Heterojunctions of Mercury Selenide Quantum Dots and Halide Perovskites with High Lattice Matching and Their Photodetection Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of HgSe CQD
2.3. Solution-Phase Ligand Exchange
2.4. Device Fabrication
2.5. Characterizations
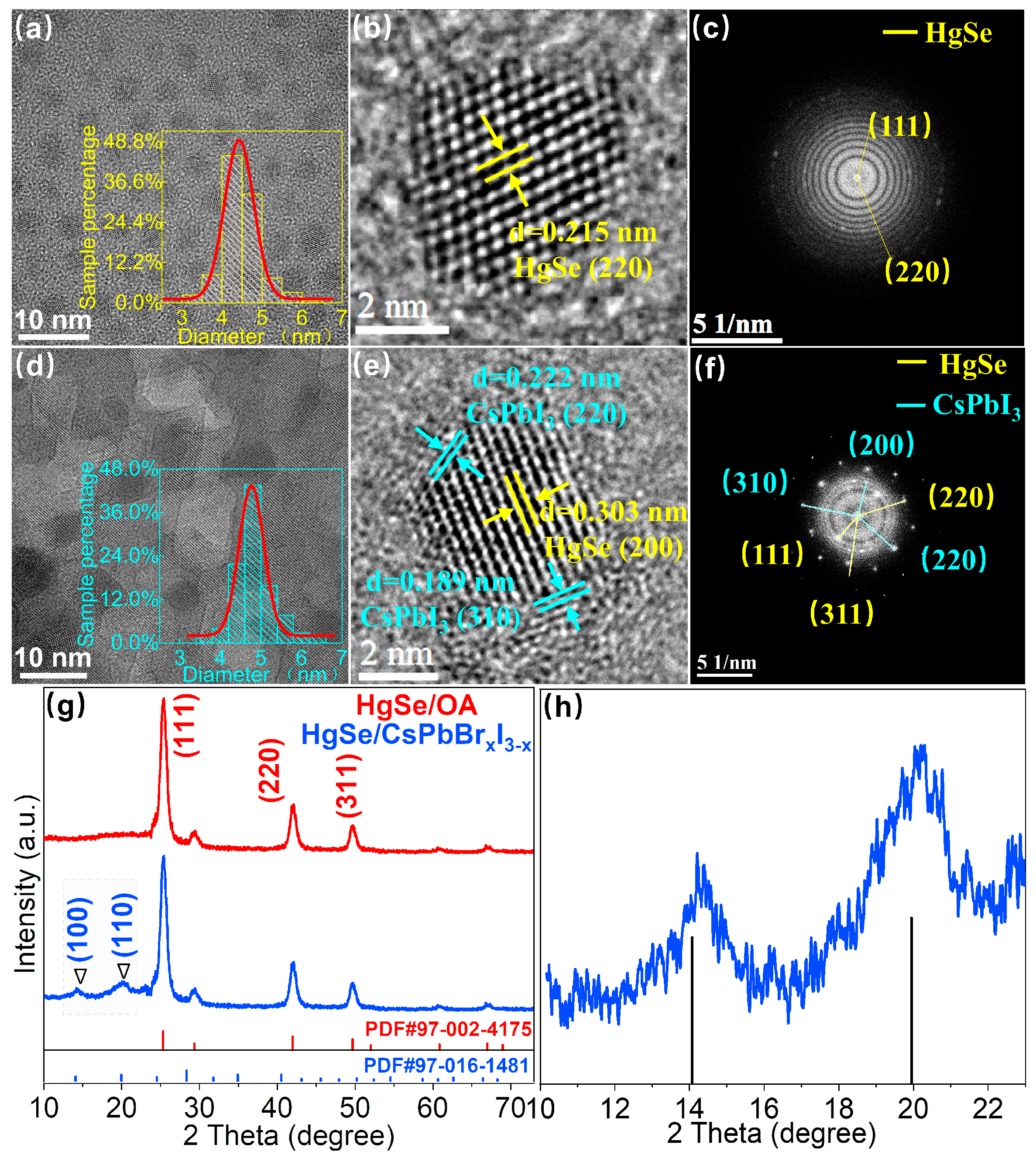
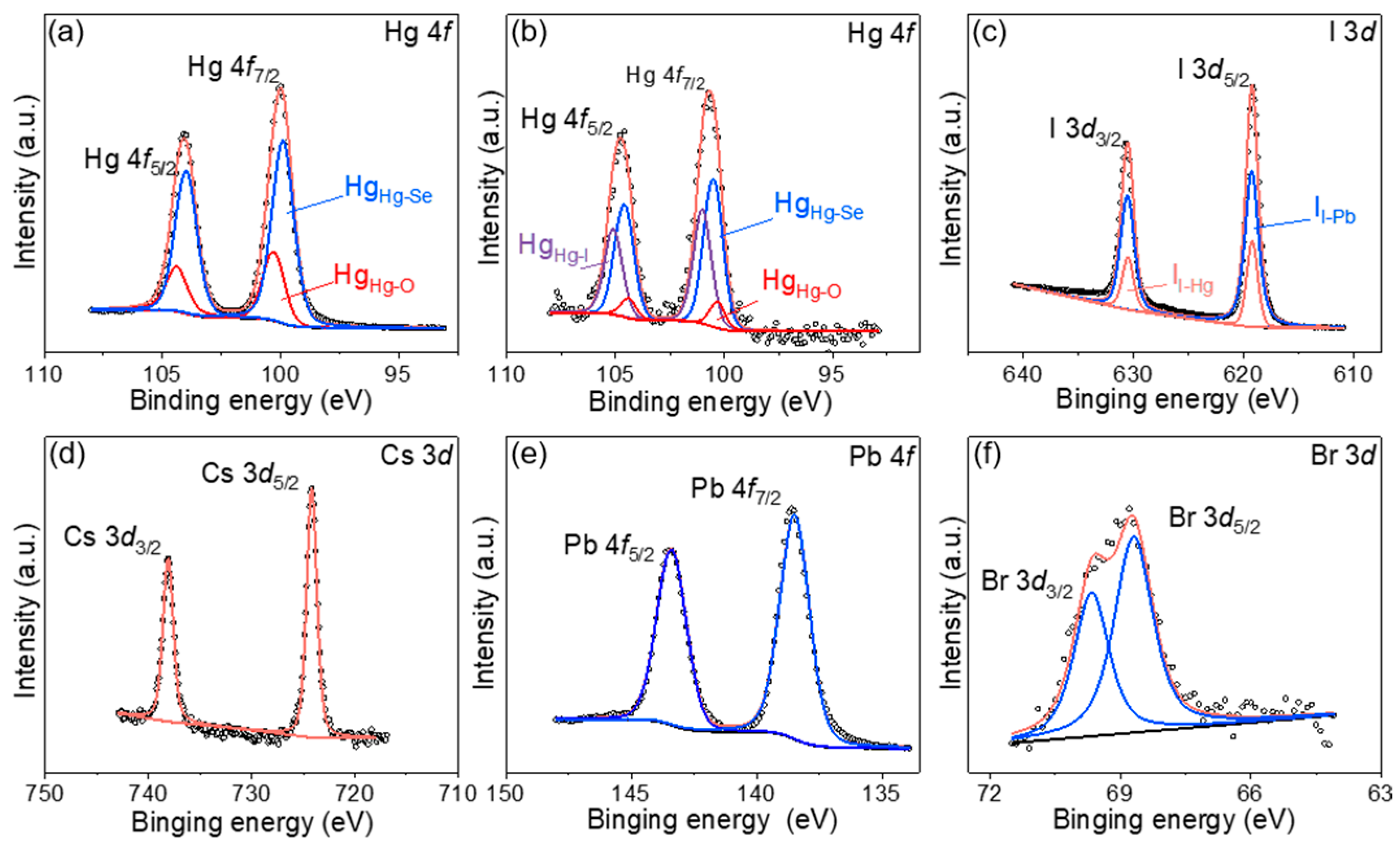
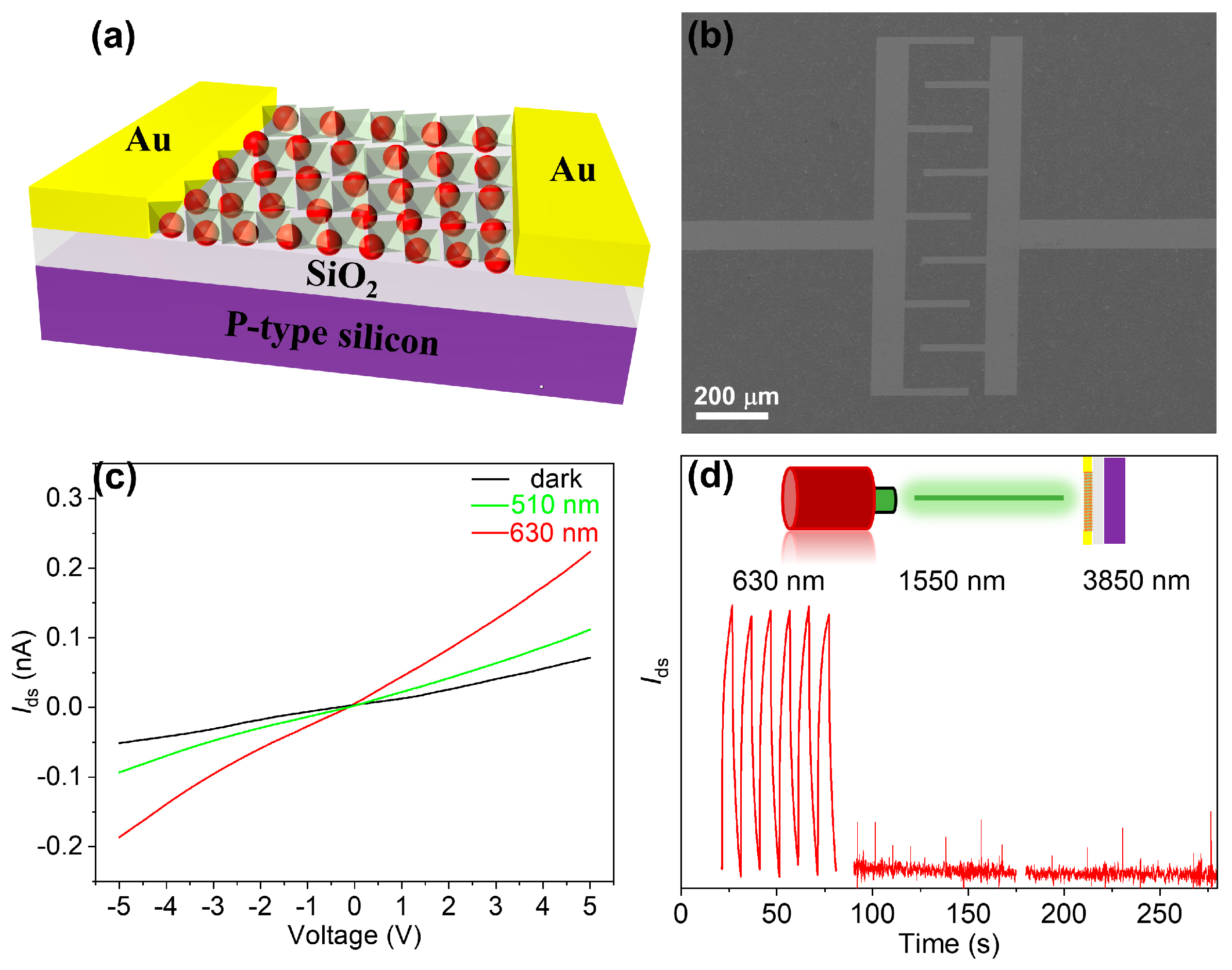
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eisner, F.; Foot, G.; Yan, J.; Azzouzi, M.; Georgiadou, D.G.; Sit, W.Y.; Firdaus, Y.; Zhang, G.; Lin, Y.H.; Yip, H.L. Emissive Charge-Transfer States at Hybrid Inorganic/Organic Heterojunctions Enable Low Non-Radiative Recombination and High-Performance Photodetectors. Adv. Mater. 2022, 34, 2104654. [Google Scholar] [CrossRef]
- Nobile, C.; Cozzoli, P.D. Synthetic Approaches to Colloidal Nanocrystal Heterostructures Based on Metal and Metal-Oxide Materials. Nanomaterials 2022, 12, 1729. [Google Scholar] [CrossRef]
- Qi, H.; Wu, W.; Chen, X. Ferroelectric Resistance Switching in Epitaxial BiFeO3/La0.7Sr0.3MnO3 Heterostructures. Materials 2023, 16, 7198. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, J.; Qi, Y.; Wang, Y.; Long, F.; Wang, M. Preparation of flower-like BiOBr/Bi2WO6 Z-scheme heterojunction through an ion exchange process with enhanced photocatalytic activity. Mater. Sci. Semicond. Process. 2022, 137, 106195. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, M.; Chen, R.; Liu, Q.; Liu, J.; Yu, J.; Zhang, H.; Liu, P.; Lin, C.; Wang, J. Polyurethane coating with heterogeneity structure induced by microphase separation: A new combination of antifouling and cavitation erosion resistance. Prog. Org. Coat. 2021, 151, 106032. [Google Scholar] [CrossRef]
- Li, Y.; Duan, J.; Berencén, Y.; Hübner, R.; Tsai, H.-S.; Kuo, C.-N.; Lue, C.S.; Helm, M.; Zhou, S.; Prucnal, S. Formation of a vertical SnSe/SnSe2 p–n heterojunction by NH3 plasma-induced phase transformation. Nanoscale Adv. 2023, 5, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Pattantyus-Abraham, A.G.; Kramer, I.J.; Barkhouse, A.R.; Wang, X.; Konstantatos, G.; Debnath, R.; Levina, L.; Raabe, I.; Nazeeruddin, M.K.; Grätzel, M.; et al. Depleted-Heterojunction Colloidal Quantum Dot Solar Cells. ACS Nano 2010, 4, 3374–3380. [Google Scholar] [CrossRef]
- Sun, L. Employing ZnS as a capping material for PbS quantum dots and bulk heterojunction solar cells. Sci. China Mater. 2016, 59, 817–824. [Google Scholar] [CrossRef]
- Ezerskyte, E.; Malikenas, M.; Sakirzanovas, S.; Katelnikovas, A.; Klimkevicius, V. Real-time observation of ion exchange dynamics during surface treatment of all-inorganic perovskite quantum dots with Zn-halogenide complexes for color tuning and enhanced quantum efficiency. RSC Adv. 2023, 13, 14370–14378. [Google Scholar] [CrossRef]
- Kagan, C.R.; Lifshitz, E.; Sargent, E.H.; Talapin, D.V. Building devices from colloidal quantum dots. Science 2016, 353, aac5523. [Google Scholar] [CrossRef]
- Green, M.; Wakefield, G.; Dobson, P.J. A simple metalorganic route to organically passivated mercury telluride nanocrystals. J. Mater. Chem. 2003, 13, 1076–1078. [Google Scholar] [CrossRef]
- Piepenbrock, M.-O.M.; Stirner, T.; Kelly, S.M.; O’Neill, M. A Low-Temperature Synthesis for Organically Soluble HgTe Nanocrystals Exhibiting Near-Infrared Photoluminescence and Quantum Confinement. J. Am. Chem. Soc. 2006, 128, 7087–7090. [Google Scholar] [CrossRef] [PubMed]
- Chiu, F.-B.; Wu, Y.-W.; Yang, S.-H. Surface Modification of ZnO Nanocrystals with Conjugated Polyelectrolytes Carrying Different Counterions for Inverted Perovskite Light-Emitting Diodes. ACS Omega 2023, 8, 19109–19118. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Luo, H.; Chen, M.; Li, C.; Kershaw, S.V.; Zhang, R.; Rogach, A.L. Mercury chalcogenide colloidal quantum dots for infrared photodetection: From synthesis to device applications. Nanoscale 2023, 15, 6476–6504. [Google Scholar] [CrossRef] [PubMed]
- Schwanninger, R.; Koepfli, S.M.; Yarema, O.; Dorodnyy, A.; Yarema, M.; Moser, A.; Nashashibi, S.; Fedoryshyn, Y.; Wood, V.; Leuthold, J. Highly Responsive Mid-Infrared Metamaterial Enhanced Heterostructure Photodetector Formed out of Sintered PbSe/PbS Colloidal Quantum Dots. ACS Appl. Mater. Interfaces 2023, 15, 10847–10857. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, M.; Ma, T.; Yan, J.; Khalaf, G.M.G.; Chen, C.; Hsu, H.-Y.; Song, H.; Tang, J. Stable PbS colloidal quantum dot inks enable blade-coating infrared solar cells. Front. Optoelectron. 2023, 16, 27. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Voznyy, O.; Sabatini, R.; García de Arquer, F.P.; Munir, R.; Balawi, A.H.; Lan, X.; Fan, F.; Walters, G.; Kirmani, A.R.; et al. Hybrid organic–inorganic inks flatten the energy landscape in colloidal quantum dot solids. Nat. Mater. 2017, 16, 258–263. [Google Scholar] [CrossRef]
- Ganeev, R.A.; Shuklov, I.A.; Zvyagin, A.I.; Dyomkin, D.V.; Smirnov, M.S.; Ovchinnikov, O.V.; Lizunova, A.A.; Perepukhov, A.M.; Popov, V.S.; Razumov, V.F. Synthesis and low-order optical nonlinearities of colloidal HgSe quantum dots in the visible and near infrared ranges. Opt. Express 2021, 29, 16710–16726. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Janmohamed, A.; Lan, X.; García de Arquer, F.P.; Voznyy, O.; Yassitepe, E.; Kim, G.-H.; Ning, Z.; Gong, X.; Comin, R.; et al. Colloidal Quantum Dot Photovoltaics Enhanced by Perovskite Shelling. Nano Lett. 2015, 15, 7539–7543. [Google Scholar] [CrossRef]
- Damasceno, E., Jr.; Barbosa, R.d.M.; da Silva, R.d.C.D.; Costa, F.d.S.; da Silva, D.R.; Viseras, C.; Perioli, L.; Fernandes, N.S. Montmorillonite–Rifampicin Nanohybrid for pH-Responsive Release of the Tuberculostatic. Pharmaceutics 2023, 15, 512. [Google Scholar] [CrossRef]
- Wang, Q.; Zheng, X.; Deng, Y.; Zhao, J.; Chen, Z.; Huang, J. Stabilizing the α-Phase of CsPbI3 Perovskite by Sulfobetaine Zwitterions in One-Step Spin-Coating Films. Joule 2017, 1, 371–382. [Google Scholar] [CrossRef]
- Gutiérrez, I.S.; Morales, V.H.; Muñoz, E.M.R.; Mendoza, R.N.; Soto, L.L.; Ledesma, C.L.P.; Casados, D.S.; Pawelec, B. Efficient Removal of Hg(II) from Water under Mildly Acidic Conditions with Hierarchical SiO2 Monoliths Functionalized with −SH Groups. Materials 2022, 15, 1580. [Google Scholar] [CrossRef] [PubMed]
- Raj, S.I.; Jaiswal, A.; Uddin, I. Ultrasmall aqueous starch-capped CuS quantum dots with tunable localized surface plasmon resonance and composition for the selective and sensitive detection of mercury(ii) ions. RSC Adv. 2020, 10, 14050–14059. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Pang, H.; Liu, X.; Li, Q.; Zhang, N.; Mao, L.; Qiu, M.; Hu, B.; Yang, H.; Wang, X. Orderly Porous Covalent Organic Frameworks-based Materials: Superior Adsorbents for Pollutants Removal from Aqueous Solutions. Innovation 2021, 2, 100076. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, M.R.A.; Elseman, A.M.; Abdelmageed, A.A.; Hashem, H.M.; Hassen, A. Synthesis and numerical simulation of formamidinium-based perovskite solar cells: A predictable device performance at NIS-Egypt. Sci. Rep. 2023, 13, 10115. [Google Scholar] [CrossRef] [PubMed]
- Judd, C.J.; Junqueira, F.L.Q.; Haddow, S.L.; Champness, N.R.; Duncan, D.A.; Jones, R.G.; Saywell, A. Structural characterisation of molecular conformation and the incorporation of adatoms in an on-surface Ullmann-type reaction. Commun. Chem. 2020, 3, 166. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Z.; Zhang, X.; Li, X.; Liu, Z.; Liao, G.; Shen, Y.; Wang, M. Achieving High Responsivity and Detectivity in a Quantum-Dot-in-Perovskite Photodetector. Nano Lett. 2023, 23, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhang, X.; Bai, X.; Wu, H.; Shen, X.; Zhang, Y.; Zhang, W.; Zheng, W.; Song, H.; Yu, W.W.; et al. Spontaneous Silver Doping and Surface Passivation of CsPbI3 Perovskite Active Layer Enable Light-Emitting Devices with an External Quantum Efficiency of 11.2%. ACS Energy Lett. 2018, 3, 1571–1577. [Google Scholar] [CrossRef]
- Yandri, V.R.; Nurunnizar, A.A.; Debora, R.; Wulandari, P.; Nursam, N.M.; Hidayat, R.; Indari, E.D.; Yamashita, Y. Crystal structures and photoluminescence characteristics of cesium lead bromide perovskite nanoplatelets depending on the antisolvent and ligand used in their syntheses. Heliyon 2024, 10, e23276. [Google Scholar] [CrossRef]
- Zhou, W.; Zheng, L.; Ning, Z.; Cheng, X.; Wang, F.; Xu, K.; Xu, R.; Liu, Z.; Luo, M.; Hu, W.; et al. Silicon: Quantum dot photovoltage triodes. Nat. Commun. 2021, 12, 6696. [Google Scholar] [CrossRef]
- Hao, Q.; Zhao, X.; Tang, X.; Chen, M. The Historical Development of Infrared Photodetection Based on Intraband Transitions. Materials 2023, 16, 1562. [Google Scholar] [CrossRef] [PubMed]
- Livache, C.; Martinez, B.; Goubet, N.; Gréboval, C.; Qu, J.; Chu, A.; Royer, S.; Ithurria, S.; Silly, M.G.; Dubertret, B.; et al. A colloidal quantum dot infrared photodetector and its use for intraband detection. Nat. Commun. 2019, 10, 2125. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Zhang, Z.; Ge, J.; Deng, Y.; Chen, X.; Zhang, J.-R.; Deng, Z.; Kambe, Y.; Talapin, D.V.; Wang, Y. Surface passivation of intensely luminescent all-inorganic nanocrystals and their direct optical patterning. Nat. Commun. 2023, 14, 49. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhang, D.; Du, Q.; Hong, W.; Liang, Y.; Duan, X.; Feng, S.; Lan, L.; Wang, L.; Chen, J.; et al. Synergistic Halide- and Ligand-Exchanges of All-Inorganic Perovskite Nanocrystals for Near-Unity and Spectrally Stable Red Emission. Nanomaterials 2023, 13, 2337. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Chen, H.; Xu, Y.; Lei, Y.; Peng, G.; Zhou, X.; Wang, Q.; Jin, Z. Double-Layer Quantum Dots as Interfacial Layer to Enhance the Performance of CsPbI3 Solar Cells. Adv. Mater. Interfaces 2022, 9, 2200813. [Google Scholar] [CrossRef]
- Liu, M.; Chen, Y.; Tan, C.-S.; Quintero-Bermudez, R.; Proppe, A.H.; Munir, R.; Tan, H.; Voznyy, O.; Scheffel, B.; Walters, G.; et al. Lattice anchoring stabilizes solution-processed semiconductors. Nature 2019, 570, 96–101. [Google Scholar] [CrossRef]
- Sanchez, R.S.; de la Fuente, M.S.; Suarez, I.; Muñoz-Matutano, G.; Martinez-Pastor, J.P.; Mora-Sero, I. Tunable light emission by exciplex state formation between hybrid halide perovskite and core/shell quantum dots: Implications in advanced LEDs and photovoltaics. Sci. Adv. 2016, 2, e1501104. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Hao, Q.; Luo, Y.; Tang, X. Mid-Infrared Intraband Photodetector via High Carrier Mobility HgSe Colloidal Quantum Dots. ACS Nano 2022, 16, 11027–11035. [Google Scholar] [CrossRef]
- Kamath, A.; Schaller, R.D.; Guyot-Sionnest, P. Bright Fluorophores in the Second Near-Infrared Window: HgSe/CdSe Quantum Dots. J. Am. Chem. Soc. 2023, 145, 10809–10816. [Google Scholar] [CrossRef]
- Chen, H.; Pina, J.M.; Hou, Y.; Sargent, E.H. Synthesis, Applications, and Prospects of Quantum-Dot-in-Perovskite Solids. Adv. Energy Mater. 2022, 12, 2100774. [Google Scholar] [CrossRef]



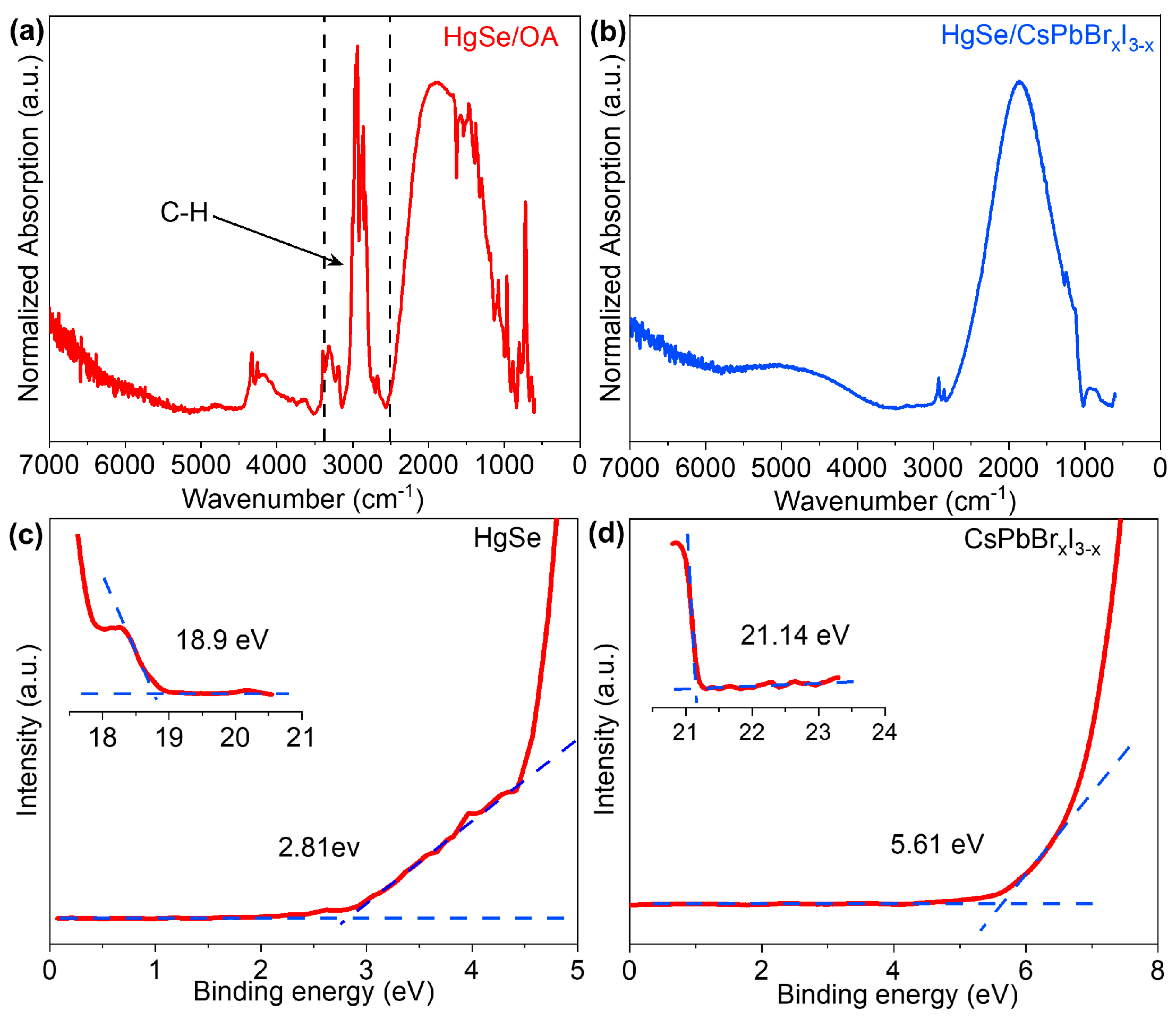

| Samples | Band Gap (eV) | Work Function (eV) | VBM (eV) | CBM (eV) |
|---|---|---|---|---|
| HgSe CQDs | 0.74 | −2.3 | −5.12 | −4.31 |
| CsPbBrxI3−x | 1.68 | −0.06 | −5.67 | −3.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.; Shan, Y.; Zhu, J.; Sun, D.; Zheng, X.; Zhang, N.; Hou, J.; Fang, Y.; Dai, N.; Liu, Y. Heterojunctions of Mercury Selenide Quantum Dots and Halide Perovskites with High Lattice Matching and Their Photodetection Properties. Materials 2024, 17, 1864. https://doi.org/10.3390/ma17081864
Yu C, Shan Y, Zhu J, Sun D, Zheng X, Zhang N, Hou J, Fang Y, Dai N, Liu Y. Heterojunctions of Mercury Selenide Quantum Dots and Halide Perovskites with High Lattice Matching and Their Photodetection Properties. Materials. 2024; 17(8):1864. https://doi.org/10.3390/ma17081864
Chicago/Turabian StyleYu, Chengye, Yufeng Shan, Jiaqi Zhu, Dingyue Sun, Xiaohong Zheng, Na Zhang, Jingshan Hou, Yongzheng Fang, Ning Dai, and Yufeng Liu. 2024. "Heterojunctions of Mercury Selenide Quantum Dots and Halide Perovskites with High Lattice Matching and Their Photodetection Properties" Materials 17, no. 8: 1864. https://doi.org/10.3390/ma17081864





