1. Introduction
Large trees play a crucial role in forest conservation, contributing significantly to the hydrological and carbon cycles, as well as sustaining biodiversity and carbon storage [
1]. Storage in large trees (≥50 cm diameter at breast height (DBH)) represents 40 to 60% of the forest’s dry aboveground biomass. Due to their specific architecture, these trees are particularly important for issues related to climate change, given their disproportionate contribution to the total forest biomass [
1,
2,
3]. The lack of data on large trees can be attributed to the high cost of sampling and to legal restrictions, because extracting these trees from the forest in Brazil is only permitted with the approval of a forest management plan [
4].
In the Brazilian Amazon, forest management plans are developed for the harvest of roundwood for sawn timber production. The true impact of this management on mitigating climate change is still debated, as it faces significant challenges in achieving effective sustainability [
5] and has both positive and negative environmental effects [
6]. The beneficial effects include the contribution of forest regeneration to carbon-stock recovery and the temporary storage of carbon in timber products. The negative aspects include the reduction of forest carbon stock due to the removal of large commercial trees, emissions from the decay of the crowns, stumps, and roots of harvested trees, and biomass loss due to collateral damage from things such as access roads, skid tracks, and log landings. Forest fires and degradation related to logging and illegal logging can turn the forest into a potential source of greenhouse gas emissions [
7,
8].
Quantifying greenhouse gas emissions from deforestation and forest degradation requires determining biomass, and for this it is essential to obtain values for wood basic density (WD), which varies according to species and spatial distribution. Wood basic density is a crucial variable in the allometric models used to estimate biomass in tropical forests [
9,
10,
11,
12,
13,
14]. These estimates are essential for assessing carbon stocks and flows, contributing to the understanding of the influence of these factors on global climate change [
15,
16].
Determining WD requires caution due to the influence of various factors, such as radial and vertical variations in relation to tree height [
3,
15,
17], differences between species and taxonomic groups [
10,
18], and spatial variations in forest typologies [
10,
11,
19,
20,
21]. Local environmental conditions, such as soil fertility and light conditions, also influence WD [
11,
22,
23].
Vertical variation along the stem must be considered when estimating forest biomass because not considering this source of systematic bias results in significant errors in estimates for vast areas, such as the Amazon [
3,
17,
24,
25,
26,
27].
Equations have been developed to correct for vertical variations within trees [
3,
15,
17] or to determine “ideal” sampling points for a single species [
28] or a group of species [
26]. However, these results, while satisfactory, cannot be generalized to all species, especially tropical ones, which have patterns of variation that differ from other regions [
3].
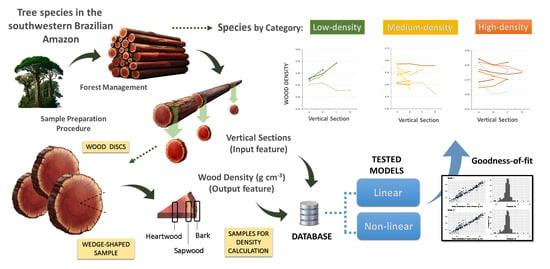
There is a knowledge gap regarding vertical variation in WD in species in the southwest region of the Brazilian Amazon [
21], especially in studies that include large trees (with diameter at breast height (DBH) ≥ 50 cm). The present study aims to determine the vertical variation of WD in the stems of large trees, to propose allometric equations and corrections for this variation, and to discuss their influences on forest biomass estimates in the southwestern Brazilian Amazon.
4. Discussion
It is well documented that there is a real influence of vertical variability on the average WD along the stem, which can differ significantly from the WD of samples taken solely at breast height (1.30 m above the ground) or the stem base [
15,
17,
25,
26,
41]. Due to this influence, some studies have suggested percentage values to correct the WD of samples only from the stem base or at breast height, such as the value of 4.3% for the central Amazon [
15] and the value of 3.2% for the southwestern Amazon [
21].
Based on the data from the current study, which was collected from 224 individuals in 20 species, there is a suggested reduction of 2.89% in the average WD value if samples are collected only at the stem base level. However, it is suggested that this correction be applied depending on the wood-type category, as the vertical variability behavior of density along the stem differs among low-, medium-, and high-density-wood categories. The use of samples from the stem bases of low-density-wood species was found to produce an underestimation of 2.89% for the WD of the stem as a whole, while the use of stem base samples from medium- and high-density-wood species caused overestimations of 4.12% and 2.46%, respectively.
This classification of wood type based on WD can contribute to better forest management by defining methodologies and management practices applicable to each group [
42]. Grouping these species and attempting to explain the different patterns of vertical density variability along the stem aids in understanding how this variable is interconnected with the life history strategies of trees and their ecological functions in the forest [
19,
43,
44,
45,
46]. Because WD is a determinant of biomass, it also affects the forest’s role in carbon storage and the global climate. Species with a high wood density have value for carbon sequestration. These species are also important for carbon in reforestation initiatives [
47,
48].
The patterns of vertical variability in the WD of the species evaluated in this study (
Figure 4,
Figure 5, and
Figure 6) align with previous findings [
3,
41,
44,
45]. When grouped by wood type, low-density-wood species (typically pioneers or early secondary species) tend to increase their WD towards the tree canopy [
3] These species have a short lifespan [
49,
50] and require high growth rates in their early growth stages [
19,
43,
51], initially producing low-density wood but later producing denser wood as growth rates decrease to maintain structural stability [
45,
52]. In contrast, species in the high-density-wood category, represented by late-secondary and climax species, generally exhibit decreasing vertical variation profiles [
6], where they initially produce a greater quantity of wood per unit volume and later this quantity decreases, contributing to a lower density at the top of the stem [
45].
The two main sets of characteristics described above represent the extremes of a continuum of possibilities where the observed variations are complex and highly dependent on the functional and strategic characteristics of each species [
41]. For example, species with intermediate characteristics, such as the medium-density-wood species in this study, exhibited vertical variations in WD that had increasing (
Ceiba samauma), highly decreasing trends (
Parkia paraensis), or even practically constant trends (
Barnebydendron riedelii), making it challenging to obtain a typical pattern without considering an average.
Vertical variability may be related to radial variation in the stem, and when analyzed together, these types of variation can better explain the trends of many species [
15,
17,
26,
44]. It was not possible to analyze radial variations in the present study, but this does not bias the results on vertical variation because the samples were taken in the form of complete disks (heartwood, sapwood, and bark). Radial variations are also related to different life strategies [
53] and can be explained by cambial age, growth-ring size, and the proportions of juvenile and mature wood, where these factors may change their proportions, increasing or decreasing with tree height [
17,
41,
54,
55] and thus also impacting vertical variation.
Vertical variation has been less studied than radial variation [
17], especially for tropical forests. The current study of vertical variability in WD along the stem is therefore important both at the taxonomic level (species) and at the functional level (ecological groups) as it contributes to a better refinement of stem biomass estimates and of carbon accounting for tropical trees [
3,
17].
In this study, equations were developed to estimate the stem WD of trees with DBH ≥ 50 cm for 20 commercial species (
Section 3.3). Regressions were run for each wood-type category, aiming to include the most significant variable (
base) and the most practical variables for field measurement (d and h). For the three wood density categories evaluated (low, medium, and high), models with only the base WD (
base) as a predictor variable provided the best fit for linear models to estimate the mean WD of the entire stem.
The findings show that
base explains more than 70% of the variations in all regressions (
Table 7,
Table 9 and
Table 10, and
Figure 7,
Figure 9 and
Figure 10). Diameter and height, although more convenient for field measurement, were not statistically significant to improve the model fit. Excluding this exception, the possible explanation for diameter and, especially, height not being significant variables is due to the lack of a consistent relationship between WD and tree size [
56]. Some studies have found positive and negative correlations, but these correlations were weak [
45,
48,
57,
58], while others have found that these variables are largely independent [
15,
59,
60]. Phillips et al. [
56] stated that tree size is not a good indicator of WD, and therefore, tree dimensions (d and h) cannot be used to infer wood density. Therefore, for the equations developed in this study for the “all species” scenario, it is recommended to use those that had only
base as the predictor variable. These equations are valuable alternatives for normalizing basic stem wood density [
3,
50] since sampling entire trees to quantify WD along the stem, as performed in this study, is expensive and rarely conducted for practical applications [
61], especially for large trees. Relying on approximate values for WD is often the only pragmatic way to obtain a more accurate estimate [
62].
Nonlinear regressions were fitted with the variables d and h (
Table 8 and
Figure 8) to be used in the estimation of biomass and, consequently, carbon. These equations can be applied to extensive areas that have forest censuses. Equations that are based solely on WD at the base of the tree (
base) as a predictor variable have greater utility for these censuses. The choice of equations provided in this study should be guided by the specific objectives of the researcher. Developing equations for different categories of the 20 species with similar characteristics and behaviors represents a significant advance in reducing uncertainties in forest biomass estimates.