Genome Survey Sequencing of Acer truncatum Bunge to Identify Genomic Information, Simple Sequence Repeat (SSR) Markers and Complete Chloroplast Genome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Illumina Sequencing Data Analysis and Assembly
2.3. Genome Size Estimation, GC Content and Genome Survey
2.4. The Genomic SSR Markers Detection
2.5. Assembly and Analysis of the Chloroplast Genome
3. Results and Discussion
3.1. Genome Sequencing and Sequence Assembly
3.2. Genomic Characteristics
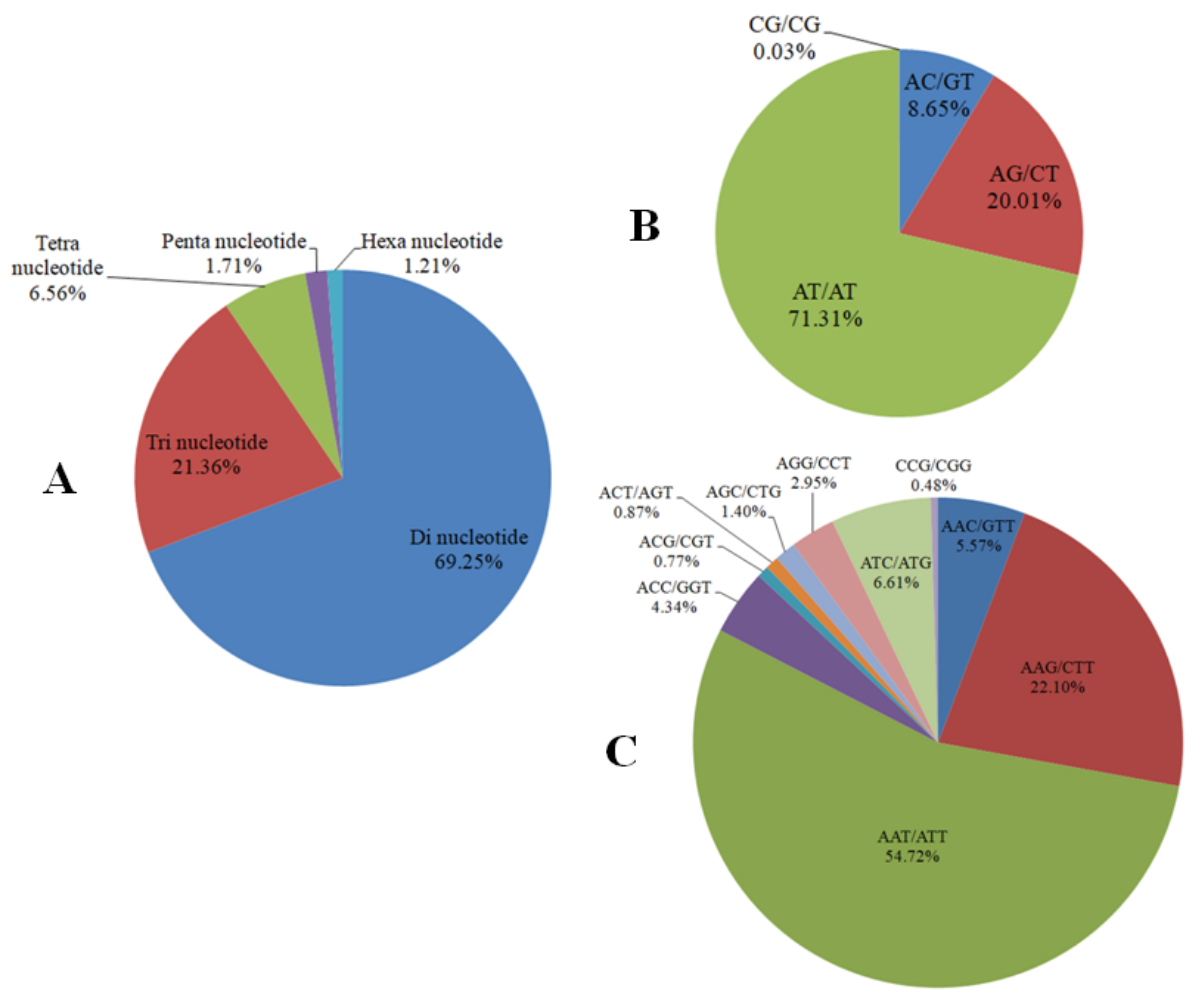
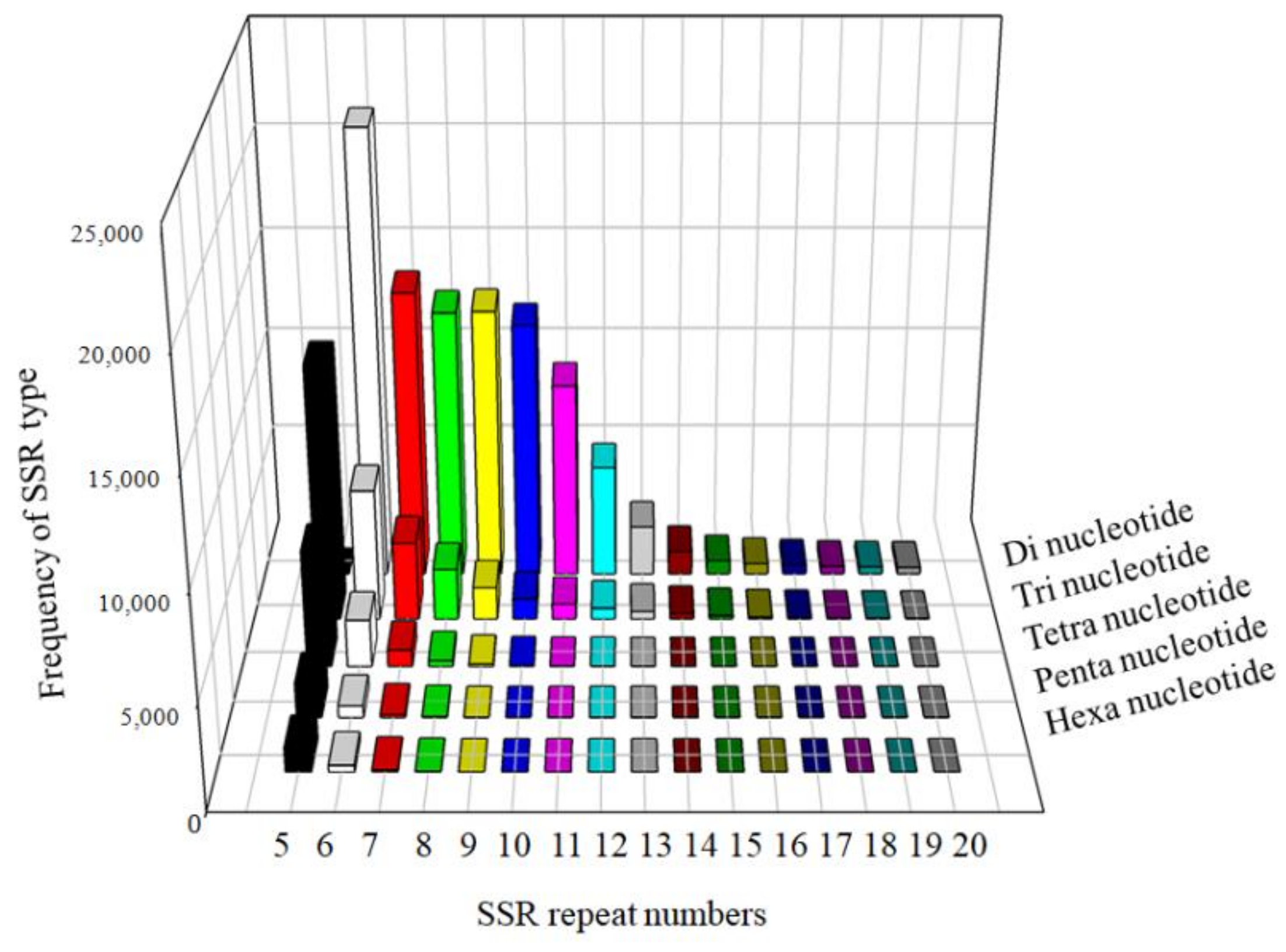
3.3. Genomic SSR Marker Development
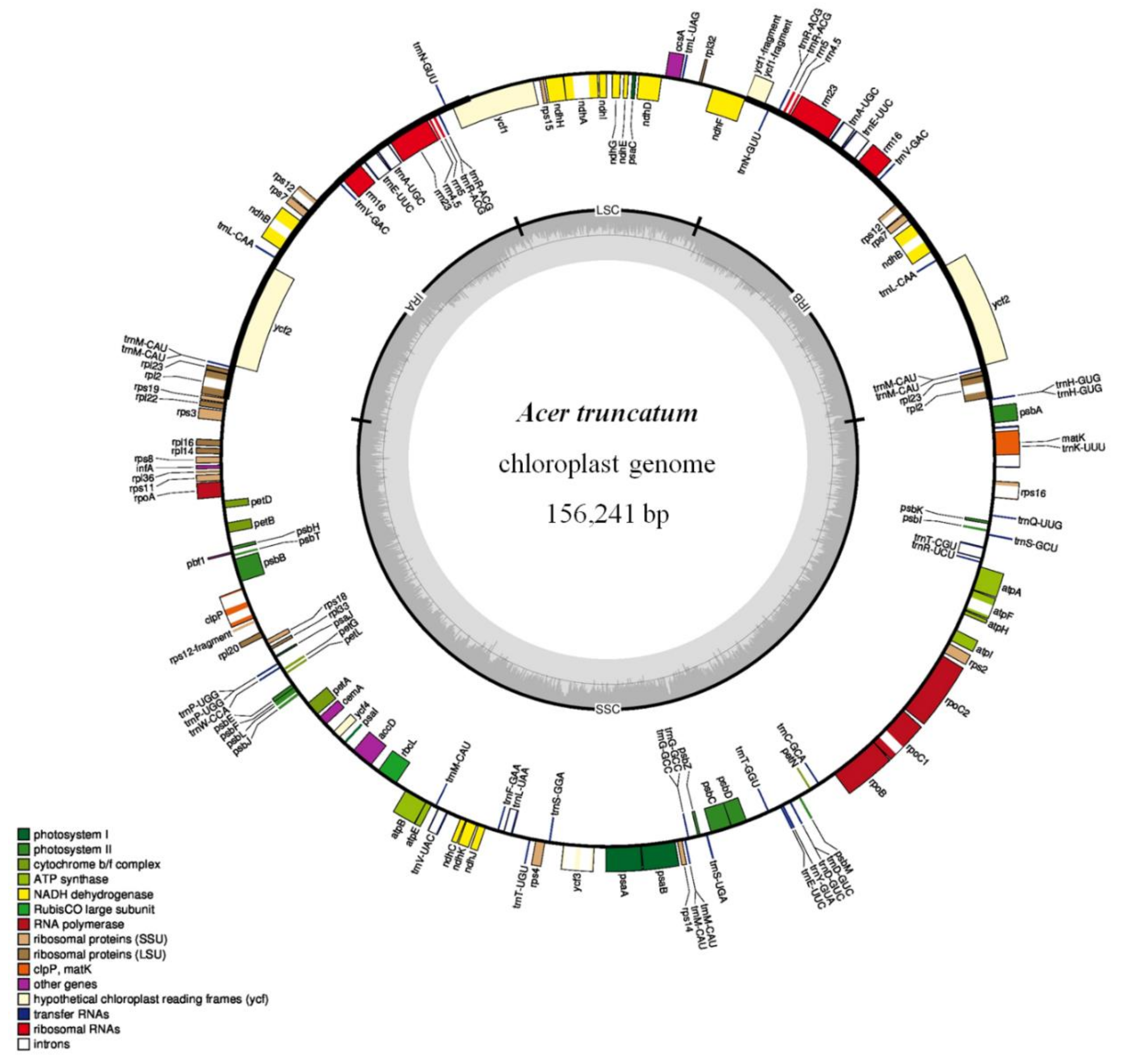
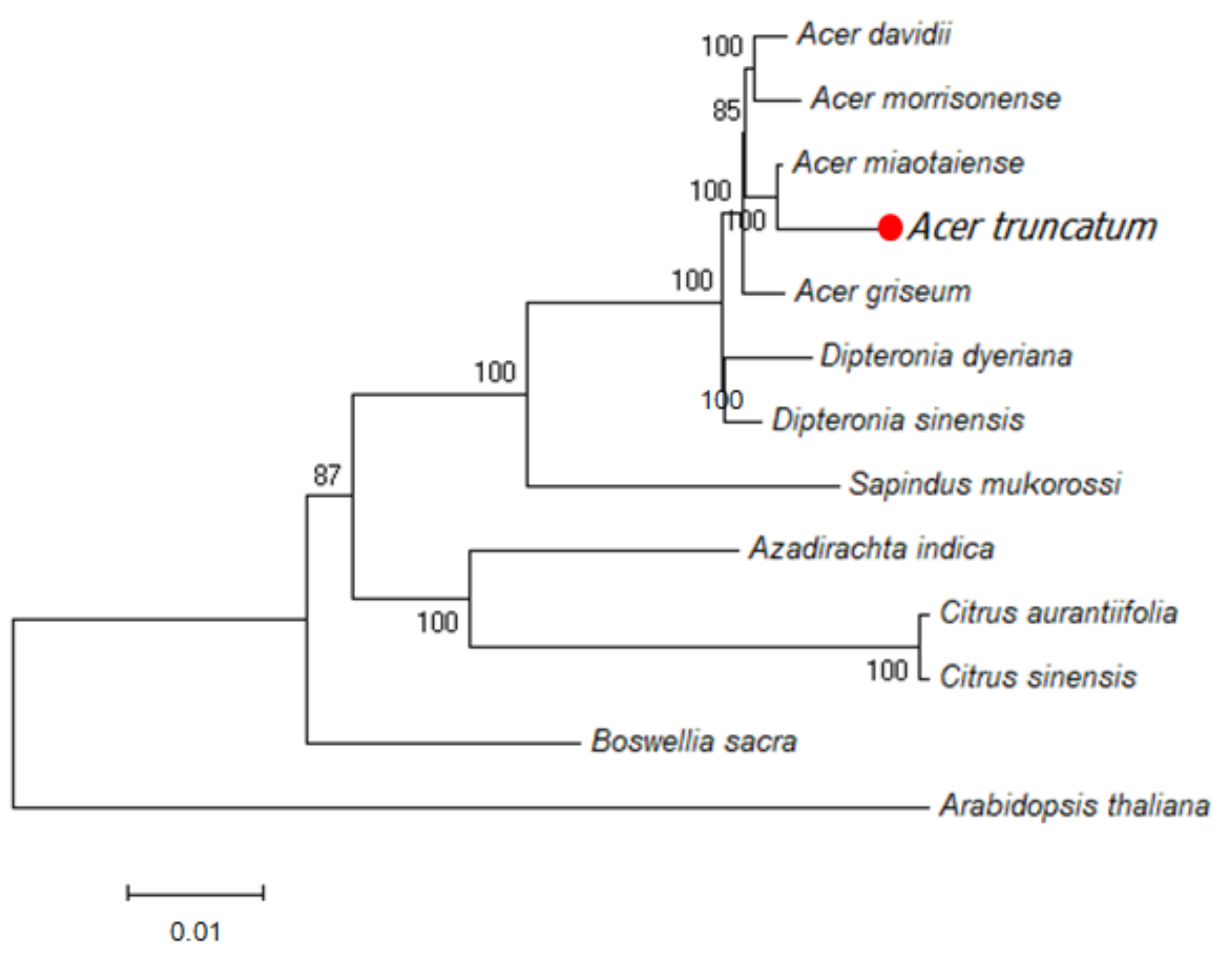
3.4. Assembly of Chloroplast Genome
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Guo, X.; Wang, R.Q.; Chang, R.Y.; Liang, X.Q.; Wang, C.D.; Luo, Y.J.; Yuan, Y.F.; Guo, W.H. Effects of nitrogen addition on growth and photosynthetic characteristics of Acer truncatum seedlings. Dendrobiology 2014, 72, 151–161. [Google Scholar] [CrossRef]
- More, D.; White, J.; More, D.; White, J. Cassell’s trees of Britain and Northern Europe. Cassells Trees Br. North. Eur. 2003. [Google Scholar]
- Wang, X.Y.; Fan, J.S.; Wang, S.Y.; Sun, R.C. A new resource of nervonic acid from purpleblow maple (Acer truncatum) seed oil. For. Prod. J. 2006, 56, 147–150. [Google Scholar]
- Poulos, A. Very long chain fatty acids in higher animals-a review. Lipids 1995, 30, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sargent, J.R.; Coupland, K.; Wilson, R. Nervonic acid and demyelinating disease. Med. Hypotheses 1994, 42, 237–242. [Google Scholar] [CrossRef]
- Guo, X.; Wang, R.Q.; Wang, C.D.; Xu, F.; Zhao, S.; Guo, W.H. Acer truncatum seedlings are more plastic than Quercus variabilis seedlings in response to different light regimes. Dendrobiology 2016, 76, 35–49. [Google Scholar] [CrossRef]
- Li, L.; Wang, X.; Niu, J.; Cui, J.; Zhang, Q.; Wan, W.; Liu, B. Effects of elevated atmospheric O3 concentrations on early and late leaf growth and elemental contents of Acer truncatum Bung under mild drought. Acta Ecol. Sin. 2017, 37, 31–34. [Google Scholar] [CrossRef]
- Yang, L.; Yin, P.; Fan, H.; Xue, Q.; Li, K.; Li, X.; Sun, L.; Liu, Y. Response Surface Methodology Optimization of Ultrasonic-Assisted Extraction of Acer Truncatum Leaves for Maximal Phenolic Yield and Antioxidant Activity. Molecules 2017, 22, 232. [Google Scholar] [CrossRef]
- Zhang, L.; Tu, Z.C.; Xie, X.; Lu, Y.; Wang, Z.X.; Wang, H.; Sha, X.M. Antihyperglycemic, antioxidant activities of two Acer palmatum cultivars, and identification of phenolics profile by UPLC-QTOF-MS/MS: New natural sources of functional constituents. Ind. Crop. Prod. 2016, 89, 522–532. [Google Scholar] [CrossRef]
- Ma, X.; Wu, L.; Ito, Y.; Tian, W. Application of preparative high-speed counter-current chromatography for separation of methyl gallate from Acer truncatum Bunge. J. Chromatogr. A 2005, 1076, 212–215. [Google Scholar] [CrossRef]
- Jiao, Y. Development of simple sequence repeat (SSR) markers from a genome survey of Chinese bayberry (Myrica rubra). BMC Genomics 2012, 13, 201. [Google Scholar] [CrossRef]
- Zhou, W.; Hu, Y.; Sui, Z.; Fu, F.; Wang, J.; Chang, L.; Guo, W.; Li, B. Genome survey sequencing and genetic background characterization of Gracilariopsis lemaneiformis (Rhodophyta) based on next-generation sequencing. PLoS ONE 2013, 8, e69909. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.; Linhai, W.; Yanxin, Z.; Xiaoqiong, Q.; Xiaoling, W.; Xia, D.; Jing, Z.; Xiurong, Z. Development of simple sequence repeat (SSR) markers of sesame (Sesamum indicum) from a genome survey. Molecules 2014, 19, 5150–5162. [Google Scholar]
- He, Y.; Xiao, H.; Deng, C.; Xiong, L.; Nie, H.; Peng, C. Survey of the genome of Pogostemon cablin provides insights into its evolutionary history and sesquiterpenoid biosynthesis. Sci. Rep. 2016, 6, 26405–26415. [Google Scholar] [CrossRef]
- An, J.; Yin, M.; Zhang, Q.; Gong, D.; Jia, X.; Guan, Y.; Hu, J. Genome Survey Sequencing of Luffa Cylindrica L. and Microsatellite High Resolution Melting (SSR-HRM) Analysis for Genetic Relationship of Luffa Genotypes. Int. J. Mol. Sci. 2017, 18, 1. [Google Scholar]
- Wang, C.; Yan, H.; Li, J.; Zhou, S.; Liu, T.; Zhang, X.; Huang, L. Genome survey sequencing of purple elephant grass (Pennisetum purpureum Schum ‘Zise’) and identification of its SSR markers. Mol. Breed. 2018, 38, 94–104. [Google Scholar] [CrossRef]
- Lu, M.; An, H.; Li, L. Genome survey sequencing for the characterization of the genetic background of Rosa roxburghii Tratt and leaf ascorbate metabolism genes. PLoS ONE 2016, 11, e0147530. [Google Scholar] [CrossRef]
- Motalebipour, E.Z.; Kafkas, S.; Khodaeiaminjan, M.; Çoban, N.; Gözel, H. Genome survey of pistachio (Pistacia vera L.) by next generation sequencing: Development of novel SSR markers and genetic diversity in Pistacia species. BMC Genomics 2016, 17, 998. [Google Scholar] [CrossRef]
- Moose, S.P.; Mumm, R.H. Molecular plant breeding as the foundation for 21st century crop improvement. Plant Physiol. 2008, 147, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Davey, J.W.; Hohenlohe, P.A.; Etter, P.D.; Boone, J.Q.; Catchen, J.M.; Blaxter, M.L. Genome-wide genetic marker discovery and genotyping using next-generation sequencing. Nat. Rev. Genet. 2011, 12, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Ekblom, R.; Galindo, J. Applications of next generation sequencing in molecular ecology of non-model organisms. Heredity 2011, 107, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Douglas, S.E. Plastid evolution: Origins, diversity, trends. Curr. Opin. Genet. Dev. 1998, 8, 655–661. [Google Scholar] [CrossRef]
- Jia, Y.; Yang, J.; He, Y.L.; He, Y.; Niu, C.; Gong, L.L.; Li, Z.H. Characterization of the whole chloroplast genome sequence of Acer davidii Franch (Aceraceae). Conserv. Genet. Resour. 2016, 8, 141–143. [Google Scholar] [CrossRef]
- Li, Z.H.; Xie, Y.S.; Zhou, T.; Jia, Y.; He, Y.L.; Yang, J. The complete chloroplast genome sequence of Acer morrisonense (Aceraceae). DNA Seq. 2015, 28, 309–310. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.C.; Chen, S.Y.; Zhang, X.Z. The complete chloroplast genome of the endangered Chinese paperbark maple, Acer griseum (Sapindaceae). Conserv. Genet. Resour. 2017, 9, 1–3. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, B.; Chen, H.; Wang, Y. Characterization of the complete chloroplast genome of Acer miaotaiense (Sapindales: Aceraceae), a rare and vulnerable tree species endemic to China. Conserv. Genet. Resour. 2016, 8, 383–385. [Google Scholar] [CrossRef]
- Luo, R.; Liu, B.; Xie, Y.; Li, Z.; Huang, W.; Yuan, J.; He, G.; Chen, Y.; Qi, P.; Liu, Y. SOAPdenovo2: An empirically improved memory-efficient short-read de novo assembler. Gigascience 2012, 1, 18–24. [Google Scholar] [CrossRef]
- Vurture, G.W.; Sedlazeck, F.J.; Nattestad, M.; Underwood, C.J.; Fang, H.; Gurtowski, J.; Schatz, M.C. GenomeScope: Fast reference-free genome profiling from short reads. Bioinformatics 2017, 33, 2202–2204. [Google Scholar] [CrossRef]
- Wang, R.; Liu, P.; Fan, J.; Li, L. Comparative transcriptome analysis two genotypes of Acer truncatum Bunge seeds reveals candidate genes that influences seed VLCFAs accumulation. Sci. Rep. 2018, 8, 15504–15512. [Google Scholar] [CrossRef]
- Li, L.; Zhang, H.; Liu, Z.; Cui, X.; Zhang, T.; Li, Y.; Zhang, L. Comparative transcriptome sequencing and de novo analysis of Vaccinium corymbosum during fruit and color development. BMC Plant Biol. 2016, 16, 223. [Google Scholar] [CrossRef]
- Dierckxsens, N.; Mardulyn, P.; Smits, G. NOVOPlasty: De novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 2017, 45, e18. [Google Scholar] [PubMed]
- Tillich, M.; Lehwark, P.; Pellizzer, T.; Ulbricht-Jones, E.S.; Fischer, A.; Bock, R.; Greiner, S. GeSeq—Versatile and accurate annotation of organelle genomes. Nucleic Acids Res. 2017, 45, W6–W11. [Google Scholar] [CrossRef]
- Marc, L.; Oliver, D.; Sabine, K.; Ralph, B. Organellar genome DRAW—A suite of tools for generating physical maps of plastid and mitochondrial genomes and visualizing expression data sets. Nucleic Acids Res. 2013, 41, W575–W581. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamidreza, C.; Yee-Greenbaum, J.L.; Glenn, T.; Mary-Jane, L.; Dupont, C.L.; Badger, J.H.; Mark, N.; Rusch, D.B.; Fraser, L.J.; Gormley, N.A. Efficient de novo assembly of single-cell bacterial genomes from short-read data sets. Nat. Biotechnol. 2011, 29, 915–921. [Google Scholar]


| Contigs | |
| Number of sequences | 3,062,684 |
| Total length (bp) | 906,602,751 |
| Max length (bp) | 25,481 |
| N50 length (bp) | 383 |
| N90 length (bp) | 136 |
| Scaffolds | |
| Number of sequences | 2,412,582 |
| Total length (bp) | 866,062,477 |
| Max length (bp) | 62,679 |
| N50 length (bp) | 735 |
| N90 length (bp) | 135 |
| G + C% (ACGT) | 34.78 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Fan, J.; Chang, P.; Zhu, L.; Zhao, M.; Li, L. Genome Survey Sequencing of Acer truncatum Bunge to Identify Genomic Information, Simple Sequence Repeat (SSR) Markers and Complete Chloroplast Genome. Forests 2019, 10, 87. https://doi.org/10.3390/f10020087
Wang R, Fan J, Chang P, Zhu L, Zhao M, Li L. Genome Survey Sequencing of Acer truncatum Bunge to Identify Genomic Information, Simple Sequence Repeat (SSR) Markers and Complete Chloroplast Genome. Forests. 2019; 10(2):87. https://doi.org/10.3390/f10020087
Chicago/Turabian StyleWang, Rongkai, Jinshuan Fan, Pan Chang, Ling Zhu, Mengran Zhao, and Lingli Li. 2019. "Genome Survey Sequencing of Acer truncatum Bunge to Identify Genomic Information, Simple Sequence Repeat (SSR) Markers and Complete Chloroplast Genome" Forests 10, no. 2: 87. https://doi.org/10.3390/f10020087





