Three-Dimensional Printed Teeth in Endodontics: A New Protocol for Microcomputed Tomography Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Natural Specimen Selection
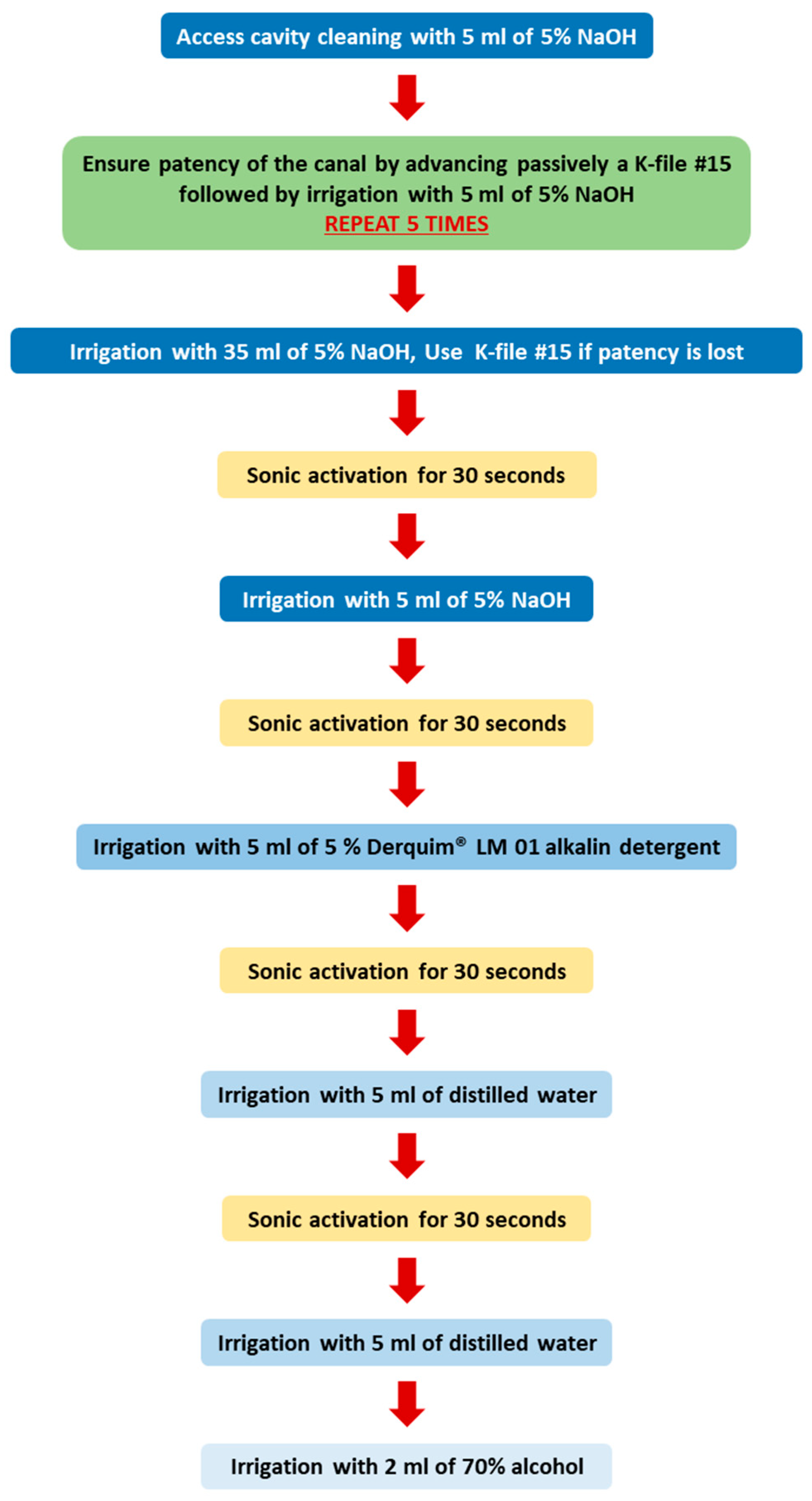
2.2. Root Canal Preparation
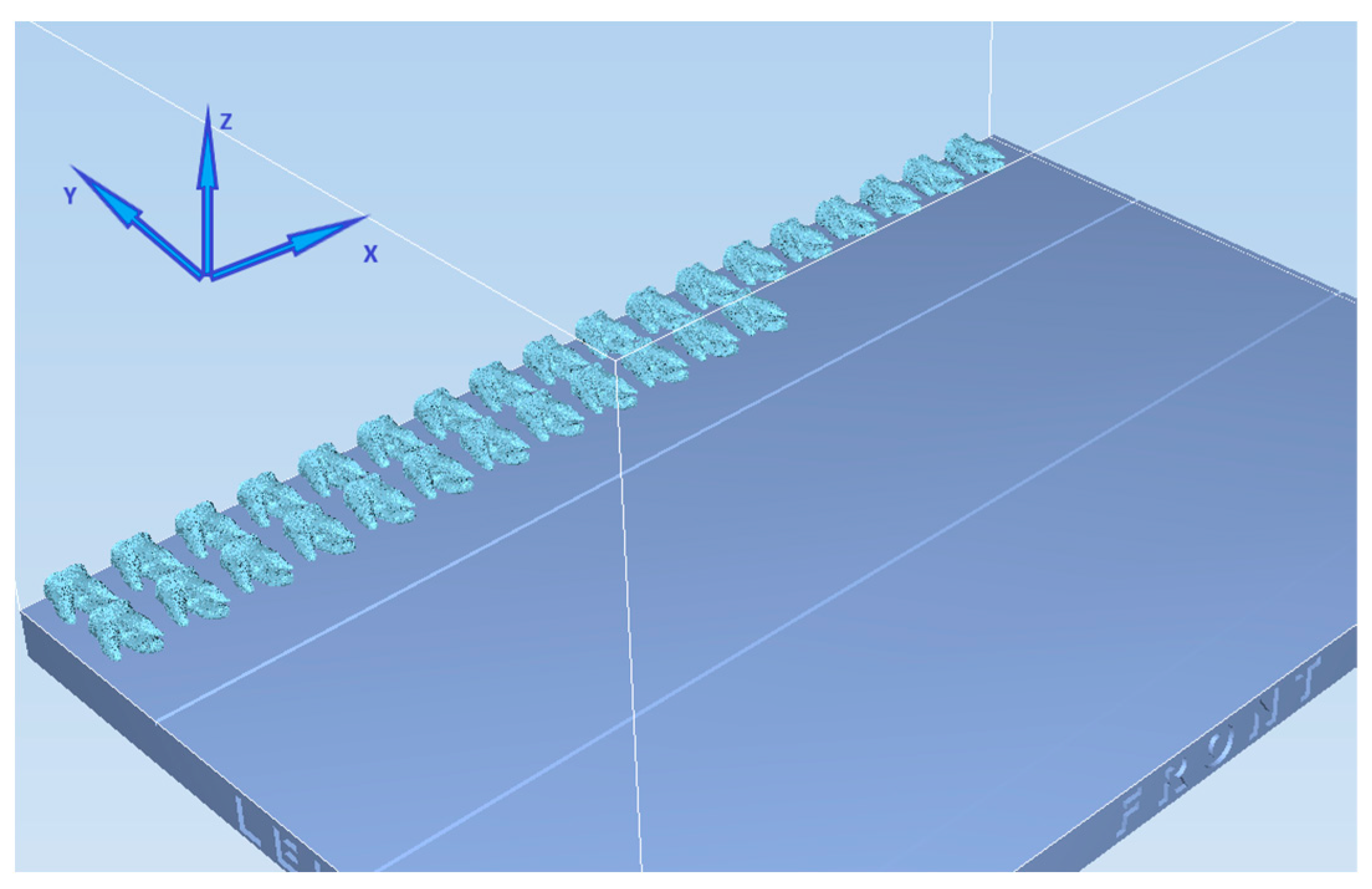
2.3. Preparation of NT and Group I with PTG
2.4. Preparation of Group II with ENDG
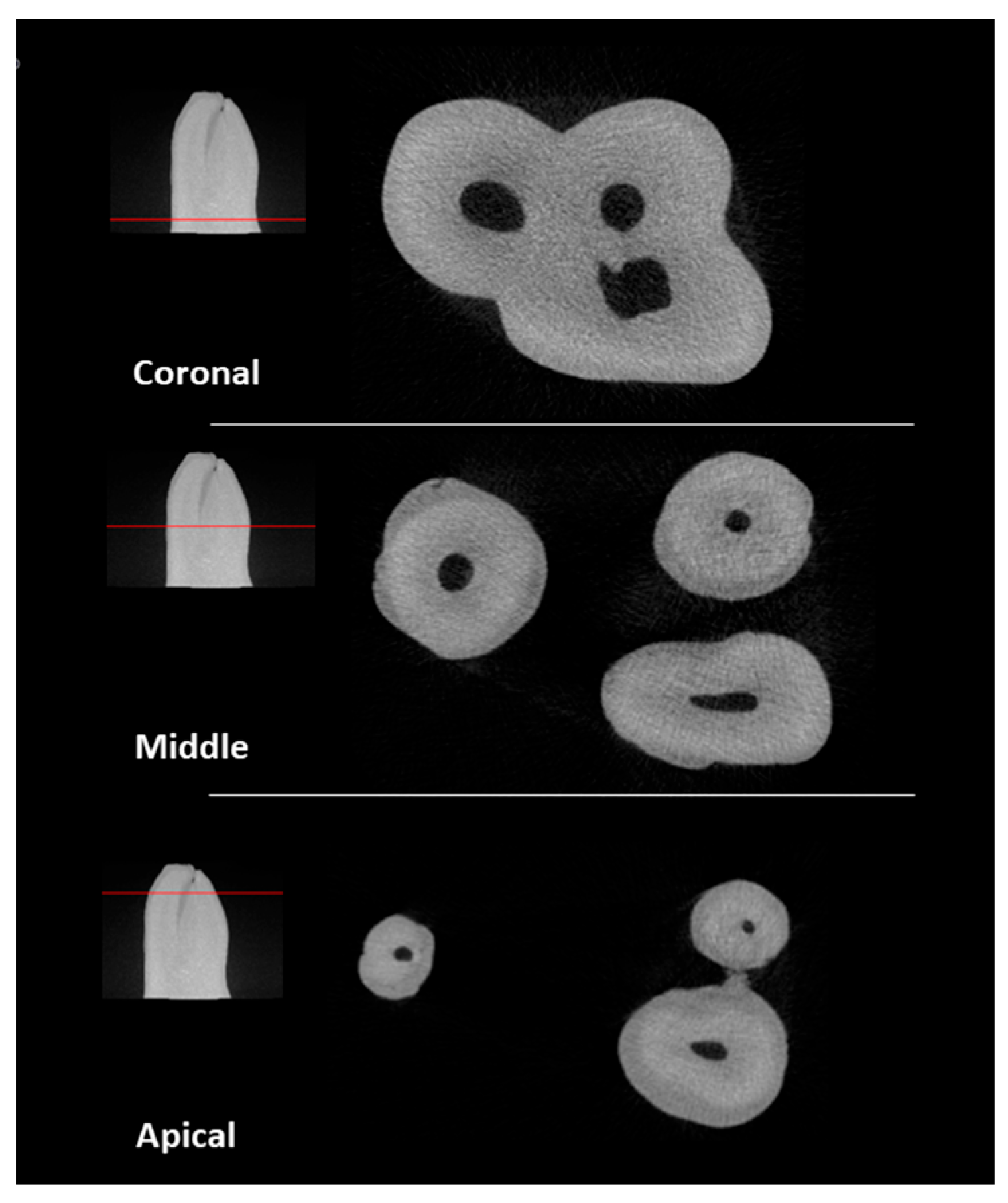
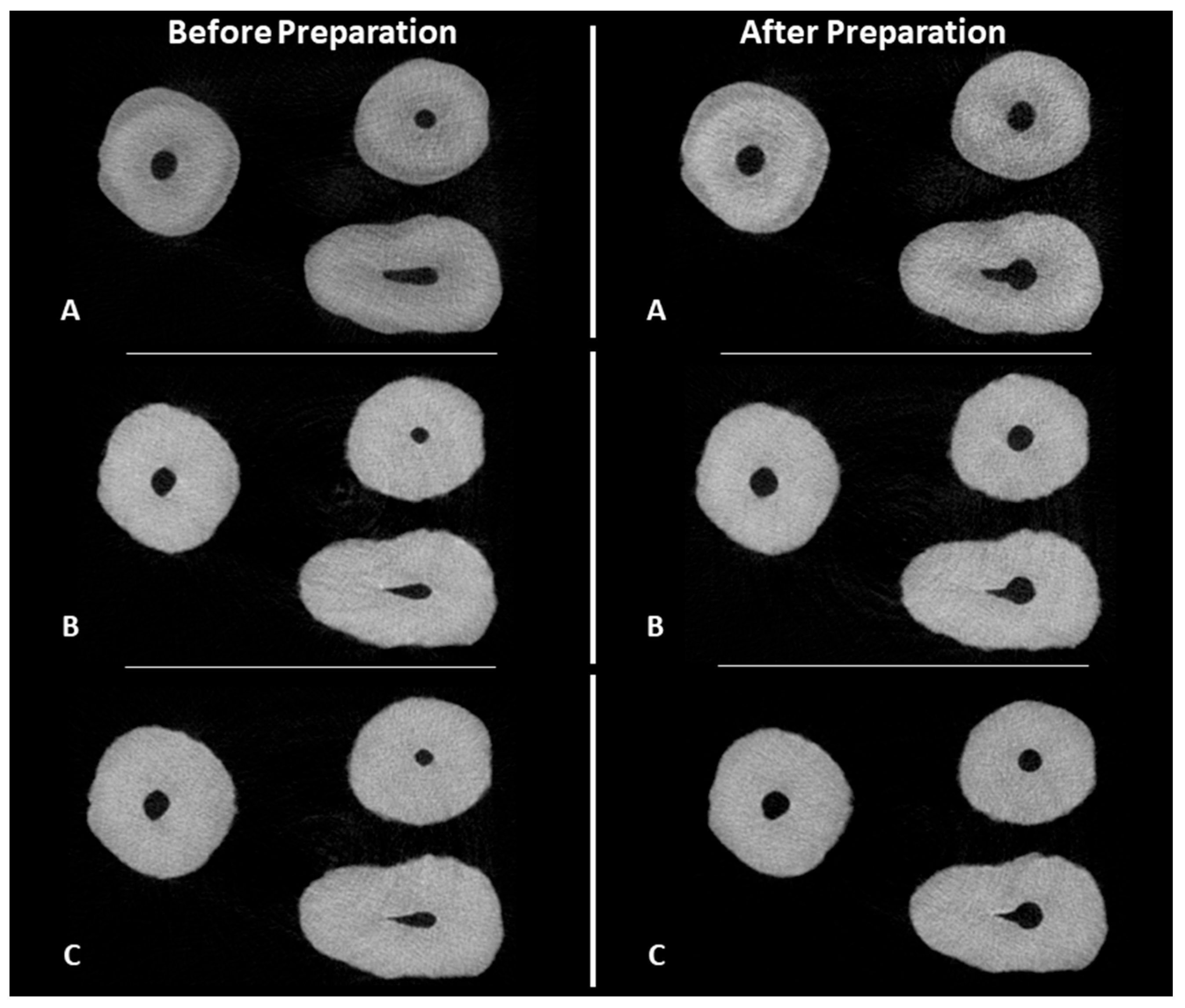
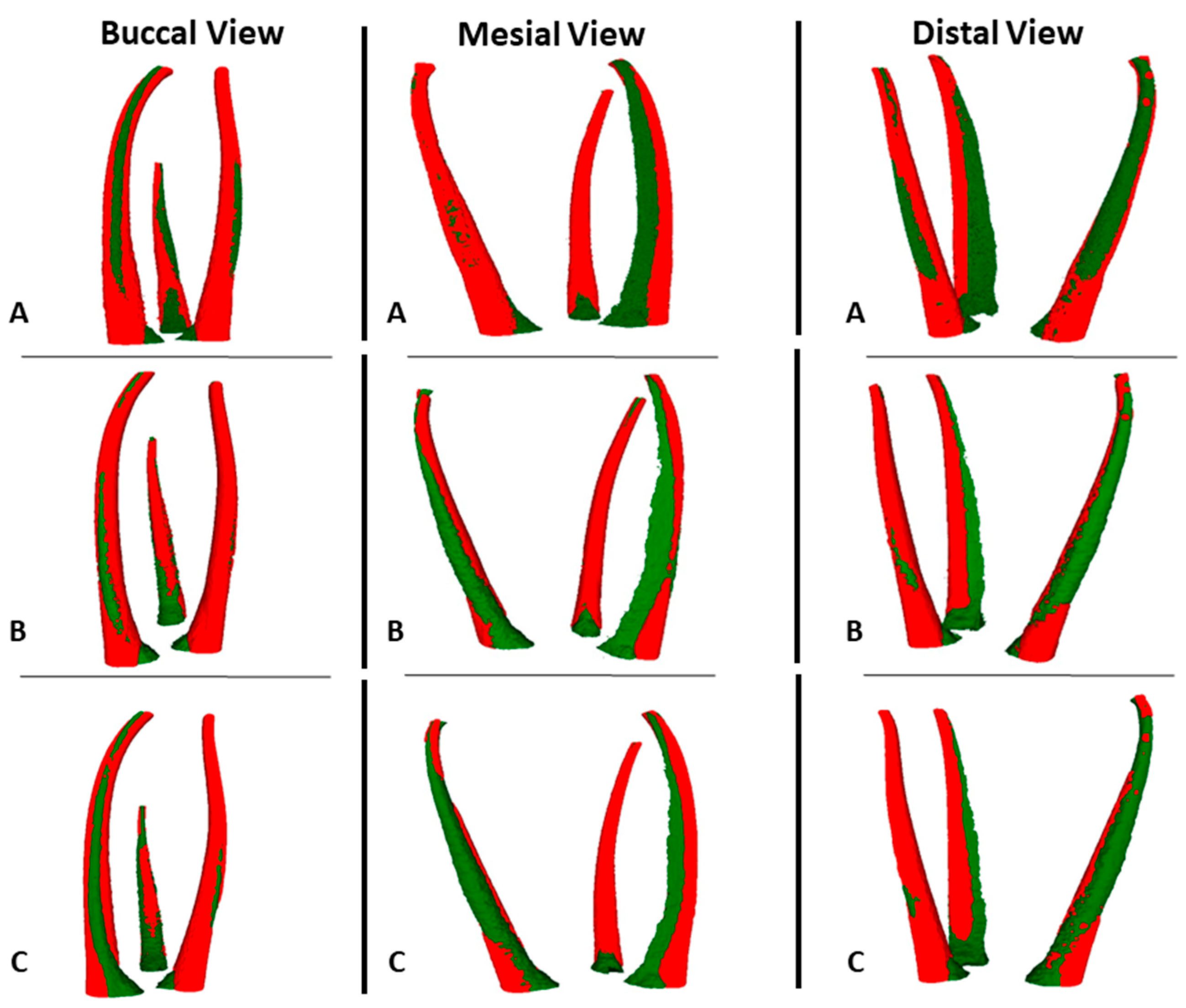
2.5. MicroCT Evaluation
2.6. Statistical Analysis
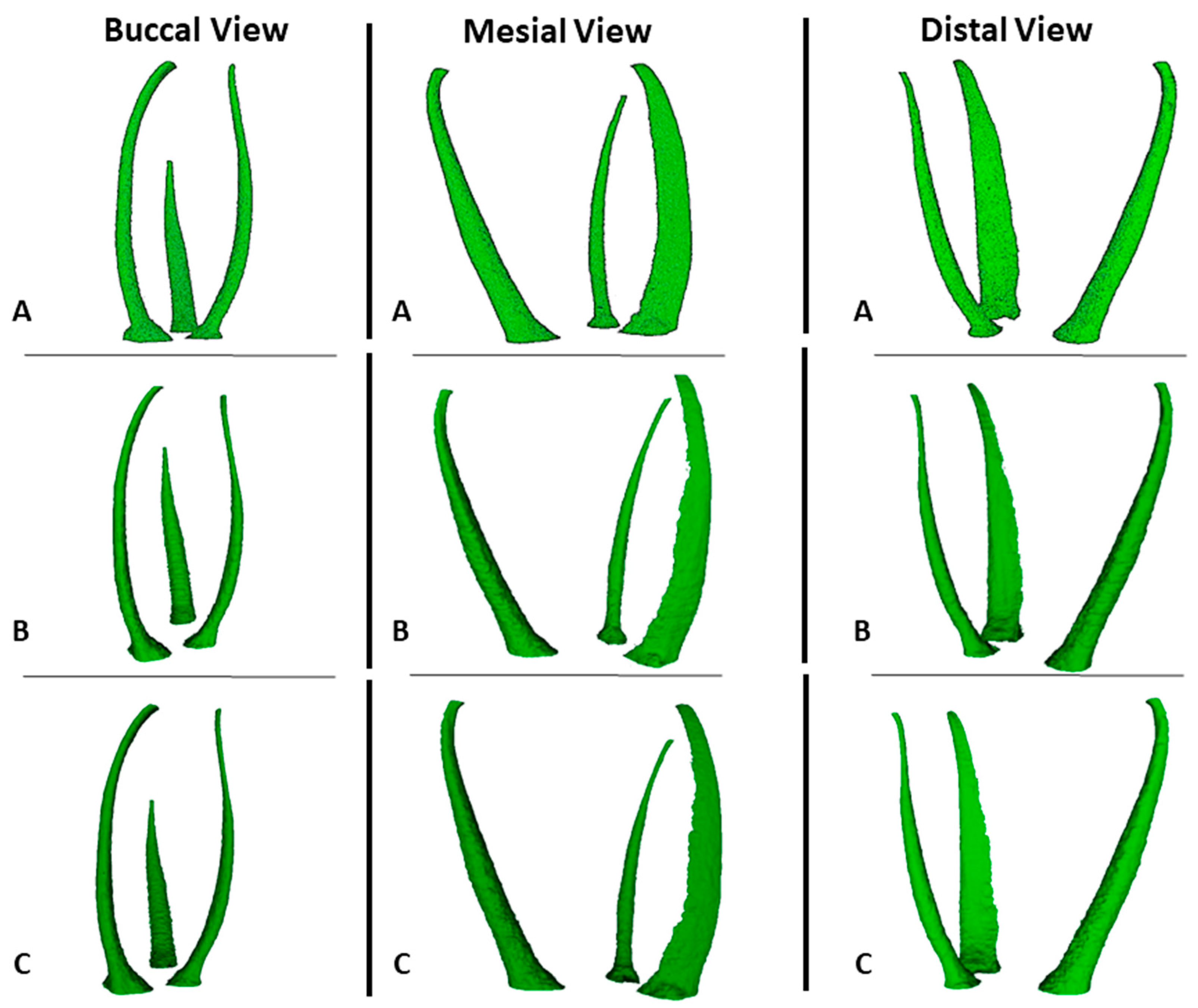
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- AAE. Glossary of Endodontic Terms. Available online: https://www.aae.org/specialty/clinical-resources/glossary-endodontic-terms/ (accessed on 4 May 2022).
- Pinheiro, S.R.; Alcalde, M.P.; Vivacqua-Gomes, N.; Bramante, C.M.; Vivan, R.R.; Duarte, M.A.H.; Vasconcelos, B.C. Evaluation of apical transportation and centring ability of five thermally treated NiTi rotary systems. Int. Endod. J. 2018, 51, 705–713. [Google Scholar] [CrossRef]
- Hulsmann, M. A critical appraisal of research methods and experimental models for studies on root canal preparation. Int. Endod. J. 2022, 55 (Suppl. S1), 95–118. [Google Scholar] [CrossRef]
- Reis, T.; Barbosa, C.; Franco, M.; Baptista, C.; Alves, N.; Castelo-Baz, P.; Martin-Cruces, J.; Martin-Biedma, B. 3D-Printed Teeth in Endodontics: Why, How, Problems and Future-A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 7966. [Google Scholar] [CrossRef]
- De-Deus, G.; Rodrigues, E.A.; Lee, J.K.; Kim, J.; Silva, E.; Belladonna, F.G.; Simoes-Carvalho, M.; Souza, E.M.; Versiani, M.A. Taper 0.06 Versus Taper 0.04: The Impact on the Danger Zone. J. Endod. 2023, 49, 536–543. [Google Scholar] [CrossRef]
- Arola, D.D.; Gao, S.; Zhang, H.; Masri, R. The Tooth: Its Structure and Properties. Dent. Clin. N. Am. 2017, 61, 651–668. [Google Scholar] [CrossRef]
- Reymus, M.; Fotiadou, C.; Kessler, A.; Heck, K.; Hickel, R.; Diegritz, C. 3D printed replicas for endodontic education. Int. Endod. J. 2019, 52, 123–130. [Google Scholar] [CrossRef]
- Orel, L.; Velea-Barta, O.-A.; Nica, L.-M.; Boscornea-Pușcu, A.-S.; Horhat, R.M.; Talpos-Niculescu, R.-M.; Sinescu, C.; Duma, V.-F.; Vulcanescu, D.-D.; Topala, F.; et al. Evaluation of the Shaping Ability of Three Thermally Treated Nickel–Titanium Endodontic Instruments on Standardized 3D-printed Dental Replicas Using Cone-Beam Computed Tomography. Medicina 2021, 57, 901. [Google Scholar] [CrossRef]
- Kolling, M.; Backhaus, J.; Hofmann, N.; Kess, S.; Krastl, G.; Soliman, S.; Konig, S. Students’ perception of three-dimensionally printed teeth in endodontic training. Eur. J. Dent. Educ. 2022, 26, 653–661. [Google Scholar] [CrossRef]
- Karatekin, A.O.; Keles, A.; Gencoglu, N. Comparison of continuous wave and cold lateral condensation filling techniques in 3D printed simulated C-shape canals instrumented with Reciproc Blue or Hyflex EDM. PLoS ONE 2019, 14, e0224793. [Google Scholar] [CrossRef]
- Kooanantkul, C.; Shelton, R.M.; Camilleri, J. Comparison of obturation quality in natural and replica teeth root-filled using different sealers and techniques. Clin. Oral Investig. 2023, 27, 2407–2417. [Google Scholar] [CrossRef]
- Ordinola-Zapata, R.; Bramante, C.M.; Duarte, M.A.; Cavenago, B.C.; Jaramillo, D.; Versiani, M.A. Shaping ability of reciproc and TF adaptive systems in severely curved canals of rapid microCT-based prototyping molar replicas. J. Appl. Oral Sci. 2014, 22, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Cho, J.W.; Chang, N.Y.; Chae, J.M.; Kang, K.H.; Kim, S.C.; Cho, J.H. Accuracy of three-dimensional printing for manufacturing replica teeth. Korean J. Orthod. 2015, 45, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Moraes, R.D.R.; Santos, T.; Marceliano-Alves, M.F.; Pintor, A.V.B.; Lopes, R.T.; Primo, L.G.; Neves, A.A. Reciprocating instrumentation in a maxillary primary central incisor: A protocol tested in a 3D printed prototype. Int. J. Paediatr. Dent. 2019, 29, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Kent, N.J.; Jolivet, L.; O’Neill, P.; Brabazon, D. An evaluation of components manufactured from a range of materials, fabricated using PolyJet technology. Adv. Mater. Process. Technol. 2017, 3, 318–329. [Google Scholar] [CrossRef]
- Herpel, C.; Tasaka, A.; Higuchi, S.; Finke, D.; Kuhle, R.; Odaka, K.; Rues, S.; Lux, C.J.; Yamashita, S.; Rammelsberg, P.; et al. Accuracy of 3D printing compared with milling—A multi-center analysis of try-in dentures. J. Dent. 2021, 110, 103681. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, N.P.; Cabot, J.M.; Smejkal, P.; Guijt, R.M.; Paull, B.; Breadmore, M.C. Comparing Microfluidic Performance of Three-Dimensional (3D) Printing Platforms. Anal. Chem. 2017, 89, 3858–3866. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Hickel, R.; Reymus, M. 3D Printing in Dentistry-State of the Art. Oper. Dent. 2020, 45, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Stratasys. SUP706 and SUP706B Support Material—EN PolyJet Best Practice. Available online: https://support.stratasys.com/en/Materials/PolyJet/PolyJet-Support (accessed on 27 January 2022).
- Smutny, M.; Kopecek, M.; Bezrouk, A. An Investigation of the Accuracy and Reproducibility of 3D Printed Transparent Endodontic Blocks. Acta Medica 2022, 65, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Reis, T.; Barbosa, C.; Franco, M.; Batista, C.; Alves, N.; Castelo-Baz, P.; Martin-Cruces, J.; Martin-Biedma, B. Root Canal Preparation of a Commercial Artificial Tooth versus Natural Tooth—A MicroCT Study. Appl. Sci. 2023, 13, 9400. [Google Scholar] [CrossRef]
- Gagliardi, J.; Versiani, M.A.; de Sousa-Neto, M.D.; Plazas-Garzon, A.; Basrani, B. Evaluation of the Shaping Characteristics of ProTaper Gold, ProTaper NEXT, and ProTaper Universal in Curved Canals. J. Endod. 2015, 41, 1718–1724. [Google Scholar] [CrossRef]
- Schneider, S.W. A comparison of canal preparations in straight and curved root canals. Oral Surg. Oral Med. Oral Pathol. 1971, 32, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.; Ajuz, N.C.; Martins, J.N.R.; Antunes, B.R.; Lima, C.O.; Vieira, V.T.L.; Braz-Fernandes, F.M.; Versiani, M.A. Multimethod analysis of three rotary instruments produced by electric discharge machining technology. Int. Endod. J. 2023, 56, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Faus-Matoses, V.; Faus-Llacer, V.; Ruiz-Sanchez, C.; Jaramillo-Vasconez, S.; Faus-Matoses, I.; Martin-Biedma, B.; Zubizarreta-Macho, A. Effect of Rotational Speed on the Resistance of NiTi Alloy Endodontic Rotary Files to Cyclic Fatigue-An In Vitro Study. J. Clin. Med. 2022, 11, 3143. [Google Scholar] [CrossRef] [PubMed]
- Perez Morales, M.L.N.; Gonzalez Sanchez, J.A.; Olivieri, J.G.; Elmsmari, F.; Salmon, P.; Jaramillo, D.E.; Terol, F.D. Micro-computed Tomographic Assessment and Comparative Study of the Shaping Ability of 6 Nickel-Titanium Files: An In Vitro Study. J. Endod. 2021, 47, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Serefoglu, B.; Piskin, B. Micro computed tomography evaluation of the Self-adjusting file and ProTaper Universal system on curved mandibular molars. Dent. Mater. J. 2017, 36, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Donnermeyer, D.; Averkorn, C.; Burklein, S.; Schafer, E. Cleaning Efficiency of Different Irrigation Techniques in Simulated Severely Curved Complex Root Canal Systems. J. Endod. 2023, 49, 1548–1552. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.M.; Verhaagen, B.; Versluis, M.; van der Sluis, L.W. Evaluation of a sonic device designed to activate irrigant in the root canal. J. Endod. 2010, 36, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Haupt, F.; Meinel, M.; Gunawardana, A.; Hulsmann, M. Effectiveness of different activated irrigation techniques on debris and smear layer removal from curved root canals: A SEM evaluation. Aust. Endod. J. 2020, 46, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Provoost, C.; Rocca, G.T.; Thibault, A.; Machtou, P.; Bouilllaguet, S. Influence of Needle Design and Irrigant Flow Rate on the Removal of Enterococcus faecalis Biofilms In Vitro. Dent. J. 2022, 10, 59. [Google Scholar] [CrossRef]
- Hartmann, R.C.; Peters, O.A.; de Figueiredo, J.A.P.; Rossi-Fedele, G. Association of manual or engine-driven glide path preparation with canal centring and apical transportation: A systematic review. Int. Endod. J. 2018, 51, 1239–1252. [Google Scholar] [CrossRef]
- Rebong, R.E.; Stewart, K.T.; Utreja, A.; Ghoneima, A.A. Accuracy of three-dimensional dental resin models created by fused deposition modeling, stereolithography, and Polyjet prototype technologies: A comparative study. Angle Orthod. 2018, 88, 363–369. [Google Scholar] [CrossRef]
- Mendřický, R. Accuracy analysis of additive technique for parts manufacturing. MM Sci. J. 2016, 2016, 1502–1508. [Google Scholar] [CrossRef]
- Kim, S.Y.; Shin, Y.S.; Jung, H.D.; Hwang, C.J.; Baik, H.S.; Cha, J.Y. Precision and trueness of dental models manufactured with different 3-dimensional printing techniques. Am. J. Orthod. Dentofac. Orthop. 2018, 153, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Rouze l’Alzit, F.; Cade, R.; Naveau, A.; Babilotte, J.; Meglioli, M.; Catros, S. Accuracy of commercial 3D printers for the fabrication of surgical guides in dental implantology. J. Dent. 2022, 117, 103909. [Google Scholar] [CrossRef]
- Kim, J.H.; Pinhata-Baptista, O.H.; Ayres, A.P.; da Silva, R.L.B.; Lima, J.F.; Urbano, G.S.; No-Cortes, J.; Vasques, M.T.; Cortes, A.R.G. Accuracy Comparison among 3D-Printing Technologies to Produce Dental Models. Appl. Sci. 2022, 12, 8425. [Google Scholar] [CrossRef]
- Bezek, L.; Meisel, N.; Williams, C. Exploring variability of orientation and aging effects in material properties of multi-material jetting parts. Rapid Prototyp. J. 2016, 22, 826–834. [Google Scholar] [CrossRef]
- Chan, C.W.; Romeo, V.R.; Lee, A.; Zhang, C.; Neelakantan, P.; Pedulla, E. Accumulated Hard Tissue Debris and Root Canal Shaping Profiles Following Instrumentation with Gentlefile, One Curve, and Reciproc Blue. J. Endod. 2023, 49, 1344–1351. [Google Scholar] [CrossRef]
- Stratasys, A.K. PolyJet™ Materials Reference Guide. Available online: https://support.stratasys.com/en/materials/polyjet/high-temp (accessed on 8 November 2023).
- Staninec, M.; Marshall, G.W.; Hilton, J.F.; Pashley, D.H.; Gansky, S.A.; Marshall, S.J.; Kinney, J.H. Ultimate tensile strength of dentin: Evidence for a damage mechanics approach to dentin failure. J. Biomed. Mater. Res. 2002, 63, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Chun, K.; Choi, H.; Lee, J. Comparison of mechanical property and role between enamel and dentin in the human teeth. J. Dent. Biomech. 2014, 5, 1758736014520809. [Google Scholar] [CrossRef]
- Plotino, G.; Grande, N.M.; Bedini, R.; Pameijer, C.H.; Somma, F. Flexural properties of endodontic posts and human root dentin. Dent. Mater. 2007, 23, 1129–1135. [Google Scholar] [CrossRef]
- Aazzouzi-Raiss, K.; Ramirez-Munoz, A.; Mendez, S.P.; Vieira, G.C.S.; Aranguren, J.; Perez, A.R. Effects of Conservative Access and Apical Enlargement on Shaping and Dentin Preservation with Traditional and Modern Instruments: A Micro-computed Tomographic Study. J. Endod. 2023, 49, 430–437. [Google Scholar] [CrossRef]
- Duque, J.A.; Vivan, R.R.; Cavenago, B.C.; Amoroso-Silva, P.A.; Bernardes, R.A.; Vasconcelos, B.C.; Duarte, M.A. Influence of NiTi alloy on the root canal shaping capabilities of the ProTaper Universal and ProTaper Gold rotary instrument systems. J. Appl. Oral Sci. 2017, 25, 27–33. [Google Scholar] [CrossRef]
- Sivakumar, M.; Nawal, R.R.; Talwar, S.; Baveja, C.P.; Kumar, R.; Yadav, S.; Kumar, S.S. Shaping, and disinfecting abilities of ProTaper Universal, ProTaper Gold, and Twisted Files: A correlative microcomputed tomographic and bacteriologic analysis. Endodontology 2023, 35, 54–59. [Google Scholar]
- Silva, E.; Martins, J.N.R.; Lima, C.O.; Vieira, V.T.L.; Braz Fernandes, F.M.; De-Deus, G.; Versiani, M.A. Mechanical Tests, Metallurgical Characterization, and Shaping Ability of Nickel-Titanium Rotary Instruments: A Multimethod Research. J. Endod. 2020, 46, 1485–1494. [Google Scholar] [CrossRef] [PubMed]
- Yalniz, H.; Koohnavard, M.; Oncu, A.; Celikten, B.; Orhan, A.I.; Orhan, K. Comparative evaluation of dentin volume removal and centralization of the root canal after shaping with the ProTaper Universal, ProTaper Gold, and One-Curve instruments using micro-CT. J. Dent. Res. Dent. Clin. Dent. Prospect. 2021, 15, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Peters, O.A.; Laib, A.; Gohring, T.N.; Barbakow, F. Changes in root canal geometry after preparation assessed by high-resolution computed tomography. J. Endod. 2001, 27, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Romeiro, K.; Brasil, S.C.; Souza, T.M.; Gominho, L.F.; Perez, A.R.; Perez, R.; Alves, F.R.F.; Rocas, I.N.; Siqueira, J.F., Jr. Influence of brushing motions on the shaping of oval canals by rotary and reciprocating instruments. Clin. Oral Investig. 2023, 27, 3973–3981. [Google Scholar] [CrossRef] [PubMed]
- Hilaly Eid, G.E.; Wanees Amin, S.A. Changes in diameter, cross-sectional area, and extent of canal-wall touching on using 3 instrumentation techniques in long-oval canals. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 112, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.H.; Park, S.S. Influence of glide path on the screw-in effect and torque of nickel-titanium rotary files in simulated resin root canals. Restor. Dent. Endod. 2012, 37, 215–219. [Google Scholar] [CrossRef]
- Ha, J.H.; Kwak, S.W.; Sigurdsson, A.; Chang, S.W.; Kim, S.K.; Kim, H.C. Stress Generation during Pecking Motion of Rotary Nickel-titanium Instruments with Different Pecking Depth. J. Endod. 2017, 43, 1688–1691. [Google Scholar] [CrossRef]
- Kyaw, M.S.; Ebihara, A.; Kasuga, Y.; Maki, K.; Kimura, S.; Htun, P.H.; Nakatsukasa, T.; Okiji, T. Influence of rotational speed on torque/force generation and shaping ability during root canal instrumentation of extracted teeth with continuous rotation and optimum torque reverse motion. Int. Endod. J. 2021, 54, 1614–1622. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Ebihara, A.; Maki, K.; Nishijo, M.; Tokita, D.; Okiji, T. Effect of Optimum Torque Reverse Motion on Torque and Force Generation during Root Canal Instrumentation with Crown-down and Single-length Techniques. J. Endod. 2020, 46, 232–237. [Google Scholar] [CrossRef] [PubMed]

| Data | Natural Tooth | 3D-Printed Teeth | |
|---|---|---|---|
| Canal Volume (mm3) | 12.08 | Mean ± SD | 11.99 ± 0.55 |
| Median | 12.07 | ||
| Centroid X (mm) | 10.92 | Mean ± SD | 10.92 ± 0.09 |
| Median | 10.93 | ||
| Centroid Y (mm) | 10.37 | Mean ± SD | 10.35 ± 0.07 |
| Median | 10.35 | ||
| Centroid Z (mm) | 8.03 | Mean ± SD | 8.02 ± 0.09 |
| Median | 8.04 | ||
| Tooth Volume Expansion (%) | Mean ± SD | 0.73 ± 0.77 | |
| Median | 0.68 | ||
| Data | Natural Tooth | 3D-Printed Teeth | ||
|---|---|---|---|---|
| Canal Volume (mm3) | Initial | 12.08 | Mean ± SD | 12.12 ± 0.42 |
| Median | 12.12 | |||
| After | 17.75 | Mean ± SD | 17.89 ± 0.69 | |
| Median | 17.66 | |||
| Volume of dentin removed | 5.68 | Mean ± SD | 5.77 ± 0.66 | |
| Median | 5.67 | |||
| % Volume increase | 46.99 | Mean ± SD | 47.68 ± 6.09 | |
| Median | 45.50 | |||
| Centroid X (mm) | Initial | 10.92 | Mean ± SD | 10.92 ± 0.07 |
| Median | 10.87 | |||
| After | 11.38 | Mean ± SD | 11.33 ± 0.13 | |
| Median | 11.32 | |||
| Alteration | 0.46 | Mean ± SD | 0.41 ± 0.17 | |
| Median | 0.41 | |||
| Centroid Y (mm) | Initial | 10.37 | Mean ± SD | 10.37 ± 0.07 |
| Median | 10.40 | |||
| After | 10.50 | Mean ± SD | 10.53 ± 0.04 | |
| Median | 10.53 | |||
| Alteration | 0.13 | Mean ± SD | 0.16 ± 0.07 | |
| Median | 0.15 | |||
| Centroid Z (mm) | Initial | 8.03 | Mean ± SD | 8.01 ± 0.11 |
| Median | 8.04 | |||
| After | 8.10 | Mean ± SD | 8.01 ± 0.10 | |
| Median | 8.02 | |||
| Alteration | 0.07 | Mean ± SD | 0.01 ± 0.015 | |
| Median | 0.02 | |||
| Transportation (mm) | 0.48 | Mean ± SD | 0.48 ± 0.14 | |
| Median | 0.45 | |||
| Unprepared area (%) | 57.64 | Mean ± SD | 54.97 ± 3.79 | |
| Median | 53.88 | |||
| Data | ProTaper Gold® | |||
|---|---|---|---|---|
| Canal Volume (mm3) | Initial | Mean ± SD | 12.12 ± 0.42 | 11.86 ± 0.66 |
| Median | 12.12 | 11.82 | ||
| After | Mean ± SD | 17.89 ± 0.69 | 17.02 ± 0.61 | |
| Median | 17.66 | 17.06 | ||
| Volume of dentin removed | Mean ± SD | 5.77 ± 0.66 | 5.24 ± 1.02 | |
| Median | 5.67 | 5.49 | ||
| % Volume increase | Mean ± SD | 47.68 ± 6.09 | 45.02 ± 10.89 | |
| Median | 45.50 | 47.25 | ||
| Centroid X (mm) | Initial | Mean ± SD | 10.92 ± 0.07 | 10.92 ± 0.11 |
| Median | 10.87 | 10.96 | ||
| After | Mean ± SD | 11.33 ± 0.13 | 11.37 ± 0.06 | |
| Median | 11.32 | 11.35 | ||
| Alteration | Mean ± SD | 0.41 ± 0.17 | 0.45 ± 0.09 | |
| Median | 0.41 | 0.43 | ||
| Centroid Y (mm) | Initial | Mean ± SD | 10.37 ± 0.07 | 10.33 ± 0.06 |
| Median | 10.40 | 10.32 | ||
| After | Mean ± SD | 10.53 ± 0.04 | 10.44 ± 0.05 | |
| Median | 10.53 | 10.45 | ||
| Alteration | Mean ± SD | 0.16 ± 0.07 | 0.11 ± 0.05 | |
| Median | 0.15 | 0.13 | ||
| Centroid Z (mm) | Initial | Mean ± SD | 8.01 ± 0.11 | 8.03 ± 0.07 |
| Median | 8.04 | 8.01 | ||
| After | Mean ± SD | 8.01 ± 0.10 | 8.07 ± 0.09 | |
| Median | 8.02 | 8.11 | ||
| Alteration | Mean ± SD | 0.01 ± 0.015 | 0.05 ± 0.09 | |
| Median | 0.02 | 0.02 | ||
| Transportation (mm) | Mean ± SD | 0.48 ± 0.14 | 0.49 ± 0.11 | |
| Median | 0.45 | 0.47 | ||
| Unprepared area (%) | Mean ± SD | 54.97 ± 3.79 | 56.41 ± 5.11 | |
| Median | 53.88 | 55.39 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis, T.; Barbosa, C.; Franco, M.; Silva, R.; Alves, N.; Castelo-Baz, P.; Martín-Cruces, J.; Martín-Biedma, B. Three-Dimensional Printed Teeth in Endodontics: A New Protocol for Microcomputed Tomography Studies. Materials 2024, 17, 1899. https://doi.org/10.3390/ma17081899
Reis T, Barbosa C, Franco M, Silva R, Alves N, Castelo-Baz P, Martín-Cruces J, Martín-Biedma B. Three-Dimensional Printed Teeth in Endodontics: A New Protocol for Microcomputed Tomography Studies. Materials. 2024; 17(8):1899. https://doi.org/10.3390/ma17081899
Chicago/Turabian StyleReis, Tiago, Cláudia Barbosa, Margarida Franco, Ruben Silva, Nuno Alves, Pablo Castelo-Baz, Jose Martín-Cruces, and Benjamín Martín-Biedma. 2024. "Three-Dimensional Printed Teeth in Endodontics: A New Protocol for Microcomputed Tomography Studies" Materials 17, no. 8: 1899. https://doi.org/10.3390/ma17081899







