The Genetic Diversity of Natural Ilex chinensis Sims (Aquifoliaceae) Populations as Revealed by SSR Markers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population Sampling
2.2. DNA Extraction and Microsatellite Genotyping
2.3. Data Analysis
3. Results
3.1. Genetic Diversity at the Species Level
3.2. Genetic Diversity at the Population Level
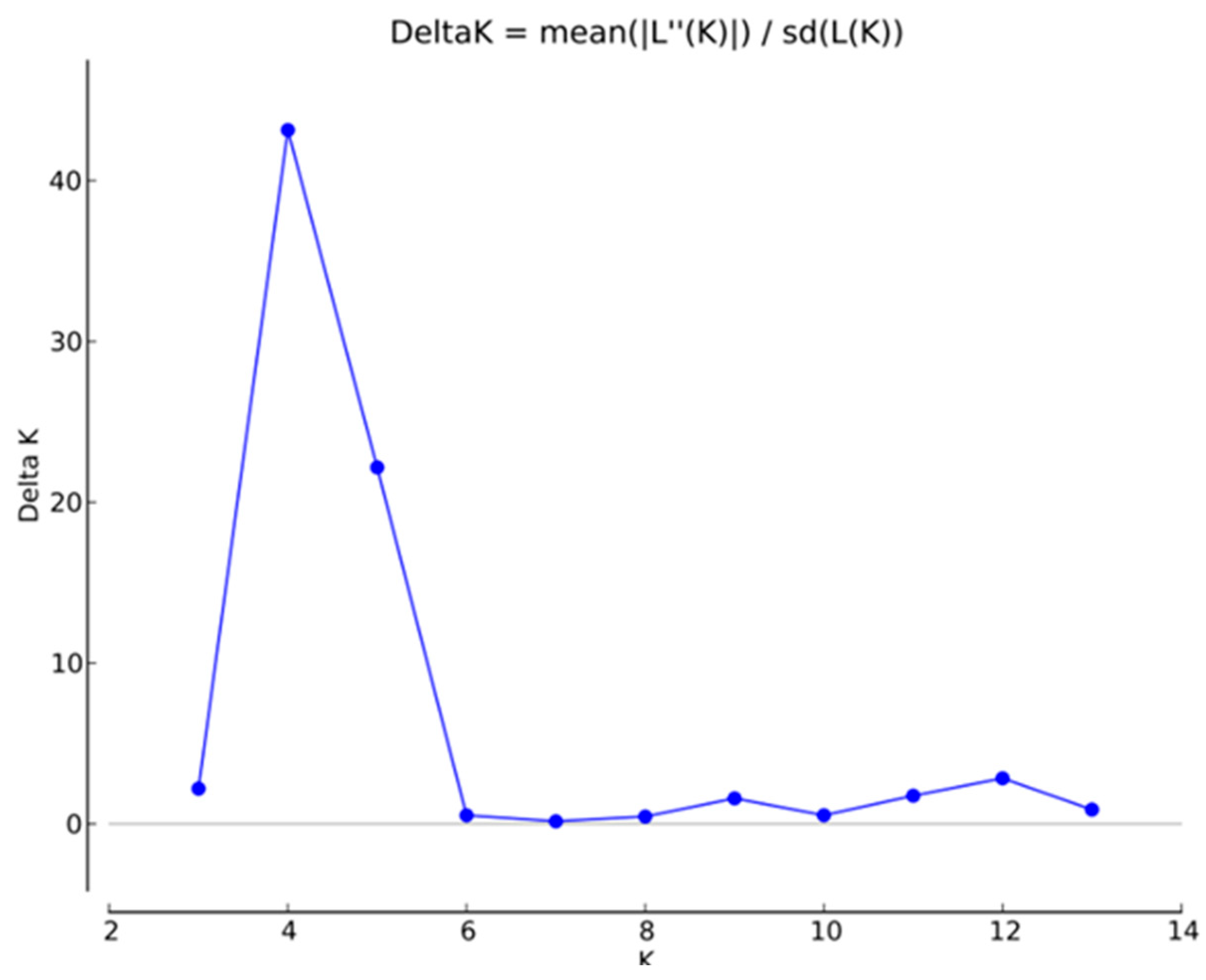
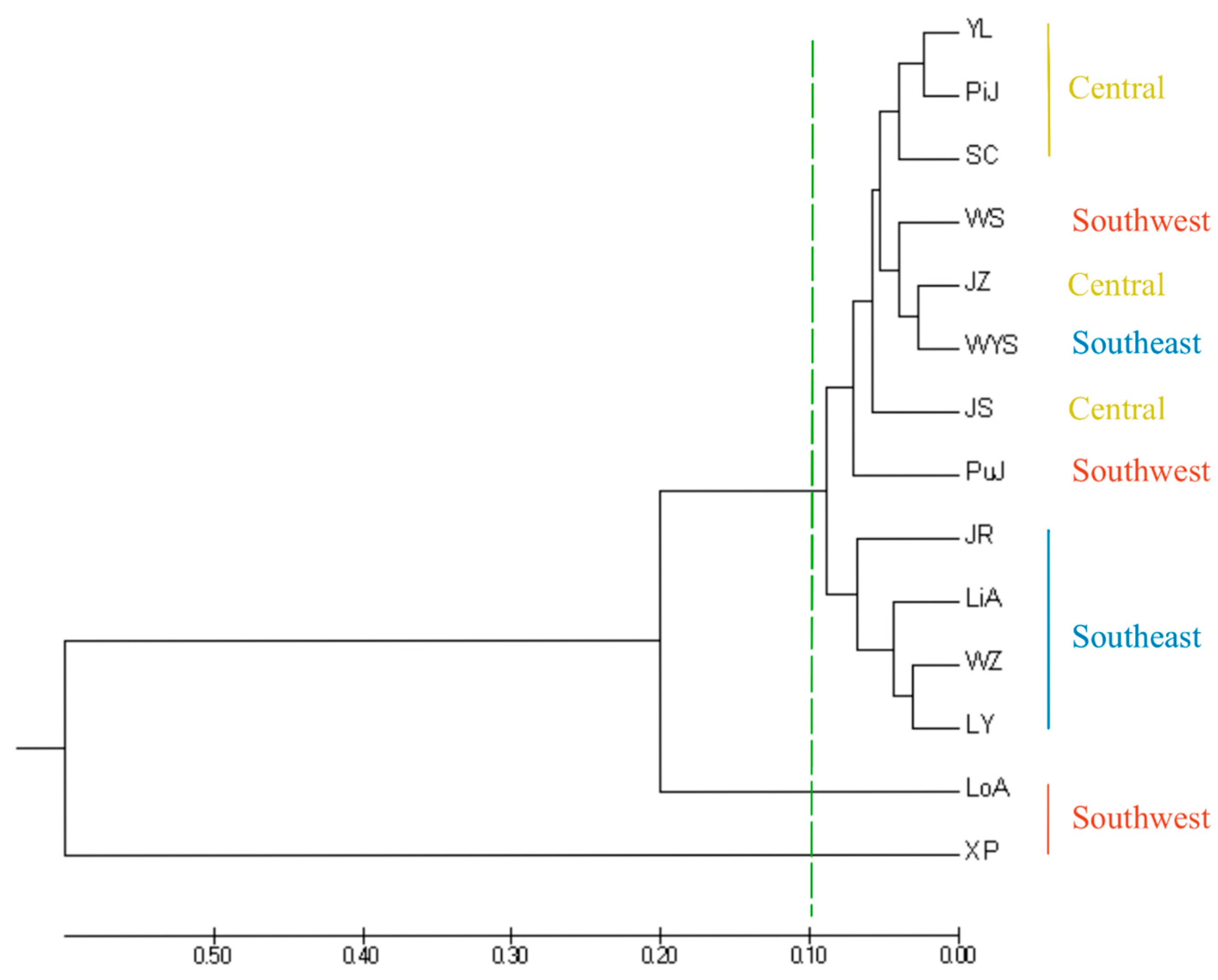
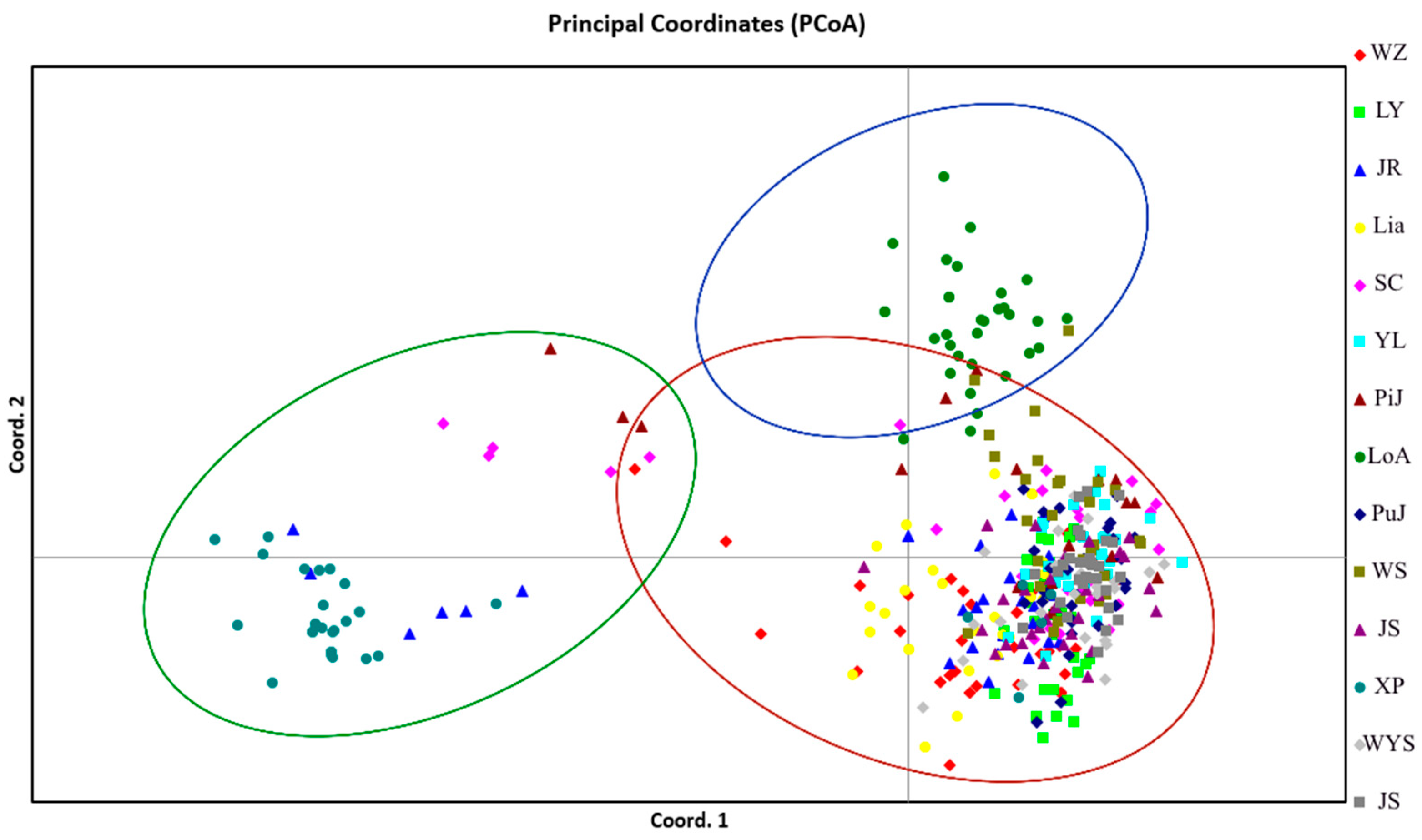
3.3. Genetic Variation and Genetic Structure
4. Discussion
4.1. Genetic Diversity
4.2. Genetic Differentiation and Genetic Structure
4.3. Intrinsic Factors Affecting Genetic Variation
4.4. Environmental Factors Affecting Genetic Variation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gottlieb, A.M.; Giberti, G.C.; Poggio, L. Molecular analyses of the genus Ilex (aquifoliaceae) in Southern south America, evidence from AFLP and ITS sequence data. Am. J. Bot. 2005, 92, 352–369. [Google Scholar] [CrossRef] [PubMed]
- Herrera, C.M.; Bazaga, P. Epigenetic correlates of plant phenotypic plasticity: DNA methylation differs between prickly and nonprickly leaves in heterophyllous Ilex aquifolium (Aquifoliaceae) trees. Bot. J. Linn. Soc. 2013, 171, 441–452. [Google Scholar] [CrossRef]
- Manen, J.-F. Are both sympatric species Ilex perado and Ilex canariensis secretly hybridizing? Indication from nuclear markers collected in Tenerife. BMC Evol. Biol. 2004, 4, 46. [Google Scholar] [CrossRef] [PubMed]
- Selbach-Schnadelbach, A.; Cavalli, S.S.; Manen, J.F.; Coelho, G.C.; De Souza-Chies, T.T. New information for Ilex phylogenetics based on the plastid psbA-trnH intergenic spacer (Aquifoliaceae). Bot. J. Linn. Soc. 2009, 159, 182–193. [Google Scholar] [CrossRef]
- Loizeau, P.A.; Barriera, G.; Manen, J.F.; Broennimann, O. Towards an understanding of the distribution of Ilex L. (Aquifoliaceae) on a worldwide scale. Biol. Skr. 2005, 55, 501–520. [Google Scholar]
- Yao, X.; Song, Y.; Yang, J.B.; Tan, Y.H.; Corlett, R.T. Phylogeny and biogeography of the hollies (Ilex L., Aquifoliaceae). J. Syst. Evol. 2021, 59, 73–82. [Google Scholar] [CrossRef]
- Cuénoud, P.; Martinez, M.A.D.P.; Loizeau, P.-A.; Spichiger, R.; Andrews, S.; Manen, J.-F. Molecular phylogeny and biogeography of the genus Ilex L. (Aquifoliaceae). Ann. Bot. 2000, 85, 111–122. [Google Scholar] [CrossRef]
- Ding, H.; Fang, Y.; Yang, Q.; Chen, X.; Yuan, F.; Xu, H.; He, L.; Yan, J.; Chen, T.; Yu, C.; et al. Community characteristics of a mid-subtropical evergreen broad-leaved forest plot in the Wuyi Mountains, Fujian Province, southeastern China. Biodivers. Sci. 2015, 23, 479–492. [Google Scholar] [CrossRef]
- Ding, H.; Fang, Y.; Yang, X.; Yuan, F.; He, L.; Yao, J.; Wu, J.; Chi, B.; Li, Y.; Chen, S.; et al. Community characteristics of a subtropical evergreen broad-leaved forest in Huangshan, Anhui Province, East China. Biodivers. Sci. 2016, 24, 875–887. [Google Scholar] [CrossRef]
- Ding, H.; Yang, Y.F.; Xu, H.; Fang, Y.; Chen, X.; Yang, Q.; Yi, X.; Xu, H.; Wen, X.; Xu, X. Species composition and community structure of the typical evergreen broad-leaved forest in the Wuyi Mountains of Southeastern China. Acta Ecol. Sin. 2015, 35, 1142–1154. [Google Scholar]
- Tang, C.Q.; Ohsawa, M. Coexistence mechanisms of evergreen, deciduous and coniferous trees in a mid-montane mixed forest on Mt. Emei, Sichuan, China. Plant Ecol. 2002, 161, 215–230. [Google Scholar] [CrossRef]
- Pavão, D.; Jevšenak, J.; Engblom, J.; Silva, L.B.; Elias, R. Tree growth-climate relationship in the Azorean holly in a temperate humid forest with low thermal amplitude. Dendrochronologia 2023, 77, 126050. [Google Scholar] [CrossRef]
- Li, L.; Xu, L.J.; Ma, G.Z.; Dong, Y.M.; Peng, Y.; Xiao, P.G. The large-leaved kudingcha (Ilex latifolia Thunb and Ilex kudingcha CJ Tseng): A traditional Chinese tea with plentiful secondary metabolites and potential biological activities. J. Nat. Med. 2013, 67, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Negrin, A.; Long, C.; Motley, T.J.; Kennelly, E.J. LC-MS metabolomics and chemotaxonomy of caffeine-containing holly (Ilex) species and related taxa in the Aquifoliaceae. J. Agric. Food Chem. 2019, 67, 5687–5699. [Google Scholar] [CrossRef]
- Jiang, S.; Cui, H.; Wu, P.; Liu, Z.; Zhao, Z. Botany, traditional uses, phytochemistry, pharmacology and toxicology of Ilex pubescens Hook et Arn. J. Ethnopharmacol. 2019, 245, 112147. [Google Scholar] [CrossRef] [PubMed]
- Noureddine, T.; Al Husseini, Z.; Nehme, A.; Massih, R.A. Antibacterial activity of Ilex paraguariensis (Yerba Mate) against Gram-positive and Gram-negative bacteria. J. Infect. Dev. Ctries. 2018, 12, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.-Y.; Li, R.-F.; Sun, H.; Li, S. New triterpenoid saponins from the leaves of Ilex chinensis and their hepatoprotective activity. Chin. J. Nat. Med. 2021, 19, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Bareke, T.; Addi, A. Bee flora diversity in different vegetation communities of Gsha-Sayilem forest in Kaffa Zone, south-western Ethiopia. Plants Environ. 2000, 2, 138–148. [Google Scholar] [CrossRef]
- Qian, Y.P.; Tian, R.N. Research advance of Ilex germplasm resources and their application to landscape. World For. Res. 2016, 29, 40–45. [Google Scholar]
- Shi, S.; Chen, S.-F.; Zhong, F.-H.; Wu, G.-X.; Liao, W.-B.; Fan, Q. Ilex sanqingshanensis sp. Nov. (Aquifoliaceae) from Jiangxi Province, China. Nord. J. Bot. 2015, 33, 662–667. [Google Scholar] [CrossRef]
- Lozada-Perez, L. A new species of Ilex (Aquifoliaceae) from Mexico. Phytotaxa 2019, 409, 296–300. [Google Scholar] [CrossRef]
- Jiang, L.; Wu, L.F.; Huang, G.Y.; Yu, J.R.; He, B.S.; Xu, K.W. A new species of Ilex sect. Ilex (Aquifoliaceae) from Guangdong, China. Phytotaxa 2020, 428, 153–158. [Google Scholar] [CrossRef]
- Manen, J.F.; Boulter, M.C.; Naciri-Graven, Y. The complex history of the genus Ilex L. (Aquifoliaceae): Evidence from the comparison of plastid and nuclear DNA sequences and from fossil data. Plant Syst. Evol. 2002, 235, 79–98. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, L.; Liu, E.D.; Liu, W.L.; Chen, L.; Kou, Y.X.; Fan, D.M.; Cheng, S.M.; Zhang, Z.Y.; Peng, H. Time to update the sectional classification of Ilex (Aquifoliaceae): New insights from Ilex phylogeny, morphology, and distribution. J. Syst. Evol. 2023, 61, 1036–1046. [Google Scholar] [CrossRef]
- Xu, K.-W.; Wei, X.-F.; Lin, C.-X.; Zhang, M.; Zhang, Q.; Zhou, P.; Fang, Y.-M.; Xue, J.-Y.; Duan, Y.-F. The chromosome-level holly (Ilex latifolia) genome reveals key enzymes in triterpenoid saponin biosynthesis and fruit color change. Front. Plant Sci. 2022, 13, 98. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Lu, Z.; Song, Y.; Hu, X.; Corlett, R.T. A chromosome-scale genome assembly for the holly (Ilex polyneura) provides insights into genomic adaptations to elevation in Southwest China. Hortic. Res. 2022, 9, 2323. [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, Q.; Li, F.; Huang, J.; Zhang, M. Assembly and comparative analysis of the complete mitochondrial genome of Ilex metabaptista (Aquifoliaceae), a Chinese endemic species with a narrow distribution. BMC Plant Biol. 2023, 23, 393. [Google Scholar] [CrossRef]
- Yao, X.; Tan, Y.-H.; Liu, Y.-Y.; Song, Y.; Yang, J.-B.; Corlett, R.T. Chloroplast genome structure in Ilex (Aquifoliaceae). Sci. Rep. 2016, 6, 28559. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.L.-H.; Park, H.-S.; Lau, T.-W.D.; Lin, Z.; Yang, T.-J.; Shaw, P.-C. Comparative analysis and phylogenetic investigation of Hong Kong Ilex chloroplast genomes. Sci. Rep. 2021, 11, 5153. [Google Scholar] [CrossRef]
- Xu, K.; Lin, C.; Lee, S.Y.; Mao, L.; Meng, K. Comparative analysis of complete Ilex (Aquifoliaceae) chloroplast genomes: Insights into evolutionary dynamics and phylogenetic relationships. BMC Genom. 2022, 23, 203. [Google Scholar] [CrossRef]
- Cascales, J.; Bracco, M.; Poggio, L.; Gottlieb, A.M. Genetic diversity of wild germplasm of ‘‘yerba mate’’ (Ilex paraguariensis St. Hil.) from Uruguay. Genetica 2014, 142, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Seoane, C.; Diaz, V.; Kageyama, P.; Moreno, M.; Tambarussi, E.; Aguiar, A.; Sebbenn, A. The neotropical tree Ilex paraguariensis A. St. Hil. (Aquifoliaceae): Pollen and seed dispersal in a fragmented landscape. Ann. For. Res. 2019, 62, 157–171. [Google Scholar] [CrossRef]
- Paiva, D.I.; Cascales, J.; Rosetti, M.E.N.; Scherer, R.A.; Gauchat, M.E.; Gottlieb, A.M. Unraveling the genetic complexity of a cultivated breeding population of “yerba mate” (Ilex paraguariensis St. Hil.). Acad. Bras. Cienc. 2020, 92, e20190113. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt, L.; e Silva, D.T.; Nascimento, B.; Saiki, F.A.; Bet Stedille, L.I.; da Costa, N.C.F.; Mantovani, A. Genetic diversity in successive age cohorts of Ilex paraguariensis in Southern Brazil. For. Sci. 2022, 68, 291–298. [Google Scholar] [CrossRef]
- Erazo-Garcia, M.P.; Guadalupe, J.J.; Rowntree, J.K.; Borja-Serrano, P.; Monteros-Silva, N.E.d.L.; Torres, M.d.L. Assessing the genetic diversity of Ilex guayusa Loes., a medicinal plant from the ecuadorian amazon. Diversity 2021, 13, 182. [Google Scholar] [CrossRef]
- Geukens, E.; Haegeman, A.; Van Meulder, J.; Van Laere, K.; Smolders, E.; Ruttink, T.; Leus, L. Exploring genetic diversity in an Ilex crenata breeding germplasm. Horticulturae 2023, 9, 485. [Google Scholar] [CrossRef]
- Torimaru, T.; Tani, N.; Tsumura, Y.; Hiraoka, K.; Tomaru, N. Development and polymorphism of simple sequence repeat DNA markers for the evergreen shrub Ilex leucoclada M. Mol. Ecol. Notes 2004, 4, 531–533. [Google Scholar] [CrossRef]
- Chen, W.-W.; Xiao, Z.-Z.; Tong, X.; Liu, Y.-P.; Li, Y.-Y. Development and characterization of 25 microsatellite primers for Ilex chinensis (Aquifoliaceae). Appl. Plant Sci. 2015, 3, 1500057. [Google Scholar] [CrossRef]
- Leng, X.; Wang, Z.; An, S.; Feng, J.; Liu, Y.; Wang, G. ISSR analysis of genetic diversity of Ilex integra, an insular endemic plant. Biodivers. Sci. 2005, 13, 546–554. [Google Scholar] [CrossRef]
- Rendell, S.; Ennos, R.A. Chloroplast DNA diversity of the dioecious European tree Ilex aquifolium L. (English holly). Mol. Ecol. 2003, 12, 2681–2688. [Google Scholar] [CrossRef]
- Setoguchi, H.; Watanabe, I. Intersectional gene flow between insular endemics of Ilex (Aquifoliaceae) on the Bonin Islands and the Ryukyu Islands. Am. J. Bot. 2000, 87, 793–810. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Li, N.; Wang, S.; Zhou, Y.; Huang, W.; Yang, Y.; Ma, Y.; Zhou, R. Molecular evidence for the hybrid origin of Ilex dabieshanensis (Aquifoliaceae). PLoS ONE 2016, 11, e0147825. [Google Scholar] [CrossRef]
- Manen, J.-F.; Barriera, G.; Loizeau, P.-A.; Naciri, Y. The history of extant Ilex species (Aquifoliaceae): Evidence of hybridization within a Miocene radiation. Mol. Phylogenet. Evol. 2010, 57, 961–977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, M.; Ding, Y.; Zhou, P.; Fang, Y. Composition of photosynthetic pigments and photosynthetic characteristics in green and yellow sectors of the variegated Aucuba japonica ‘Variegata’ leaves. Flora 2018, 240, 25–33. [Google Scholar] [CrossRef]
- Peakall, R.O.D.; Smouse, P.E.; GENALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Liu, K.; Muse, S.V. PowerMarker: An integrated analysis environment for genetic marker analysis. Bioinformatics 2005, 21, 2128–2129. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2015, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.T. Application of ArcGIS in geography teaching of secondary school: A case study in the practice of map teaching. Wirel. Pers. Commun. Int. J. 2018, 102, 2543–2553. [Google Scholar]
- Costa, V.; Angelini, C.; De Feis, I.; Ciccodicola, A. Uncovering the complexity of transcriptomes with RNA-SEQ. In The Role of Bioinformatics in Agriculture; Apple Academic Press: Palm Bay, FL, USA, 2014. [Google Scholar]
- Schielzeth, H.; Wolf, J.B.W. Community genomics: A community-wide perspective on within-species genetic diversity. Am. J. Bot. 2021, 108, 2108–2111. [Google Scholar] [CrossRef]
- Sun, Y.P.; Zhang, D.L.; Geng, F. Genetic diversity and taxon delineation of Ilex glabra using AFLP markers. Acta Hortic. 2010, 859, 261–270. [Google Scholar]
- Wang, Y.-S.; Zhao, L.-M.; Wang, H.; Wang, J.; Huang, D.-M.; Hong, R.-M.; Teng, X.-H.; Miki, N. Molecular genetic variation in a clonal plant population of Leymus chinensis (Trin.) Tzvel. J. Integr. Plant Biol. 2005, 47, 1055–1064. [Google Scholar] [CrossRef]
- Yan, H.; Qi, H.; Li, Y.; Wu, Y.; Wang, Y.; Chen, J.; Yu, J. Assessment of the genetic relationship and population structure in Oil-Tea Camellia species using simple sequence repeat (SSR) markers. Genes 2022, 13, 2162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Y.-H.; Wang, Y.-H.; Shen, S.-K. Genetic diversity and population structure of Rhododendron rex Subsp. rex inferred from microsatellite markers and chloroplast DNA sequences. Plants 2020, 9, 338. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.L., Jr.; Cabral, R.L.R.; Sartori, L.; de Miranda, F.D.; Caldeira, M.V.W.; Moreira, S.O.; Godinho, T.d.O.; de Oliveira, F.S. Molecular markers applied to the genetic characterization of Dalbergia nigra: Implications for conservation and management. Trees 2022, 36, 1539–1557. [Google Scholar] [CrossRef]
- Wang, F.; Cheng, X.; Cheng, S.; Li, W.; Huang, X. Genetic diversity of the wild ancient tea tree (Camellia taliensis) populations at different altitudes in Qianjiazhai. PLoS ONE 2023, 18, e0283189. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Hao, X.; Han, L.; Zhai, Y.; Zhou, S.; Chen, S.; Ren, D.; An, X. Unraveling the genetic diversity and structure of Quercus liaotungensis population through analysis of microsatellite markers. PeerJ 2021, 9, e10922. [Google Scholar] [CrossRef]
- Li, N.; Yang, Y.; Xu, F.; Chen, X.; Wei, R.; Li, Z.; Pan, W.; Zhang, W. Genetic diversity and population structure analysis of Castanopsis hystrix and construction of a core collection using phenotypic traits and molecular markers. Genes 2022, 13, 2383. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Zhang, P.; Hu, C.; Chachar, S.; Riaz, A.; Wang, R.; Yang, Y. Genetic diversity, germplasm identification and population structure of Diospyros kaki Thunb. from different geographic regions in China using SSR markers. Sci. Hortic. 2019, 251, 233–240. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, H.; Huang, H.; Vargas-Mendoza, C.F. Chloroplast diversity and population differentiation of Castanopsis fargesii (Fagaceae): A dominant tree species in evergreen broad-leaved forest of subtropical China. Tree Genet. Genomes 2014, 10, 1531–1539. [Google Scholar] [CrossRef]
- Wang, S.; Luo, Y.; Yang, T.; Zhang, Y.; Li, Z.; Jin, W.; Fang, Y. Genetic diversity of Rhododendron simsii Planch. natural populations at different altitudes in Wujiashan Mountain. Caryologia 2019, 72, 41–51. [Google Scholar]
- Yang, A.; Zhong, Y.; Liu, S.; Liu, L.; Liu, T.; Li, Y.; Yu, F. New insight into the phylogeographic pattern of Liriodendron chinense (Magnoliaceae) revealed by chloroplast DNA: East–west lineage split and genetic mixture within western subtropical China. PeerJ 2019, 7, e6355. [Google Scholar] [CrossRef]
- Hamrick, L.; Godt, M.J.W. Effects of life history traits on genetic diversity in plant species. Philosophical Transactions of the Royal Society of London. Ser. B Biol. Sci. 1996, 351, 1291–1298. [Google Scholar]
- Matsuhisa, S.; Ushimaru, A. Sexual dimorphism in floral longevity and flowering synchrony in relation to pollination and mating success in three dioecious Ilex species. Am. J. Bot. 2015, 102, 1187–1197. [Google Scholar] [CrossRef]
- Tsang, A.C.W.; Corlett, R.T. Reproductive biology of the Ilex species (Aquifoliaceae) in Hong Kong, China. Can. J. Bot. 2005, 83, 1645–1654. [Google Scholar] [CrossRef]
- Kuang, Z.Y.; Peng, Y.; Fang, Y.M. Preliminary observation on the effect of volatile organic components of Ilex rotunda on its pollinating insect Apis cerana. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2023. Available online: https://kns.cnki.net/kcms/detail/32.1161.S.20230327.1323.006.html (accessed on 10 January 2024).
- Carr, D.E. Sexual dimorphism and fruit production in a dioecious understory tree, Ilex opaca Ait. Oecologia 1991, 85, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Torimaru, T.; Tomaru, N.; Nishimura, N.; Yamamoto, S. Clonal diversity and genetic differentiation in Ilex leucoclada M. patches in an old-growth beech forest. Mol. Ecol. 2003, 12, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Torimaru, T.; Tomaru, N. Fine-scale clonal structure and diversity within patches of a clone-forming dioecious shrub, Ilex leucoclada (Aquifoliaceae). Ann. Bot. 2005, 95, 295–304. [Google Scholar] [CrossRef]
- Takagi, E.; Togashi, K. Evidence of sex change in Ilex integra. Botany 2012, 90, 75–78. [Google Scholar] [CrossRef]
- Obeso, J.R.; Fernández-Calvo, I.C. Fruit removal, pyrene dispersal, post-dispersal predation and seedling establishment of a bird-dispersed tree. Plant Ecol. 2002, 165, 223–233. [Google Scholar] [CrossRef]
- Arrieta, S.; Suarez, F. Spatial dynamics of Ilex aquifolium populations seed dispersal and seed bank: Understanding the first steps of regeneration. Plant Ecol. 2005, 177, 237–248. [Google Scholar] [CrossRef]
- Obeso, J.R. Patterns of variation in Ilex aquifolium fruit traits related to fruit consumption by birds and seed predation by rodents. Ecoscience 1998, 5, 463–469. [Google Scholar] [CrossRef]
- Martínez, I.; García, D.; Obeso, J.R. Differential seed dispersal patterns generated by a common assemblage of vertebrate frugivores in three fleshy-fruited trees. Écoscience 2008, 15, 189–199. [Google Scholar] [CrossRef]
- Salvande, M.; Mulet, M.; Gonzalez, L.A.G. Ilex canariensis Poir. (Aquifoliaceae) post-dispersal seed predation in the Canary Islands. Plant Ecol. 2006, 187, 143–151. [Google Scholar] [CrossRef]
- Karubian, J.; Browne, L.; Bosque, C.; Carlo, T.; Galetti, M.; Loiselle, B.A.; Blake, J.G.; Cabrera, D.; Durães, R.; Labecca, F.M.; et al. Seed dispersal by neotropical birds: Emerging patterns and underlying processes. Ornitol. Neotrop. 2012, 23, 9–24. [Google Scholar]
- Parejo-Farnés, C.; Albaladejo, R.G.; Camacho, C.; Aparicio, A. From species to individuals: Combining barcoding and microsatellite analyses from non-invasive samples in plant ecology studies. Plant Ecol. 2018, 219, 1151–1158. [Google Scholar] [CrossRef]
- Sosa, P.A.; González-González, E.A.; González-Pérez, M.A.; de Paz, P.L.P. Contrasting patterns of genetic differentiation in Macaronesian lineages of Ilex (Aquifoliaceae). Bot. J. Linn. Soc. 2013, 173, 258–268. [Google Scholar] [CrossRef]
- Ohsawa, T.; Ide, Y. Global patterns of genetic variation in plant species along vertical and horizontal gradients on mountains. Glob. Ecol. Biogeogr. 2008, 17, 152–163. [Google Scholar] [CrossRef]
- Meng, H.J.; Wei, X.Z.; Jiang, M.X. Contrasting elevational patterns of genetic variation in Euptelea pleiospermum along mountains at the core and edges of its latitudinal range. Plant Ecol. 2019, 220, 13–28. [Google Scholar] [CrossRef]
- Hahn, T.; Kettle, C.J.; Ghazoul, J.; Frei, E.R.; Matter, P.; Pluess, A.R. Patterns of genetic variation across altitude in three plant species of semi-dry grasslands. PLoS ONE 2012, 7, e41608. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.H.; Su, T.; Gao, X.Y.; Li, J.; Jiang, X.L.; Sun, H.; Zhou, Z.K. Warm–cold colonization: Response of oaks to uplift of the Himalaya–Hengduan Mountains. Mol. Ecol. 2017, 26, 3276–3294. [Google Scholar] [CrossRef] [PubMed]
- Tong, G.B.; Zhang, J.P.; Yang, X.D.; Luo, B.X.; Wang, Y.Z.; Chen, P.Y. Late cenozoic palynoflora and environment changes in Yunnan-Guizhou Plateau. Mar. Geol. Quat. Geol. 1994, 14, 91–104. [Google Scholar]
- Vega, C.; Fernández, V.; Gil, L.; Valbuena-Carabaña, M. Clonal diversity and fine-scale genetic structure of a keystone species: Ilex aquifolium. Forests 2022, 13, 1431. [Google Scholar] [CrossRef]
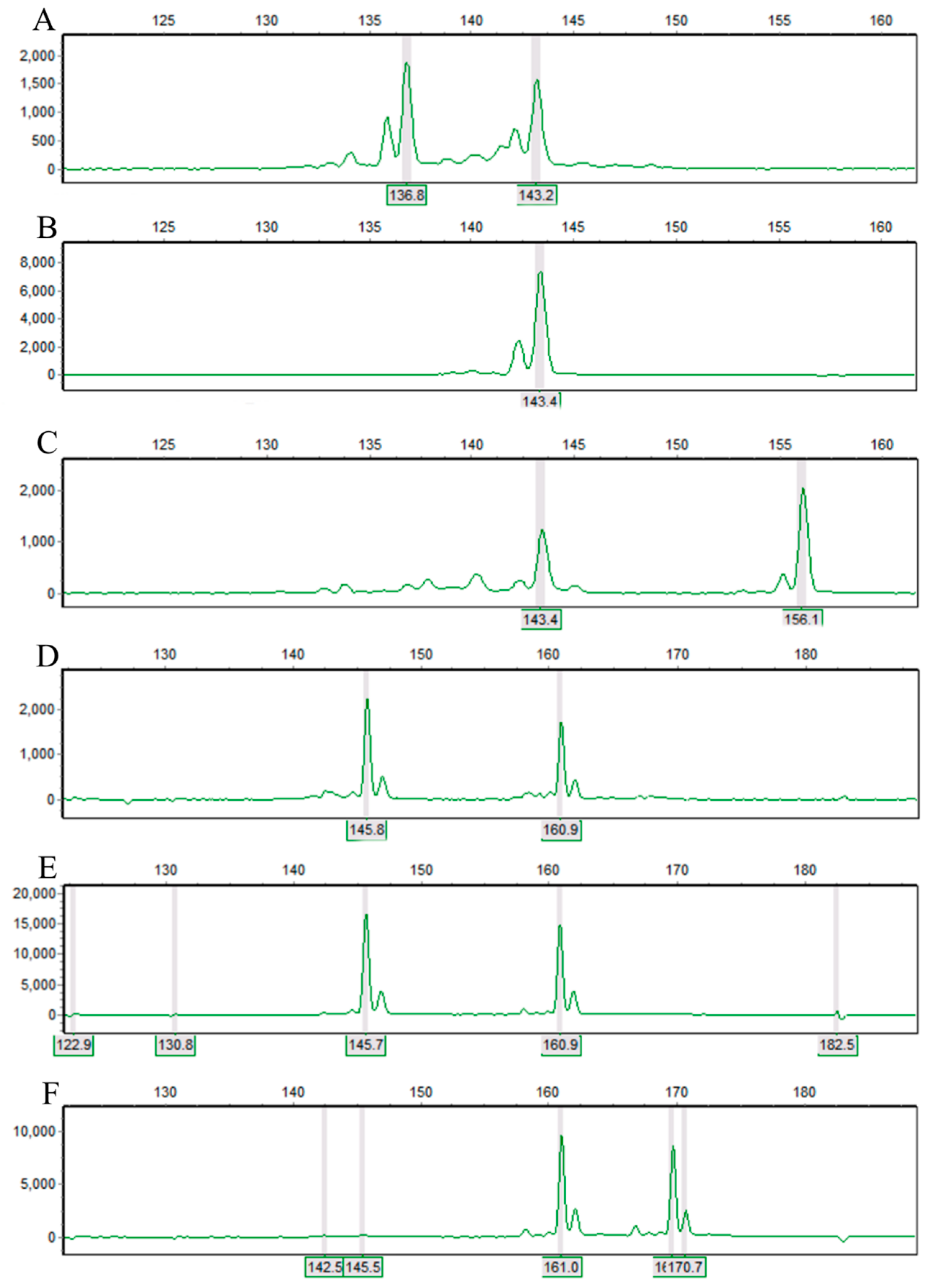


| Na | Ne | I | Ho | He | F | Fis | Fit | Fst | PIC | Nm | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ilex-SSR-01 | 5.429 | 2.804 | 1.165 | 0.582 | 0.587 | 0.008 | 0.008 | 0.193 | 0.187 | 0.715 | 1.089 |
| Ilex-SSR-28 | 3.714 | 1.972 | 0.800 | 0.368 | 0.438 | 0.136 | 0.159 | 0.333 | 0.207 | 0.538 | 0.960 |
| Ilex-SSR-29 | 4.429 | 2.221 | 0.966 | 0.425 | 0.541 | 0.215 | 0.213 | 0.302 | 0.113 | 0.557 | 1.967 |
| Ilex-SSR-32 | 2.000 | 1.116 | 0.181 | 0.037 | 0.090 | 0.468 | 0.590 | 0.795 | 0.500 | 0.176 | 0.250 |
| Ilex-SSR-34 | 2.786 | 1.550 | 0.573 | 0.232 | 0.330 | 0.291 | 0.297 | 0.535 | 0.339 | 0.490 | 0.488 |
| Ilex-SSR-41 | 2.500 | 1.271 | 0.327 | 0.121 | 0.170 | 0.204 | 0.289 | 0.508 | 0.308 | 0.243 | 0.561 |
| Ilex-SSR-68 | 5.071 | 2.531 | 1.112 | 0.516 | 0.580 | 0.103 | 0.110 | 0.251 | 0.158 | 0.647 | 1.329 |
| Ilex-SSR-69 | 7.000 | 3.798 | 1.522 | 0.705 | 0.715 | 0.018 | 0.014 | 0.130 | 0.118 | 0.810 | 1.871 |
| Ilex-SSR-74 | 4.357 | 2.158 | 0.924 | 0.468 | 0.487 | 0.049 | 0.039 | 0.263 | 0.232 | 0.627 | 0.826 |
| Ilex-SSR-75 | 10.786 | 6.102 | 1.991 | 0.796 | 0.822 | 0.032 | 0.031 | 0.123 | 0.094 | 0.911 | 2.397 |
| Ilex-SSR-79 | 5.071 | 2.777 | 1.181 | 0.585 | 0.611 | 0.036 | 0.042 | 0.232 | 0.198 | 0.737 | 1.010 |
| Mean | 4.831 | 2.573 | 0.977 | 0.440 | 0.488 | 0.142 | 0.163 | 0.333 | 0.223 | 0.587 | 0.870 |
| Population Code | Na | Ne | I | Ho | He |
|---|---|---|---|---|---|
| Southeast | |||||
| WZ | 5.091 | 2.442 | 0.985 | 0.490 | 0.499 |
| LY | 4.000 | 2.221 | 0.835 | 0.468 | 0.454 |
| JR | 5.727 | 2.903 | 1.198 | 0.510 | 0.595 |
| LiA | 5.091 | 2.970 | 1.088 | 0.535 | 0.550 |
| WYS | 5.364 | 2.939 | 0.999 | 0.403 | 0.478 |
| Mean | 5.055 | 2.695 | 1.021 | 0.481 | 0.515 |
| Central | |||||
| JZ | 4.636 | 2.342 | 0.933 | 0.436 | 0.464 |
| JS | 4.273 | 2.198 | 0.809 | 0.380 | 0.402 |
| YL | 4.909 | 2.698 | 0.956 | 0.423 | 0.458 |
| PiJ | 5.818 | 2.932 | 1.141 | 0.469 | 0.535 |
| SC | 6.182 | 2.729 | 1.176 | 0.433 | 0.558 |
| Mean | 5.164 | 2.580 | 1.003 | 0.428 | 0.483 |
| Southwest | |||||
| WS | 4.636 | 2.286 | 0.880 | 0.363 | 0.444 |
| PuJ | 3.636 | 2.338 | 0.774 | 0.385 | 0.412 |
| XP | 4.364 | 2.518 | 1.000 | 0.435 | 0.518 |
| LoA | 3.909 | 2.500 | 0.899 | 0.426 | 0.468 |
| Mean | 4.136 | 2.410 | 0.888 | 0.402 | 0.461 |
| Source | Degree of Freedom (df) | The Sum of Squares (SS) | The Mean of Squares (MS) | Est. Var. | Participation/% |
|---|---|---|---|---|---|
| Among Pops | 13 | 515.129 | 39.625 | 0.635 | 18.5% |
| Within Pops | 401 | 939.000 | 2.342 | 2.342 | 81.5% |
| Total | 414 | 1454.129 | 41.967 | 2.977 | 100% |
| FST = 0.185 *** | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, S.; Zhou, P.; Fang, Y.; Wang, X.; Zhang, M.; Zhang, Q. The Genetic Diversity of Natural Ilex chinensis Sims (Aquifoliaceae) Populations as Revealed by SSR Markers. Forests 2024, 15, 763. https://doi.org/10.3390/f15050763
Hou S, Zhou P, Fang Y, Wang X, Zhang M, Zhang Q. The Genetic Diversity of Natural Ilex chinensis Sims (Aquifoliaceae) Populations as Revealed by SSR Markers. Forests. 2024; 15(5):763. https://doi.org/10.3390/f15050763
Chicago/Turabian StyleHou, Sixuan, Peng Zhou, Yanming Fang, Xuejie Wang, Min Zhang, and Qiang Zhang. 2024. "The Genetic Diversity of Natural Ilex chinensis Sims (Aquifoliaceae) Populations as Revealed by SSR Markers" Forests 15, no. 5: 763. https://doi.org/10.3390/f15050763






