Simulation and Study of Influencing Factors on the Solidification Microstructure of Hazelett Continuous Casting Slabs Using CAFE Model
Abstract
:1. Introduction
2. Establishing the Calculation Model
2.1. Establishing the CAFE Heat Transfer and Micro-Kinetic Model
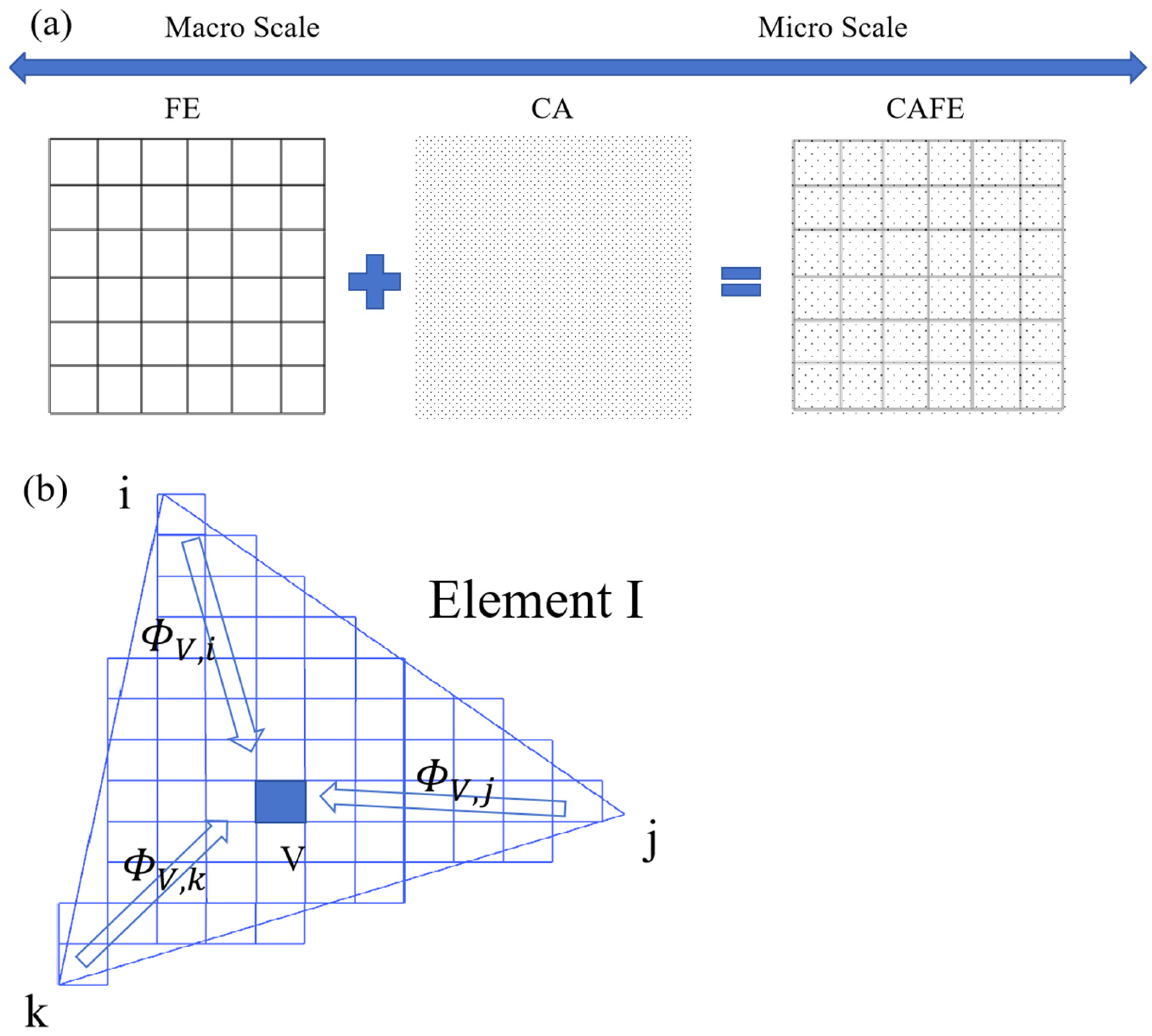
2.2. Coupled CA and FE Calculation Model
3. Constructing a Finite Element Model and Selecting Parameters
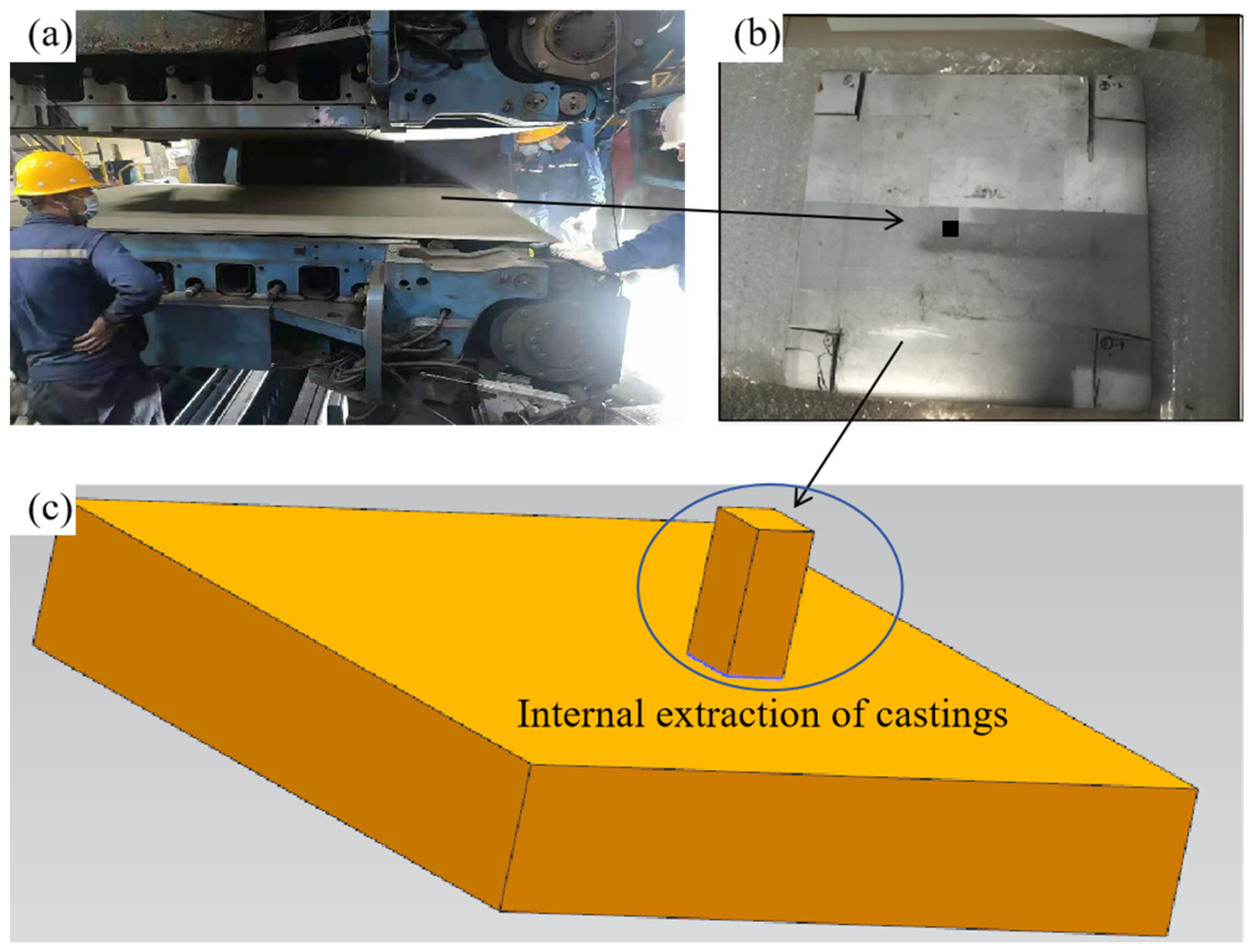
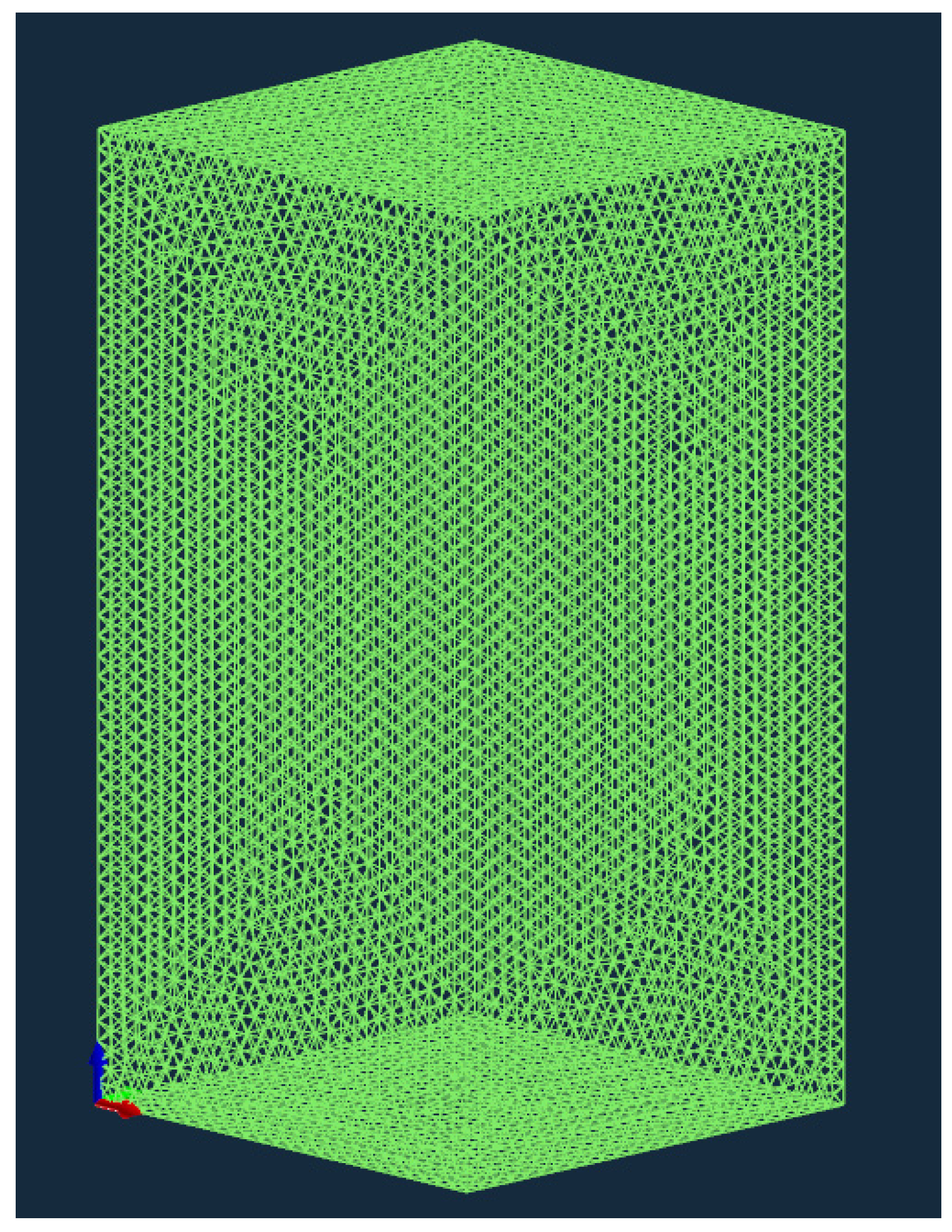
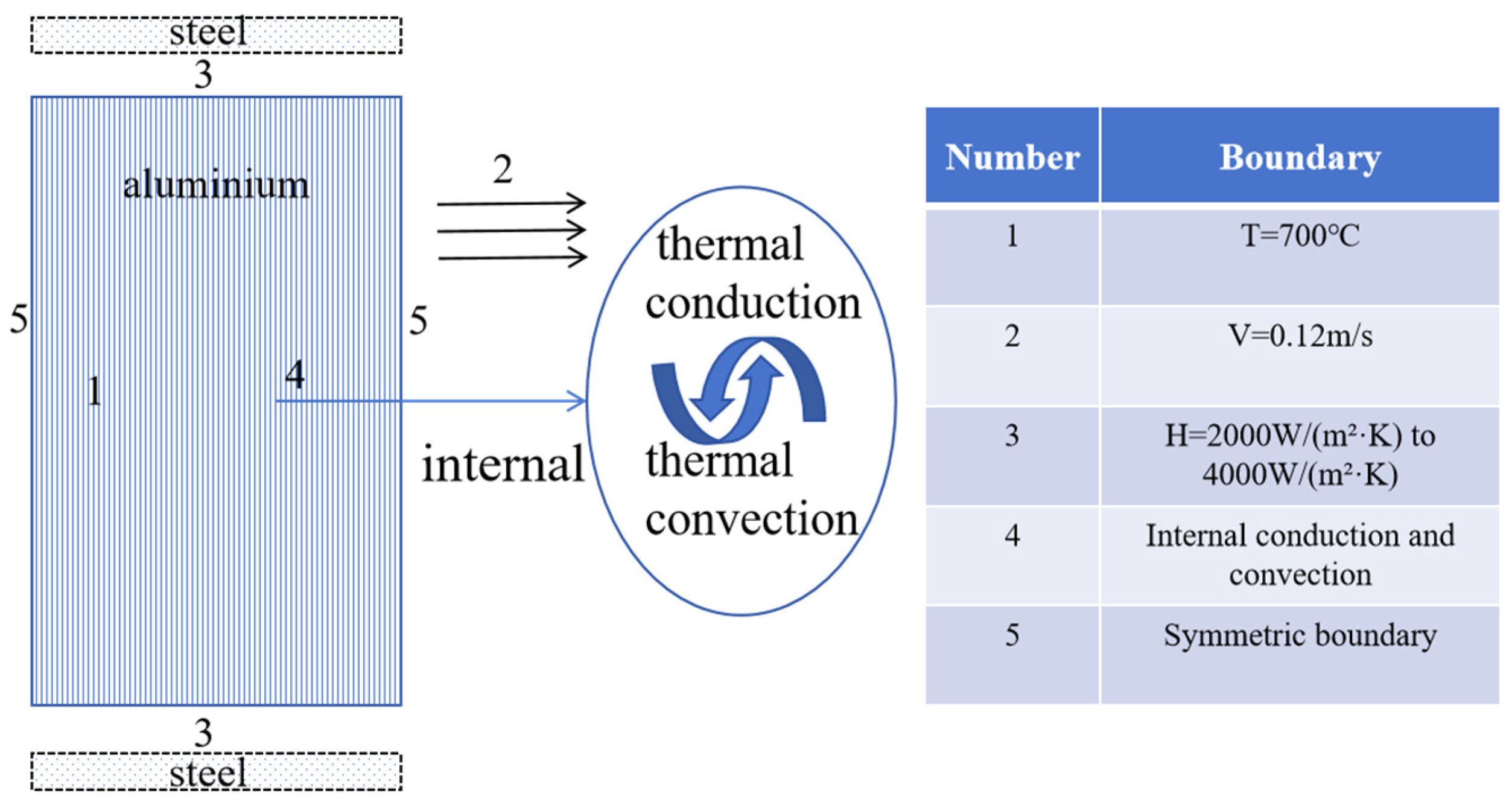
3.1. Construction of Finite Element Model
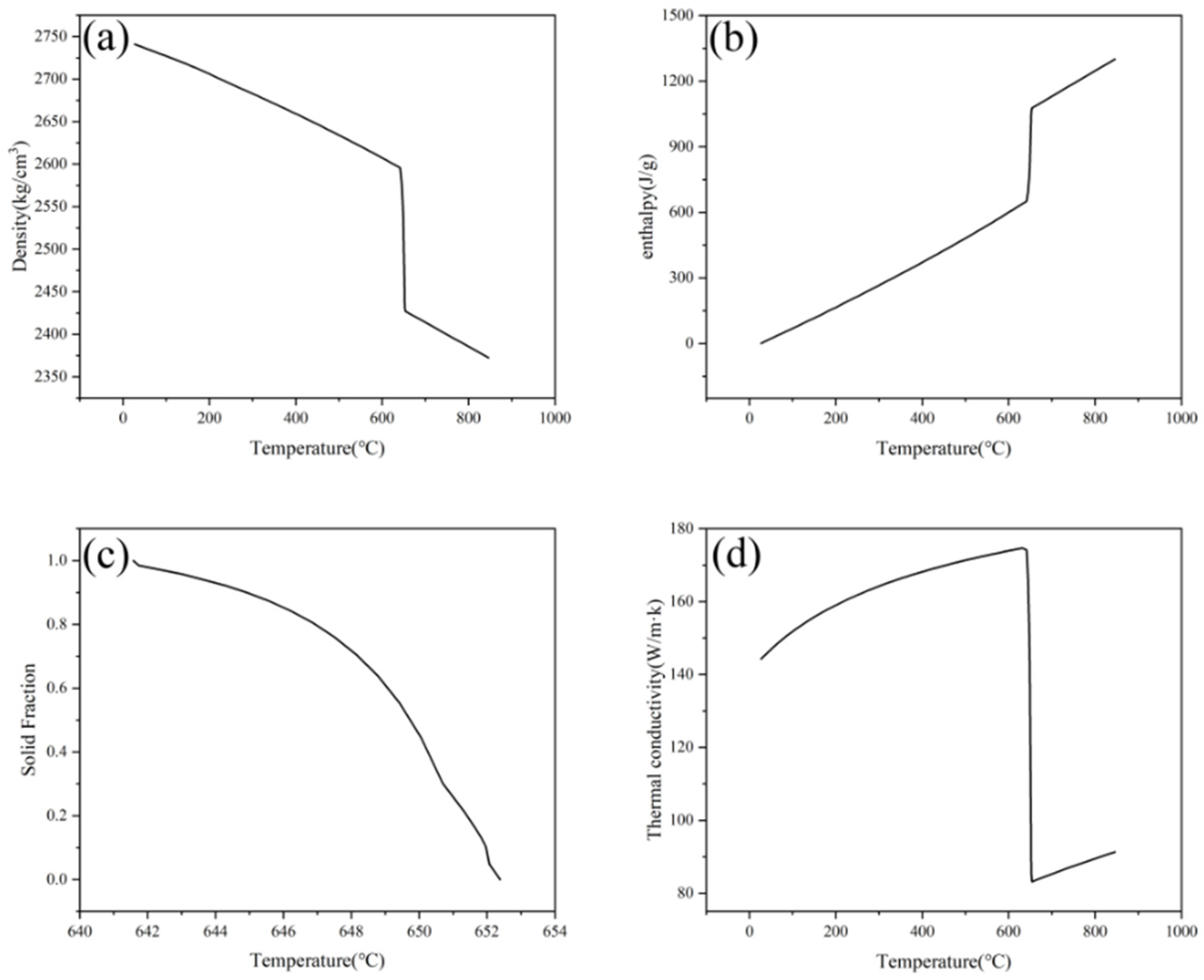
3.2. Selecting Physical Parameters and Establishing Initial and Boundary Conditions for the Solidification Model
4. Determination of Nucleation Parameters and Their Effects on the Solidification Structure
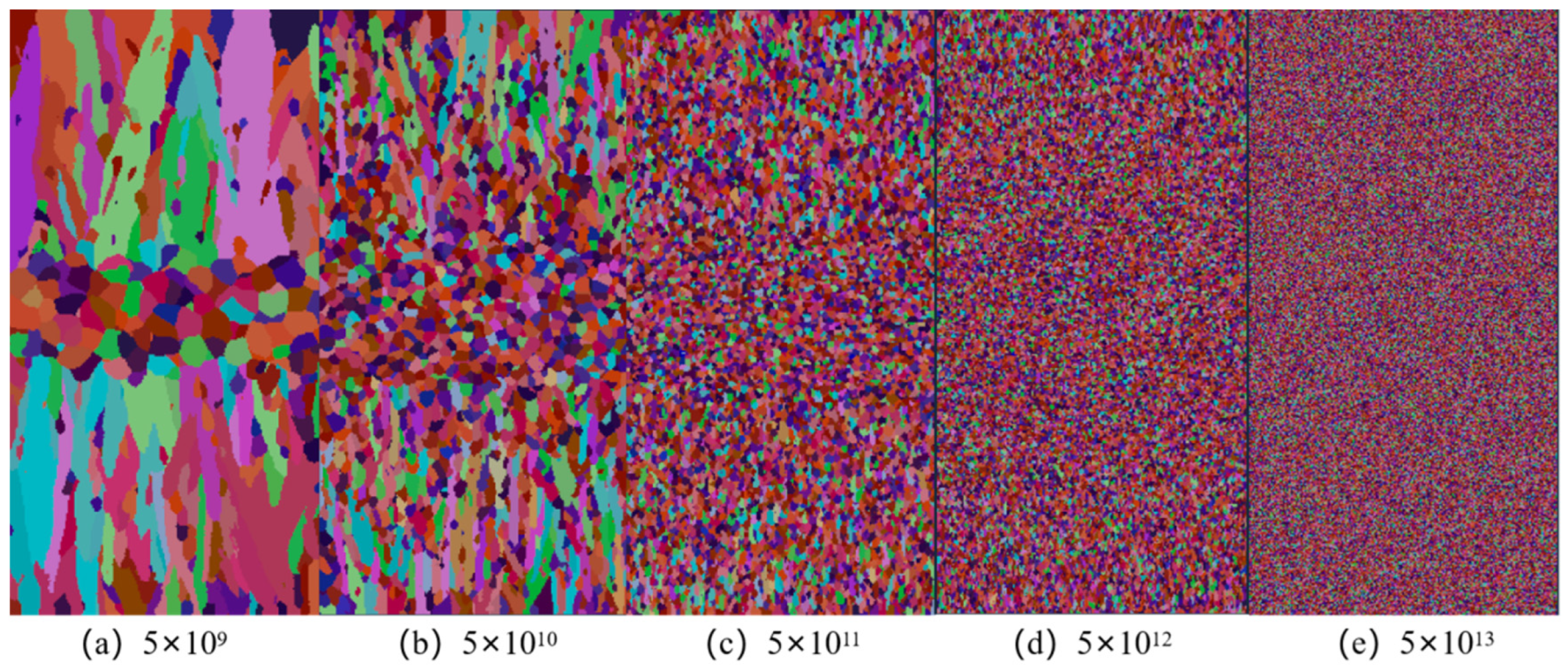
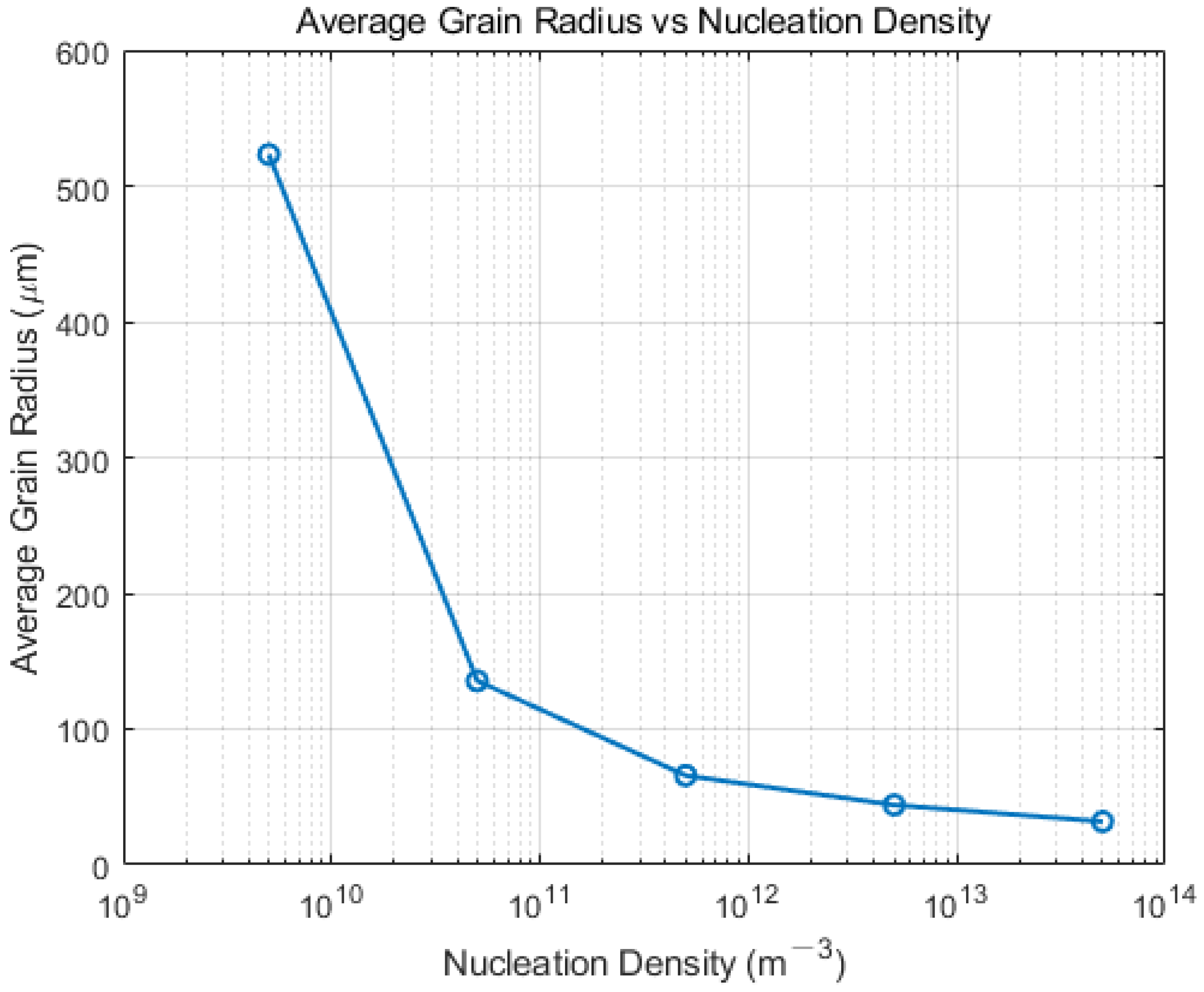
4.1. Effect of Nucleation Density on Solidification Structure
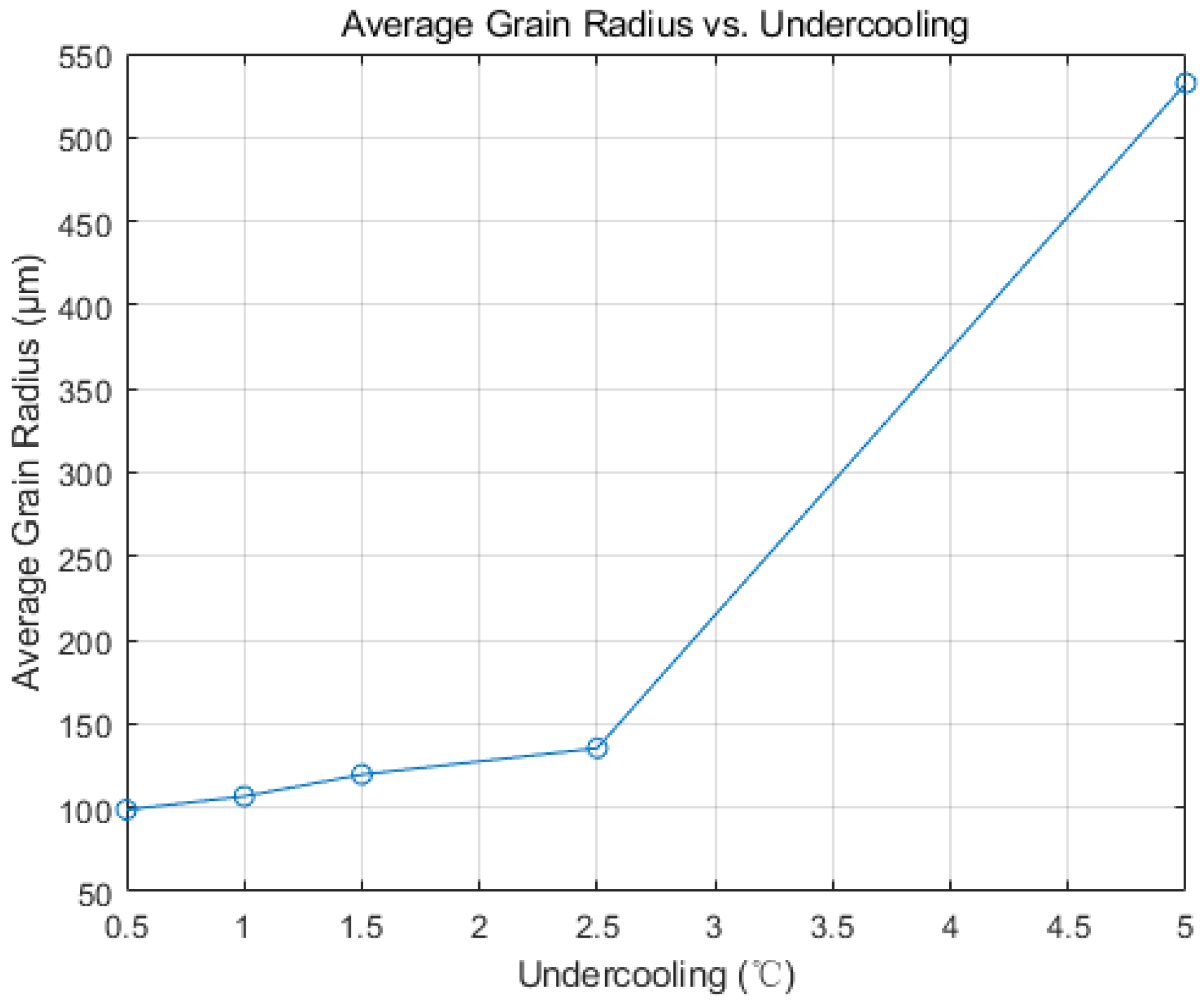
4.2. Effect of Undercooling Rate on Solidification Structure
4.3. The General Law Governing the Influence of Nucleation Parameters on Solidification Structure
5. Analyzing and Verifying CAFE Simulations
5.1. Experimental Verification
5.2. Effect of Process Parameters on Solidification Structure
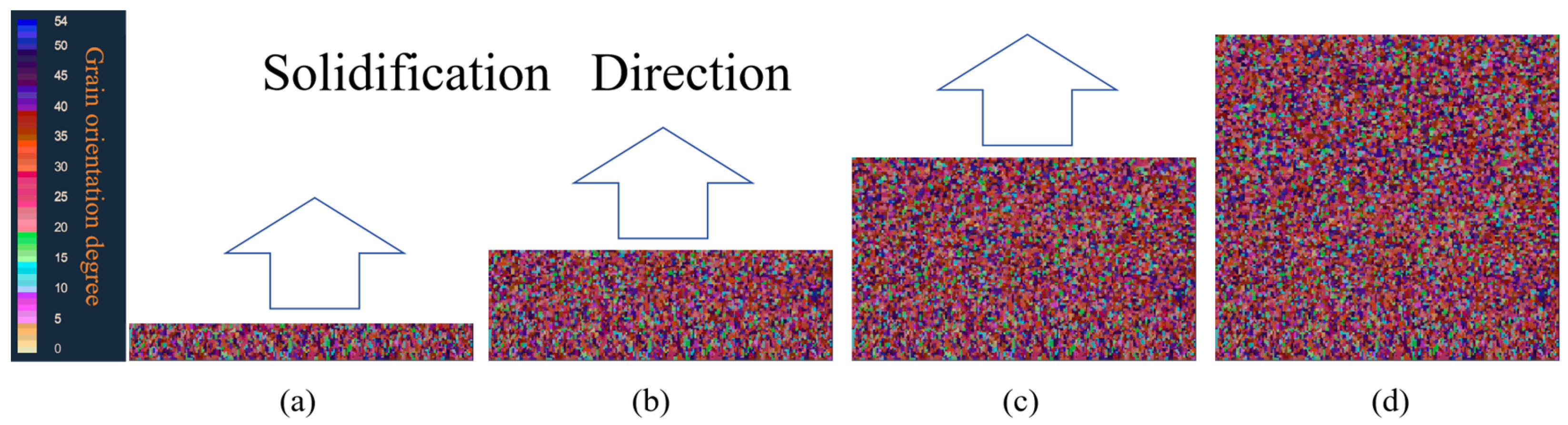
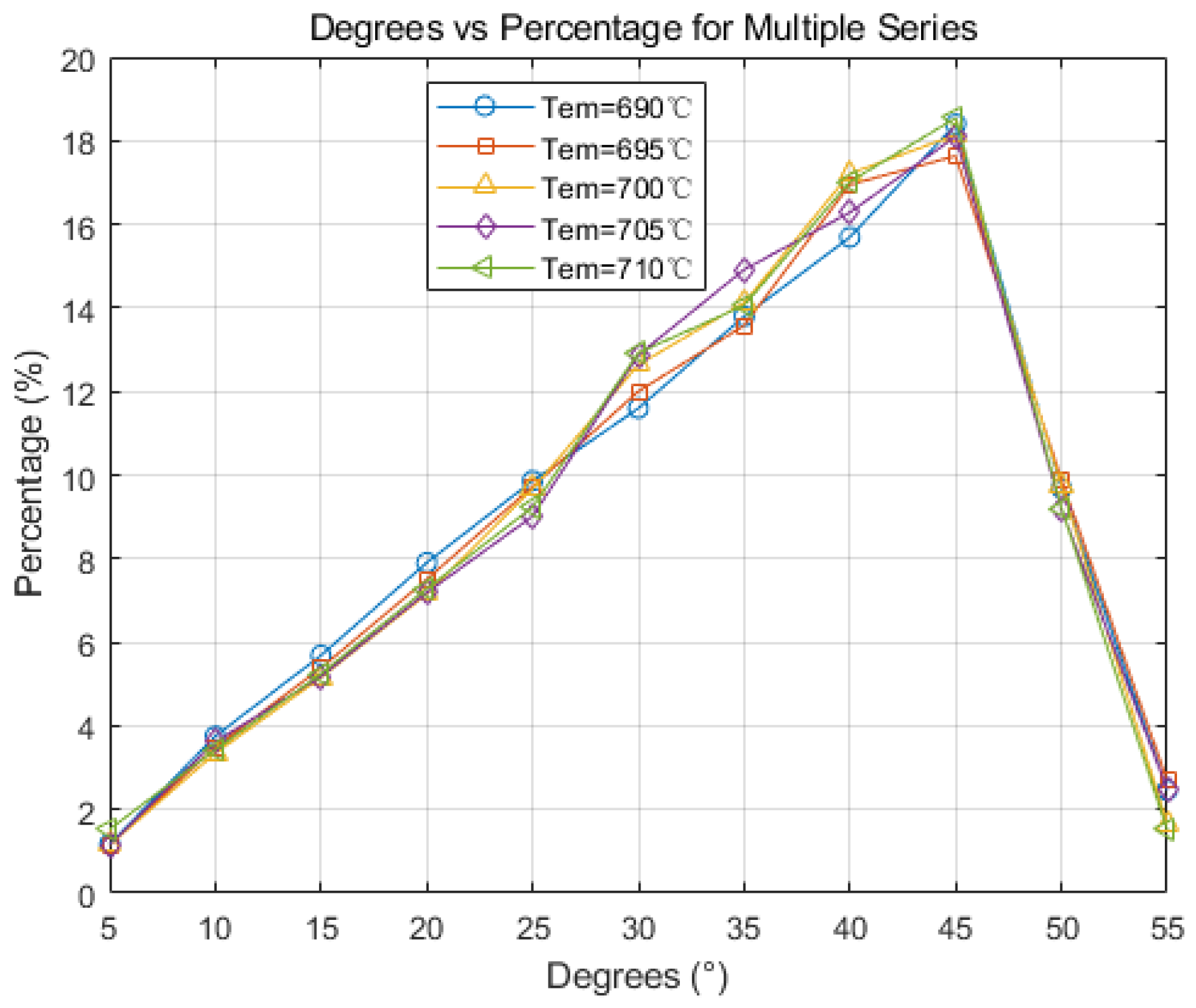
5.2.1. Effect of Initial Temperature on Solidification Structure
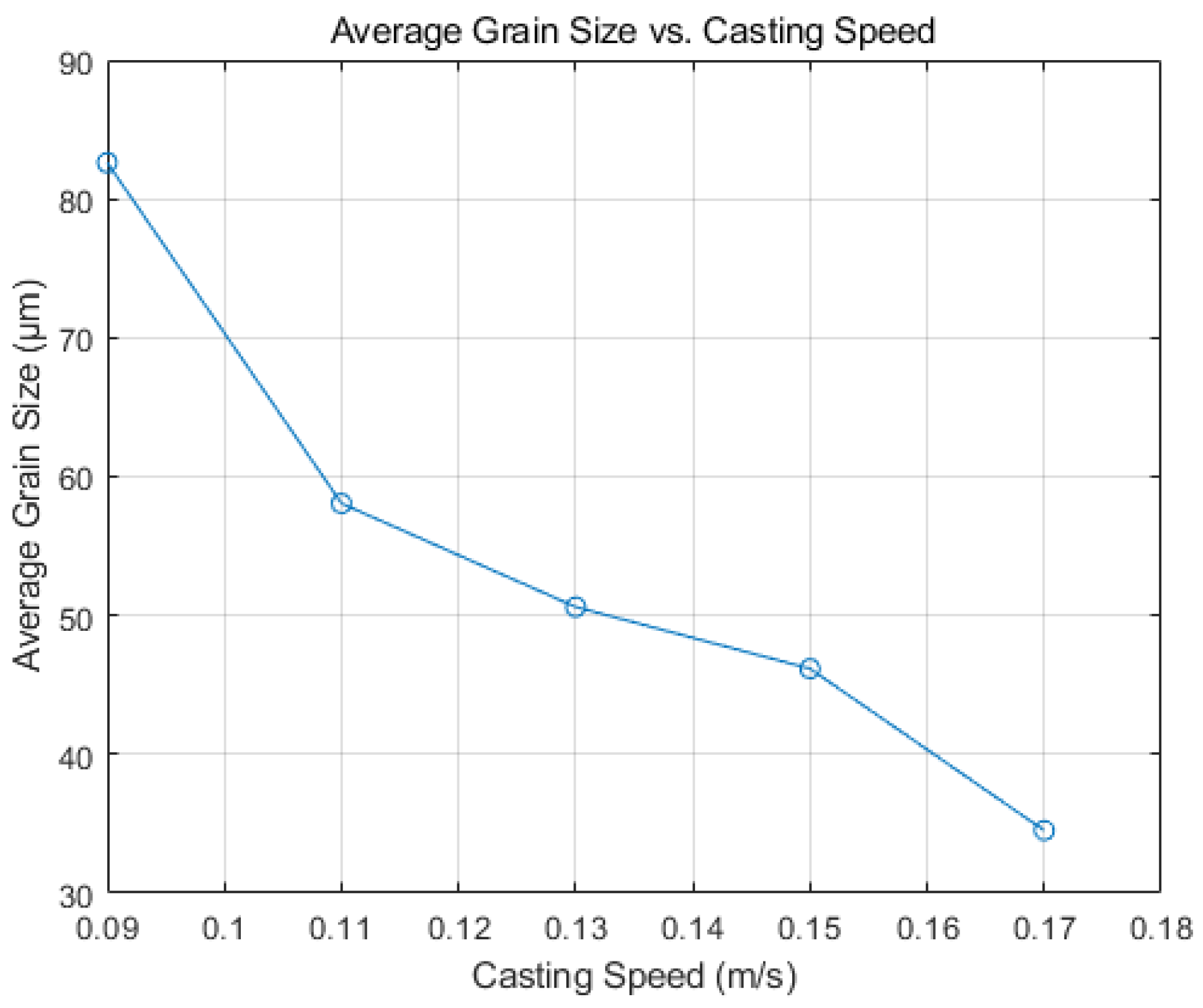
5.2.2. Effect of Continuous Casting on Solidification Structure
6. Conclusions
- (1)
- Based on the CAFE methodology, a comprehensive computational model for the solidification structure of the Hazelett continuous casting process was established. Simulation analysis demonstrated that adjusting the cooling rate and controlling the nucleation rate, particularly when increasing nucleation density, resulted in the slab’s grain structure manifesting a distinct equiaxed morphology, with an increase in average grain size. Observed trends were consistent across differing levels of undercooling. Metallographic analysis determined the nucleation density of the Hazelett continuous casting aluminum alloy to span between 1011 m−3 and 1013 m−3, with undercooling ranging from 1 °C to 2.5 °C. Within these nucleation parameters, the grain sizes were observed between 35 μm and 72 μm, and were primarily characterized by equiaxed grains.
- (2)
- The initial pouring temperature significantly impacts the solidification structure of the continuous casting billet. With an increase in the initial continuous casting temperature, the grain size correspondingly enlarges. Equiaxed crystal sizes incrementally expand from 57 μm to 61.3 μm. An increase in pouring temperature leads to a higher proportion of high-angle grain boundaries, while the proportion of low-angle grain boundaries remains relatively unchanged. Higher casting temperatures diminish dendrite spacing, inhibiting the swift expansion of columnar crystals, subsequently leading to their melting and transformation into equiaxed crystals.
- (3)
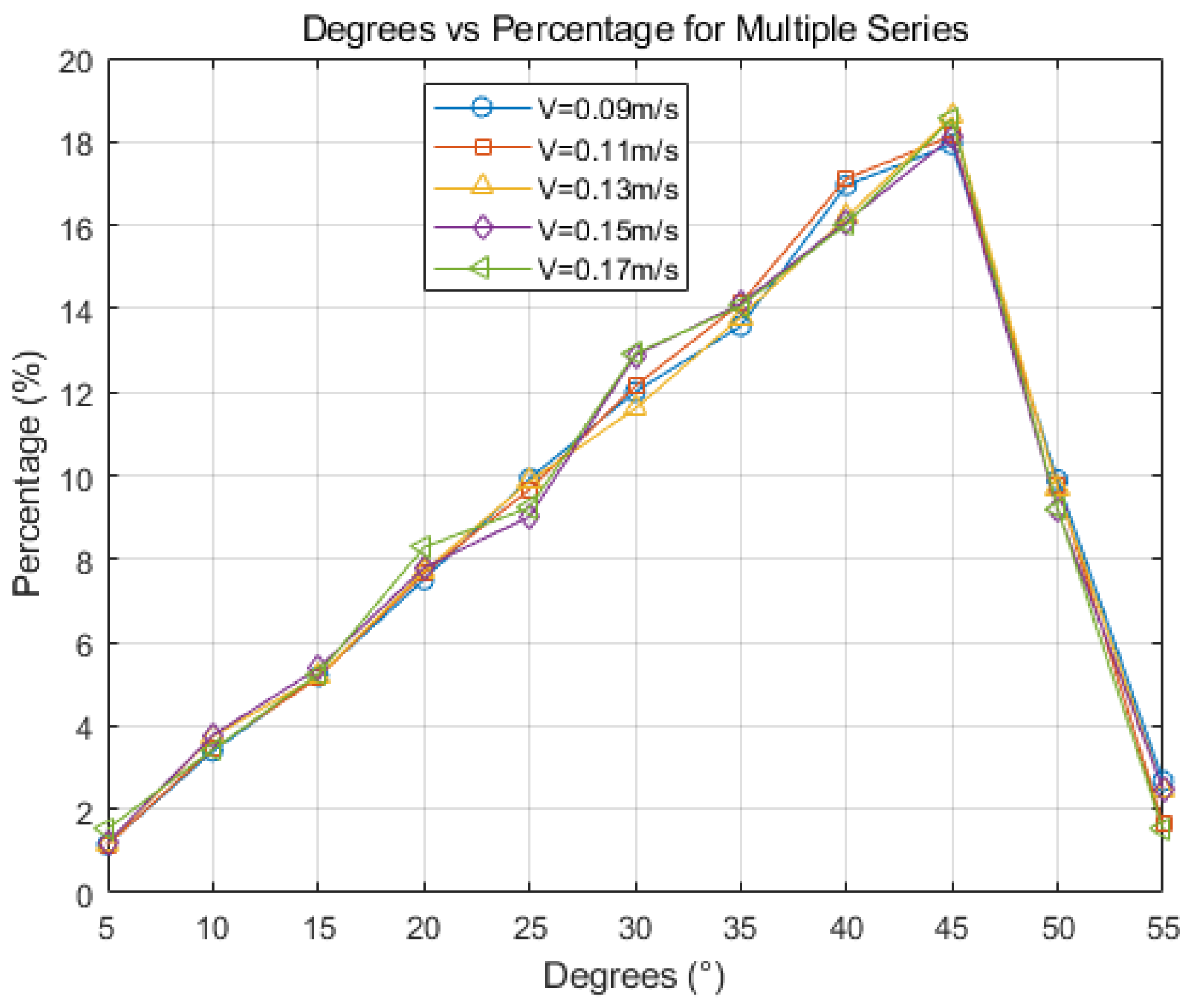
- Increasing casting speed results in a larger equiaxed grain area within the slab, and the grain size decreases accordingly, from 83 μm to 35 μm, and minimal changes in grain boundary orientation are observed. In actual production, changes in the casting speed of the continuous casting machine are harmonized with corresponding cooling conditions. The optimal range of adjustments suggests that the actual casting speed has a minimal influence on the solidification structure. The simulation results suggest that a Hazelett continuous casting speed ranging from 0.11 m/s to 0.13 m/s is optimal.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salvador, M.D.; Amigó, V.; Reig, L.; Bloem, C.; Carsi, M.; Caruana, G. Mechanical and microstructural evolution of a 3xxx aluminium alloy made by hazelett process. Rev. Metal. 2007, 43, 424–433. [Google Scholar] [CrossRef]
- Sanders, R.E. Continuous Casting for Aluminum Sheet: A Product Perspective. JOM 2012, 64, 291–301. [Google Scholar] [CrossRef]
- Tian, N.; Zhang, G.F.; Yan, P.; Li, P.C.; Feng, Z.H.; Wang, X.L. Simulation and Experimental Study on the Effect of Superheat on Solidification Microstructure Evolution of Billet in Continuous Casting. Materials 2024, 17, 682. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, Y.F.; Zhang, H.B.; Yin, Z.H.; Liu, H.N.; Li, R.X.; Li, L.J.; Xu, Z.S.; Zhong, H.G.; Zhai, Q.J. Optimization of Process Parameters for 40Cr Steel Continuous Casting Round Bloom with Pulsed Magneto-Oscillation Treatment. Steel Res. Int. 2024, 95, 2300660. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, J.; Guo, F.; Zhou, C. Flow field simulation of the thin metal ribbon formed by twin-belt rapid solidification. J. Univ. Sci. Technol. Beijing Miner. Metall. Mater. 2008, 15, 155–160. [Google Scholar] [CrossRef]
- Chen, B. Numerical Simulation of Solidification and Heat Transfer of Continuous Casting Aluminum Alloy Strip in Hazelett. Master’s Thesis, Zhengzhou University, Zhengzhou, China, 2011. [Google Scholar]
- Penumakala, P.K.; Nallathambi, A.K.; Specht, E.; Urlau, U.; Hamilton, D.; Dykes, C. Influence of process parameters on solidification length of twin-belt continuous casting. Appl. Therm. Eng. 2018, 134, 275–286. [Google Scholar] [CrossRef]
- Noii, N.; Khodadadian, A.; Ulloa, J.; Aldakheel, F.; Wick, T.; François, S.; Wriggers, P. Bayesian Inversion with Open-Source Codes for Various One-Dimensional Model Problems in Computational Mechanics. Arch. Comput. Methods Eng. 2022, 29, 4285–4318. [Google Scholar] [CrossRef]
- Khodadadian, A.; Noii, N.; Parvizi, M.; Abbaszadeh, M.; Wick, T.; Heitzinger, C. A Bayesian estimation method for variational phase-field fracture problems. Comput. Mech. 2020, 66, 827–849. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhang, D.; Pan, F.; Yang, M. Thermal-stress simulation of direct-chill casting of AZ31 magnesium alloy billets. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2009, 24, 376–382. [Google Scholar] [CrossRef]
- Rappaz, M.G.; Gandin, C.A. Probabilistic Modelling of Microstructure Formation in Solidification Processes. Acta Metall. Mater. 1993, 41, 345–360. [Google Scholar] [CrossRef]
- Bazzaro, G.; De Bona, F. Effectiveness of Travelling Slice Modeling in Representing the Continuous Casting Process of Large Product Sections. Metals 2023, 13, 1505. [Google Scholar] [CrossRef]
- Andersson, C.; Lundbäck, A. Modeling the Evolution of Grain Texture during Solidification of Laser-Based Powder Bed Fusion Manufactured Alloy 625 Using a Cellular Automata Finite Element Model. Metals 2023, 13, 1846. [Google Scholar] [CrossRef]
- Yang, W.; He, P.; Chang, L.; Li, T.; Bai, X.; Luo, Z.; Zhao, N.; Liu, Q. Numerical Analysis of Slag–Steel–Air Four-Phase Flow in Steel Continuous Casting Model Using CFD-DBM-VOF Model. Metals 2023, 13, 1943. [Google Scholar] [CrossRef]
- Wang, S.; Ma, M.; Wei, Z.; Liu, J. The effect of cold rolling process on the microstructure and properties of cast rolled 3003 aluminum alloy. Nonferrous Met. Process. 2022, 51, 17–23+43. [Google Scholar]
- Chen, Z.; Li, Y.; Zhao, F.; Li, S.; Zhang, J. Progress in numerical simulation of casting process. Meas. Control 2022, 55, 257–264. [Google Scholar] [CrossRef]
- Sadegh, M.M.; Vasily, P. Implementation of nucleation in cellular automaton simulation of microstructural evolution during additive manufacturing of Al alloys. Addit. Manuf. 2020, 36, 101726. [Google Scholar]
- Pan, D.; Zhong, H.; Guo, Q.; Li, Y.; Xiao, Y.; Zhang, K. Research on solidification behavior of a high Mn steel during continuous casting based on solidification characteristic unit analysis. Mater. Lett. 2022, 327, 133028. [Google Scholar] [CrossRef]
- Chen, S.-D.; Chen, J.-C. Simulation of microstructures in solidification of aluminum twin-roll casting. Trans. Nonferrous Met. Soc. China 2012, 22, 1452–1456. [Google Scholar] [CrossRef]
- Lorbiecka, A.Z.; Vertnik, R.; Gjerkeš, H.; Manojlovic, G.; Sencic, B.; Cesar, J.; Šarler, B. Numerical Modeling of Grain Structure in Continuous Casting of Steel. CMC Comput. Mater. Contin. 2009, 8, 195–208. [Google Scholar]
- Wang, L.J. The effect of homogenization annealing and holding time on the microstructure and properties of 3003 aluminum alloy. Light Alloy Fabr. Technol. 2010, 38, 5–7. [Google Scholar] [CrossRef]
- Zhao, R.; Yan, M.; Jiao, Z.; Guo, D.; Huang, H. Variation Law of Thickness Fraction of Three-Laminated Aluminum Composite Plate by Solid–Liquid–Solid and Liquid–Solid–Liquid Twin-Roll Casting. Metals 2022, 12, 1710. [Google Scholar] [CrossRef]
- Xu, M.-G.; Guthrie, R.; Isac, M. Transport phenomena in meniscus region of horizontal single belt casting. Trans. Nonferrous Met. Soc. China 2020, 30, 3124–3132. [Google Scholar] [CrossRef]
- Penumakala, P.K.; Nallathambi, A.K.; Specht, E.; Urlau, U.; Hamilton, D.; Dykes, C. Feasibility Study of Continuous Casting of Steel Billets in Twin-Belt Caster. Metall. Mater. Trans. B 2018, 50, 42–51. [Google Scholar] [CrossRef]
- Luo, S.; Yang, G.; Chen, J.; Xiao, L.; Huang, W.; Yuan, Q.; Jie, W. In-situ studies and simulation of equiaxed solidification in Al–20Cu alloy. Mater. Sci. Technol. 2021, 37, 59–65. [Google Scholar] [CrossRef]
- Yang, Y.; Guan, S.; Chen, B. Study on microstructure and properties of Hazelett continuous casting and rolling 3003 aluminum alloy plates. Light Alloy Fabr. Technol. 2010, 38, 15–18+26. [Google Scholar] [CrossRef]
- Wen, X.Y.; Wen, W.; Liu, Y.; Long, Z.D.; Ningileri, S.; Zhai, T.; Li, Z. Textures and microstructures of AA3xxx hotbands from two different continuous casting processes. Mater. Sci. Technol. 2024, 31, 1495–1500. [Google Scholar] [CrossRef]
- Ashtari, P.; Gatenby, K. Formation of inverse segregation on the surface of belt-cast Al–Fe–Si and Al–Fe–Si–Mn alloys. Scr. Mater. 2007, 57, 627–630. [Google Scholar] [CrossRef]
- Xiao, Z.; Dong, S.; Song, N.; Ma, Z.; Lv, Z. Simulation of the solidification organization of X15CrNiSi20-12 alloy castings during investment casting based on the CAFE model. J. Phys. Conf. Ser. 2023, 2419, 012075. [Google Scholar] [CrossRef]
- Li, Y.; Chang, X.; Yang, G.; Sun, L.-G.; Zhang, Q.-J.; Xiao, P.-C.; Chen, W. Numerical simulation of solidification structures in continuous casting of a thin slab at high casting speed. Ironmak. Steelmak. 2023, 50, 21–29. [Google Scholar] [CrossRef]
- Spinelli, J.E.; Cheung, N.; Goulart, P.R.; Quaresma, J.M.V.; Garcia, A. Design of mechanical properties of Al-alloys chill castings based on the metal/mold interfacial heat transfer coefficient. Int. J. Therm. Sci. 2011, 51, 145–154. [Google Scholar] [CrossRef]
- St. John, D.H.; Easton, M.A.; Cao, P.; Bermingham, M.; Qian, M. A Brief History of the Grain Refinement of Cast Light Alloys. Mater. Sci. Forum 2013, 765, 123–129. [Google Scholar] [CrossRef]





| Element | Si | Fe | Cu | Mn | Zn | Impurity | Al |
|---|---|---|---|---|---|---|---|
| Content | 0.6 | 0.7 | 0.05~0.20 | 1.0~1.5 | 0.1 | 0.15 | 97.4~96.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Q.; Jin, W.; Huang, S.; Guo, Y.; Jiang, M.; Li, X. Simulation and Study of Influencing Factors on the Solidification Microstructure of Hazelett Continuous Casting Slabs Using CAFE Model. Materials 2024, 17, 1869. https://doi.org/10.3390/ma17081869
Pan Q, Jin W, Huang S, Guo Y, Jiang M, Li X. Simulation and Study of Influencing Factors on the Solidification Microstructure of Hazelett Continuous Casting Slabs Using CAFE Model. Materials. 2024; 17(8):1869. https://doi.org/10.3390/ma17081869
Chicago/Turabian StylePan, Qiuhong, Wei Jin, Shouzhi Huang, Yufeng Guo, Mingyuan Jiang, and Xuan Li. 2024. "Simulation and Study of Influencing Factors on the Solidification Microstructure of Hazelett Continuous Casting Slabs Using CAFE Model" Materials 17, no. 8: 1869. https://doi.org/10.3390/ma17081869




