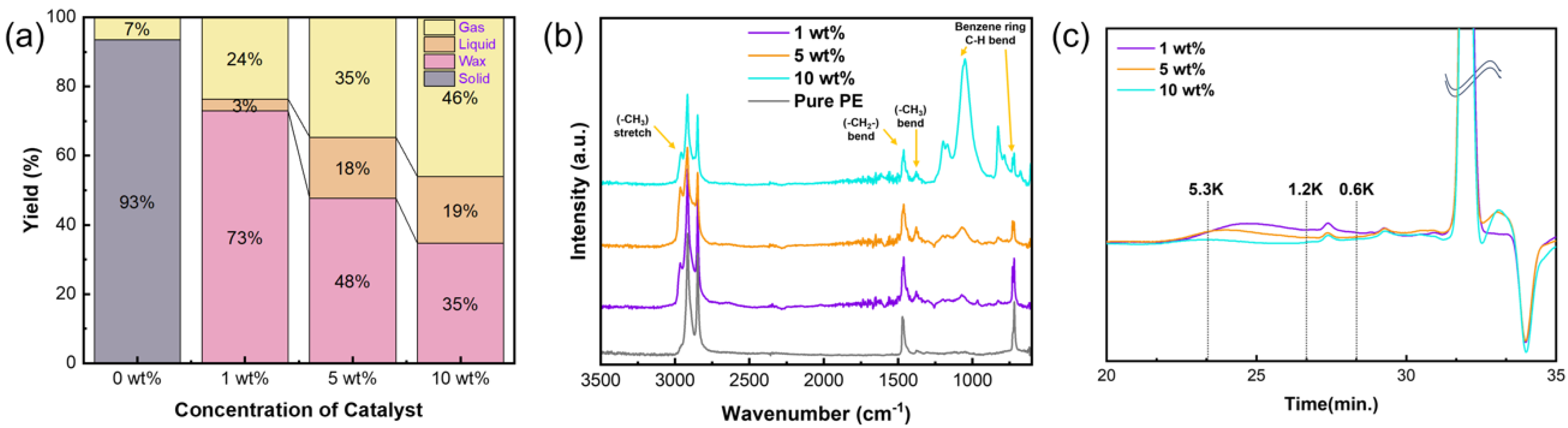
3.2.2. Effect of Catalyst Amount on PE Upcycling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jia, C.; Xie, S.; Zhang, W.; Intan, N.N.; Sampath, J.; Pfaendtner, J.; Lin, H. Deconstruction of High-Density Polyethylene into Liquid Hydrocarbon Fuels and Lubricants by Hydrogenolysis over Ru Catalyst. Chem. Catal. 2021, 1, 437–455. [Google Scholar] [CrossRef]
- Barbosa, A.S.; Biancolli, A.L.; Lanfredi, A.J.C.; Rodrigues, O.; Fonseca, F.C.; Santiago, E.I. Enhancing the Durability and Performance of Radiation-Induced Grafted Low-Density Polyethylene-Based Anion-Exchange Membranes by Controlling Irradiation Conditions. J. Membr. Sci. 2022, 659, 120804. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Kamal, R.S.; Shaban, M.M.; Raju, G.; Farag, R.K. High-Density Polyethylene Waste (Hdpe)-Waste-Modified Lube Oil Nanocomposites as Pour Point Depressants. ACS Omega 2021, 6, 31926–31934. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological Degradation of Plastics: A Comprehensive Review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef] [PubMed]
- Kunwar, B.; Cheng, H.N.; Chandrashekaran, S.R.; Sharma, B.K. Plastics to Fuel: A Review. Renew. Sustain. Energy Rev. 2016, 54, 421–428. [Google Scholar] [CrossRef]
- Diaz-Silvarrey, L.S.; Zhang, K.; Phan, A.N. Monomer Recovery through Advanced Pyrolysis of Waste High Density Polyethylene (HDPE). Green Chem. 2018, 20, 1813–1823. [Google Scholar] [CrossRef]
- Bagri, R.; Williams, P.T. Catalytic Pyrolysis of Polyethylene. J. Anal. Appl. Pyrolysis 2002, 63, 29–41. [Google Scholar] [CrossRef]
- Panda, A.K.; Singh, R.K.; Mishra, D.K. Thermolysis of Waste Plastics to Liquid Fuela Suitable Method for Plastic Waste Management and Manufacture of Value Added Products—A World Prospective. Renew. Sustain. Energy Rev. 2010, 14, 233–248. [Google Scholar] [CrossRef]
- Li, L.; Luo, H.; Shao, Z.; Zhou, H.; Lu, J.; Chen, J.; Huang, C.; Zhang, S.; Liu, X.; Xia, L.; et al. Converting Plastic Wastes to Naphtha for Closing the Plastic Loop. J. Am. Chem. Soc. 2023, 145, 1847–1854. [Google Scholar] [CrossRef]
- Hussein, Z.A.; Shakor, Z.M.; Alzuhairi, M.; Al-Sheikh, F. The Yield of Gasoline Range Hydrocarbons from Plastic Waste Pyrolysis. Energy Sources Part A Recovery Util. Environ. Eff. 2022, 44, 718–731. [Google Scholar] [CrossRef]
- Vance, B.C.; Najmi, S.; Kots, P.A.; Wang, C.; Jeon, S.; Stach, E.A.; Zakharov, D.N.; Marinkovic, N.; Ehrlich, S.N.; Ma, L.; et al. Structure–Property Relationships for Nickel Aluminate Catalysts in Polyethylene Hydrogenolysis with Low Methane Selectivity. JACS Au 2023, 3, 2156–2165. [Google Scholar] [CrossRef] [PubMed]
- Schyns, Z.O.; Shaver, M.P. Mechanical Recycling of Packaging Plastics: A Review. Macromol. Rapid Commun. 2020, 42, e2000415. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Delva, L.; Van Geem, K. Mechanical and Chemical Recycling of Solid Plastic Waste. Waste Manag. 2017, 69, 24–58. [Google Scholar]
- Rahimi, A.; García, J.M. Chemical Recycling of Waste Plastics for New Materials Production. Nat. Rev. Chem. 2017, 1, 0046. [Google Scholar] [CrossRef]
- Jehanno, C.; Alty, J.W.; Roosen, M.; De Meester, S.; Dove, A.P.; Chen, E.Y.-X.; Leibfarth, F.A.; Sardon, H. Critical Advances and Future Opportunities in Upcycling Commodity Polymers. Nature 2022, 603, 803–814. [Google Scholar] [CrossRef]
- Jin, H.; Gonzalez-Gutierrez, J.; Oblak, P.; Zupančič, B.; Emri, I. The Effect of Extensive Mechanical Recycling on the Properties of Low Density Polyethylene. Polym. Degrad. Stab. 2012, 97, 2262–2272. [Google Scholar] [CrossRef]
- Onwudili, J.A.; Insura, N.; Williams, P.T. Composition of Products from the Pyrolysis of Polyethylene and Polystyrene in a Closed Batch Reactor: Effects of Temperature and Residence Time. J. Anal. Appl. Pyrolysis 2009, 86, 293–303. [Google Scholar] [CrossRef]
- Kiran, N.; Ekinci, E.; Snape, C.E. Recyling of Plastic Wastes via Pyrolysis. Resour. Conserv. Recycl. 2000, 29, 273–283. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and Recovery Routes of Plastic Solid Waste (PSW): A Review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef]
- Rauert, C.; Pan, Y.; Okoffo, E.D.; O’Brien, J.W.; Thomas, K.V. Extraction and Pyrolysis-GC-MS Analysis of Polyethylene in Samples with Medium to High Lipid Content. J. Environ. Expo. Assess. 2022, 1, 13. [Google Scholar] [CrossRef]
- Zhang, W.; Kim, S.; Wahl, L.; Khare, R.; Hale, L.; Hu, J.; Camaioni, D.M.; Gutiérrez, O.Y.; Liu, Y.; Lercher, J.A. Low-Temperature Upcycling of Polyolefins into Liquid Alkanes via Tandem Cracking-Alkylation. Science 2023, 379, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zeng, M.; Yappert, R.D.; Sun, J.; Lee, Y.-H.; LaPointe, A.M.; Peters, B.; Abu-Omar, M.M.; Scott, S.L. Polyethylene Upcycling to Long-Chain Alkylaromatics by Tandem Hydrogenolysis/Aromatization. Science 2020, 370, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xie, T.; Kots, P.A.; Vance, B.C.; Yu, K.; Kumar, P.; Fu, J.; Liu, S.; Tsilomelekis, G.; Stach, E.A.; et al. Polyethylene Hydrogenolysis at Mild Conditions over Ruthenium on Tungstated Zirconia. JACS Au 2021, 1, 1422–1434. [Google Scholar] [CrossRef] [PubMed]
- Celik, G.; Kennedy, R.M.; Hackler, R.A.; Ferrandon, M.; Tennakoon, A.; Patnaik, S.; LaPointe, A.M.; Ammal, S.C.; Heyden, A.; Perras, F.A.; et al. Upcycling Single-Use Polyethylene into High-Quality Liquid Products. ACS Cent. Sci. 2019, 5, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Y.; Tennakoon, A.; Wu, X.; Sahasrabudhe, C.; Qi, L.; Peters, B.G.; Sadow, A.D.; Huang, W. Catalytic Hydrogenolysis of Polyethylene under Reactive Separation. ACS Catal. 2024, 14, 2084–2094. [Google Scholar] [CrossRef]
- Vance, B.C.; Kots, P.A.; Wang, C.; Granite, J.E.; Vlachos, D.G. Ni/SiO2 Catalysts for Polyolefin Deconstruction via the Divergent Hydrogenolysis Mechanism. Appl. Catal. B Environ. 2023, 322, 122138. [Google Scholar] [CrossRef]
- Medford, A.J.; Vojvodic, A.; Hummelshøj, J.S.; Voss, J.; Abild-Pedersen, F.; Studt, F.; Bligaard, T.; Nilsson, A.; Nørskov, J.K. From the Sabatier Principle to a Predictive Theory of Transition-Metal Heterogeneous Catalysis. J. Catal. 2015, 328, 36–42. [Google Scholar] [CrossRef]
- Arroyave, A.; Cui, S.; Lopez, J.C.; Kocen, A.L.; LaPointe, A.M.; Delferro, M.; Coates, G.W. Catalytic Chemical Recycling of Post-Consumer Polyethylene. J. Am. Chem. Soc. 2022, 144, 23280–23285. [Google Scholar] [CrossRef]
- Ding, W.B.; Tuntawiroon, W.; Liang, J.; Anderson, L.L. Depolymerization of Waste Plastics with Coal over Metal-Loaded Silica-Alumina Catalysts. Fuel Process. Technol. 1996, 49, 49–63. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Z.; Zhang, X.; Li, T.; Li, Y.; Chen, X.; Wang, K. Catalytic Hydrogenolysis of Plastic to Liquid Hydrocarbons over a Nickel-Based Catalyst. Environ. Pollut. 2022, 313, 120154. [Google Scholar] [CrossRef]
- Si, X.; Chen, J.; Wang, Z.; Hu, Y.; Ren, Z.; Lu, R.; Liu, L.; Zhang, J.; Pan, L.; Cai, R.; et al. Ni-Catalyzed Carbon–Carbon Bonds Cleavage of Mixed Polyolefin Plastics Waste. J. Energy Chem. 2023, 85, 562–569. [Google Scholar] [CrossRef]
- Singh, S.B.; Tandon, P.K. Catalysis: A brief review on nano-catalyst. J. Energy Chem. Eng. 2014, 2, 106–115. [Google Scholar]
- Akubo, K.; Nahil, M.A.; Williams, P.T. Aromatic Fuel Oils Produced from the Pyrolysis-Catalysis of Polyethylene Plastic with Metal-Impregnated Zeolite Catalysts. J. Energy Inst. 2019, 92, 195–202. [Google Scholar] [CrossRef]
- Susastriawan, A.A.P.; Purnomo; Sandria, A. Experimental Study the Influence of Zeolite Size on Low-Temperature Pyrolysis of Low-Density Polyethylene Plastic Waste. Therm. Sci. Eng. Prog. 2020, 17, 100497. [Google Scholar]
- Jiang, C.; Wang, Y.; Luong, T.; Robinson, B.; Liu, W.; Hu, J. Low Temperature Upcycling of Polyethylene to Gasoline Range Chemicals: Hydrogen Transfer and Heat Compensation to Endothermic Pyrolysis Reaction over Zeolites. J. Environ. Chem. Eng. 2022, 10, 107492. [Google Scholar] [CrossRef]
- Somorjai, G.A.; Blakely, D.W. Mechanism of Catalysis of Hydrocarbon Reactions by Platinum Surfaces. Nature 1975, 258, 580–583. [Google Scholar] [CrossRef]
- Tennakoon, A.; Wu, X.; Paterson, A.L.; Patnaik, S.; Pei, Y.; LaPointe, A.M.; Ammal, S.C.; Hackler, R.A.; Heyden, A.; Slowing, I.I.; et al. Catalytic Upcycling of High-Density Polyethylene via a Processive Mechanism. Nat. Catal. 2020, 3, 893–901. [Google Scholar] [CrossRef]
- Cho, H.; Lee, N.; Kim, B.H. Synthesis of Highly Monodisperse Nickel and Nickel Phosphide Nanoparticles. Nanomaterials 2022, 12, 3198. [Google Scholar] [CrossRef]
- Park, J.; Kang, E.; Son, U.; Park, M.; Lee, K.; Kim, J.; Kim, W.; Noh, H.-J.; Park, J.-H.; Bae, C.J.; et al. Monodisperse Nanoparticles of Ni and NiO: Synthesis, Characterization, Self-assembled Superlattices, and Catalytic Applications in the Suzuki Coupling Reaction. Adv. Mater. 2005, 17, 429–434. [Google Scholar] [CrossRef]
- Pang, H.; Yang, G.; Li, L.; Yu, J. Toward the Production of Renewable Diesel over Robust Ni Nanoclusters Highly Dispersed on a Two-Dimensional Zeolite. NPG Asia Mater. 2023, 15, 24. [Google Scholar] [CrossRef]
- Barton, R.R.; Carrier, M.; Segura, C.; Fierro, J.L.G.; Escalona, N.; Peretti, S.W. Ni/HZSM-5 Catalyst Preparation by Deposition-Precipitation. Part 1. Effect of Nickel Loading and Preparation Conditions on Catalyst Properties. Appl. Catal. A Gen. 2017, 540, 7–20. [Google Scholar] [CrossRef]
- Escobar, J.; Núñez, S.; Montesinos-Castellanos, A.; de los Reyes, J.A.; Rodríguez, Y.; González, O.A. Dibenzothiophene Hydrodesulfurization over PdPt/Al2O3–TiO2. Influence of Ti-Addition on Hydrogenating Properties. Mater. Chem. Phys. 2016, 171, 185–194. [Google Scholar]
- Rios-Escobedo, R.; Ortiz-Santos, E.; Colín-Luna, J.A.; de León, J.N.D.; del Angel, P.; Escobar, J.; de los Reyes, J.A. Anisole Hydrodeoxygenation: A Comparative Study of Ni/TiO2-ZrO2 and Commercial TiO2 Supported Ni and NiRu Catalysts. Top. Catal. 2022, 65, 1448–1461. [Google Scholar] [CrossRef]
- Beck, I.E.; Bukhtiyarov, V.I.; Pakharukov, I.Y.; Zaikovsky, V.I.; Kriventsov, V.V.; Parmon, V.N. Platinum Nanoparticles on Al2O3: Correlation between the Particle Size and Activity in Total Methane Oxidation. J. Catal. 2009, 268, 60–67. [Google Scholar] [CrossRef]
- Marcilla, A.; Beltrán, M.I.; Navarro, R. TG/FT-IR Analysis of HZSM5 and HUSY Deactivation during the Catalytic Pyrolysis of Polyethylene. J. Anal. Appl. Pyrolysis 2006, 76, 222–229. [Google Scholar] [CrossRef]
- Wang, S.; Tang, Y.; Schobert, H.H.; Guo, Y.; Gao, W.; Lu, X. FTIR and Simultaneous TG/MS/FTIR Study of Late Permian Coals from Southern China. J. Anal. Appl. Pyrolysis 2013, 100, 75–80. [Google Scholar] [CrossRef]
- Sarıkoç, S. Fuels of the Diesel-Gasoline Engines and Their Properties. Diesel Gasol. Engines 2020, 31–45. [Google Scholar] [CrossRef]
- Palčić, A.; Valtchev, V. Analysis and Control of Acid Sites in Zeolites. Appl. Catal. A Gen. 2020, 606, 117795. [Google Scholar] [CrossRef]
- Hideshi, O.; Wintzer, M.E.; Nakamura, R. Non-Zero Binding Enhances Kinetics of Catalysis: Machine Learning Analysis on the Experimental Hydrogen Binding Energy of Platinum. ACS Catal. 2021, 11, 6298–6303. [Google Scholar]
- Niu, J.; Rao, B.K.; Jena, P. Binding of Hydrogen Molecules by a Transition-Metal Ion. Phys. Rev. Lett. 1992, 68, 2277–2280. [Google Scholar] [CrossRef]
- Laursen, A.B.; Varela, A.S.; Dionigi, F.; Fanchiu, H.; Miller, C.; Trinhammer, O.L.; Rossmeisl, J.; Dahl, S. Electrochemical Hydrogen Evolution: Sabatier’s Principle and the Volcano Plot. J. Chem. Educ. 2012, 89, 1595–1599. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, H.; Jin, A.; Kim, S.J.; Kwon, Y.; Lee, E.; Shin, J.J.; Kim, B.H. Conversion of Polyethylene to Low-Molecular-Weight Oil Products at Moderate Temperatures Using Nickel/Zeolite Nanocatalysts. Materials 2024, 17, 1863. https://doi.org/10.3390/ma17081863
Cho H, Jin A, Kim SJ, Kwon Y, Lee E, Shin JJ, Kim BH. Conversion of Polyethylene to Low-Molecular-Weight Oil Products at Moderate Temperatures Using Nickel/Zeolite Nanocatalysts. Materials. 2024; 17(8):1863. https://doi.org/10.3390/ma17081863
Chicago/Turabian StyleCho, Hyungjin, Ahyeon Jin, Sun Ju Kim, Youngmin Kwon, Eunseo Lee, Jaeman J. Shin, and Byung Hyo Kim. 2024. "Conversion of Polyethylene to Low-Molecular-Weight Oil Products at Moderate Temperatures Using Nickel/Zeolite Nanocatalysts" Materials 17, no. 8: 1863. https://doi.org/10.3390/ma17081863





