Plant Species and Functional Diversity of Novel Forests Growing on Coal Mine Heaps Compared with Managed Coniferous and Deciduous Mixed Forests
Abstract
:1. Introduction
2. Materials and Methods
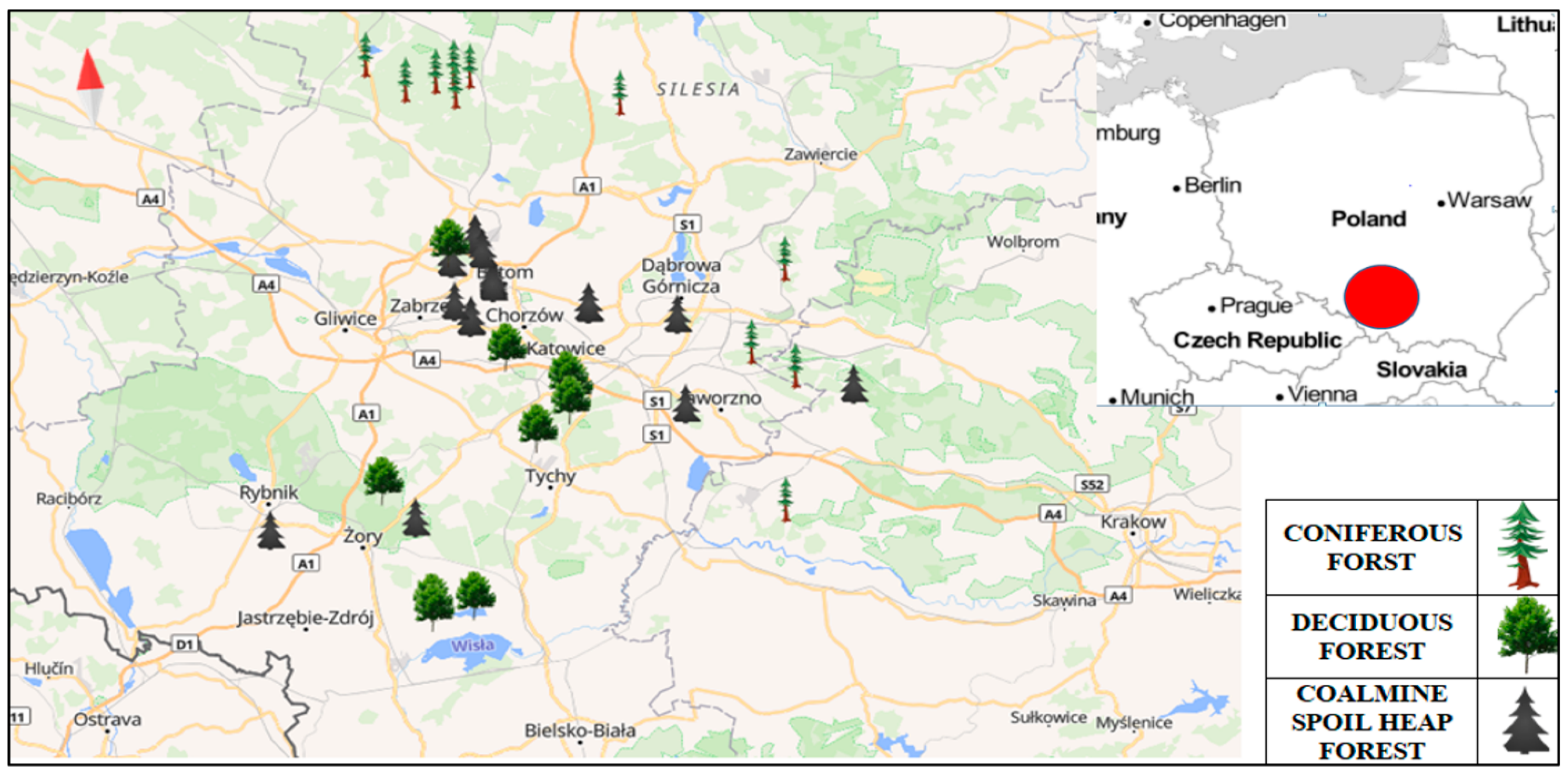
2.1. Study Area
2.2. Study Design and Plant Composition
2.3. Soil/Substrate Sampling, Fieldwork, and Laboratory Analysis
2.4. Measurement of the Soil/Substrate Respiration (Sr)
2.5. Plant Functional Traits
2.6. Data Analysis
3. Results
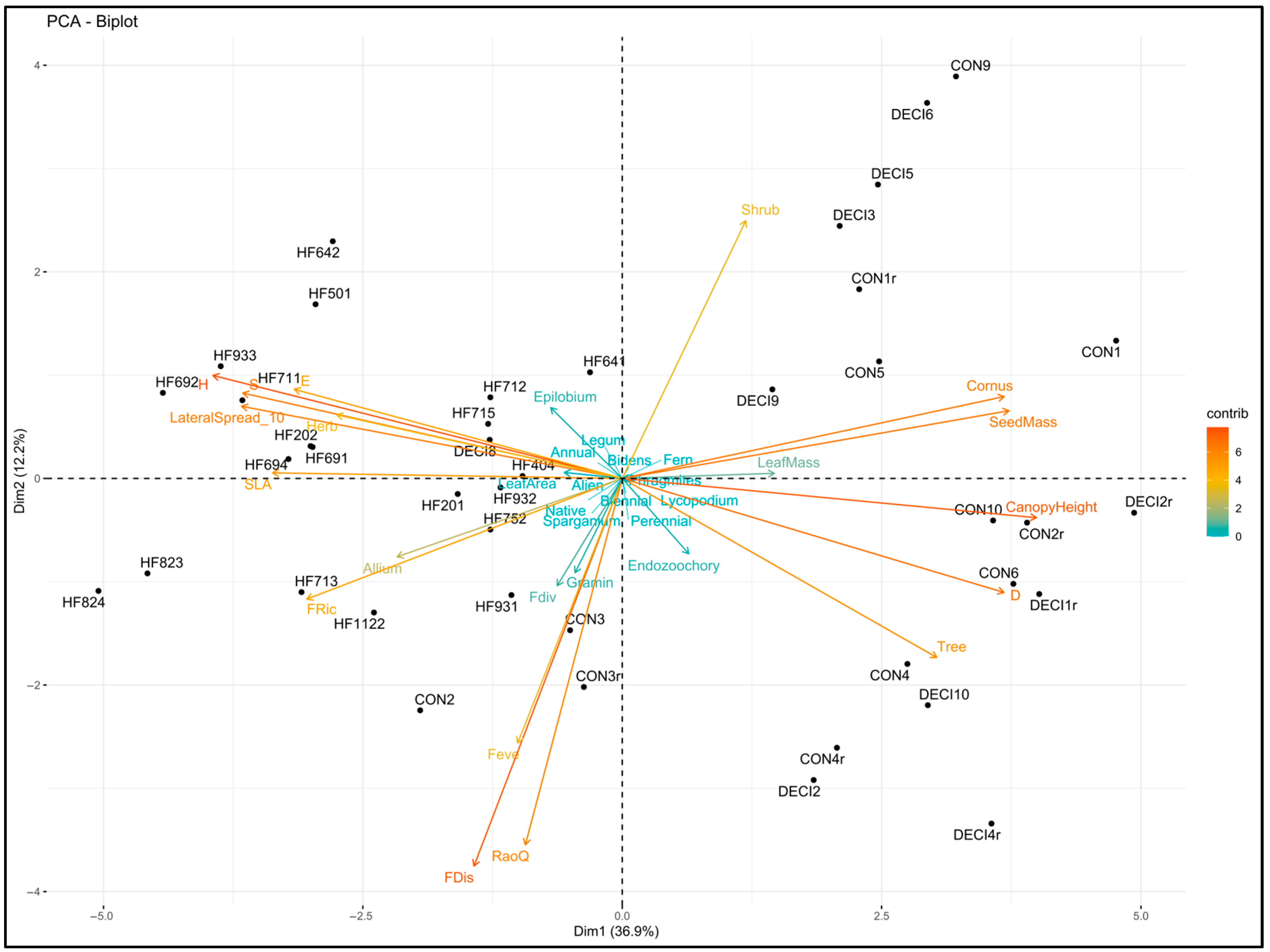
3.1. Classification of the Forest Vegetation Patches
3.2. Alpha Diversity
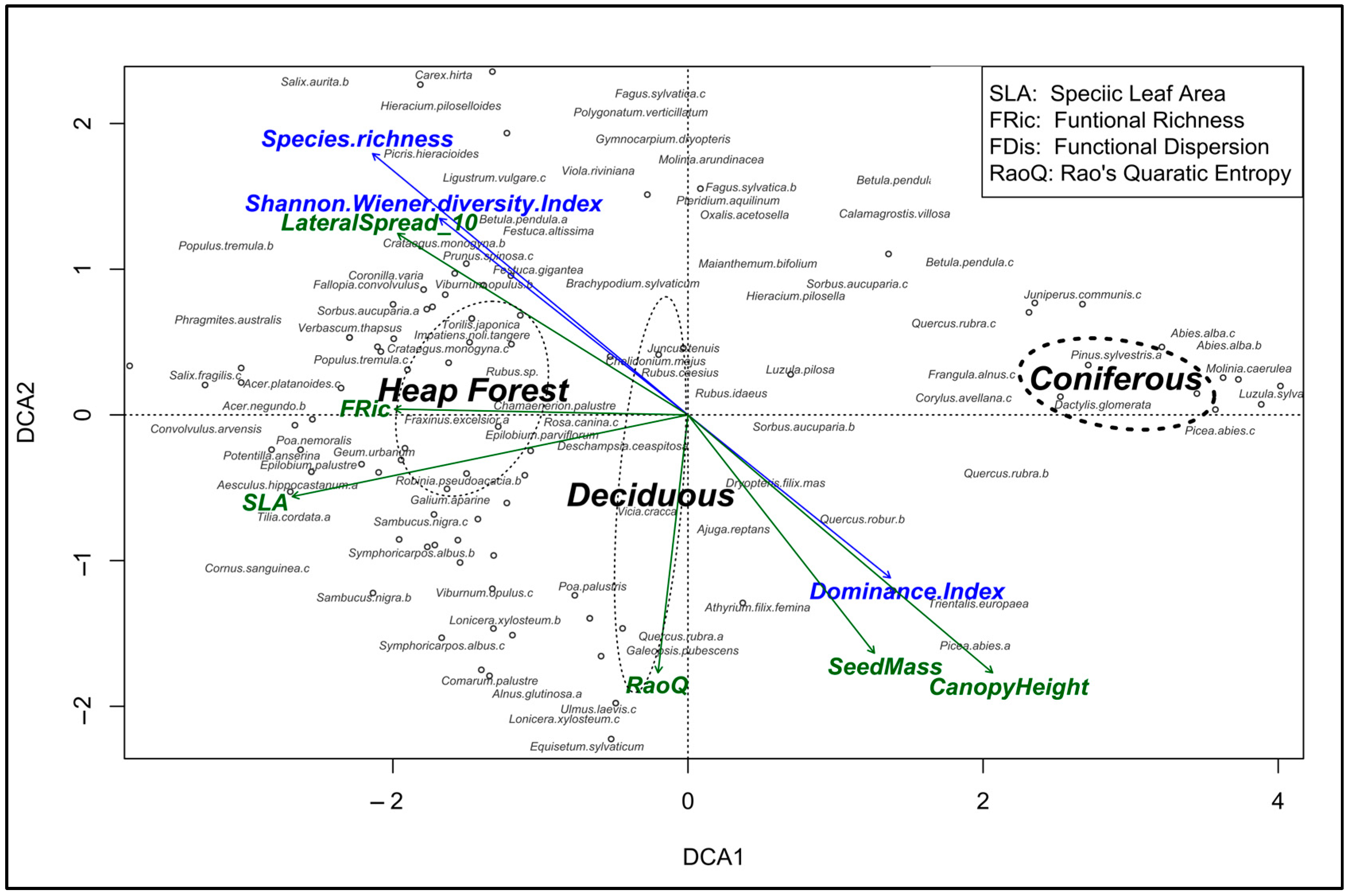
3.3. Taxonomic and Functional Diversity
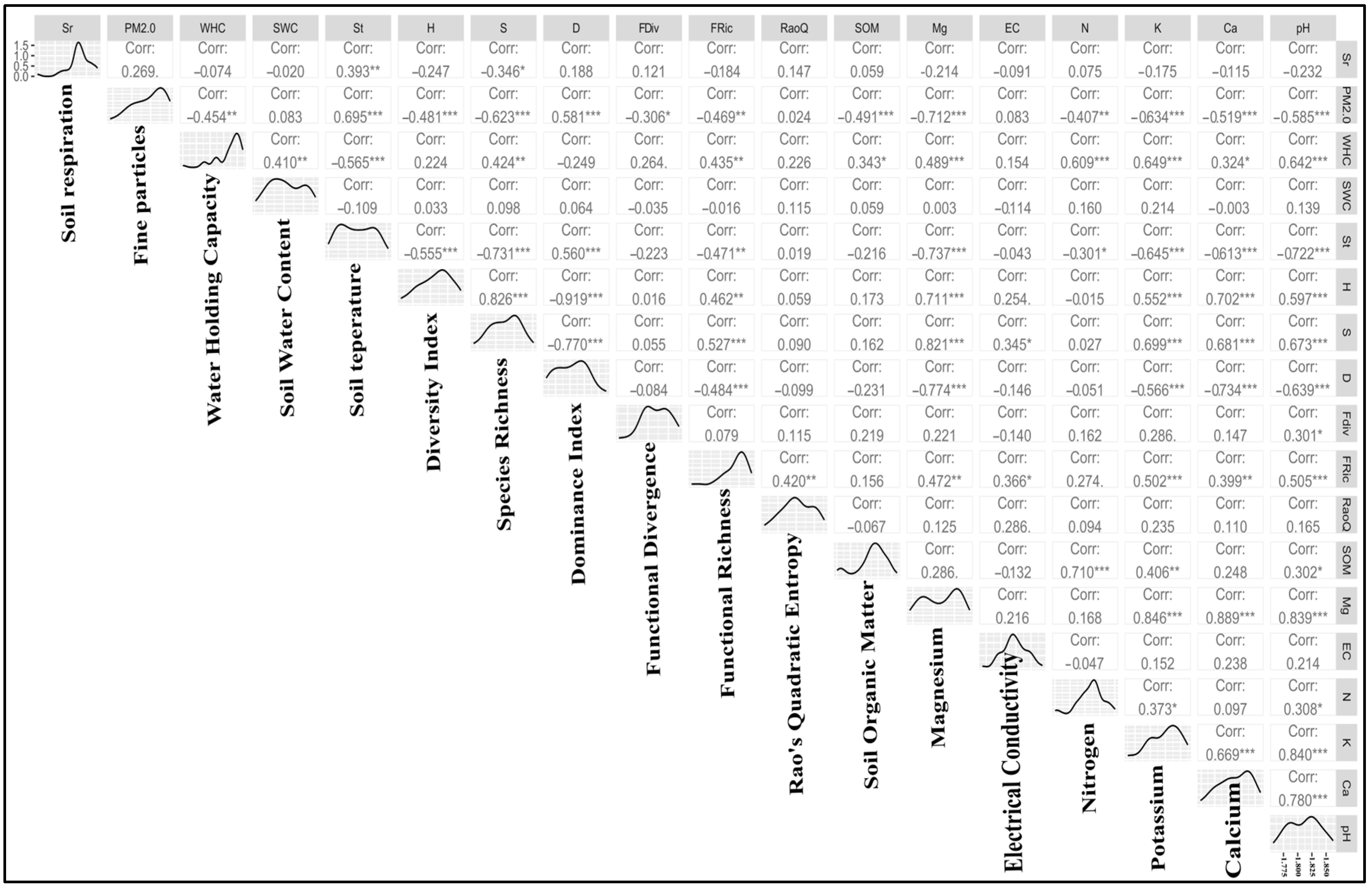
3.4. Correlation between Variables
4. Discussion
4.1. Comparison of Species Diversity in the Studied Forest Types
4.2. Functional Diversity in the Studied Forests
4.3. Environmental Relevance of Plant Traits in Novel and Managed Forests
4.4. Invasive Plants and the Novel Forests of Post-Coal Mine Heaps
4.5. Soil Respiration in the Heap Forests versus Managed and Mixed Deciduous Forest Types
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Coniferous | A Layer% | B Layer% | C Layer% | Total% | |
| Pinus sylvestris | 58 | 2.75 | 1.8 | 63 | |
| Vaccinium mytrillus | 26 | ||||
| V. vitis-idaea | 11.5 | ||||
| Deciduous | Quercus robur | 27.8 | 04 | 3 | 31 |
| Carex brizoides | 21.3 | ||||
| Fagus sylvatica | 14 | 3 | 1 | 18 | |
| Heap Forest | Betula pendula | 17.8 | 1.5 | 0.33 | 19 |
| Robinia pseudoacacia | 14.6 | 2.32 | 0.25 | 17 | |
| Acer pseudoplatanus | 6.68 | 3.4 | 1.2 | 11 | |
| Tilia cordata | 6.68 | 2.6 | 0.91 | 10 | |
| Pupulus tremula | 5.56 | 0.26 | 5.82 | ||
| Impatiens parviflora | 5.37 |
References
- Jactel, H.; Bauhus, J.; Boberg, J.; Bonal, D.; Castagneyrol, B.; Gardiner, B.; Gonzalez-Olabarria, J.R.; Koricheva, J.; Meurisse, N.; Brockerhoff, E.G. Tree Diversity Drives Forest Stand Resistance to Natural Disturbances. Curr. For. Rep. 2017, 3, 223–243. [Google Scholar] [CrossRef]
- Barlow, J.; Gardner, T.A.; Araujo, I.S.; Bonaldo, A.B.; Costa, J.E.; Esposito, M.C.; Ferreira, L.V.; Hawes, J.; M Hernandez, M.I.; Hoogmoed, M.S.; et al. Quantifying the Biodiversity Value of Tropical Primary, Secondary, and Plantation Forests. Proc. Natl. Acad. Sci. USA 2007, 46, 18555–18560. [Google Scholar] [CrossRef] [PubMed]
- Brockerhoff, E.G.; Jactel, H.; Parrotta, J.A.; Quine, C.P.; Sayer, J. Plantation Forests and Biodiversity: Oxymoron or Opportunity? Biodivers. Conserv. 2008, 5, 925–951. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Phillips, O.L.; Jackson, R.B. The Structure, Distribution, and Biomass of the World’s Forests. In Annual Review of Ecology, Evolution, and Systematics; Annual Reviews Inc.: San Mateo, CA, USA, 2013; pp. 593–622. [Google Scholar]
- Prach, K.; Walker, L.R. Comparative Plant Succession among Terrestrial Biomes of the World; Cambridge University Press: Cambridge, UK, 2020. [Google Scholar]
- Prach, K.; Lencová, K.; Řehounková, K.; Dvořáková, H.; Jírová, A.; Konvalinková, P.; Mudrák, O.; Novák, J.; Trnková, R. Spontaneous Vegetation Succession at Different Central European Mining Sites: A Comparison across Seres. Environ. Sci. Pollut. Res. 2013, 11, 7680–7685. [Google Scholar] [CrossRef] [PubMed]
- Peltzer, D.A.; Wardle, D.A.; Allison, V.J.; Troy Baisden, W.; Bardgett, R.D.; Chadwick, O.A.; Condron, L.M.; Parfitt, R.L.; Porder, S.; Richardson, S.J.; et al. Concepts & Synthesis Emphasizing New Ideas to Stimulate Research in Ecology Understanding Ecosystem Retrogression. Ecol. Monogr. 2010, 4, 509–529. [Google Scholar]
- Wardle, D.A.; Zackrisson, O.; Hörnberg, G.; Gallet, C. The Influence of Island Area on Ecosystem Properties. Science 1997, 5330, 1296–1299. [Google Scholar] [CrossRef]
- Elias, F.; Ferreira, J.; Lennox, G.D.; Berenguer, E.; Ferreira, S.; Schwartz, G.; Melo, L.d.O.; Reis Júnior, D.N.; Nascimento, R.O.; Ferreira, F.N.; et al. Assessing the Growth and Climate Sensitivity of Secondary Forests in Highly Deforested Amazonian Landscapes. Ecology 2020, 101, e02954. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, R.J.; Arico, S.; Aronson, J.; Baron, J.S.; Bridgewater, P.; Cramer, V.A.; Epstein, P.R.; Ewel, J.J.; Klink, C.A.; Lugo, A.E.; et al. Novel Ecosystems: Theoretical and Management Aspects of the New Ecological World Order. Glob. Ecol. Biogeogr. 2006, 1, 1–7. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Higgs, E.; Harris, J.A. Novel Ecosystems: Implications for Conservation and Restoration. Trends Ecol. Evol. 2009, 11, 599–605. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Higgs, E.S.; Hall, C.M. (Eds.) Defining Novel Ecosystems. In Novel Ecosystems: Intervening in the New Ecological World Order; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 58–60. [Google Scholar]
- Morse, N.B.; Pellissier, P.A.; Cianciola, E.N.; Brereton, R.L.; Sullivan, M.M.; Shonka, N.K.; Wheeler, T.B.; McDowell, W.H. Novel Ecosystems in the Anthropocene: A Revision of the Novel Ecosystem Concept for Pragmatic Applications. Ecol. Soc. 2014, 2, 12. [Google Scholar] [CrossRef]
- Kowarik, I. Novel Urban Ecosystems, Biodiversity, and Conservation. Environ. Pollut. 2011, 8–9, 1974–1983. [Google Scholar] [CrossRef] [PubMed]
- Tropek, R.; Kadlec, T.; Hejda, M.; Kocarek, P.; Skuhrovec, J.; Malenovsky, I.; Vodka, S.; Spitzer, L.; Banar, P.; Konvicka, M. Technical Reclamations Are Wasting the Conservation Potential of Post-Mining Sites. A Case Study of Black Coal Spoil Dumps. Ecol. Eng. 2012, 43, 13–18. [Google Scholar]
- Violle, C.; Navas, M.-L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the Concept of Trait Be Functional! Oikos 2007, 5, 882–892. [Google Scholar] [CrossRef]
- Goswami, M.; Bhattacharyya, P.; Mukherjee, I.; Tribedi, P. Functional Diversity: An Important Measure of Ecosystem Functioning. Adv. Microbiol. 2017, 1, 82–93. [Google Scholar] [CrossRef]
- Kefi, S.K.; Holmgren, M.; Scheffer, M. When Can Positive Interactions Cause Alternative Stable States in Ecosystems? Funct. Ecol. 2015, 30, 88–97. [Google Scholar] [CrossRef]
- Ville’ger, S.; Mason, N.W.H.; Mouillot, D. New Multidimensional Functional Diversity Indices for a Multifaceted Framework in Functional Ecology. Ecology 2008, 8, 2290–2301. [Google Scholar] [CrossRef] [PubMed]
- Botta-Dukát, Z. Rao’s Quadratic Entropy as a Measure of Functional Diversity Based on Multiple Traits. J. Veg. Sci. 2005, 16, 533–540. [Google Scholar] [CrossRef]
- Grime, J.P. Benefits of Plant Diversity to Ecosystems: Immediate, Filter and Founder Effects. J. Ecol. 1998, 86, 902–910. [Google Scholar] [CrossRef]
- Sonkoly, J.; Kelemen, A.; Valkó, O.; Deák, B.; Kiss, R.; Tóth, K.; Miglécz, T.; Tóthmérész, B.; Török, P. Both Mass Ratio Effects and Community Diversity Drive Biomass Production in a Grassland Experiment. Sci. Rep. 2019, 9, 1848. [Google Scholar] [CrossRef]
- Aarssen, L.W. Nordic Society Oikos High Productivity in Grassland Ecosystems: Effected by Species Diversity or Productive Species? Oikos 1997, 1, 183–184. [Google Scholar] [CrossRef]
- Huston, M.A. Hidden Treatments in Ecological Experiments: Re-Evaluating the Ecosystem Function of Biodiversity. Oecologia 1997, 110, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Finegan, B.; Peña-Claros, M.; de Oliveira, A.; Ascarrunz, N.; Bret-Harte, M.S.; Carreño-Rocabado, G.; Casanoves, F.; Díaz, S.; Eguiguren Velepucha, P.; Fernandez, F.; et al. Does Functional Trait Diversity Predict Above-Ground Biomass and Productivity of Tropical Forests? Testing Three Alternative Hypotheses. J. Ecol. 2015, 1, 191–201. [Google Scholar] [CrossRef]
- Conti, G.; Díaz, S. Plant Functional Diversity and Carbon Storage—An Empirical Test in Semi-Arid Forest Ecosystems. J. Ecol. 2013, 1, 18–28. [Google Scholar] [CrossRef]
- Kirby, K.R.; Potvin, C. Variation in Carbon Storage among Tree Species: Implications for the Management of a Small-Scale Carbon Sink Project. For. Ecol. Manag. 2007, 2–3, 208–221. [Google Scholar] [CrossRef]
- Lange, M.; Eisenhauer, N.; Sierra, C.A.; Bessler, H.; Engels, C.; Griffiths, R.I.; Mellado-Vázquez, P.G.; Malik, A.A.; Roy, J.; Scheu, S.; et al. Plant Diversity Increases Soil Microbial Activity and Soil Carbon Storage. Nat. Commun. 2015, 6, 6707. [Google Scholar] [CrossRef] [PubMed]
- Aslam, T.J.; Benton, T.G.; Nielsen, U.N.; Johnson, S.N. Impacts of Eucalypt Plantation Management on Soil Faunal Communities and Nutrient Bioavailability: Trading Function for Dependence? Biol. Fertil. Soils 2015, 5, 637–644. [Google Scholar] [CrossRef]
- De Vries, F.T.; Thébault, E.; Liiri, M.; Birkhofer, K.; Tsiafouli, M.A.; Bjørnlund, L.; Jørgensen, H.B.; Brady, M.V.; Christensen, S.; De Ruiter, P.C.; et al. Soil Food Web Properties Explain Ecosystem Services across European Land Use Systems. Proc. Natl. Acad. Sci. USA 2013, 35, 14296–14301. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wan, S.; Fu, S.; Wang, X.; Wang, M.; Liang, C.; Chen, Y.; Zhu, X. Effects of Understory Removal and Nitrogen Fertilization on Soil Microbial Communities in Eucalyptus Plantations. For. Ecol. Manag. 2013, 310, 80–86. [Google Scholar] [CrossRef]
- Gliński, J.; Stepniewski, W. Soil Aeration and Its Role for Plants; CRC Press: Boca Raton, FL, USA, 1985. [Google Scholar]
- Szczepańska, J. Zwałowiska Odpadów Górnictwa Węgla Kamiennego Jako Ogniska Zanieczyszczeń Środowiska Wodnego. In Technologia Wody; Wydawnictwo 2K Technologie s.c.: Kraków, Poland, 1987. [Google Scholar]
- Cabala, J.M.; Cmiel, S.R.; Idziak, A.F. Environmental Impact of Mining Activity in the Upper Silesian Coal Basin (Poland). Geol. Belgica 2004, 3–4, 225–229. [Google Scholar]
- Woźniak, G.; Dyderski, M.K.; Kompała-Bąba, A.; Jagodziński, A.M.; Pasierbiński, A.; Błońska, A.; Bierza, W.; Magurno, F.; Sierka, E. Use of Remote Sensing to Track Postindustrial Vegetation Development. Land Degrad. Dev. 2021, 3, 1426–1439. [Google Scholar] [CrossRef]
- Wożniak, G.; Pasierbiński, A.; Rostański, A. The Diversity of Spontaneous Woodland Vegetation on Coals Mine Heaps of Upper-Silesian Industrial Region. Arch. Environ. Prot. 2003, 2, 93–105. [Google Scholar]
- Bradshaw, A. The Use of Natural Processes in Reclamation—Advantages and Difficulties. Landsc. Urban Plan. 2000, 2–4, 89–100. [Google Scholar] [CrossRef]
- Londo, G. The Decimal Scale for Releves of Permanent Quadrats. Plant Ecol. 1976, 33, 61–64. [Google Scholar] [CrossRef]
- Hristov, B.; Filcheva, E.; Ivanov, P. Organic Matter Content and Composition of Soils with Stagnic Properties from Bulgaria. Bulg. J. Soil Sci. 2016, 1, 25–32. [Google Scholar]
- Rutherford, P.M.; McGill, W.; Arocena, J.M.; Figueiredo, C.T. Total Nitrogen. In Soil Sampling and Methods of Analysis; Carter, M.R., Gregoric, E.G., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 267–278. [Google Scholar]
- Ziadi, N.; Tran, T.S. Mehlich 3-Extractable Elements. In Soil Sampling and Methods of Analysis; Carter, M.R., Gregorich, E.G., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 81–88. [Google Scholar]
- López-Marcos, D.; Martínez-Ruiz, C.; Turrión, M.B.; Jonard, M.; Titeux, H.; Ponette, Q.; Bravo, F. Soil Carbon Stocks and Exchangeable Cations in Monospecific and Mixed Pine Forests. Eur. J. For. Res. 2018, 137, 831–847. [Google Scholar] [CrossRef]
- Kleyer, M.; Bekker, R.M.; Knevel, I.C.; Bakker, J.P.; Thompson, K.; Sonnenschein, M.; Poschlod, P.; Van Groenendael, J.M.; Klime, L.; Klime S Ová, J.; et al. The LEDA Traitbase: A Database of Life-History Traits of the Northwest European Flora. J. Ecol. 2008, 96, 1266–1274. [Google Scholar] [CrossRef]
- Maitner, B.S.; Boyle, B.; Casler, N.; Condit, R.; Donoghue, J.; Durán, S.M.; Guaderrama, D.; Hinchliff, C.E.; Jørgensen, P.M.; Kraft, N.J.; et al. The Bien r Package: A Tool to Access the Botanical Information and Ecology Network (BIEN) Database. Methods Ecol. Evol. 2018, 9, 373. [Google Scholar] [CrossRef]
- Chytrý, M.; Danihelka, J.; Kaplan, Z.; Wild, J.; Holubová, D.; Novotný, P.; Reznícková, M.; Rohn, M.; Drevojan, P.; Grulich, V.; et al. Pladias Database of the Czech Flora and Vegetation. Preslia 2021, 1, 1–87. [Google Scholar] [CrossRef]
- Klotz, S.; Kühn, I.; Durka, W. Eine Datenbank Mit Biologisch-Ökologischen Merkmalen Zur Flora von Deutschland; Bundesamt für Naturschutz: Bonn, Germany, 2002; Volume 38. [Google Scholar]
- Kattge, J.; Diaz, S.; Lavorel, S.; Prentice, I.C.; Leadley, P.; Bönisch, G.; Garnier, E.; Westoby, M.; Reich, P.B.; Wright, I.J.; et al. TRY-a Global Database of Plant Traits. Glob. Chang. Biol. 2011, 17, 2905–2935. [Google Scholar] [CrossRef]
- Guerra, J.G.; Cabello, F.; Fernández-Quintanilla, C.; Dorado, J. A Trait-Based Approach in a Mediterranean Vineyard: Effects of Agricultural Management on the Functional Structure of Plant Communities. Agric. Ecosyst. Environ. 2021, 316, 107465. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New Handbook for Standardised Measurement of Plant Functional Traits Worldwide. Aust. J. Bot. 2016, 3, 167–234. [Google Scholar] [CrossRef]
- Westoby, M.; Falster, D.S.; Moles, A.T.; Vesk, P.A.; Wright, I.J. Plant Ecological Strategies: Some Leading Dimensions of Variation between Species. Annu. Rev. Ecol. Syst. 2002, 33, 125–159. [Google Scholar] [CrossRef]
- Piekarska-Stachowiak, A.; Szary, M.; Ziemer, B.; Besenyei, L.; Woz’niak, G.W. An Application of the Plant Functional Group Concept to Restoration Practice on Coal Mine Spoil Heaps; An Application of the Plant Functional Group Concept to Restoration Practice on Coal Mine Spoil Heaps. Ecol. Res. 2014, 29, 843–853. [Google Scholar] [CrossRef]
- Klimeš, L.; Klimešová, J. CLO-PLA2-a Database of Clonal Plants in Central Europe. Plant Ecol. 1999, 141, 9–19. [Google Scholar] [CrossRef]
- Klimešová, J.; Martínková, J.; Herben, T. Horizontal Growth: An Overlooked Dimension in Plant Trait Space. Perspect. Plant Ecol. Evol. Syst. 2018, 32, 18–21. [Google Scholar] [CrossRef]
- Sádlo, J.; Chytrý, M.; Pergl, J.; Pyšek, P. Plant Dispersal Strategies: A New Classification Based on the Multiple Dispersal Modes of Individual Species. Preslia 2018, 1, 1–22. [Google Scholar] [CrossRef]
- Mirek, Z.; Piękoś-Mirek, H.; Zając, A.; Zając, M. Flowering Plants and Pteridophytes of Poland a Checklist; W. Szafer Institute of Botany, Polihs Academy of Science: Kraków, Poland, 2002. [Google Scholar]
- Tokarska-Guzik, B. The Establishment and Spread of Alien Plant Species (Kenophytes) in the Flora of Poland; Migula, P., Ed.; Wydawnictwo Uniwersytetu Śląskiego: Katowice, Poland, 2005. [Google Scholar]
- Oksanen, J.; Minchin, P.R. Instability of Ordination Results under Changes in Input Data Order: Explanations and Remedies. J. Veg. Sci. 1997, 3, 447–454. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Package “vegan” Title Community Ecology Package Version 2.6-2. 2022. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 6 March 2024).
- Garnier, E.; Cortez, J.; Bille‘sbille‘bille‘s, G.; Navas, M.-L.; Roumet, C.; Debussche, M.; Laurent, G.R.; Blanchard, A.; Aubry, D.; Bellmann, A.; et al. Plant Functional Markers Capture Ecosystem Properties during Secondary Succession. Ecology 2004, 9, 2630–2637. [Google Scholar] [CrossRef]
- Laliberté, E.; Legendre, P. A Distance-Based Framework for Measuring Functional Diversity from Multiple Traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Laliberté, E.; Legendre, P.; Shipley, B. Measuring Functional Diversity (FD) from Multiple Traits, and Other Tools for Functional Ecology R package version 1.0-12.3. 2022. Available online: https://cran.r-project.org/web/packages/FD/index.html (accessed on 6 March 2024).
- Mason, N.; Mouillot, D.; Lee, W.G.; Bastow Wilson Mason, J.; H Mason, N.W.; Wilson, J.B. Functional Richness, Functional Evenness and Functional Divergence: The Primary Components of Functional Diversity. Oikos 2005, 111, 112–118. [Google Scholar] [CrossRef]
- Royston, P. Remark AS R94: A Remark on Algorithm AS 181: The W-Test for Normality. Appl. Stat. 1995, 4, 547–551. [Google Scholar] [CrossRef]
- Wheeler, B. Permutation Tests for Linear Models. R Package. Version 2.1.0. 2022. Available online: http://CRAN.R-project.org/package=lmPerm (accessed on 6 March 2023).
- Becker, R.A.; Chambers, J.M.; Wilks, A.R. The New S Language: A Programming Environment for Data Analysis and Graphics. In Wadsworth & Brooks/Cole. Advanced Books and Software; Springer: Berlin, Germany, 1998. [Google Scholar]
- Woźniak, G.; Chmura, D.; Dyderski, M.K.; Błońska, A.; Jagodziński, A.M. How Different Is the Forest on Post-Coal Mine Heap Regarded as Novel Ecosystem? For. Ecol. Manag. 2022, 1, 120205. [Google Scholar] [CrossRef]
- Matuszkiewicz, W. Przewodnik Do Oznaczania Zbiorowisk Roślinnych Polski, 3rd ed.; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2006. [Google Scholar]
- Bierza, W.; Czarnecka, J.; Błońska, A.; Kompała-Bąba, A.; Hutniczak, A.; Jendrzejek, B.; Bakr, J.; Jagodziński, A.M.; Prostański, D.; Woźniak, G. Plant Diversity and Species Composition in Relation to Soil Enzymatic Activity in the Novel Ecosystems of Urban–Industrial Landscapes. Sustainability 2023, 15, 7284. [Google Scholar] [CrossRef]
- Weiss, J.; Burghardt, W.; Gausmann, P.; Haag, R.; Haeupler, H.-N.; Hamann, M.; Leder, B.; Schulte, A.; Stempelmann, I. Nature Returns to Abandoned Industrial Land: Monitoring Succession in Urban-Industrial Woodlands in the German Ruhr. In Wild Urban Woodlands; Kowarik, I., Körner, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 143–162. [Google Scholar]
- Peterken, G.F.; Game, M. Historical Factors Affecting the Number and Distribution of Vascular Plant Species in the Woodlands of Central Lincolnshire. J. Ecol. 1984, 1, 155–182. [Google Scholar] [CrossRef]
- Kidawa, J.; Chmura, D.; Molenda, T. The Hydrological-Hydrochemical Factors That Control the Invasion of the Black Locust (Robinia pseudoacacia L.) in Succession in Areas with Opencast Mines. Plants 2021, 1, 40. [Google Scholar] [CrossRef] [PubMed]
- Řehounková, K.; Prach, K. Spontaneous Vegetation Succession in Gravel-Sand Pits: A Potential for Restoration. Restor. Ecol. 2008, 2, 305–312. [Google Scholar] [CrossRef]
- Mudrák, O.; Frouz, J.; Velichová, V. Understory Vegetation in Reclaimed and Unreclaimed Post-Mining Forest Stands. Ecol. Eng. 2010, 6, 783–790. [Google Scholar] [CrossRef]
- Rawlik, M.; Kasprowicz, M.; Jagodziński, A.M. Differentiation of Herb Layer Vascular Flora in Reclaimed Areas Depends on the Species Composition of Forest Stands. For. Ecol. Manag. 2018, 409, 541–551. [Google Scholar] [CrossRef]
- Rawlik, M.; Kasprowicz, M.; Jagodziński, A.M.; Kaźmierowski, C.; Łukowiak, R.; Grzebisz, W. Canopy Tree Species Determine Herb Layer Biomass and Species Composition on a Reclaimed Mine Spoil Heap. Sci. Total Environ. 2018, 635, 1205–1214. [Google Scholar] [CrossRef]
- Dzwonko, Z. Relations between the Floristic Composition of Isolated Young Woods and Their Proximity to Ancient Woodland. J. Veg. Sci. 1993, 5, 693–698. [Google Scholar] [CrossRef]
- Dzwonko, Z.; Gawrofiski, S. The Role of Woodland Fragments, Soil Types, and Dominant Species in Secondary Succession on the Western Carpathian Foothills. Vegetatio 1994, 111, 149–160. [Google Scholar] [CrossRef]
- Dzwonko, Z. Effect of Proximity to Ancient Deciduous Woodland on Restoration of the Field Layer Vegetation in a Pine Plantation. Ecography 2001, 24, 198–204. [Google Scholar] [CrossRef]
- Verheyen, K.; Hermy, M. Recruitment and Growth of Herb-Layer Species with Different Colonizing Capacities-125. J. Veg. Sci. 2004, 15, 125–134. [Google Scholar] [CrossRef]
- Woźniak, G.; Bakr, J.; Dyczko, A.; Jarosz, J.; Ryś, K.; Radosz, Ł.; Kaul, S.; Adamik, K.; Besenyei, L.; Prostański, D. The Diversity and Plant Species Composition of the Spontaneous Vegetation on Coal Mine Spoil Heaps in Relation to the Area Size. Min. Mach. 2023, 41, 68–84. [Google Scholar]
- Díaz, S.; Cabido, M. Vive La Différence: Plant Functional Diversity Matters to Ecosystem Processes. Trends Ecol. Evol. 2001, 11, 446–455. [Google Scholar]
- Hooper, D.U.; Solan, M.; Symstad, A.; Diaz, S.; Gessner, M.O.; Buchmann, N.; Degrange, P.G.; Hulot, F.; Mermillod-Blondin, F.; Roy, J.; et al. Species Diversity, Functional Diversity and Ecosystem Functioning. In Biodiversity and Ecosystem Functioning: Synthesis and Perspectives; Oxford University Press: Oxford, UK, 2002; pp. 195–208. [Google Scholar]
- Buzzard, V.; Hulshof, C.M.; Birt, T.; Violle, C.; Enquist, B.J. Re-Growing a Tropical Dry Forest: Functional Plant Trait Composition and Community Assembly during Succession. Funct. Ecol. 2016, 6, 1006–1013. [Google Scholar] [CrossRef]
- Enquist, B.J.; West, G.B.; Charnov, E.L.; Brown, J.H. Allometric Scaling of productionand Life-History variationin Vascular Plants. Nature 1999, 401, 907–911. [Google Scholar] [CrossRef]
- Lambers, H.; Poorter, H. Inherent Variation in Growth Rate Between Higher Plants: A Search for Physiological Causes and Ecological Consequences. Adv. Ecol. Res. 1992, 23, 188–261. [Google Scholar]
- Enquist, B.J.; Norberg, J.; Bonser, S.P.; Violle, C.; Webb, C.T.; Henderson, A.; Sloat, L.L.; Savage, V.M. Scaling from Traits to Ecosystems: Developing a General Trait Driver Theory via Integrating Trait-Based and Metabolic Scaling Theories. In Advances in Ecological Research; Academic Press Inc.: Cambridge, MA, USA, 2015; Volume 52, pp. 249–318. [Google Scholar]
- Norberg, J.; Swaney, D.P.; Dushoff, J.; Lin, J.; Casagrandi, R.; Levin, S.A. Phenotypic Diversity and Ecosystem Functioning in Changing Environments: A Theoretical Framework. Proc. Natl. Acad. Sci. USA 2001, 20, 11376–11381. [Google Scholar] [CrossRef]
- Kobe, R.K. Light Gradient Partitioning among Tropical Tree Species through Differential Seedling Mortality and Growth. Source Ecol. 1999, 1, 187–201. [Google Scholar] [CrossRef]
- Pacala, S.W.; Canham, C.D.; Saponara, J.; Silander, J.A.; Kobe, R.K.; Ribbens, E. Forest Models Defined by Field Measurements: Estimation, Error Analysis and Dynamics. Ecol. Monogr. 1996, 1, 1–43. [Google Scholar] [CrossRef]
- Tilman, D. The Resource-Ratio Hypothesis of Plant Succession. Am. Nat. 1985, 6, 827–852. [Google Scholar] [CrossRef]
- León, J.D.; Osorio, N.W. Role of Litter Turnover in Soil Quality in Tropical Degraded Lands of Colombia. Sci. World J. 2014, 2014, 693981. [Google Scholar] [CrossRef] [PubMed]
- Mason, N.W.H.; Carswell, F.E.; Richardson, S.J.; Burrows, L.E. Leaf Palatability and Decomposability Increase during a 200-Year-Old Post-Cultural Woody Succession in New Zealand. J. Veg. Sci. 2011, 1, 6–17. [Google Scholar] [CrossRef]
- Niklas, K.J.; Owens, T.; Reich, P.B.; Cobb, E.D. Nitrogen/Phosphorus Leaf Stoichiometry and the Scaling of Plant Growth. Ecol. Lett. 2005, 6, 636–642. [Google Scholar] [CrossRef]
- Poorter, H. Is Interspecific Variation in Relative Growth Rate Positively Correlated with Biomass Allocation to the Leaves? Am. Nat. 1991, 5, 1264–1268. [Google Scholar] [CrossRef]
- Reich, P.B.; Walters, M.B.; Ellsworth, D.S. From Tropics to Tundra: Global Convergence in Plant Functioning. Proc. Natl. Acad. Sci. USA 1997, 94, 13730–13734. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.J.; Reich, P.B.; Cornelissen, J.H.C.; Falster, D.S.; Garnier, E.; Hikosaka, K.; Lamont, B.B.; Lee, W.; Oleksyn, J.; Osada, N.; et al. Assessing the Generality of Global Leaf Trait Relationships. New Phytol. 2005, 166, 485–496. [Google Scholar] [CrossRef]
- Aspelmeier, S.; Leuschner, C. Genotypic Variation in Drought Response of Silver Birch (Betula Pendula Roth): Leaf and Root Morphology and Carbon Partitioning. Trees—Struct. Funct. 2006, 1, 42–52. [Google Scholar] [CrossRef]
- Stuart Chapin, F.; Matson, P.A.; Mooney, H.A. Principles of Terrestrial Ecosystem Ecology; Springer: New York, NY, USA, 2002. [Google Scholar]
- Grime, J.P. Trait Convergence and Trait Divergence in Herbaceous Plant Communities: Mechanisms and Consequences. J. Veg. Sci. 2006, 17, 255–260. [Google Scholar] [CrossRef]
- Poorter, L.; Bongers, F.; Sterck, F.; Wöll, H. Beyond the Regeneration Phase: Differentiation of Height-Light Trajectories among Tropical Tree Species. J. Ecol. 2005, 93, 256–267. [Google Scholar] [CrossRef]
- Lohbeck, M.; Poorter, L.; Martínez-Ramos, M.; Rodriguez-Velázquez, J.; van Breugel, M.; Bongers, F. Changing Drivers of Species Dominance during Tropical Forest Succession. Funct. Ecol. 2014, 4, 1052–1058. [Google Scholar] [CrossRef]
- Verheyen, K.; Bossuyt, B.; Hermy, M.; Tack, G. The Land Use History (1278–1990) of a Mixed Hardwood Forest in Western Belgium and Its Relationship with Chemical Soil Characteristics. J. Biogeogr. 1999, 5, 1115–1128. [Google Scholar] [CrossRef]
- Koerner, W.; Dupouey, J.L.; Dambrine, E.; Benoit, M.; Dupouey, J.L.; Dambrinet, E. Influence of Past Land Use on the Vegetation and Soils of Present Day Forest in the Vosges Mountains, France. J. Ecol. 1997, 3, 351–358. [Google Scholar] [CrossRef]
- Dzwonko, Z. Assessment of Light and Soil Conditions in Ancient and Recent Woodlands by Ellenberg Indicator Values. J. Appl. Ecol. 2001, 5, 942–951. [Google Scholar] [CrossRef]
- Bossuyt, B.; Hermy, M.; Deckers, J. Migration of Herbaceous Plant Species across Ancient–Recent Forest Ecotones in Central Belgium. J. Ecol. 1999, 87, 628–638. [Google Scholar] [CrossRef]
- Pietrzykowski, M. Tree Species Selection and Reaction to Mine Soil Reconstructed at Reforested Post-Mine Sites: Central and Eastern European Experiences. Ecol. Eng. 2019, 142, 100012. [Google Scholar] [CrossRef]
- Jagodziński, A.M.; Kałucka, I.; Horodecki, P.; Oleksyn, J. Aboveground Biomass Allocation and Accumulation in a Chronosequence of Young Pinus Sylvestris Stands Growing on a Lignite Mine Spoil Heap. Dendrobiology 2014, 72, 139–150. [Google Scholar] [CrossRef]
- Kowarik, I. Time Lags in Biological Invasions with Regard to the Success and Failure of Alien Species. In Plant Invasions—General Apsects and Special Problems; SPB Academic Publishing: Amsterdam The Netherlands, 1995; pp. 15–38. [Google Scholar]
- Tischew, S.; Lorenz, A. Spontaneous Development of Peri-Urban Woodlands in Lignite Mining Areas of Eastern Germany. In Wild Urban Woodlands; Kowarik, I., Körner, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 163–180. [Google Scholar]
- Dettmar, J. Verhandlungen Der Gesellschaft Für Ökologie Industrietypische Flora Im Ruhrgebiet. Verhandlungen Der Ges. Für Okol. 1992, 21, 49–52. [Google Scholar]
- Dyderski, M.K.; Jagodziński, A.M. Drivers of Invasive Tree and Shrub Natural Regeneration in Temperate Forests. Biol. Invasions 2018, 20, 2363–2379. [Google Scholar] [CrossRef]
- Vítková, M.; Sádlo, J.; Roleček, J.; Petřík, P.; Sitzia, T.; Müllerová, J.; Pyšek, P. Robinia Pseudoacacia-Dominated Vegetation Types of Southern Europe: Species Composition, History, Distribution and Management. Sci. Total Environ. 2020, 707, 134857. [Google Scholar] [CrossRef] [PubMed]
- Gentili, R.; Ferrè, C.; Cardarelli, E.; Montagnani, C.; Bogliani, G.; Citterio, S.; Comolli, R. Comparing Negative Impacts of Prunus Serotina, Quercus Rubra and Robinia pseudoacacia on Native Forest Ecosystems. Forests 2019, 10, 842. [Google Scholar] [CrossRef]
- Chmura, D.; Sierka, E. The Invasibility of Deciduous Forest Communities after Disturbance: A Case Study of Carex Brizoides and Impatiens Parviflora Invasion. For. Ecol. Manag. 2007, 2–3, 487–495. [Google Scholar] [CrossRef]
- Pruchniewicz, D.; Żołnierz, L.; Andonovski, V. Habitat Factors Influencing the Competitive Ability of Calamagrostis Epigejos (L.) Roth in Mountain Plant Communities. Turk. J. Botany 2017, 6, 579–587. [Google Scholar] [CrossRef]
- Kompała-Bąba, A.; Bierza, W.; Błońska, A.; Sierka, E.; Magurno, F.; Chmura, D.; Besenyei, L.; Radosz, Ł.; Woźniak, G. Vegetation Diversity on Coal Mine Spoil Heaps—How Important Is the Texture of the Soil Substrate? Biologia 2019, 74, 419–436. [Google Scholar] [CrossRef]
- Pietrzykowski, M.; Woś, B.; Haus, N. Scots Pine Needles Macronutrient (N, P, K, CA, MG, and S) Supply at Different Reclaimed Mine Soil Substrates-as an Indicator of the Stability of Developed Forest Ecosystems. Environ. Monit. Assess. 2013, 1852, 7445–7457. [Google Scholar] [CrossRef] [PubMed]
- Pietrzykowski, M.; Socha, J.; Van Doorn, N.S.; Pietrzykowski, M.; Socha, J.; Van Doorn, N.S. Scots Pine (Pinus sylvestris L.) Site Index in Relation to Physico-Chemical and Biological Properties in Reclaimed Mine Soils. New For. 2015, 46, 247–266. [Google Scholar] [CrossRef]
- Ryan, M.G. Temperature and Tree Growth. Tree Physiol. 2010, 30, 667–668. [Google Scholar] [CrossRef]
- Way, D.A.; Oren, R. Differential Responses to Changes in Growth Temperature between Trees from Different Functional Groups and Biomes: A Review and Synthesis of Data. Tree Physiol. 2010, 30, 669–688. [Google Scholar] [CrossRef]
- Pell, M.; Stenström, J.; Granhall, U. Soil Respiration. In Microbial Methods for Assessing Soil Quality; Bloem, J., Benedetti, A., Hopkins, D., Eds.; CABI: London, UK, 2006. [Google Scholar]



| Plant Traits | Code | Data Type | Unit | Mean Value | (Min.–Max.) |
|---|---|---|---|---|---|
| Specific Leaf Area | SLA | Numerical | mm2 mg−1 | 3.09 | 2.28–4.08 |
| Leaf Mass | LeafMass | Numerical | mg | 4.48 | 2.67–5.61 |
| Leaf Area | LeafArea | Numerical | mm2 | 7.42 | 5.63–8.54 |
| Seed Mass | SeedMass | Numerical | mg | 2.60 | 0.18–6.44 |
| Canopy Height | CanopyHeight | Numerical | m | 1.36 | 0.33–3.42 |
| Lateral Spread | LateralSpread | Numerical | m/y | 0.03 | 0.00–0.12 |
| Functional Group | FG | Categorical | Four categories: Trees, Legumes, Graminoids, Herbs | ||
| Life Span | Life_span | Categorical | Three categories: Perennial, Biennial, Annual | ||
| Origin of Species | Alien_stat | Categorical | Two categories: Native, Alien | ||
| Life Form | Life_form | Categorical | Six categories: Megaphanerophytes, Nanophanerophytes, Hemicryptophyte, Chamaephytes, Geophytes, Therophytes | ||
| Dispersal Mode | Dispersal_Mode | Categorical | Seven categories: Allium, Cornus, Bidens, Lycopodium, Phragmites, Epilobium, Sparganium | ||
| Coniferous Forest | Deciduous Forest | Heap Forest | p < 0.05 | ||
|---|---|---|---|---|---|
| Soil Parameters | Substrate Water Content | 16.9 b ± 2.7 | 33.4 a ± 7.1 | 21.8 b ± 10 | 1.5 × 10−4 * |
| Water-Holding Capacity | 0.16 c ± 0.06 | 0.39 a ± 0.05 | 0.33 b ± 0.04 | 2.5 × 10−13 | |
| Fine Particles (PM < 2.0) | 0.38 a ± 0.09 | 0.51 b ± 0.10 | 0.58 c ± 0.10 | 3.1 × 10−8 | |
| Substrate Temperature | 14.77 a ± 1 | 12.70 b ± 1.4 | 10.64 c ± 1.4 | 0.050 * | |
| Soil Organic Matter% | 9.56 ± 4 | 10.5 ± 3 | 12.2 ± 5 | 0.3069 ns | |
| pH | 5.92 c ± 0.1 | 6.07 b ± 0.1 | 6.26 a ± 0.1 | 2.2 × 10−13 | |
| Electrical Conductivity | 5.33 ± 0.5 | 5.39 ± 0.2 | 5.52 ± 0.5 | 0.4666 ns | |
| Sodium ppm | 20.47 ± 4 | 21.69 ± 4 | 20.19 ± 4 | 0.6296 ns | |
| Calcium ppm | 324 b ± 173 | 357 b ± 250 | 1974 a ± 961 | 1.1 × 10−8 | |
| Potassium ppm | 85 c ± 25 | 149 b ± 43 | 215 a ± 55 | 5.9 × 10−9 | |
| Phosphorus ppm | 36.1 ± 9 | 32.5 ± 5 | 30.6 ± 5 | 0.2125464 ns | |
| Magnesium ppm | 30 c ± 10 | 50 b ± 20 | 172 a ± 48 | 2.4 × 10−14 | |
| Total Nitrogen% | 0.37 b ± 0.1 | 0.60 a ± 0.2 | 0.50 a ± 0.2 | 0.01463 | |
| Soil Respiration (mg CO2/h/m2) | 0.90 b ± 0.11 | 0.96 a ± 0.27 | 0.76 c ± 0.30 | 0.0272 | |
| Coniferous Forest | Deciduous Forest | Heap Forest | p < 0.05 | ||
|---|---|---|---|---|---|
| Taxonomic and Functional Indices | Species Richness | 18.3 b ± 5 | 21.6 b ± 6 | 36.0 a ± 8 | 9.9 × 10−9 |
| Shannon–Wiener Index | 2.03 b ± 0.3 | 1.90 b ± 0.4 | 2.71 a ± 0.3 | 4.5 × 10−8 | |
| Dominance Index | 0.21 a ± 0.04 | 0.25 a ± 0.10 | 0.12 b ± 0.05 | 2.3 × 10−6 * | |
| Evenness Index | 0.71 b ± 0.04 | 0.62 b ± 0.09 | 0.76 a ± 0.07 | 0.0001209 | |
| Functional Evenness | 0.65 a ± 0.13 | 0.55 b ± 0.11 | 0.63 a ± 0.08 | 0.059 ns | |
| Functional Divergence | 0.69 b ± 0.07 | 0.71 ab ± 0.10 | 0.76 a ± 0.09 | 0.058 ns | |
| Functional Dispersion | 4.4 a ± 0.02 | 4.5 a ± 0.05 | 4.6 a ± 0.02 | 0.67 ns | |
| Functional Richness | 35.0 b ± 21 | 44.3 b ± 25 | 65.8 a ± 21 | 0.026 * | |
| Rao’s Quadratic Entropy | 24.5 a ± 9 | 27.3 a ± 11 | 26.9 a ± 7 | 0.62 ns | |
| CWM Traits | Specific Leaf Area | 2.68 c ± 0.2 | 2.99 b ± 0.2 | 3.35 a ± 0.3 | 1.5 × 10−6 * |
| Seed Mass | 3.6 a ± 2 | 4.4 a ± 2 | 1.2 b ± 1 | 9.6 × 10−5 * | |
| Canopy Height | 2.4 a ± 1 | 2.3 a ± 0 | 0.4 b ± 0 | 7.4 × 10−7 * | |
| Lateral Spread 0_10 | 0.007 b ± 0.01 | 0.017 b ± 0.01 | 0.055 a ± 0.03 | 9.1 × 10−6 * | |
| Leaf Area | 6.8 c ± 0.6 | 7.9 a ± 0.4 | 7.5 b ± 0.5 | 1.0 × 10−5 * | |
| Leaf Mass | 4.30 b ± 0.4 | 4.93 a ± 0.3 | 4.37 b ± 0.6 | 4.2 × 10−3 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakr, J.; Kompała-Bąba, A.; Bierza, W.; Hutniczak, A.; Błońska, A.; Chmura, D.; Magurno, F.; Jagodziński, A.M.; Besenyei, L.; Bacler-Żbikowska, B.; et al. Plant Species and Functional Diversity of Novel Forests Growing on Coal Mine Heaps Compared with Managed Coniferous and Deciduous Mixed Forests. Forests 2024, 15, 730. https://doi.org/10.3390/f15040730
Bakr J, Kompała-Bąba A, Bierza W, Hutniczak A, Błońska A, Chmura D, Magurno F, Jagodziński AM, Besenyei L, Bacler-Żbikowska B, et al. Plant Species and Functional Diversity of Novel Forests Growing on Coal Mine Heaps Compared with Managed Coniferous and Deciduous Mixed Forests. Forests. 2024; 15(4):730. https://doi.org/10.3390/f15040730
Chicago/Turabian StyleBakr, Jawdat, Agnieszka Kompała-Bąba, Wojciech Bierza, Agnieszka Hutniczak, Agnieszka Błońska, Damian Chmura, Franco Magurno, Andrzej M. Jagodziński, Lynn Besenyei, Barbara Bacler-Żbikowska, and et al. 2024. "Plant Species and Functional Diversity of Novel Forests Growing on Coal Mine Heaps Compared with Managed Coniferous and Deciduous Mixed Forests" Forests 15, no. 4: 730. https://doi.org/10.3390/f15040730







