Mixed-Species Stands Improve the Coordination between Leaf and Fine Root Traits in a Common Garden Experiment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Study Plots
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Variations in Leaf and Root Traits among Different Trait Categories at Different Species Richness Levels
3.1.1. Variations in Leaf Traits across Different Trait Categories at Different Species Richness Levels
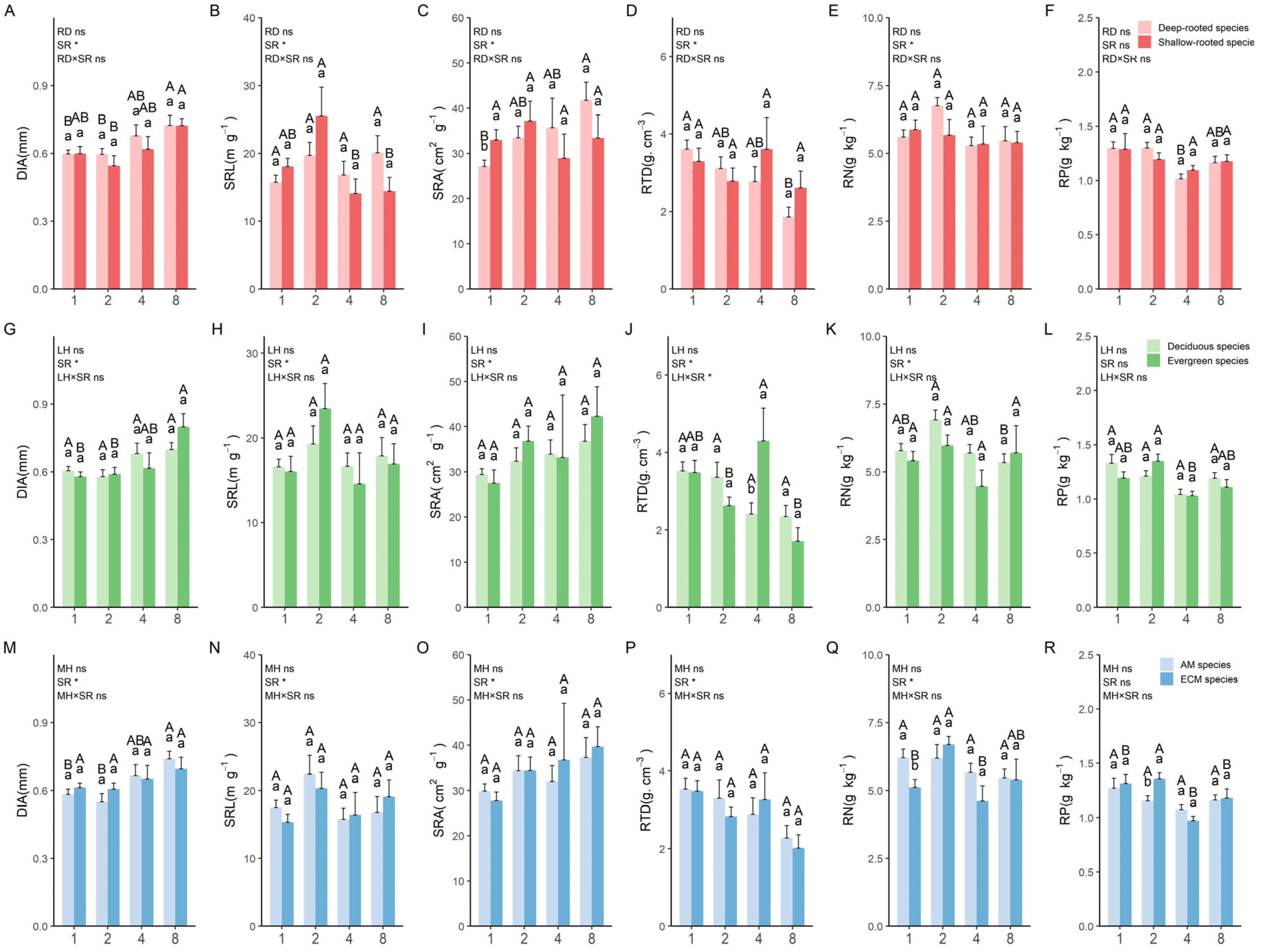
3.1.2. Variations in Absorptive Root Traits across Different Trait Categories at Different Species Richness Levels
3.1.3. Variations in Transport Root Traits across Different Trait Categories at Different Species Richness Levels
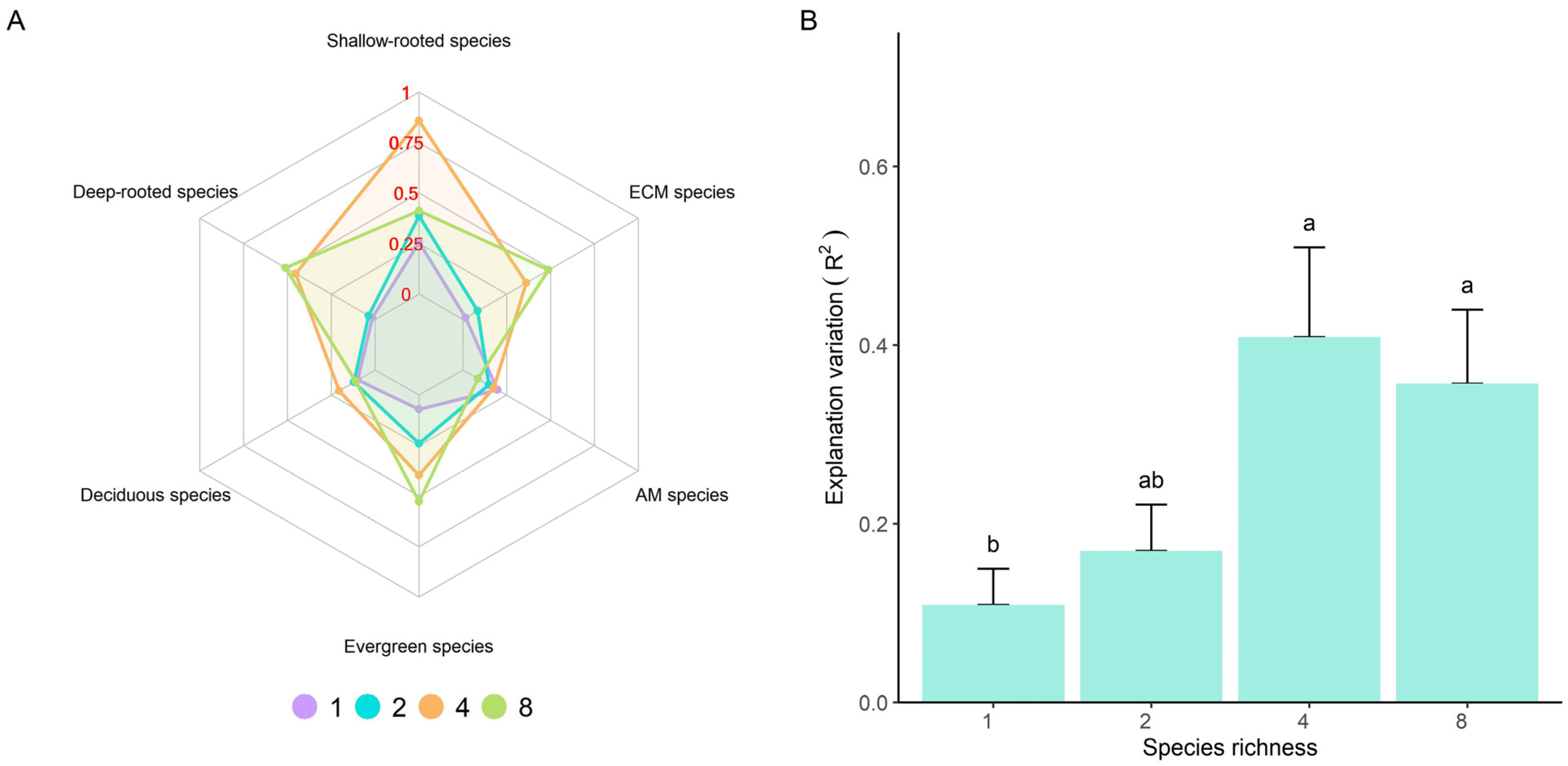
3.2. The Coordination between Leaf and Fine Root Traits
4. Discussion
4.1. Effects of Trait Categories and Species Richness on Leaf and Root Traits
4.1.1. Effects of Trait Categories and Species Richness on Leaf Traits
4.1.2. Effects of Trait Categories and Species Richness on Root Traits
4.2. The Coordination between Leaf and Fine Root Traits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Escudero, A.; Valladares, F. Trait-Based Plant Ecology: Moving towards a Unifying Species Coexistence Theory: Features of the Special Section. Oecologia 2016, 180, 919–922. [Google Scholar] [CrossRef]
- Mason, C.M.; Goolsby, E.W.; Humphreys, D.P.; Donovan, L.A. Phylogenetic Structural Equation Modelling Reveals No Need for an ‘Origin’ of the Leaf Economics Spectrum. Ecol. Lett. 2016, 19, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Gilbert, G.S.; Li, W.; Fang, M.; Lu, H.; Yu, S. Linking Aboveground Traits to Root Traits and Local Environment: Implications of the Plant Economics Spectrum. Front. Plant Sci. 2019, 10, 1412. [Google Scholar] [CrossRef]
- Weemstra, M.; Zambrano, J.; Allen, D.; Umaña, M.N. Tree Growth Increases through Opposing Above-ground and Below-ground Resource Strategies. J. Ecol. 2021, 109, 3502–3512. [Google Scholar] [CrossRef]
- Fortunel, C.; Fine, P.V.A.; Baraloto, C. Leaf, Stem and Root Tissue Strategies across 758 Neotropical Tree Species. Funct. Ecol. 2012, 26, 1153–1161. [Google Scholar] [CrossRef]
- Paź-Dyderska, S.; Dyderski, M.K.; Szwaczka, P.; Brzezicha, M.; Bigos, K.; Jagodziński, A.M. Leaf Traits and Aboveground Biomass Variability of Forest Understory Herbaceous Plant Species. Ecosystems 2020, 23, 555–569. [Google Scholar] [CrossRef]
- Niinemets, Ü. Is There a Species Spectrum within the World-Wide Leaf Economics Spectrum? Major Variations in Leaf Functional Traits in the Mediterranean Sclerophyll Quercus Ilex. New Phytol. 2015, 205, 79–96. [Google Scholar] [CrossRef]
- Reich, P.B. The World-Wide ‘Fast-Slow’ Plant Economics Spectrum: A Traits Manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Zhao, Y.-T.; Ali, A.; Yan, E.-R. The Plant Economics Spectrum Is Structured by Leaf Habits and Growth Forms across Subtropical Species. Tree Physiol. 2017, 37, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Croft, H.; Chen, J.M.; Wang, R.; Mo, G.; Luo, S.; Luo, X.; He, L.; Gonsamo, A.; Arabian, J.; Zhang, Y.; et al. The Global Distribution of Leaf Chlorophyll Content. Remote Sens. Environ. 2020, 236, 111479. [Google Scholar] [CrossRef]
- Rawat, M.; Arunachalam, K.; Arunachalam, A.; Alatalo, J.M.; Pandey, R. Assessment of Leaf Morphological, Physiological, Chemical and Stoichiometry Functional Traits for Understanding the Functioning of Himalayan Temperate Forest Ecosystem. Sci. Rep. 2021, 11, 23807. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, K.A.; Ogle, K.; Cornelissen, J.H.C.; Cornwell, W.K.; Bönisch, G.; Craine, J.M.; Jackson, B.G.; Kattge, J.; Peltzer, D.A.; Penuelas, J.; et al. Global Relationship of Wood and Leaf Litter Decomposability: The Role of Functional Traits within and across Plant Organs. Glob. Ecol. Biogeogr. 2014, 23, 1046–1057. [Google Scholar] [CrossRef]
- Polley, H.W.; Yang, C.; Wilsey, B.J.; Fay, P.A. Spectrally Derived Values of Community Leaf Dry Matter Content Link Shifts in Grassland Composition with Change in Biomass Production. Remote Sens. Ecol. Conserv. 2020, 6, 344–353. [Google Scholar] [CrossRef]
- Li, J.; Chen, X.; Niklas, K.J.; Sun, J.; Wang, Z.; Zhong, Q.; Hu, D.; Cheng, D. A Whole-plant Economics Spectrum Including Bark Functional Traits for 59 Subtropical Woody Plant Species. J. Ecol. 2022, 110, 248–261. [Google Scholar] [CrossRef]
- Pan, Y.; Cieraad, E.; Armstrong, J.; Armstrong, W.; Clarkson, B.R.; Colmer, T.D.; Pedersen, O.; Visser, E.J.W.; Voesenek, L.A.C.J.; van Bodegom, P.M. Global Patterns of the Leaf Economics Spectrum in Wetlands. Nat. Commun. 2020, 11, 4519. [Google Scholar] [CrossRef]
- Freschet, G.T.; Cornelissen, J.H.C.; van Logtestijn, R.S.P.; Aerts, R. Evidence of the ‘Plant Economics Spectrum’ in a Subarctic Flora. J. Ecol. 2010, 98, 362–373. [Google Scholar] [CrossRef]
- Liu, B.; He, J.; Zeng, F.; Lei, J.; Arndt, S.K. Life Span and Structure of Ephemeral Root Modules of Different Functional Groups from a Desert System. New Phytol. 2016, 211, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Díaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; ter Steege, H.; Morgan, H.D.; van der Heijden, M.G.A.; et al. A Handbook of Protocols for Standardised and Easy Measurement of Plant Functional Traits Worldwide. Aust. J. Bot. 2003, 51, 335. [Google Scholar] [CrossRef]
- McCormack, M.L.; Dickie, I.A.; Eissenstat, D.M.; Fahey, T.J.; Fernandez, C.W.; Guo, D.; Helmisaari, H.; Hobbie, E.A.; Iversen, C.M.; Jackson, R.B.; et al. Redefining Fine Roots Improves Understanding of Below-ground Contributions to Terrestrial Biosphere Processes. New Phytol. 2015, 207, 505–518. [Google Scholar] [CrossRef]
- Guerrero-Ramírez, N.R.; Mommer, L.; Freschet, G.T.; Iversen, C.M.; McCormack, M.L.; Kattge, J.; Poorter, H.; Van Der Plas, F.; Bergmann, J.; Kuyper, T.W.; et al. Global Root Traits (GRooT) Database. Global Ecol. Biogeogr. 2021, 30, 25–37. [Google Scholar] [CrossRef]
- de la Riva, E.G.; Querejeta, J.I.; Villar, R.; Pérez-Ramos, I.M.; Marañón, T.; Galán Díaz, J.; de Tomás Marín, S.; Prieto, I. The Economics Spectrum Drives Root Trait Strategies in Mediterranean Vegetation. Front. Plant Sci. 2021, 12, 773118. [Google Scholar] [CrossRef] [PubMed]
- Addo-Danso, S.D.; Prescott, C.E.; Adu-Bredu, S.; Duah-Gyamfi, A.; Moore, S.; Guy, R.D.; Forrester, D.I.; Owusu-Afriyie, K.; Marshall, P.L.; Malhi, Y. Fine-Root Exploitation Strategies Differ in Tropical Old Growth and Logged-over Forests in Ghana. Biotropica 2018, 50, 606–615. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Chen, H.Y.H.; Reich, P.B. Global-Scale Latitudinal Patterns of Plant Fine-Root Nitrogen and Phosphorus. Nat. Commun. 2011, 2, 344. [Google Scholar] [CrossRef] [PubMed]
- Prieto, I.; Roumet, C.; Cardinael, R.; Dupraz, C.; Jourdan, C.; Kim, J.H.; Maeght, J.L.; Mao, Z.; Pierret, A.; Portillo, N.; et al. Root Functional Parameters along a Land-Use Gradient: Evidence of a Community-Level Economics Spectrum. J. Ecol. 2015, 103, 361–373. [Google Scholar] [CrossRef]
- Ma, Z.; Guo, D.; Xu, X.; Lu, M.; Bardgett, R.D.; Eissenstat, D.M.; McCormack, M.L.; Hedin, L.O. Evolutionary History Resolves Global Organization of Root Functional Traits. Nature 2018, 555, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Isaac, M.E.; Martin, A.R.; de Melo Virginio Filho, E.; Rapidel, B.; Roupsard, O.; Van den Meersche, K. Intraspecific Trait Variation and Coordination: Root and Leaf Economics Spectra in Coffee across Environmental Gradients. Front. Plant Sci. 2017, 8, 1196. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, J.S.; Burns, J.H.; Nicholson, J.; Rogers, L.; Valverde-Barrantes, O. Decoupled Leaf and Root Carbon Economics Is a Key Component in the Ecological Diversity and Evolutionary Divergence of Deciduous and Evergreen Lineages of Genus Rhododendron. Am. J. Bot. 2017, 104, 803–816. [Google Scholar] [CrossRef]
- Hu, Y.; Pan, X.; Yang, X.; Liu, G.; Liu, X.; Song, Y.; Zhang, M.; Cui, L.; Dong, M. Is There Coordination of Leaf and Fine Root Traits at Local Scales? A Test in Temperate Forest Swamps. Ecol. Evol. 2019, 9, 8714–8723. [Google Scholar] [CrossRef] [PubMed]
- Osnas, J.L.D.; Lichstein, J.W.; Reich, P.B.; Pacala, S.W. Global Leaf Trait Relationships: Mass, Area, and the Leaf Economics Spectrum. Science 2013, 340, 741–744. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Q.; Zhao, N.; Yu, G.; He, N. Complex Trait Relationships between Leaves and Absorptive Roots: Coordination in Tissue N Concentration but Divergence in Morphology. Ecol. Evol. 2017, 7, 2697–2705. [Google Scholar] [CrossRef]
- de la Riva, E.G.; Marañón, T.; Pérez-Ramos, I.M.; Navarro-Fernández, C.M.; Olmo, M.; Villar, R. Root Traits across Environmental Gradients in Mediterranean Woody Communities: Are They Aligned along the Root Economics Spectrum? Plant Soil. 2018, 424, 35–48. [Google Scholar] [CrossRef]
- Butterfield, B.J.; Bradford, J.B.; Munson, S.M.; Gremer, J.R. Aridity Increases Below-Ground Niche Breadth in Grass Communities. Plant Ecol. 2017, 218, 385–394. [Google Scholar] [CrossRef]
- Mueller, K.E.; Kray, J.A.; Blumenthal, D.M. Coordination of Leaf, Root, and Seed Traits Shows the Importance of Whole Plant Economics in Two Semiarid Grasslands. New Phytol. 2024, 241, 2410–2422. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, Z.; Zhang, J.; Song, H.; Liang, Q.; Tao, J.; Cornelissen, J.H.C.; Liu, J. Do Shallow Soil, Low Water Availability, or Their Combination Increase the Competition between Grasses with Different Root Systems in Karst Soil? Environ. Sci. Pollut. Res. 2017, 24, 10640–10651. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.L.; Revermann, R.; Meller, P.; Gonçalves, F.M.P.; Aidar, M.P.M.; Lages, F.; Finckh, M. Functional Traits and Symbiotic Associations of Geoxyles and Trees Explain the Dominance of Detarioid Legumes in Miombo Ecosystems. New Phytol. 2021, 230, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Smith, F.A. Roles of Arbuscular Mycorrhizas in Plant Nutrition and Growth: New Paradigms from Cellular to Ecosystem Scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, H.; Li, S.; Fu, X.; Dai, X.; Yan, H.; Kou, L. Mycorrhizal and Environmental Controls over Root Trait–Decomposition Linkage of Woody Trees. New Phytol. 2021, 229, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Schwinning, S. The Ecohydrology of Roots in Rocks. Ecohydrology 2010, 3, 238–245. [Google Scholar] [CrossRef]
- Jose, S.; Williams, R.; Zamora, D. Belowground Ecological Interactions in Mixed-Species Forest Plantations. For. Ecol. Manag. 2006, 233, 231–239. [Google Scholar] [CrossRef]
- Bussotti, F.; Pollastrini, M. Evaluation of Leaf Features in Forest Trees: Methods, Techniques, Obtainable Information and Limits. Ecol. Indic. 2015, 52, 219–230. [Google Scholar] [CrossRef]
- Felix, J.A.; Stevenson, P.C.; Koricheva, J. Plant Neighbourhood Diversity Effects on Leaf Traits: A Meta-Analysis. Funct. Ecol. 2023, 37, 3150–3163. [Google Scholar] [CrossRef] [PubMed]
- Milla, R.; Reich, P.B. The Scaling of Leaf Area and Mass: The Cost of Light Interception Increases with Leaf Size. Proc. R. Soc. B 2007, 274, 2109–2115. [Google Scholar] [CrossRef] [PubMed]
- Fynn, R.W.S.; Morris, C.D.; Kirkman, K.P. Plant Strategies and Trait Trade-Offs Influence Trends in Competitive Ability along Gradients of Soil Fertility and Disturbance. J. Ecol. 2005, 93, 384–394. [Google Scholar] [CrossRef]
- Bu, W.; Schmid, B.; Liu, X.; Li, Y.; Härdtle, W.; von Oheimb, G.; Liang, Y.; Sun, Z.; Huang, Y.; Bruelheide, H.; et al. Interspecific and Intraspecific Variation in Specific Root Length Drives Aboveground Biodiversity Effects in Young Experimental Forest Stands. J. Plant Ecol. 2017, 10, 158–169. [Google Scholar] [CrossRef]
- Moloney, K.A.; Holzapfel, C.; Tielbörger, K.; Jeltsch, F.; Schurr, F.M. Rethinking the Common Garden in Invasion Research. Perspect. Plant Ecol. Evol. Syst. 2009, 11, 311–320. [Google Scholar] [CrossRef]
- Cadotte, M.W.; Arnillas, C.A.; Livingstone, S.W.; Yasui, S.-L.E. Predicting Communities from Functional Traits. Trends Ecol. Evol. 2015, 30, 510–511. [Google Scholar] [CrossRef] [PubMed]
- Bruelheide, H.; Nadrowski, K.; Assmann, T.; Bauhus, J.; Both, S.; Buscot, F.; Chen, X.-Y.; Ding, B.; Durka, W.; Erfmeier, A.; et al. Designing Forest Biodiversity Experiments: General Considerations Illustrated by a New Large Experiment in Subtropical China. Methods Ecol. Evol. 2014, 5, 74–89. [Google Scholar] [CrossRef]
- Deng, M.; Hu, S.; Guo, L.; Jiang, L.; Huang, Y.; Schmid, B.; Liu, C.; Chang, P.; Li, S.; Liu, X.; et al. Tree Mycorrhizal Association Types Control Biodiversity-Productivity Relationship in a Subtropical Forest. Sci. Adv. 2023, 9, eadd4468. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New Handbook for Standardised Measurement of Plant Functional Traits Worldwide. Aust. J. Bot. 2013, 61, 167. [Google Scholar] [CrossRef]
- Chang, Y.; Zhong, Q.; Yang, H.; Xu, C.; Hua, W.; Li, B. Patterns and Driving Factors of Leaf C, N, and P Stoichiometry in Two Forest Types with Different Stand Ages in a Mid-Subtropical Zone. For. Ecosyst. 2022, 9, 100005. [Google Scholar] [CrossRef]
- Legendre, P.; Oksanen, J.; ter Braak, C.J.F. Testing the Significance of Canonical Axes in Redundancy Analysis. Methods Ecol. Evol. 2011, 2, 269–277. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Davrinche, A.; Haider, S. Intra-Specific Leaf Trait Responses to Species Richness at Two Different Local Scales. Basic Appl. Ecol. 2021, 55, 20–32. [Google Scholar] [CrossRef]
- Pérez-Ramos, I.M.; Roumet, C.; Cruz, P.; Blanchard, A.; Autran, P.; Garnier, E. Evidence for a ‘Plant Community Economics Spectrum’ Driven by Nutrient and Water Limitations in a Mediterranean Rangeland of Southern France. J. Ecol. 2012, 100, 1315–1327. [Google Scholar] [CrossRef]
- Yu, K.; D’Odorico, P. Climate, Vegetation, and Soil Controls on Hydraulic Redistribution in Shallow Tree Roots. Adv. Water Resour. 2014, 66, 70–80. [Google Scholar] [CrossRef]
- de Vries, F.T.; Brown, C.; Stevens, C.J. Grassland Species Root Response to Drought: Consequences for Soil Carbon and Nitrogen Availability. Plant Soil 2016, 409, 297–312. [Google Scholar] [CrossRef]
- Wang, L.; He, Y.; Umer, M.; Guo, Y.; Tan, Q.; Kang, L.; Fang, Z.; Shen, K.; Xia, T.; Wu, P.; et al. Strategic Differentiation of Subcommunities Composed of Evergreen and Deciduous Woody Species Associated with Leaf Functional Traits in the Subtropical Mixed Forest. Ecol. Indic. 2023, 150, 110281. [Google Scholar] [CrossRef]
- Yao, L.; Ding, Y.; Yao, L.; Ai, X.; Zang, R. Trait Gradient Analysis for Evergreen and Deciduous Species in a Subtropical Forest. Forests 2020, 11, 364. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M. Mycorrhizal Types Differ in Ecophysiology and Alter Plant Nutrition and Soil Processes. Biol. Rev. 2019, 94, 1857–1880. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wang, Y.; He, X.; Zhang, S.; Song, X.; Zhang, N. Plant–Soil Feedback Is Dependent on Tree Mycorrhizal Types and Tree Species Richness in a Subtropical Forest. Geoderma 2024, 442, 116780. [Google Scholar] [CrossRef]
- Kilpeläinen, J.; Barbero-López, A.; Adamczyk, B.; Aphalo, P.J.; Lehto, T. Morphological and Ecophysiological Root and Leaf Traits in Ectomycorrhizal, Arbuscular-Mycorrhizal and Non-Mycorrhizal Alnus Incana Seedlings. Plant Soil 2019, 436, 283–297. [Google Scholar] [CrossRef]
- Weemstra, M.; Kuyper, T.W.; Sterck, F.J.; Umaña, M.N. Incorporating Belowground Traits: Avenues towards a Whole-Tree Perspective on Performance. Oikos 2023, 2023, e08827. [Google Scholar] [CrossRef]
- Valverde-Barrantes, O.J.; Smemo, K.A.; Blackwood, C.B. Fine Root Morphology Is Phylogenetically Structured, but Nitrogen Is Related to the Plant Economics Spectrum in Temperate Trees. Funct. Ecol. 2015, 29, 796–807. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Keenan, T.F.; Hallik, L. A Worldwide Analysis of Within-canopy Variations in Leaf Structural, Chemical and Physiological Traits across Plant Functional Types. New Phytol. 2015, 205, 973–993. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Xiao, W.; Dijkstra, F.A.; Zhu, B.; Wang, P.; Cheng, W. Linking Absorptive Roots and Their Functional Traits with Rhizosphere Priming of Tree Species. Soil Biol. Biochem. 2020, 150, 107997. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Wang, Z.; Gu, J. Functional Trait Plasticity but Not Coordination Differs in Absorptive and Transport Fine Roots in Response to Soil Depth. Forests 2019, 11, 42. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, X.; Wang, H.; Wang, Z.; Gu, J. Root Tip Morphology, Anatomy, Chemistry and Potential Hydraulic Conductivity Vary with Soil Depth in Three Temperate Hardwood Species. Tree Physiol. 2016, 36, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Uria, P.; Körner, C. Low Temperature Limits of Root Growth in Deciduous and Evergreen Temperate Tree Species. Funct. Ecol. 2007, 21, 211–218. [Google Scholar] [CrossRef]
- Dalle Fratte, M.; Montagnoli, A.; Anelli, S.; Lipreri, E.; Cerabolini, B.E.L. Mulching in Lowland Hay Meadows Drives an Adaptive Convergence of Above- and below-Ground Traits Reducing Plasticity and Improving Biomass: A Possible Tool for Enhancing Phytoremediation. Front. Plant Sci. 2022, 13, 1062911. [Google Scholar] [CrossRef]
- de la Riva, E.G.; Tosto, A.; Pérez-Ramos, I.M.; Navarro-Fernández, C.M.; Olmo, M.; Anten, N.P.R.; Marañón, T.; Villar, R. A Plant Economics Spectrum in Mediterranean Forests along Environmental Gradients: Is There Coordination among Leaf, Stem and Root Traits? J. Veg. Sci. 2016, 27, 187–199. [Google Scholar] [CrossRef]
- Lebrija-Trejos, E.; Pérez-García, E.A.; Meave, J.A.; Bongers, F.; Poorter, L. Functional Traits and Environmental Filtering Drive Community Assembly in a Species-Rich Tropical System. Ecology 2010, 91, 386–398. [Google Scholar] [CrossRef]
- Homulle, Z.; George, T.S.; Karley, A.J. Root Traits with Team Benefits: Understanding Belowground Interactions in Intercropping Systems. Plant Soil 2022, 471, 1–26. [Google Scholar] [CrossRef]
- Zhu, H.; Zhou, S.; Yan, L.; Shi, J.; Shen, Y. Studies on the Evergreen Broad-Leaved Forests of Yunnan, Southwestern China. Bot. Rev. 2019, 85, 131–148. [Google Scholar] [CrossRef]
- Hicks Pries, C.E.; Lankau, R.; Ingham, G.A.; Legge, E.; Krol, O.; Forrester, J.; Fitch, A.; Wurzburger, N. Differences in Soil Organic Matter between EcM- and AM-Dominated Forests Depend on Tree and Fungal Identity. Ecology 2023, 104, e3929. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, H.L.; Stavridou, E. Deep Root Growth and Nitrogen Uptake by Rocket (Diplotaxis tenuifolia L.) as Affected by Nitrogen Fertilizer, Plant Density and Leaf Harvesting on a Coarse Sandy Soil. Soil Use Manag. 2017, 33, 62–71. [Google Scholar] [CrossRef]
- Fargione, J.E.; Tilman, D. Diversity Decreases Invasion via Both Sampling and Complementarity Effects: Diversity Causes Invader Underyielding. Ecol. Lett. 2005, 8, 604–611. [Google Scholar] [CrossRef]
- Guo, L.; Deng, M.; Yang, S.; Liu, W.; Wang, X.; Wang, J.; Liu, L. The Coordination between Leaf and Fine Root Litter Decomposition and the Difference in Their Controlling Factors. Glob. Ecol. Biogeogr. 2021, 30, 2286–2296. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Q.; Zhou, D.; Xu, W.; Gao, J.; Wang, Z. How Evergreen and Deciduous Trees Coexist during Secondary Forest Succession: Insights into Forest Restoration Mechanisms in Chinese Subtropical Forest. Glob. Ecol. Conserv. 2021, 25, e01418. [Google Scholar] [CrossRef]
- Seyedi, N.; Costa, C.; Máguas, C.; Correia, O. The Contribution of Leaf Life Span to Sexual Dimorphism in Deciduous and Evergreen Pistacia Species under Mediterranean Conditions. Flora 2019, 251, 114–121. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, J.; Xiao, H.; Liu, J.; Wang, L. Variations in Leaf Functional Traits among Plant Species Grouped by Growth and Leaf Types in Zhenjiang, China. J. For. Res. 2017, 28, 241–248. [Google Scholar] [CrossRef]
- Comas, L.H.; Callahan, H.S.; Midford, P.E. Patterns in Root Traits of Woody Species Hosting Arbuscular and Ectomycorrhizas: Implications for the Evolution of Belowground Strategies. Ecol. Evol. 2014, 4, 2979–2990. [Google Scholar] [CrossRef]
- Valverde-Barrantes, O.J.; Smemo, K.A.; Feinstein, L.M.; Kershner, M.W.; Blackwood, C.B. Patterns in Spatial Distribution and Root Trait Syndromes for Ecto and Arbuscular Mycorrhizal Temperate Trees in a Mixed Broadleaf Forest. Oecologia 2018, 186, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Lutz, J.A.; Guo, Q.; Hao, Z.; Wang, X.; Gilbert, G.S.; Mao, Z.; Orwig, D.A.; Parker, G.G.; Sang, W.; et al. Mycorrhizal Type Influences Plant Density Dependence and Species Richness across 15 Temperate Forests. Ecology 2021, 102, e03259. [Google Scholar] [CrossRef] [PubMed]
- Repáč, I.; Balanda, M.; Vencurik, J.; Kmet, J.; Krajmerová, D.; Paule, L. Effects of Substrate and Ectomycorrhizal Inoculation on the Development of Two-Years-Old Container-Grown Norway Spruce (Picea abies Karst.) Seedlings. Iforest 2014, 8, 487. [Google Scholar] [CrossRef]
- Phillips, R.P.; Brzostek, E.; Midgley, M.G. The Mycorrhizal-associated Nutrient Economy: A New Framework for Predicting Carbon–Nutrient Couplings in Temperate Forests. New Phytol. 2013, 199, 41–51. [Google Scholar] [CrossRef]


| Species Name | Abbreviation | Family Name | Root Depth | Leaf Habit | Mycorrhizal Type |
|---|---|---|---|---|---|
| Castanea henryi (Skan) Rehd. et Wils. | CaHe | Fagaceae | deep-rooted | deciduous | ECM |
| Castanopsis sclerophylla (Lindley & Paxton) Schottky | CaSc | Fagaceae | deep-rooted | evergreen | ECM |
| Choerospondias axillaris (Roxb.) Burtt et Hill | ChAx | Anacardiaceae | shallow-rooted | deciduous | AM |
| Cyclobalanopsis glauca (Thunberg) Oersted | CyGl | Fagaceae | deep-rooted | evergreen | ECM |
| Cyclobalanopsis myrsinifolia (Blume) Oersted | CyMy | Fagaceae | deep-rooted | evergreen | ECM |
| Koelreuteria bipinnata Franch. | KoBi | Sapindaceae | shallow-rooted | deciduous | AM |
| Liquidambar formosana Hance | LiFo | Hamamelidaceae | deep-rooted | deciduous | AM |
| Lithocarpus glaber (Thunb.) Nakai | LiGl | Fagaceae | deep-rooted | evergreen | ECM |
| Nyssa sinensis Oliver | NySi | Nyssaceae | deep-rooted | deciduous | AM |
| Quercus fabri Hance | QuFa | Fagaceae | deep-rooted | deciduous | ECM |
| Quercus serrata Murray | QuSe | Fagaceae | deep-rooted | deciduous | ECM |
| Sapindus saponaria L. | SaSa | Sapindaceae | shallow-rooted | deciduous | AM |
| Schima superba Gardn. et Champ. | ScSu | Theaceae | shallow-rooted | evergreen | AM |
| Triadica sebifera (Linnaeus) Small | TrSe | Euphorbiaceae | deep-rooted | deciduous | AM |
| Species Richness | Tree Height (m) | Basal Diameter (cm) | Basal Area (cm2) |
|---|---|---|---|
| 1 | 5.36 ± 2.36 a | 7.84 ± 4.17 a | 61.84 ± 64.44 a |
| 2 | 4.69 ± 2.85 a | 7.24 ± 4.43 a | 56.48 ± 65.38 a |
| 4 | 5.49 ± 2.50 a | 8.51 ± 5.28 a | 78.52 ± 84.79 a |
| 8 | 4.98 ± 2.21 a | 7.16 ± 4.33 a | 54.74 ± 58.85 a |
| Parameters | Abbreviation | Parameters | Abbreviation |
|---|---|---|---|
| Species richness | SR | Leaf habit | LH |
| Root depth | RD | Mycorrhizal type | MT |
| Chlorophyll | CHL | Root diameter | DIA |
| Specific leaf area | SLA | Specific root length | SRL |
| Leaf area | LA | Specific root surface area | SRA |
| Leaf dry matter content | LDMC | Root tissue density | RTD |
| Leaf nitrogen | LN | Root nitrogen | RN |
| Leaf nitrogen | LP | Root phosphorus | RP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, C.; Cheng, Y.; Zeng, S.; Yang, S.; Lin, X.; Shi, J.; Bu, W. Mixed-Species Stands Improve the Coordination between Leaf and Fine Root Traits in a Common Garden Experiment. Forests 2024, 15, 744. https://doi.org/10.3390/f15050744
Li Y, Zhang C, Cheng Y, Zeng S, Yang S, Lin X, Shi J, Bu W. Mixed-Species Stands Improve the Coordination between Leaf and Fine Root Traits in a Common Garden Experiment. Forests. 2024; 15(5):744. https://doi.org/10.3390/f15050744
Chicago/Turabian StyleLi, Yuxin, Cancan Zhang, Yiqing Cheng, Shiqi Zeng, Shiyun Yang, Xiaofan Lin, Jianmin Shi, and Wensheng Bu. 2024. "Mixed-Species Stands Improve the Coordination between Leaf and Fine Root Traits in a Common Garden Experiment" Forests 15, no. 5: 744. https://doi.org/10.3390/f15050744





