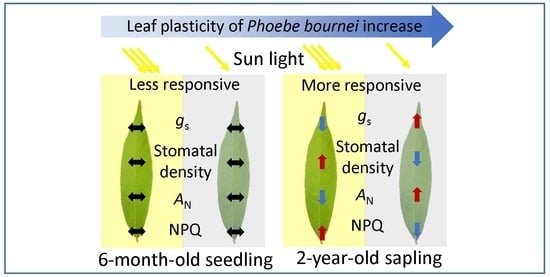
Leaf Plasticity of the Subtropical Evergreen Tree Phoebe bournei Increases with Ontogeny in Response to Sun and Shade
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Plant Morphology Measurements and Leaf Structural Analysis
2.3. Gas Exchange Measurements
2.4. Chlorophyll Fluorescence Measurements
2.5. Light and Transmission Electron Microscopy
2.6. Carbohydrate, Chlorophyll, and Oxidative Parameter Analyses
2.7. Statistical Analysis
3. Results
3.1. Growth and Morphological Variation Due to Sun and Shade
3.2. Anatomical Differences of P. bournei Plants Due to Sun and Shade
3.3. Transmission Electron Micrographs of Chloroplasts
3.4. ROS and Chlorophyll Changes in Response to Sun and Shade H2O2
3.5. Chlorophyll Fluorescence
3.6. Leaf Photosynthetic Capacity Changes by Gas Exchange Analysis
3.7. Nonstructural Carbohydrate (NSC) Accumulation
3.8. Plasticity Index
4. Discussion
4.1. Investments for Constructing New Leaves Increase with Light Intensity and Age of Juvenile P. bournei
4.2. Full Sun Condition Increases Stress Risks Instead of Carbon Gain in Seedlings of P. bournei
4.3. Leaf Plasticity of P. bournei Increases with Ontogeny
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ntawuhiganayo, E.B.; Uwizeye, F.K.; Zibera, E.; Dusenge, M.E.; Ziegler, C.; Ntirugulirwa, B.; Nsabimana, D.; Wallin, G.; Uddling, J. Traits controlling shade tolerance in tropical montane trees. Tree Physiol. 2020, 40, 183–197. [Google Scholar] [CrossRef]
- Lusk, C.H.; Falster, D.S.; Jara-Vergara, C.K.; Jimenez-Castillo, M.; Saldaña-Mendoza, A. Ontogenetic variation in light requirements of juvenile rainforest evergreens. Funct. Ecol. 2008, 22, 454–459. [Google Scholar] [CrossRef]
- Henry, H.A.L.; Aarssen, L.W. On the relationship between shade tolerance and shade avoidance strategies in woodland plants. Oikos 1997, 80, 575. [Google Scholar] [CrossRef]
- Franklin, K.A. Photomorphogenesis: Plants Feel Blue in the Shade. Curr. Biol. 2016, 26, R1275–R1276. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Milmanda, G.L.; Ballare, C.L. Shade Avoidance: Expanding the Color and Hormone Palette. Trends Plant Sci. 2021, 26, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Ballare, C.L.; Pierik, R. The shade-avoidance syndrome: Multiple signals and ecological consequences. Plant Cell Environ. 2017, 40, 2530–2543. [Google Scholar] [CrossRef]
- Lusk, C.H.; Perez-Millaqueo, M.M.; Piper, F.I.; Saldana, A. Ontogeny, understorey light interception and simulated carbon gain of juvenile rainforest evergreens differing in shade tolerance. Ann. Bot. 2011, 108, 419–428. [Google Scholar] [CrossRef]
- He, P.; Wright, I.J.; Zhu, S.; Onoda, Y.; Liu, H.; Li, R.; Liu, X.; Hua, L.; Oyanoghafo, O.O.; Ye, Q. Leaf mechanical strength and photosynthetic capacity vary independently across 57 subtropical forest species with contrasting light requirements. New Phytol. 2019, 223, 607–618. [Google Scholar] [CrossRef]
- Way, D.A.; Pearcy, R.W. Sunflecks in trees and forests: From photosynthetic physiology to global change biology. Tree Physiol. 2012, 32, 1066–1081. [Google Scholar] [CrossRef] [PubMed]
- Kono, M.; Noguchi, K.; Terashima, I. Roles of the cyclic electron flow around PSI (CEF-PSI) and O2-dependent alternative pathways in regulation of the photosynthetic electron flow in short-term fluctuating light in Arabidopsis thaliana. Plant Cell Environ. 2014, 55, 990–1004. [Google Scholar] [CrossRef]
- Allahverdiyeva, Y.; Suorsa, M.; Tikkanen, M.; Aro, E.M. Photoprotection of photosystems in fluctuating light intensities. J. Exp. Bot. 2015, 66, 2427–2436. [Google Scholar] [CrossRef] [PubMed]
- Osunkoya, O.O.; Ash, J.E.; Hopkins, M.S.; Graham, A.W. Influence of seed size and seedling ecological attributes on shade-tolerance of rain-forest tree species in northern Queensland. J. Ecol. 1994, 82, 149–163. [Google Scholar] [CrossRef]
- Niinemets, U.L.O. The controversy over traits conferring shade-tolerance in trees: Ontogenetic changes revisited. J. Ecol. 2006, 94, 464–470. [Google Scholar] [CrossRef]
- Piovesan, G.; Lüttge, U. Tree growth dynamics during early ontogenetic stages in closed forests. Trees 2018, 32, 671–673. [Google Scholar] [CrossRef]
- Tariq, A.; Pan, K.; Olatunji, O.A.; Graciano, C.; Li, Z.; Sun, F.; Sun, X.; Song, D.; Chen, W.; Zhang, A.; et al. Phosphorous application improves drought tolerance of Phoebe zhennan. Front. Plant Sci. 2017, 8, 1561. [Google Scholar] [CrossRef]
- Ensminger, I.; Schmidt, L.; Lloyd, J. Soil temperature and intermittent frost modulate the rate of recovery of photosynthesis in Scots pine under simulated spring conditions. New Phytol. 2008, 177, 428–442. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Klughammer, C.; Schreiber, U. Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the Saturation Pulse method. PAM Appl. Notes 2008, 1, 27–35. [Google Scholar]
- Zhang, S.; Jiang, H.; Peng, S.; Korpelainen, H.; Li, C. Sex-related differences in morphological, physiological, and ultrastructural responses of Populus cathayana to chilling. J. Exp. Bot. 2011, 62, 675–686. [Google Scholar] [CrossRef]
- Lin, X.Y.; Wang, X.X.; Zeng, Q.Y.; Yang, Q. Leaf structure and photosynthesis in Populus alba under naturally fluctuating environments. Photosynthetica 2022, 60, 240–250. [Google Scholar] [CrossRef]
- Lundell, R.; Saarinen, T.; Åström, H.; Hänninen, H. The boreal dwarf shrub Vaccinium vitis-idaea retains its capacity for photosynthesis through the winter. Botany 2008, 86, 491–500. [Google Scholar] [CrossRef]
- Tóth, V.R.; Mészáros, I.; Veres, S.; Nagy, J. Effects of the available nitrogen on the photosynthetic activity and xanthophyll cycle pool of maize in field. J. Plant Physiol. 2002, 159, 627–634. [Google Scholar] [CrossRef]
- Wellburn, A.R.; Lichtenthaler, H. Formulae and program to determine total carotenoids and chlorophylls A and B of leaf extracts in different solvents. In Advances in Photosynthesis Research; Sybesma, C., Ed.; Springer: Dordrecht, The Netherlands, 1984; Volume 12, pp. 9–12. [Google Scholar]
- Valladares, F.; Wright, S.J.; Lasso, E.; Kitajima, K.; Pearcy, R.W. Plastic phenotypic response to light of 16 congeneric shrubs from a panamanian rainforest. Ecology 2000, 81, 1925–1936. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, Y.-J.; Zeng, Q.-Y. Overexpression of three orthologous glutathione S-transferases from Populus increased salt and drought resistance in Arabidopsis. Biochem. Syst. Ecol. 2019, 83, 57–61. [Google Scholar] [CrossRef]
- Westoby, M.; Schrader, J.; Falster, D. Trait ecology of startup plants. New Phytol. 2022, 235, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Houter, N.C.; Pons, T.L. Ontogenetic changes in leaf traits of tropical rainforest trees differing in juvenile light requirement. Oecologia 2012, 169, 33–45. [Google Scholar] [CrossRef]
- Kitajima, K. Relative importance of photosynthetic traits and allocation patterns as correlates of seedling shade tolerance of 13 tropical trees. Oecologia 1994, 98, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Hughes, N.M.; Burkey, K.O.; Cavender-Bares, J.; Smith, W.K. Xanthophyll cycle pigment and antioxidant profiles of winter-red (anthocyanic) and winter-green (acyanic) angiosperm evergreen species. J. Exp. Bot. 2012, 63, 1895–1905. [Google Scholar] [CrossRef] [PubMed]
- Kardiman, R.; Raebild, A. Relationship between stomatal density, size and speed of opening in Sumatran rainforest species. Tree Physiol. 2018, 38, 696–705. [Google Scholar] [CrossRef]
- Sewelam, N.; Jaspert, N.; Van Der Kelen, K.; Tognetti, V.B.; Schmitz, J.; Frerigmann, H.; Stahl, E.; Zeier, J.; Van Breusegem, F.; Maurino, V.G. Spatial H2O2 signaling specificity: H2O2 from chloroplasts and peroxisomes modulates the plant transcriptome differentially. Mol. Plant 2014, 7, 1191–1210. [Google Scholar] [CrossRef]
- Hieno, A.; Naznin, H.A.; Inaba-Hasegawa, K.; Yokogawa, T.; Hayami, N.; Nomoto, M.; Tada, Y.; Yokogawa, T.; Higuchi-Takeuchi, M.; Hanada, K.; et al. Transcriptome analysis and identification of a transcriptional regulatory network in the response to H2O2. Plant Physiol. 2019, 180, 1629–1646. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Y.; Chao, D.Y.; Gao, J.P.; Zhu, M.Z.; Shi, M.; Lin, H.X. A previously unknown zinc finger protein, DST, regulates drought and salt tolerance in rice via stomatal aperture control. Genes Dev. 2009, 23, 1805–1817. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wakao, S.; Fischer, B.B.; Niyogi, K.K. Sensing and Responding to Excess Light. Annu. Rev. Plant Biol. 2009, 60, 239–260. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Hu, Y.-Y.; Yu, W.-W.; Song, L.-L.; Wu, J.-S. Growth, photosynthetic and physiological responses of Torreya grandis seedlings to varied light environments. Trees 2015, 29, 1011–1022. [Google Scholar] [CrossRef]







| Leaf Traits | 6 mon | 1 yr | 2 yr |
|---|---|---|---|
| LMA | 0.26 | 0.12 | 0.22 |
| Stomatal density | 0.09 | 0.18 | 0.15 |
| Leaf thickness | 0.22 | 0.19 | 0.10 |
| Chloroplast number | 0.05 | 0.24 | 0.35 |
| Plastoglobule number | 0.33 | 0.51 | 0.36 |
| Fv/Fm | 0.10 | 0.10 | 0.08 |
| YII | 0.25 | 0.27 | 0.31 |
| Y(NO) | 0.05 | 0.07 | 0.11 |
| Y(NPQ) | 0.26 | 0.36 | 0.37 |
| AN | 0.21 | 0.51 | 0.36 |
| Ci | 0.10 | 0.16 | 0.02 |
| gs | 0.12 | 0.60 | 0.44 |
| E | 0.42 | 0.44 | 0.09 |
| iWUE | 0.28 | 0.11 | 0.41 |
| Stomatal limitations | 0.28 | 0.49 | 0.35 |
| Total | 0.20 | 0.29 | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.-B.; Liu, C.-C.; Chen, J.-X.; Zhang, M.-M.; Zhang, J.-H.; Tong, Z.-K.; Yang, Q. Leaf Plasticity of the Subtropical Evergreen Tree Phoebe bournei Increases with Ontogeny in Response to Sun and Shade. Forests 2023, 14, 1683. https://doi.org/10.3390/f14081683
Li X-B, Liu C-C, Chen J-X, Zhang M-M, Zhang J-H, Tong Z-K, Yang Q. Leaf Plasticity of the Subtropical Evergreen Tree Phoebe bournei Increases with Ontogeny in Response to Sun and Shade. Forests. 2023; 14(8):1683. https://doi.org/10.3390/f14081683
Chicago/Turabian StyleLi, Xin-Bei, Cheng-Cheng Liu, Jia-Xin Chen, Meng-Meng Zhang, Jun-Hong Zhang, Zai-Kang Tong, and Qi Yang. 2023. "Leaf Plasticity of the Subtropical Evergreen Tree Phoebe bournei Increases with Ontogeny in Response to Sun and Shade" Forests 14, no. 8: 1683. https://doi.org/10.3390/f14081683