Transcriptomic and Metabolomic Analysis of the Heat-Stress Response of Populus tomentosa Carr.
Abstract
:1. Introduction
2. Materials and Methods
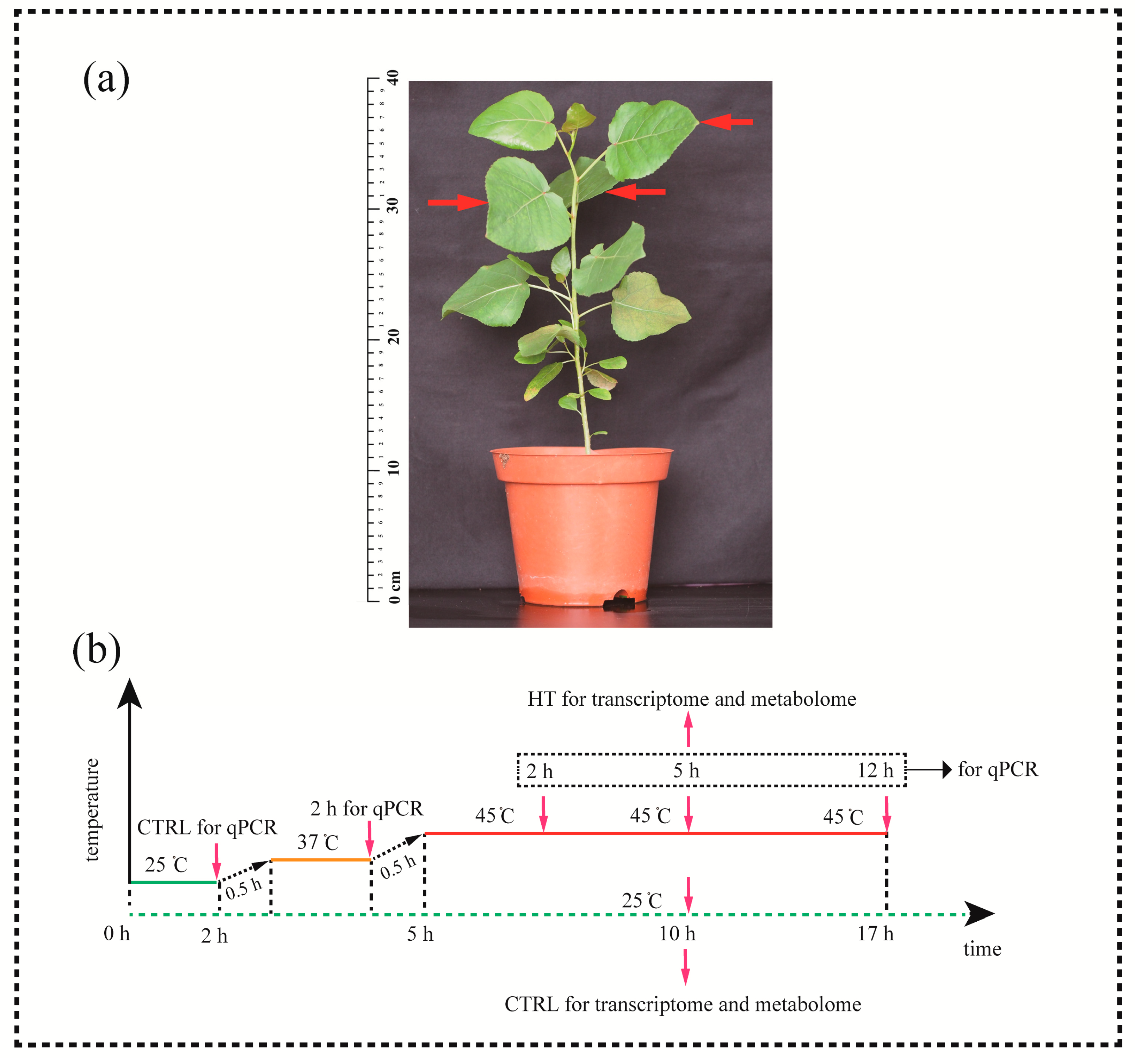
2.1. Plant Materials and Treatments
2.2. RNA Isolation and Illumina Sequencing
2.3. Metabolite Preparation
2.4. Metabolomic Analysis
2.5. Scanning Electron Microscopy
2.6. Assay of Antioxidant Enzyme Activity
2.7. Soluble Sugar and Soluble Protein Assays
2.8. H2O2 Assay
2.9. Real-Time Reverse Transcription PCR
2.10. Statistical Analysis
3. Results
3.1. Stomatal Variation and Physiological Changes under Heat Stress
3.2. Transcriptomic Changes in Response to Heat Stress
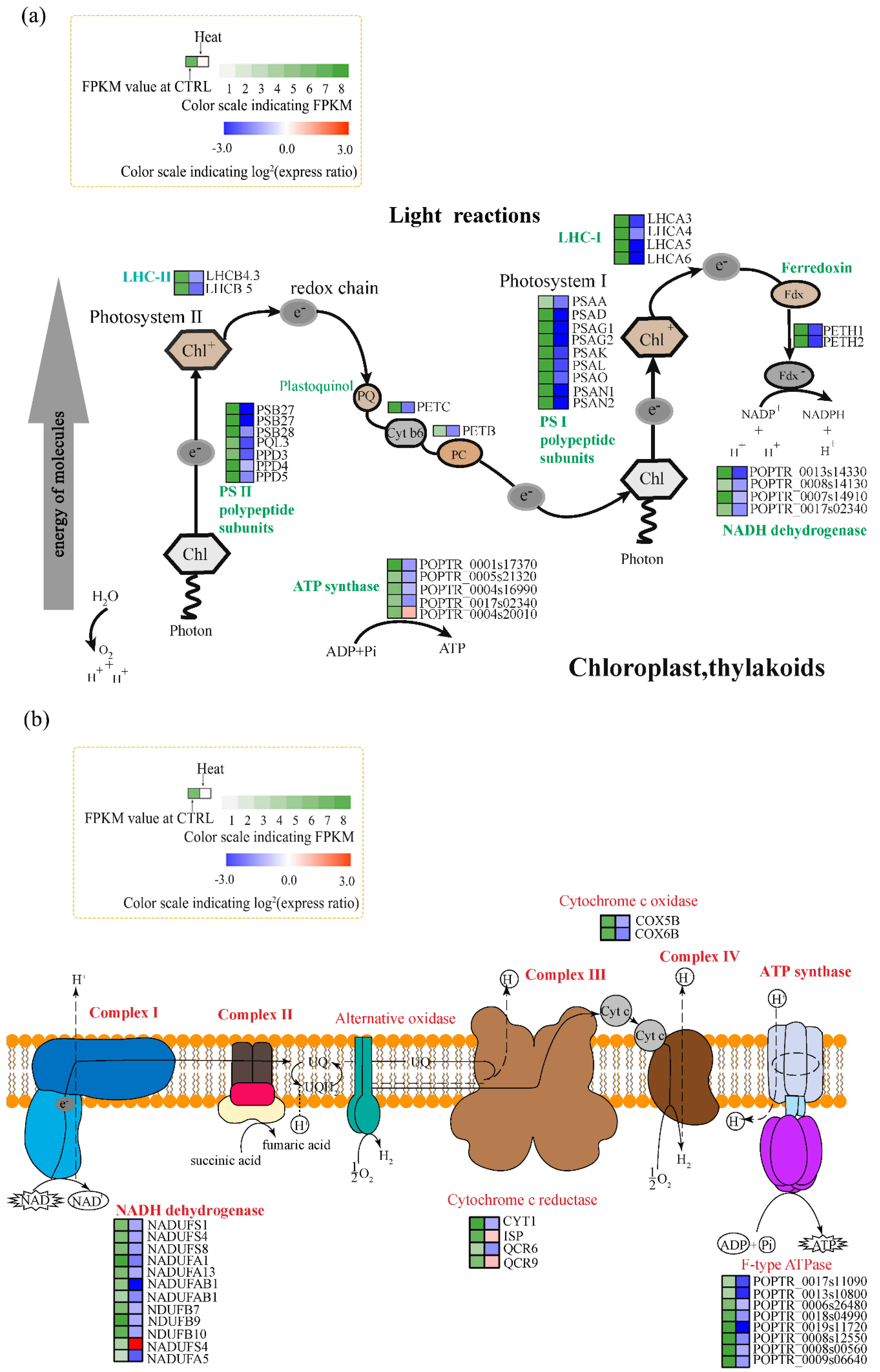
3.3. Genes Associated with Photosynthesis and Respiration
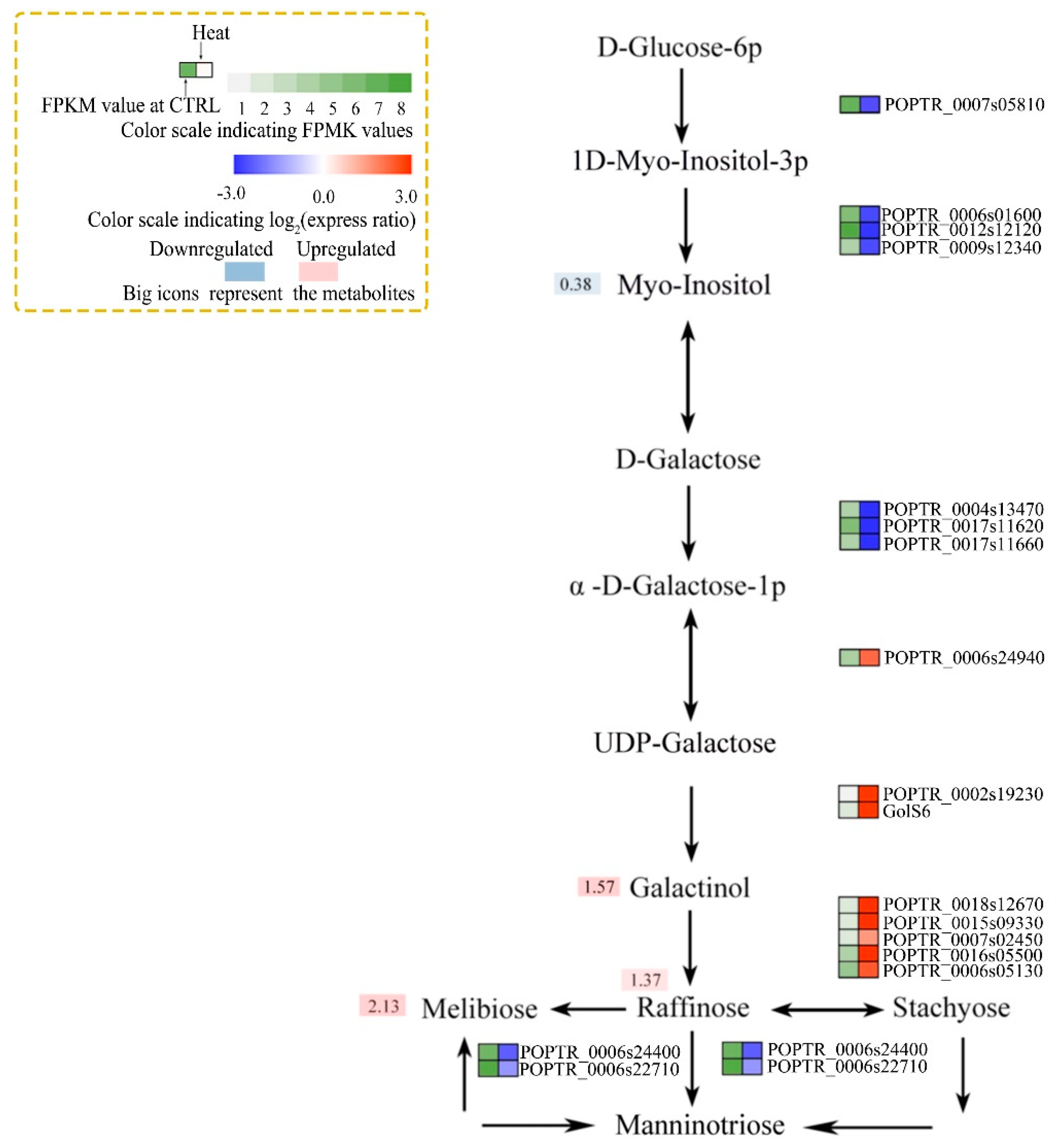
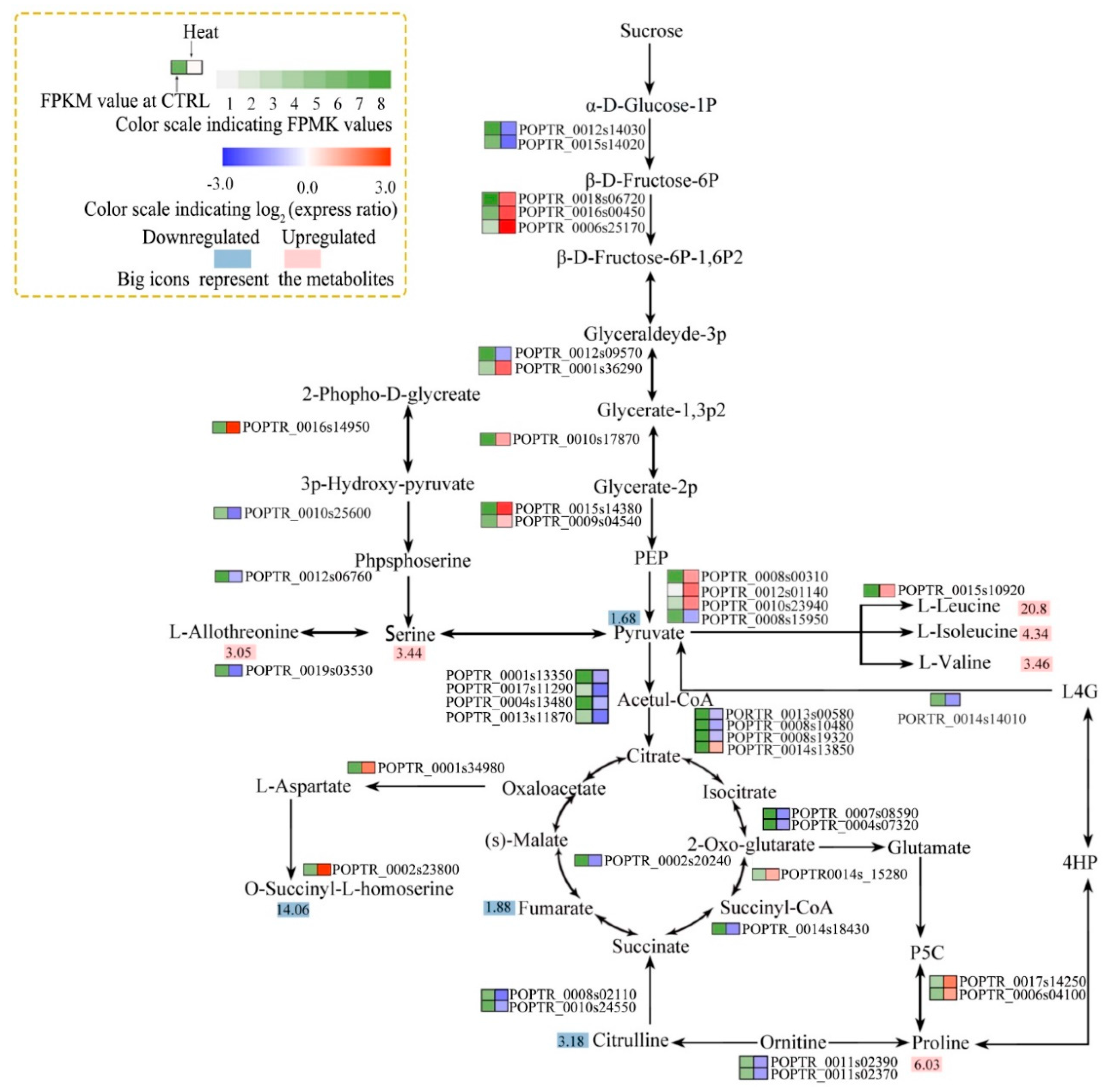
3.4. Integrated Analysis of Osmotic and Resistant Substance Metabolism-associated Gene Expression and Metabolite Levels
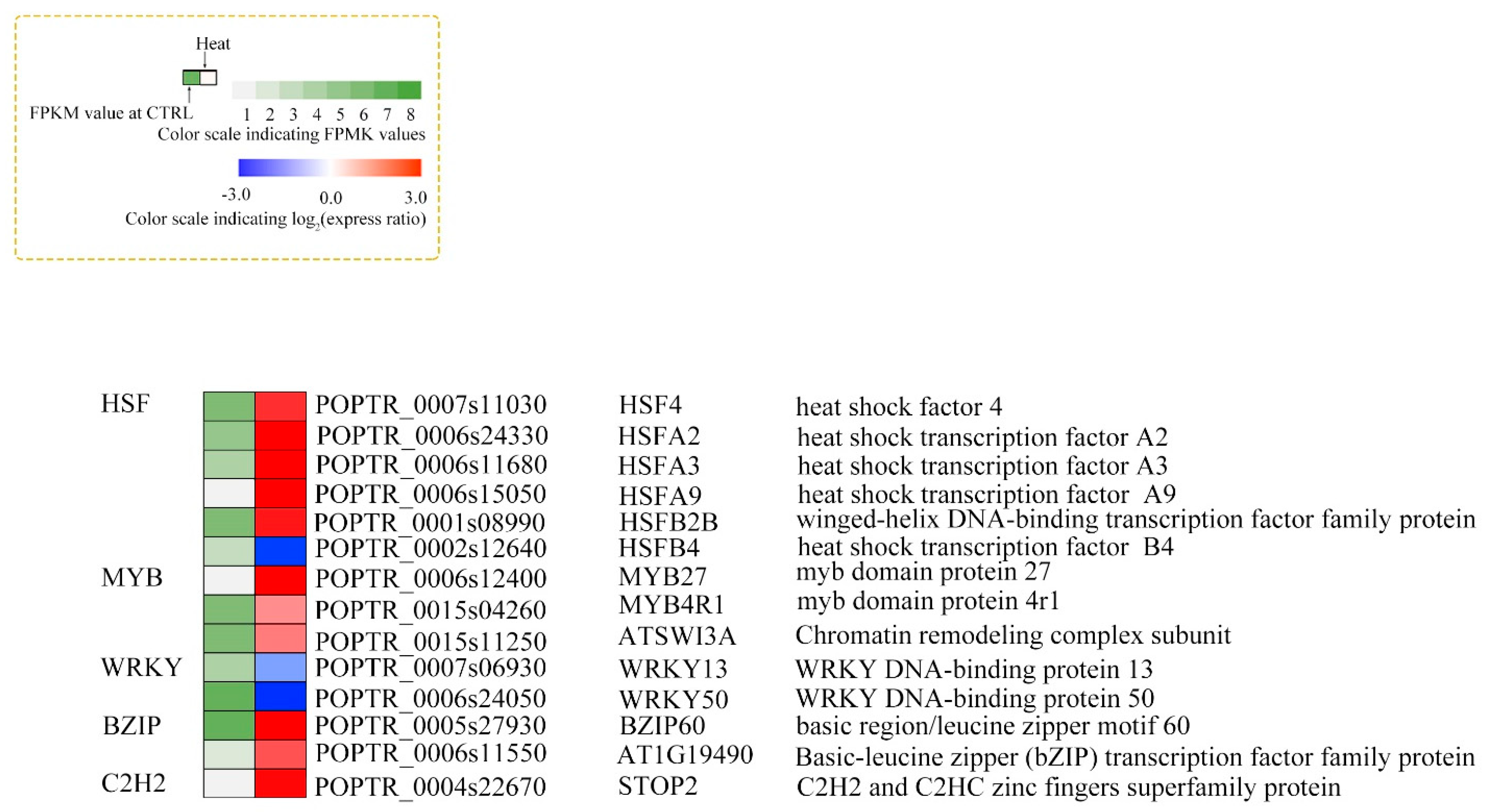
3.5. Transcription Factors Involved in Heat Treatment
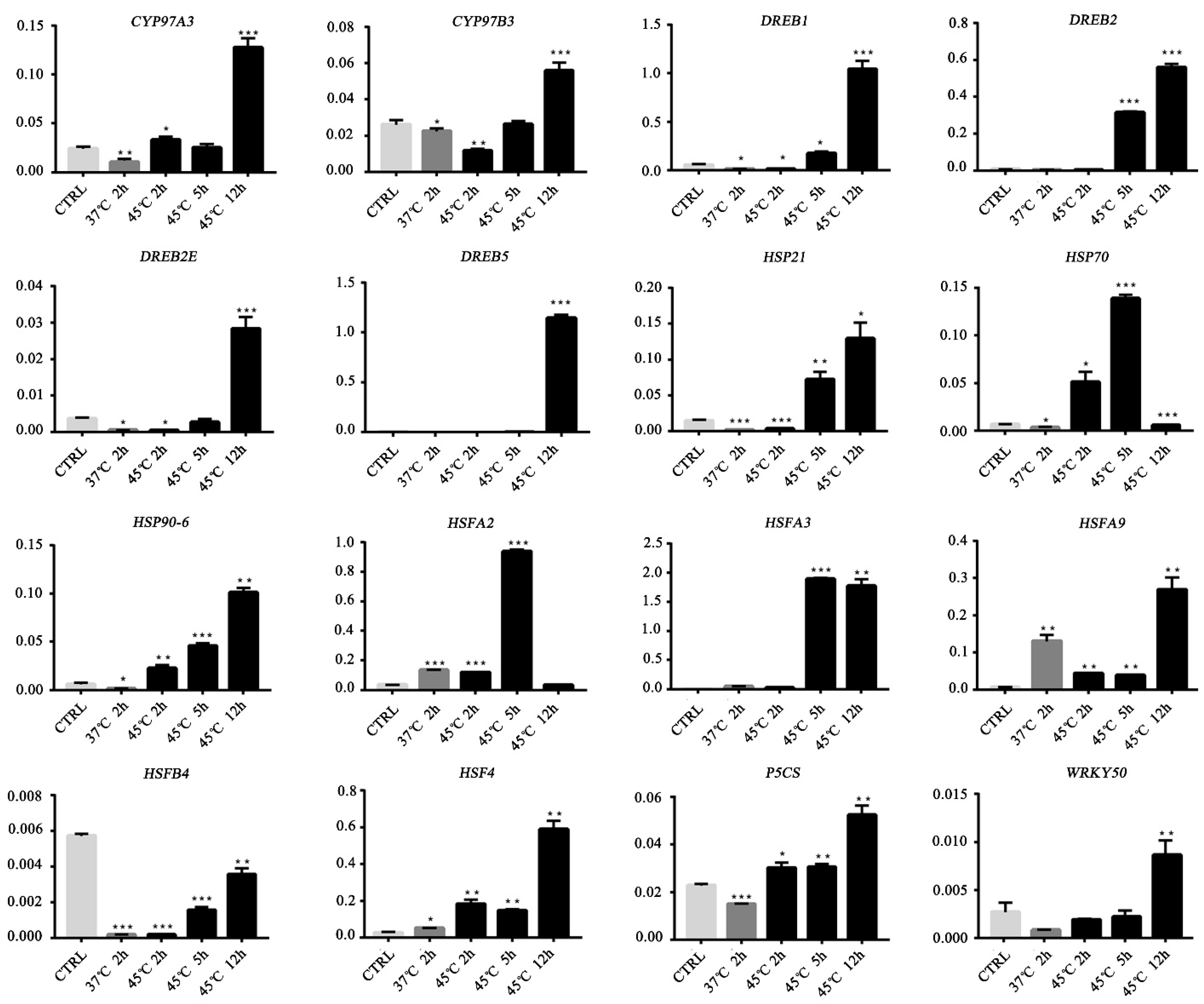
3.6. Validation of Gene Expression by qRT-PCR
4. Discussion
4.1. Effects of Heat Stress on Antioxidant Activity
4.2. Effects of Heat Stress on Photosynthesis
4.3. Effects of Heat Stress on Respiration
4.4. Osmotic and Resistant Substance Metabolism in Response to Heat Stress
4.5. Transcription Factors Involved in the Response to Heat Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Easterling, D.R.; Meehl, G.A.; Parmesan, C.; Changnon, S.A.; Karl, T.R.; Mearns, L.O. Climate extremes: Observations, modeling, and impacts. Science 2000, 289, 2068–2074. [Google Scholar] [CrossRef]
- Ohama, N.; Sato, H.; Shinozaki, K.; Yamaguchi-Shinozak, K. Transcriptional regulatory network of plant heat stress response. Trends Plant Sci. 2017, 22, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Bita, C.E.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Sharma, A.; Mishra, M.; Mishra, R.K.; Chowdhuri, D.K. Heat shock proteins in toxicology: How close and how far? Life Sci. 2010, 86, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Bita, C.E.; Zenoni, S.; Vriezen, W.H.; Mariani, C.; Pezzotti, M.; Gerats, T. Temperature stress differentially modulates transcription in meiotic anthers of heat-tolerant and heat-sensitive tomato plants. BMC Genom. 2011, 12, 384. [Google Scholar] [CrossRef] [PubMed]
- Rienth, M.; Torregrosa, L.; Luchaire, N.; Chatbanyong, R.; Lecourieux, D.; Kelly, M.T.; Romieu, C. Day and night heat stress trigger different transcriptomic responses in green and ripening grapevine (Vitis vinifera) fruit. BMC Plant Biol. 2014, 14, 108. [Google Scholar] [CrossRef]
- Yang, J.; Chen, X.; Zhu, C.; Peng, X.; He, X.; Fu, J.; Ouyang, L.; Bian, J.; Hu, L.; Sun, X.; et al. RNA-seq reveals differentially expressed genes of rice (Oryza sativa) spikelet in response to temperature interacting with nitrogen at meiosis stage. BMC Genom. 2015, 16, 959. [Google Scholar] [CrossRef]
- Ohama, N.; Kusakabe, K.; Mizoi, J.; Zhao, H.; Kidokoro, S.; Koizumi, S.; Takahashi, F.; Ishida, T.; Yanagisawa, S.; Shinozaki, K.; et al. The transcriptional cascade in the heat stress response of Arabidopsis is strictly regulated at the level of transcription factor expression. Plant Cell 2016, 28, 181–201. [Google Scholar] [CrossRef]
- Kaplan, F.; Kopka, J.; Haskell, D.W.; Zhao, W.; Schiller, K.C.; Gatzke, N.; Sung, D.Y.; Guy, C.L. Exploring the temperature-stress metabolome of Arabidopsis. Plant Physiol. 2004, 136, 4159–4168. [Google Scholar] [CrossRef]
- Luengwilai, K.; Saltveit, M.; Beckles, D.M. Metabolite content of harvested Micro-Tom tomato (Solanum lycopersicum L.) fruit is altered by chilling and protective heat-shock treatments as shown by GC–MS metabolic profiling. Postharvest Biol. Technol. 2012, 63, 116–122. [Google Scholar] [CrossRef]
- Obata, T.; Witt, S.; Lisec, J.; Palacios-Rojas, N.; Florez-Sarasa, I.; Yousfi, S.; Araus, J.L.; Cairns, J.E.; Fernie, A.R. Metabolite profiles of maize leaves in drought, heat and combined stress field trials reveal the relationship between metabolism and grain yield. Plant Physiol. 2015, 169, 2665–2683. [Google Scholar] [CrossRef] [PubMed]
- De Leonardis, A.M.; Fragasso, M.; Beleggia, R.; Ficco, D.B.M.; De Vita, P.; Mastrangelo, A.M. Effects of heat stress on metabolite accumulation and composition, and nutritional properties of durum wheat grain. Int. J. Mol. Sci. 2015, 16, 30382–30404. [Google Scholar] [CrossRef] [PubMed]
- Glaubitz, U.; Li, X.; Schaedel, S.; Erban, A.; Sulpice, R.; Kopka, J.; Hincha, D.K.; Zuther, E. Integrated analysis of rice transcriptomic and metabolomic responses to elevated night temperatures identifies sensitivity- and tolerance-related profiles. Plant Cell Environ. 2017, 40, 121–137. [Google Scholar] [CrossRef]
- Janz, D.; Lautner, S.; Wildhagen, H.; Behnke, K.; Schnitzler, J.P.; Rennenberg, H.; Fromm, J.; Polle, A. Salt stress induces the formation of a novel type of ‘pressure wood’ in two Populus species. New Phytol. 2012, 194, 129–141. [Google Scholar] [CrossRef]
- Chen, J.; Yin, W.; Xia, X. Transcriptome profiles of Populus euphratica upon heat shock stress. Curr. Genom. 2014, 15, 326–340. [Google Scholar] [CrossRef]
- Song, Y.; Chen, Q.; Ci, D.; Shao, X.; Zhang, D. Effects of high temperature on photosynthesis and related gene expression in poplar. BMC Plant Biol. 2014, 14, 111. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Jiang, H.; Zhang, S.; Chen, L.; Li, X.; Korpelainen, H.; Li, C. Transcriptional profiling reveals sexual differences of the leaf transcriptomes in response to drought stress in Populus yunnanensis. Tree Physiol. 2012, 32, 1541–1555. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Li, S.; Cao, X.; Li, H.; Shi, W.; Polle, A.; Liu, T.X.; Peng, C.; Luo, Z.B. Physiological and transcriptional regulation in poplar roots and leaves during acclimation to high temperature and drought. Physiol. Plant 2016, 157, 38–53. [Google Scholar] [CrossRef]
- Jia, J.; Zhou, J.; Shi, W.; Cao, X.; Luo, J.; Polle, A.; Luo, Z.B. Comparative transcriptomic analysis reveals the roles of overlapping heat-/drought-responsive genes in poplars exposed to high temperature and drought. Sci. Rep. 2017, 7, 43215. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.W.; Lu, W.C.; Lu, Z.G.; Ren, S.X.; Zhao, B.B.; Wang, L.; Teng, N.J.; Jin, B. Identification and analysis of microRNAs in the SAM and leaves of Populus tomentosa. Forests 2019, 10, 130. [Google Scholar] [CrossRef]
- Lu, Z.; Xu, J.; Li, W.; Zhang, L.; Cui, J.; He, Q.; Wang, L.; Jin, B. Transcriptomic analysis reveals mechanisms of sterile and fertile flower differentiation and development in Viburnum macrocephalum f. keteleeri. Front. Plant Sci. 2017, 8, 261. [Google Scholar] [CrossRef] [PubMed]
- Populus trichocarpa Genome. Available online: ftp://ftp.ensemblgenomes.org/pub/release-40/plants/fasta/populus_trichocarpa/ (accessed on 29 April 2019).
- Trapnell, C.; Pachter, L.; Salzberg, S.L. TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics 2009, 25, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Cai, T.; Olyarchuk, J.G.; Wei, L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef] [Green Version]
- Kind, T.; Wohlgemuth, G.; Lee, D.Y.; Lu, Y.; Palazoglu, M.; Shahbaz, S.; Fiehn, O. FiehnLib–mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal. Chem. 2009, 81, 10038–10048. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, L.; Cheng, F.; Zhao, J.; Cui, J.; Li, W.; Wang, L.; Jin, B. The morphology, ultrastructure, element distribution and motion behaviour in pollen of Ginkgo biloba L. Trees 2016, 30, 2189–2201. [Google Scholar] [CrossRef]
- Bournonville, C.F.; Díaz-Ricci, J.C. Quantitative determination of superoxide in plant leaves using a modified NBT staining method. Phytochem. Anal. 2011, 22, 268–271. [Google Scholar] [CrossRef]
- Allen, P.J.; Bennett, K. PASW Statistics by SPSS: A Practical Guide: Version 18.0; Cengage Learning: South Melbourne, Austria, 2010. [Google Scholar]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signalling. J. Exp. Bot. 2013, 65, 1229–1240. [Google Scholar] [CrossRef] [Green Version]
- Jin, B.; Wang, L.; Wang, J.; Jiang, K.Z.; Wang, Y.; Jiang, X.X.; Ni, C.Y.; Wang, Y.L.; Teng, N.J. The effect of experimental warming on leaf functional traits, leaf structure and leaf biochemistry in Arabidopsis thaliana. BMC Plant Biol. 2011, 11, 35. [Google Scholar] [CrossRef]
- Ciou, J.Y.; Lin, H.H.; Chiang, P.Y.; Wang, C.C.; Charles, A.L. The role of polyphenol oxidase and peroxidase in the browning of water caltrop pericarp during heat treatment. Food Chem. 2011, 127, 523–527. [Google Scholar] [CrossRef]
- Silva, E.N.; Ferreira-Silva, S.L.; Fontenele, A.V.; Ribeiro, R.V.; Viégas, R.A.; Silveira, J.A.G. Photosynthetic changes and protective mechanisms against oxidative damage subjected to isolated and combined drought and heat stresses in Jatropha curcas plants. J. Plant Physiol. 2010, 167, 1157–1164. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Kurek, I.; Chang, T.K.; Bertain, S.M.; Madrigal, A.; Liu, L.; Lassner, M.W.; Zhu, G. Enhanced thermostability of Arabidopsis Rubisco activase improves photosynthesis and growth rates under moderate heat stress. Plant Cell 2007, 19, 3230–3241. [Google Scholar] [CrossRef]
- Sharkey, T.D.; Zhang, R. High temperature effects on electron and proton circuits of photosynthesis. J. Integr. Plant Biol. 2010, 52, 712–722. [Google Scholar] [CrossRef]
- Liu, H.; Huang, R.Y.C.; Chen, J.; Gross, M.L.; Pakrasi, H.B. Psb27, a transiently associated protein, binds to the chlorophyll binding protein CP43 in photosystem II assembly intermediates. Proc. Natl. Acad. Sci. USA 2011, 108, 18536–18541. [Google Scholar] [CrossRef]
- Sakata, S.; Mizusawa, N.; Kubota-Kawai, H.; Sakurai, I.; Wada, H. Psb28 is involved in recovery of photosystem II at high temperature in Synechocystis sp. PCC 6803. Biochim. Biophys. Acta 2013, 1827, 50–59. [Google Scholar] [CrossRef]
- Alboresi, A.; Caffarri, S.; Nogue, F.; Bassi, R.; Morosinotto, T. In silico and biochemical analysis of Physcomitrella patens photosynthetic antenna: Identification of subunits which evolved upon land adaptation. PLoS ONE 2008, 3, e2033. [Google Scholar] [CrossRef]
- Downs, C.A.; Heckathorn, S.A. The mitochondrial small heat-shock protein protects NADH: Ubiquinone oxidoreductase of the electron transport chain during heat stress in plants. FEBS Lett. 1998, 430, 246–250. [Google Scholar] [CrossRef]
- Zhou, H.H.; Chen, Y.N.; Li, W.H.; Chen, Y.P. Photosynthesis of Populus euphratica in relation to groundwater depths and high temperature in arid environment, northwest China. Photosynthetica 2010, 48, 257–268. [Google Scholar] [CrossRef]
- Bolton, M.D. Primary metabolism and plant defense—Fuel for the fire. Mol. Plant Microbe Interact. 2009, 22, 487–497. [Google Scholar] [CrossRef]
- Yamakawa, H.; Hakata, M. Atlas of rice grain filling-related metabolism under high temperature: Joint analysis of metabolome and transcriptome demonstrated inhibition of starch accumulation and induction of amino acid accumulation. Plant Cell Physiol. 2010, 51, 795–809. [Google Scholar] [CrossRef]
- Araujo, W.L.; Nunes-Nesi, A.; Nikoloski, Z.; Sweetlove, L.J.; Fernie, A.R. Metabolic control and regulation of the tricarboxylic acid cycle in photosynthetic and heterotrophic plant tissues. Plant Cell Environ. 2012, 35, 1–21. [Google Scholar] [CrossRef]
- Sicher, R. Combined effects of CO2 enrichment and elevated growth temperatures on metabolites in soybean leaflets: Evidence for dynamic changes of TCA cycle intermediates. Planta 2013, 238, 369–380. [Google Scholar] [CrossRef]
- Hamanishi, E.T.; Barchet, G.L.H.; Dauwe, R.; Mansfield, S.D.; Campbell, M.M. Poplar trees reconfigure the transcriptome and metabolome in response to drought in a genotype-and time-of-day-dependent manner. BMC Genom. 2015, 16, 329. [Google Scholar] [CrossRef]
- Nishizawa, A.; Yabuta, Y.; Shigeoka, S. Galactinol and raffinose constitute a novel function to protect plants from oxidative damage. Plant Physiol. 2008, 147, 1251–1263. [Google Scholar] [CrossRef]
- Lv, W.T.; Lin, B.; Zhang, M.; Xue, J.H. Proline accumulation is inhibitory to Arabidopsis seedlings during heat stress. Plant Physiol. 2011, 156, 1921–1933. [Google Scholar] [CrossRef]
- Szabados, L.; Savoure, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Hahn, A.; Bublak, D.; Schleiff, E.; Scharf, K.D. Crosstalk between Hsp90 and Hsp70 chaperones and heat stress transcription factors in tomato. Plant Cell 2011, 23, 741–755. [Google Scholar] [CrossRef]
- Grover, A.; Mittal, D.; Negi, M.; Lavania, D. Generating high temperature tolerant transgenic plants: Achievements and challenges. Plant Sci. 2013, 205–206, 38–47. [Google Scholar] [CrossRef]
- Tripp, J.; Mishra, S.K.; Scharf, K.D. Functional dissection of the cytosolic chaperone network in tomato mesophyll protoplasts. Plant Cell Environ. 2009, 32, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Li, Y.; Xing, D.; Gao, C. Characterization of mitochondrial dynamics and subcellular localization of ROS reveal that HsfA2 alleviates oxidative damage caused by heat stress in Arabidopsis. J. Exp. Bot. 2009, 60, 2073–2091. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, L.; Wang, A.; Xu, X.; Li, J. Ectopic overexpression of SlHsfA3, a heat stress transcription factor from tomato, confers increased thermotolerance and salt hypersensitivity in germination in transgenic Arabidopsis. PLoS ONE 2013, 8, e54880. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Fu, Q.; Huang, W.; Yu, D. Functional analysis of an Arabidopsis transcription factor WRKY25 in heat stress. Plant Cell Rep. 2009, 28, 683–693. [Google Scholar] [CrossRef]
- Li, S.; Zhou, X.; Chen, L.; Huang, W.; Yu, D. Functional characterization of Arabidopsis thaliana WRKY39 in heat stress. Mol. Cells 2010, 29, 475–483. [Google Scholar] [CrossRef]



| Index Type | Indexes |
|---|---|
| ROS scavenging | SOD ↑ H2O2 ↑ CAT ↑ |
| Stomata activity | Stomata opening ↓ |
| Photosynthesis | PSB27↓ PSB28 ↓ PQL3 ↓ PPD3 ↓ PSAD ↓ CB4.3 ↓ LCB5↓ LHA3 ↓ LHCA4 ↓ LCA5 ↓ LCA6 ↓ PETB ↓ PTEC↓ |
| Respiration (glycolysis) | Phosphofructokinase ↑ Phosphoglyceratekinase ↑ Enolase ↑ Pyruvatekinase ↑ Phosphoglucomutase ↓ |
| Respiration (TCA cycle) | Dihydrolipoamide Acetyltransferase ↓ Isocitrate dehydrogenase ↓ ATP citrate lyase ↓ Succinate—CoA ligaseligase ↓ Fumarate hydratase ↓ Aconitate hydratase ↑ |
| Respiration (ETC) | NADUAFB1↓ NADUFS4 ↑ COX5B↓ COX6B ↓ F-type ATPase ↓ |
| Lipids Amino acid | L-allothreonine ↑ Pelargonic acid ↑ Itaconic acid ↓Androstanediol ↓ Valine ↑ Leucine ↑ Isoleucine ↑ |
| Osmoprotectants | Proline ↑ Raffinose ↑ Melibose ↑ |
| TFs and HSPs | HSFA2 ↑ HSFA3 ↑ HSF4 ↑ HSFB4 ↑ HSFA9 ↑ HSP70 ↑ HSP90-6 ↑ DREB2 ↑ WRKY13↓ WRKY50 ↓ bZIP60↑ MYB27 ↑ MYB4R1 ↑ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, S.; Ma, K.; Lu, Z.; Chen, G.; Cui, J.; Tong, P.; Wang, L.; Teng, N.; Jin, B. Transcriptomic and Metabolomic Analysis of the Heat-Stress Response of Populus tomentosa Carr. Forests 2019, 10, 383. https://doi.org/10.3390/f10050383
Ren S, Ma K, Lu Z, Chen G, Cui J, Tong P, Wang L, Teng N, Jin B. Transcriptomic and Metabolomic Analysis of the Heat-Stress Response of Populus tomentosa Carr. Forests. 2019; 10(5):383. https://doi.org/10.3390/f10050383
Chicago/Turabian StyleRen, Shixiong, Kaibiao Ma, Zhaogeng Lu, Gang Chen, Jiawen Cui, Peixi Tong, Li Wang, Nianjun Teng, and Biao Jin. 2019. "Transcriptomic and Metabolomic Analysis of the Heat-Stress Response of Populus tomentosa Carr." Forests 10, no. 5: 383. https://doi.org/10.3390/f10050383





