Influence of Etchants on Etched Surfaces of High-Strength and High-Conductivity Cu Alloy of Different Processing States
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Materials
2.2. Etching and Characterization Methods
3. Results and Discussion
3.1. Grain Structure of Specimens Annealed at Different Temperatures
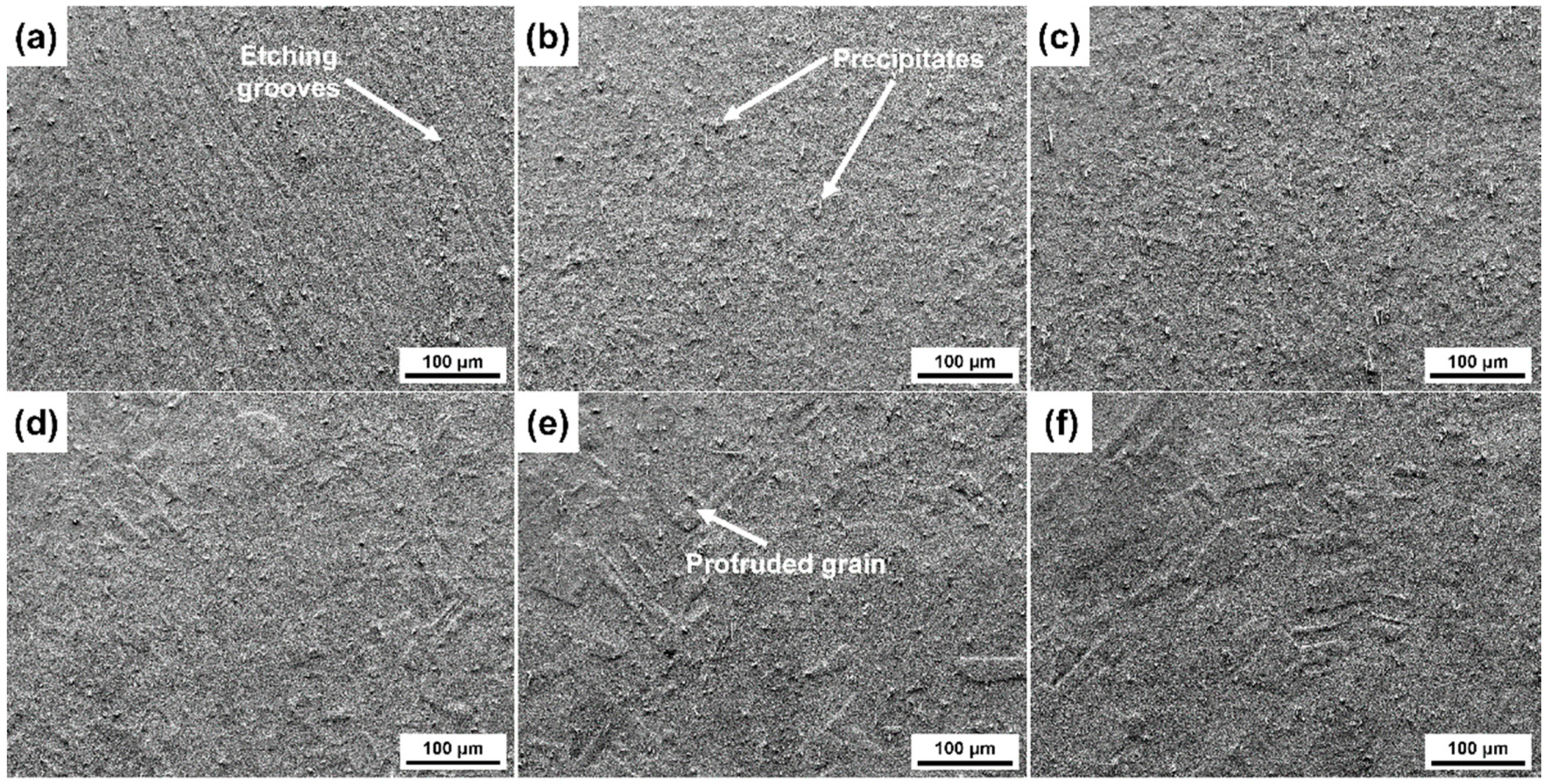
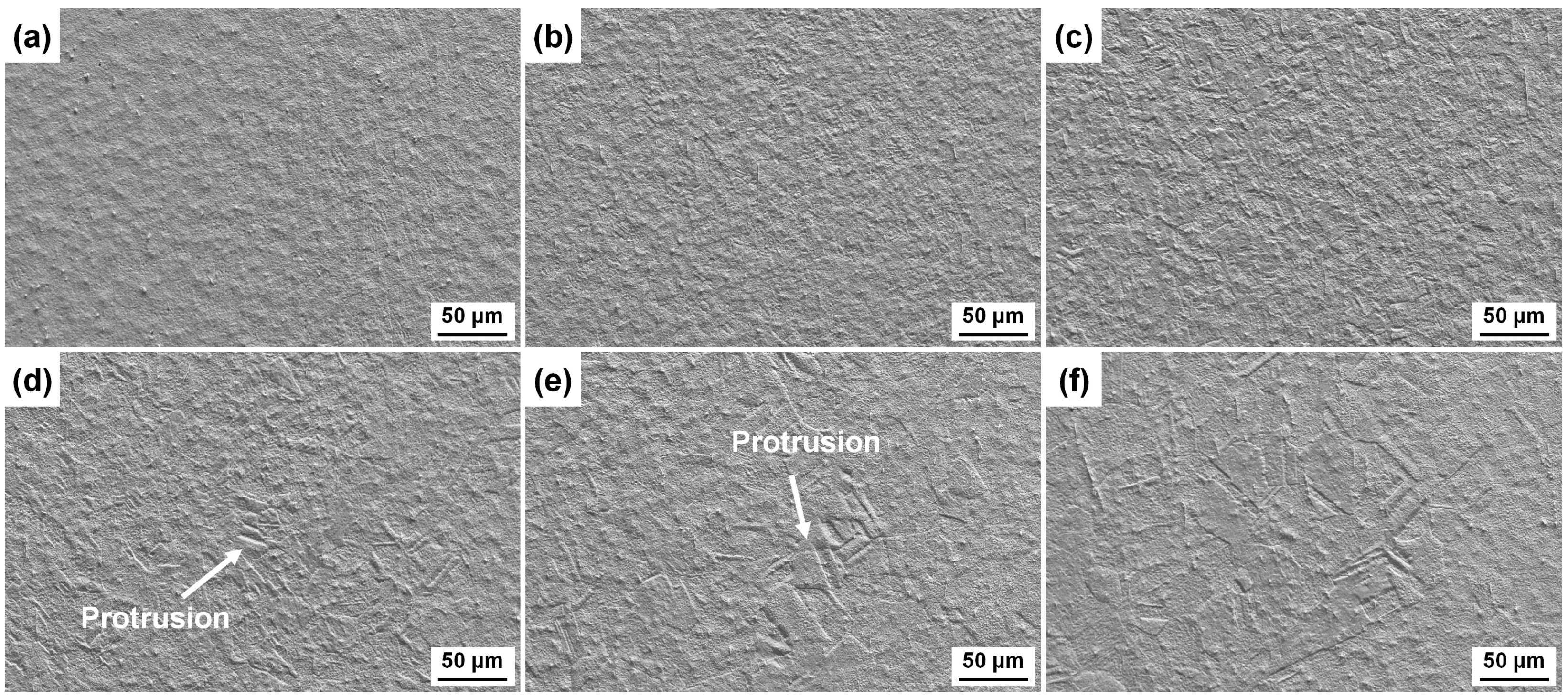
3.2. Surface Morphologies after Etching with Different Etchants
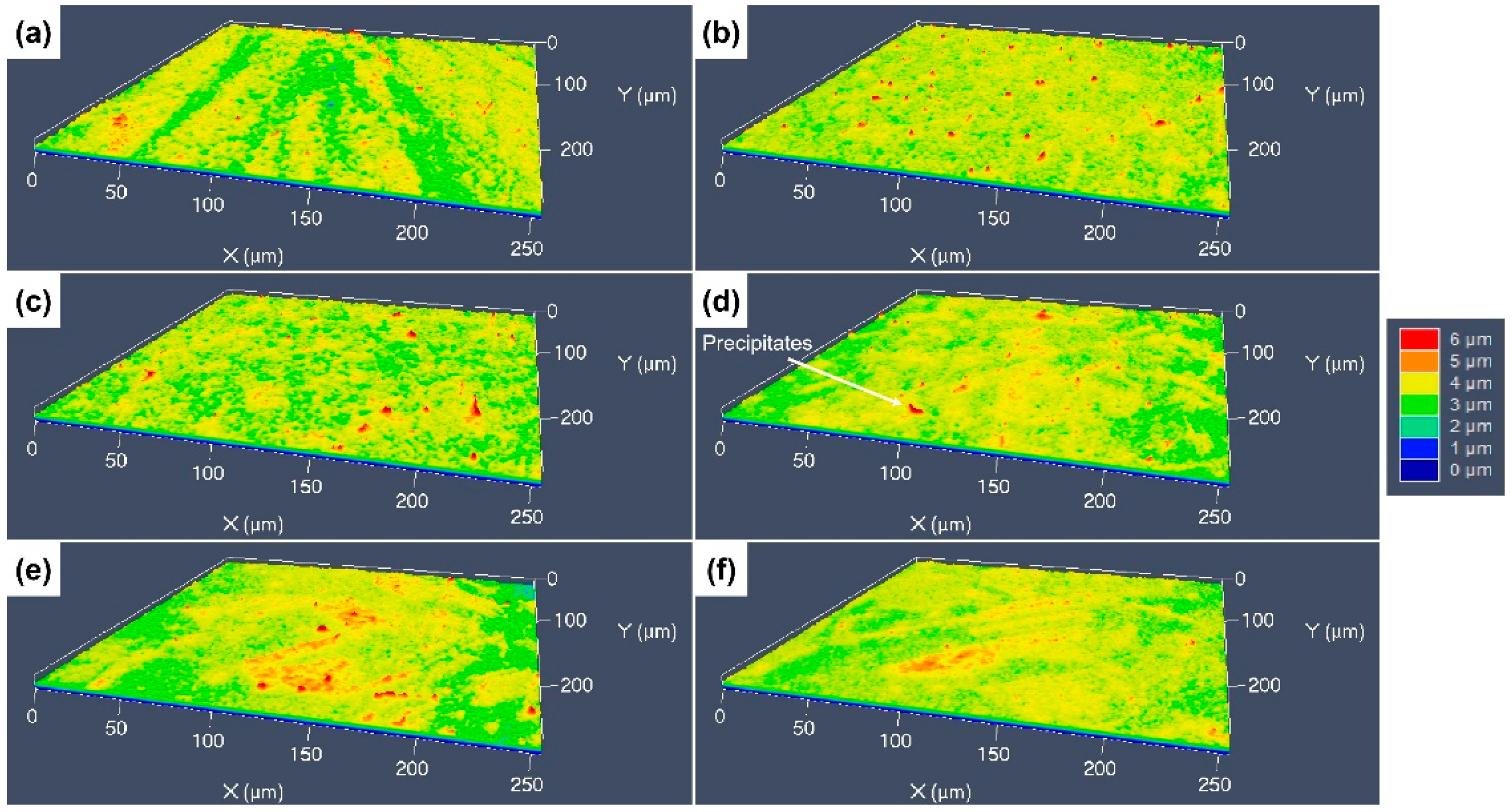
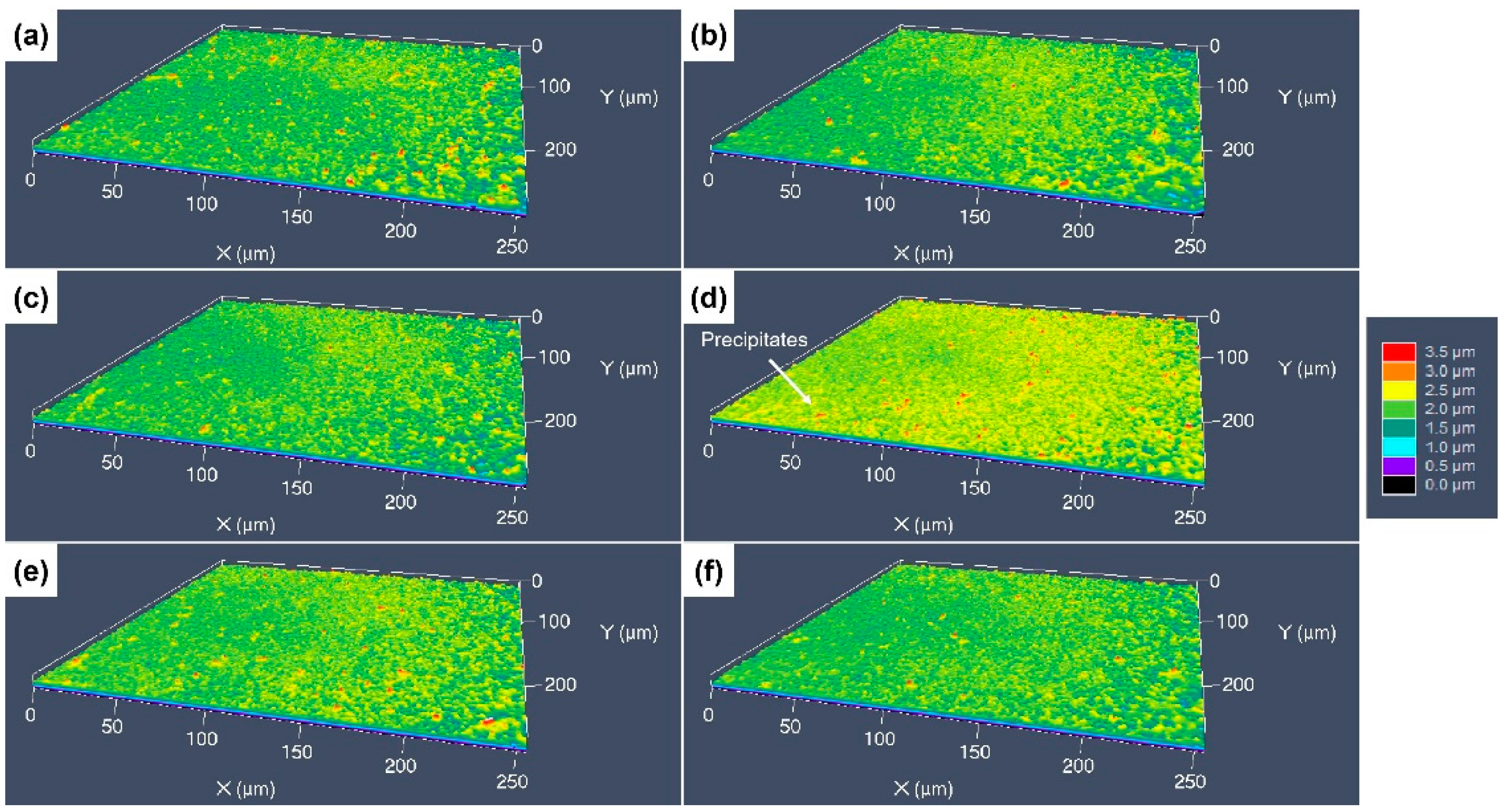
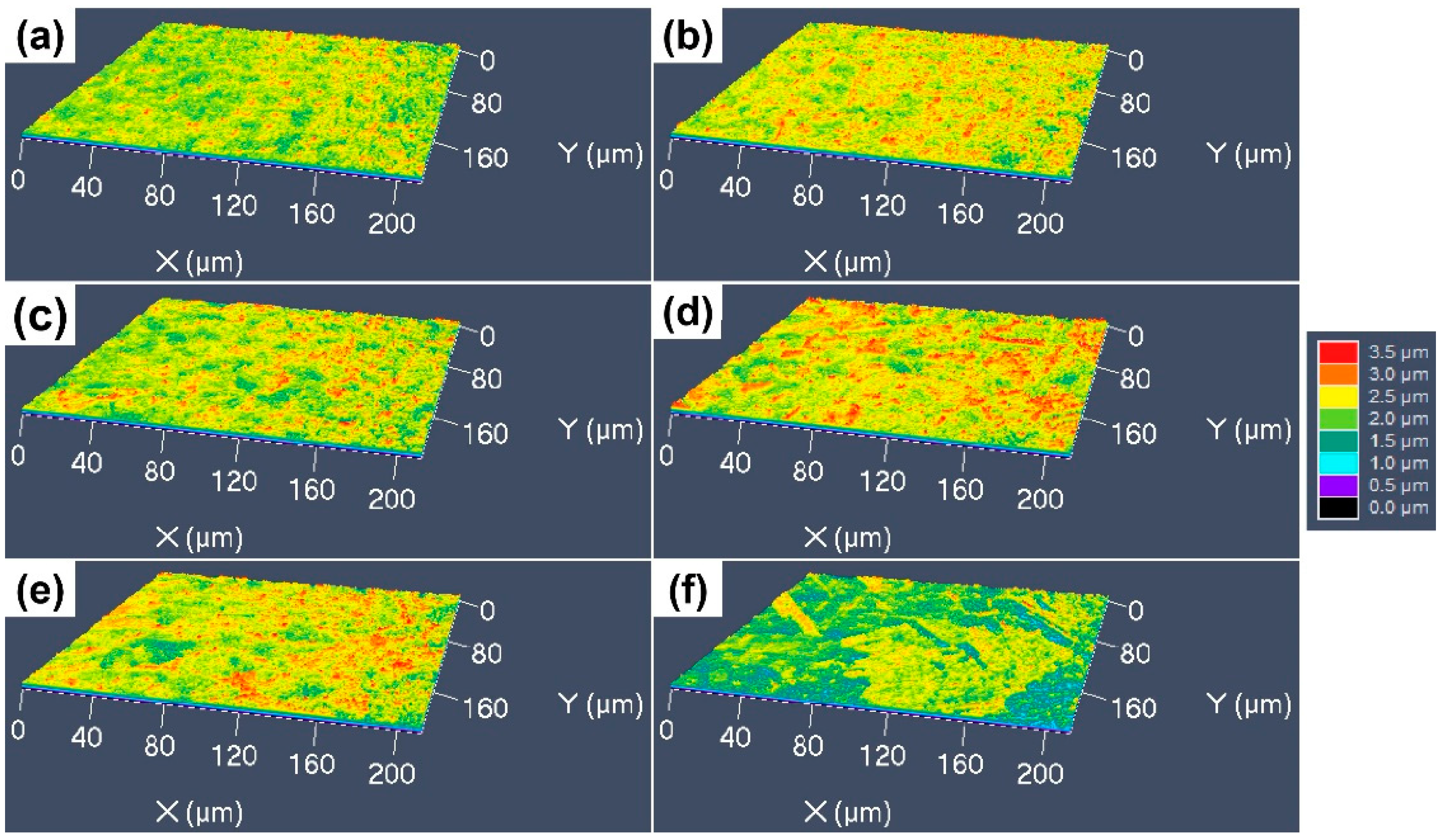
3.3. 3D Surface Undulations after Etching with Different Etchants
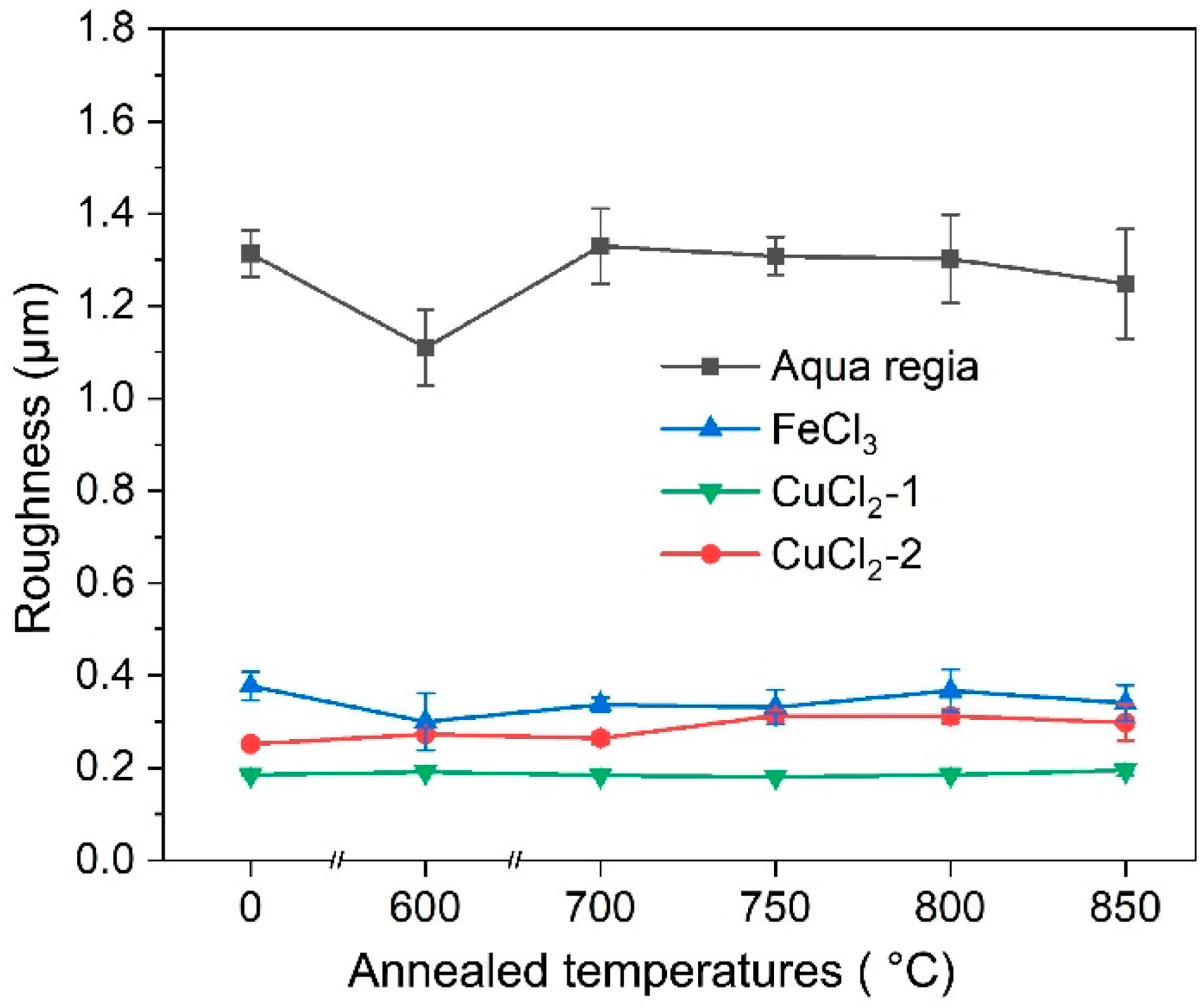
3.4. Surface Roughness
3.5. Discussion
4. Conclusions
- The etching rate in aqua regia is high, and the grain orientation, GBs and dislocations have significant influences on the etching rate, the height difference between the etch-resistant grains and the surrounding grains of about 5–6 μm. The preferential etching of some atomic planes forms steps at some GBs, and preferential etching around the GB and dislocation group forms grooves. For etching with the FeCl3 and CuCl2-2 etchants, such steps and grooves become blurred and almost invisible on the surface etched by the CuCl2-1 etchant.
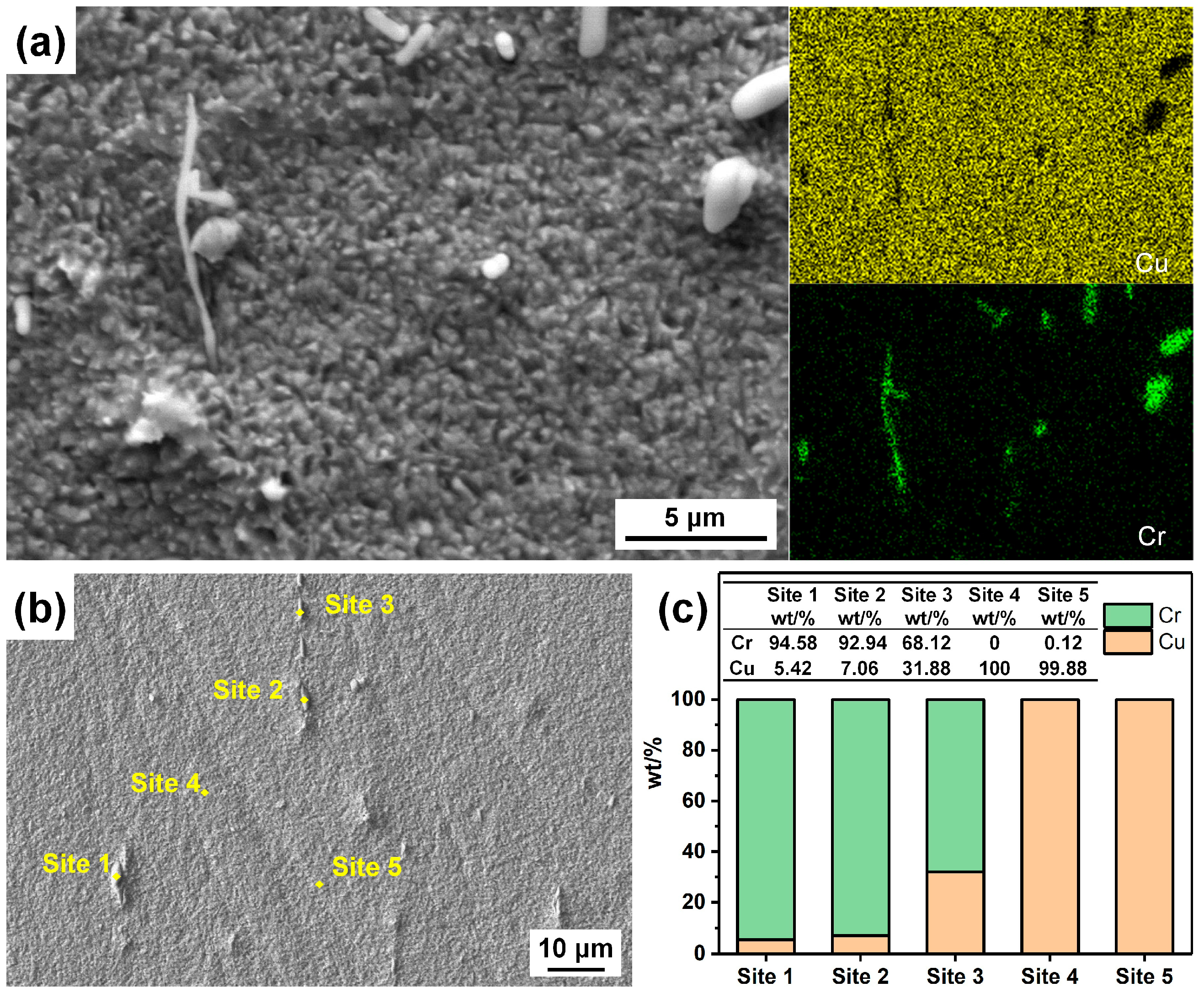
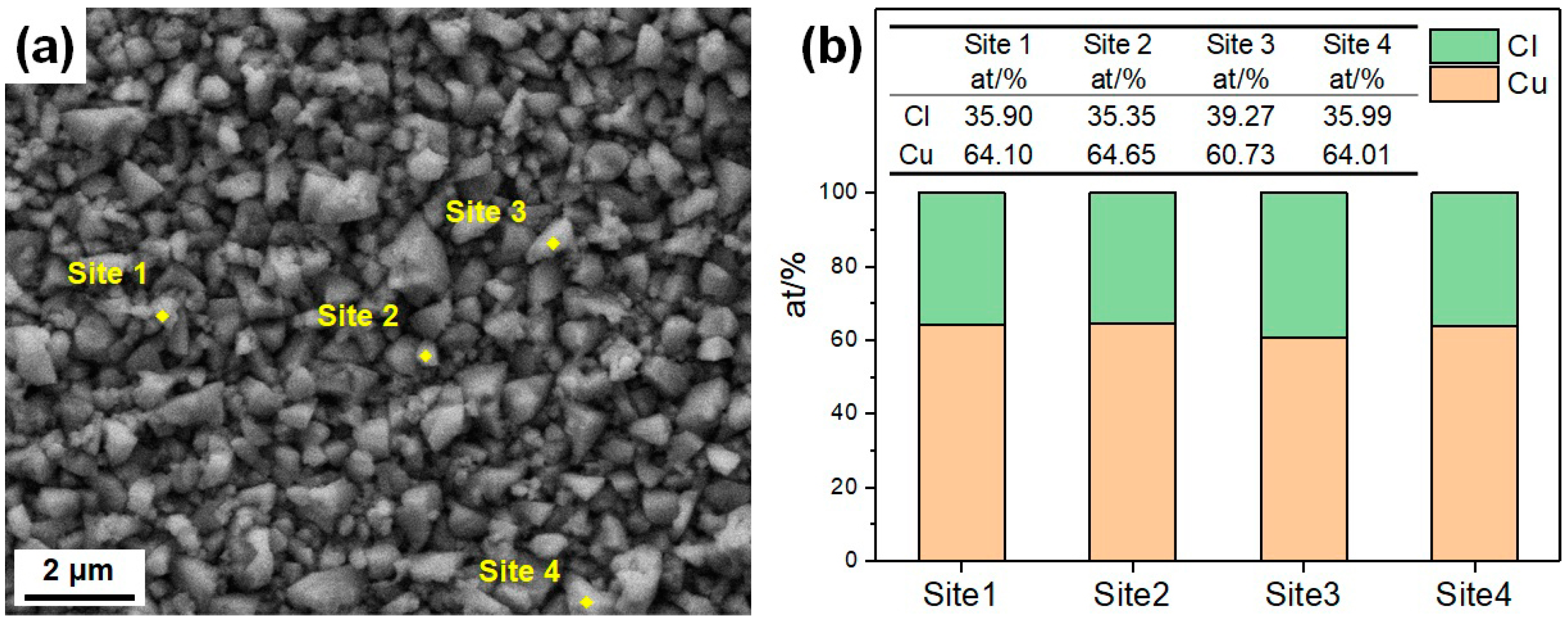
- The CuCrSn alloy surface etched by aqua regia is clean, with very little Cr-rich particles. For the specimens etched with the FeCl3 and CuCl2 etchants, high-density Cr-rich particles remained on the surfaces; thus, etching in these two etchants is more likely to be affected by the alloy composition and the precipitations.
- Due to the serious difference in etching rate at the different locations, the surface roughness of the specimens etched by aqua regia is about 1.27 μm. There is a little fluctuation on the surfaces etched with the FeCl3 and CuCl2 etchants; thus, the surface roughness are only around 0.2~0.3 μm. For the same kind of etchant, the ion concentration can affect the etched surface morphology, although not fundamentally.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Visser, A.; Buhlert, M. Theoretical and practical aspects of the miniaturization of lead frames by double sided asymmetrical spray etching. J. Mater. Process. Technol. 2001, 115, 108–113. [Google Scholar] [CrossRef]
- Zhao, W.C.; Feng, R.; Wang, X.W.; Wang, Y.X.; Pan, Y.K.; Gong, B.K.; Han, X.J.; Feng, T.J. Effect of grain boundaries and crystal orientation on etching behavior of 12 µm thick rolled copper foil. Mater. Today Commun. 2023, 34, 105029. [Google Scholar] [CrossRef]
- Kim, B.J.; Jeon, H.I.; Kim, G.J.; Cho, N.H.; Khim, J.Y.; Kim, Y.K. Wettable flank routable thin Micro Lead Frame for automotive applications. Microelectron. Reliab. 2022, 135, 114602. [Google Scholar] [CrossRef]
- Yang, H.Y.; Ma, Z.C.; Lei, C.H.; Meng, L.; Fang, Y.T.; Liu, J.B.; Wang, H.T. High strength and high conductivity Cu alloys: A review. Sci. China Technol. Sci. 2020, 63, 2505–2517. [Google Scholar] [CrossRef]
- Yang, K.; Wang, Y.H.; Guo, M.X.; Wang, H.; Mo, Y.D.; Dong, X.G.; Lou, H.F. Recent development of advanced precipitation-strengthened Cu alloys with high strength and conductivity: A review. Prog. Mater. Sci. 2023, 138, 101141. [Google Scholar] [CrossRef]
- Williams, K.R.; Gupta, K.; Wasilik, M. Etch rates for micromachining processing-Part II. J. Microelectromech. Syst. 2003, 12, 761–778. [Google Scholar] [CrossRef]
- Çakır, O. Review of etchants for copper and its alloys in wet etching processes. Key Eng. Mater. 2008, 364, 460–465. [Google Scholar]
- Zhang, Y.; Zhang, Z.T.; Yang, J.L.; Yue, Y.K.; Zhang, H.F. Fabrication of superhydrophobic surface on stainless steel by two-step chemical etching. Chem. Phys. Lett. 2022, 797, 139567. [Google Scholar] [CrossRef]
- Allen, D.M.; Almond, H.J.A. Characterization of aqueous ferric chloride etchants used in industrial photochemical machining. J. Mater. Process. Technol. 2004, 149, 238–245. [Google Scholar] [CrossRef]
- Çakır, O. Chemical etching of aluminum. J. Mater. Process. Technol. 2008, 199, 337–340. [Google Scholar] [CrossRef]
- Sheng, J.Z.; Li, H.; Shen, S.N.; Ming, R.J.; Sun, B.; Wang, J.; Zhang, D.D.; Tang, Y.G. Investigation on chemical etching process of FPCB with 18 μm line pitch. IEEE Access 2021, 9, 50872–50879. [Google Scholar] [CrossRef]
- Çakır, O.; Temel, H.; Kiyak, M. Chemical etching of Cu-ETP copper. J. Mater. Process. Technol. 2005, 162, 275–279. [Google Scholar] [CrossRef]
- Choi, J.C.; Lee, J.H. Etching behaviors of Cu and invar for metal core PCB applications. J. Nanosci. Nanotechnol. 2017, 17, 7358–7361. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Huang, C.D.; Ji, X.Q.; Wang, Y.X. A new electrolytic method for on-site regeneration of acidic copper (II) chloride etchant in printed circuit board production. Int. J. Electrochem. Sci. 2013, 8, 6258–6268. [Google Scholar] [CrossRef]
- Darchen, A.; Drissi-Daoudi, R.; Irzho, A. Electrochemical investigations of copper etching by Cu (NH3) 4Cl2 in ammoniacal solutions. J. Appl. Electrochem. 1997, 27, 448–454. [Google Scholar] [CrossRef]
- Wang, S.F.; Ding, F.; Wang, F.W.; Wang, X.; Zou, H.L. Study on reducing side etching of Copper microelectrode by multi-step etching process. Mater. Res. Express 2019, 6, 126411. [Google Scholar] [CrossRef]
- Kondo, K.; Kurihara, H.; Murakami, H. Etching morphology of single-crystal copper. Electrochem. Solid State 2005, 9, C36. [Google Scholar] [CrossRef]
- Köllensperger, P.A.; Karl, W.J.; Ahmad, M.M.; Pike, W.T.; Green, M. Patterning of platinum (Pt) thin films by chemical wet etching in Aqua Regia. J. Micromech. Microeng. 2012, 22, 067001. [Google Scholar] [CrossRef]
- Seo, B.H.; Lee, S.H.; Park, I.S.; Seo, J.H.; Choe, H.H.; Jeon, J.H.; Hong, M.P. Effect of nitric acid on wet etching behavior of Cu/Mo for TFT application. Curr. Appl. Phys. 2011, 11, S262–S265. [Google Scholar] [CrossRef]
- Ralston, K.D.; Birbilis, N. Effect of grain size on corrosion: A review. Corrosion 2010, 66, 075005. [Google Scholar] [CrossRef]
- Fang, J.Y.; Li, C.F.; Liu, F.; Hou, H.L.; Zhang, X.L.; Zhang, Q.K.; Yang, L.J.; Xu, C.; Song, Z.L. Effects of grain orientation and grain size on etching behaviors of high-strength and high-conductivity Cu alloy. Mater. Today Commun. 2024, 38, 108111. [Google Scholar] [CrossRef]
- Peng, L.J.; Xie, H.F.; Huang, G.J.; Xu, G.L.; Yin, X.Q.; Feng, X.; Mi, X.J.; Yang, Z. The phase transformation and strengthening of a Cu-0.71 wt% Cr alloy. J. Alloys Compd. 2017, 708, 1096–1102. [Google Scholar] [CrossRef]
- Li, J.Z.; Ding, H.; Li, B.M.; Gao, W.L.; Bai, J.; Sha, G. Effect of Cr and Sn additions on microstructure, mechanical-electrical properties and softening resistance of Cu-Cr-Sn alloy. Mater. Sci. Eng. A 2021, 802, 140628. [Google Scholar] [CrossRef]
- Yamashita, M.; Mimaki, T.; Hashimoto, S.; Miura, S. Intergranular corrosion of copper and α-Cu-Al alloy bicrystals. Philos. Mag. A 1991, 63, 695–705. [Google Scholar] [CrossRef]
- Miyamoto, H.; Yoshimura, K.; Mimaki, T.; Yamashita, M. Behavior of intergranular corrosion of <011> tilt grain boundaries of pure copper bicrystals. Corros. Sci. 2002, 44, 1835–1846. [Google Scholar]
- Carlson, R.K.; Yang, P.; Clegg, S.M.; Batista, E.R. Mechanistic Study of the Production of NOx Gases from the Reaction of Copper with Nitric Acid. Inorg. Chem. 2020, 59, 16833–16842. [Google Scholar] [CrossRef]
- Atta, R.M. Effect of applying air pressure during wet etching of micro copper PCB tracks with ferric chloride. Int. J. Mater. Res. 2022, 113, 795–808. [Google Scholar] [CrossRef]
- Choi, J.C.; Lee, Y.S.; Lee, J.; Kwon, H.W.; Lee, J.H. Etching behaviors of galvanic coupled metals in PCB applications. In Proceedings of the 2018 Pan Pacific Microelectronics Symposium (Pan Pacific), Big Island, HI, USA, 5–8 February 2018; IEEE: Piscataway, NJ, USA, 2018; pp. 1–4. [Google Scholar]
- Jian, C.; Jusheng, M.; Gangqiang, W.; Xiangyun, T. Effects on etching rates of copper in ferric chloride solutions. In Proceedings of the 2nd 1998 IEMT/IMC Symposium (IEEE Cat. No. 98EX225), Tokyo, Japan, 15–17 August 2002; IEEE: Piscataway, NJ, USA, 2002; pp. 144–148. [Google Scholar]
- Braun, M.; Nobe, K. Electrodissolution kinetics of copper in acidic chloride solutions. J. Electrochem. Soc. 1979, 126, 1666. [Google Scholar] [CrossRef]






| Alloy Composition | Treatments of Specimens | The Four Etchants | Characterization |
|---|---|---|---|
| Cu-0.437Cr-0.253 (wt%) |
| Aqua regia |
|
| Acidic FeCl3 etchant: 45 g FeCl3·6H2O + 100 mL H2O + 10 mL concentrated HCl | |||
| CuCl2-1: 20 g CuCl2·2H2O + 100 mL H2O + 15 mL concentrated HCl | |||
| CuCl2-2: 28.5 g CuCl2·2H2O + 112 mL H2O + 10 mL concentrated HCl |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, J.; Zhang, Q.; Zhang, X.; Liu, F.; Li, C.; Yang, L.; Xu, C.; Song, Z. Influence of Etchants on Etched Surfaces of High-Strength and High-Conductivity Cu Alloy of Different Processing States. Materials 2024, 17, 1966. https://doi.org/10.3390/ma17091966
Fang J, Zhang Q, Zhang X, Liu F, Li C, Yang L, Xu C, Song Z. Influence of Etchants on Etched Surfaces of High-Strength and High-Conductivity Cu Alloy of Different Processing States. Materials. 2024; 17(9):1966. https://doi.org/10.3390/ma17091966
Chicago/Turabian StyleFang, Jinyang, Qingke Zhang, Xinli Zhang, Feng Liu, Chaofeng Li, Lijing Yang, Cheng Xu, and Zhenlun Song. 2024. "Influence of Etchants on Etched Surfaces of High-Strength and High-Conductivity Cu Alloy of Different Processing States" Materials 17, no. 9: 1966. https://doi.org/10.3390/ma17091966





