Pre-Infection Stages of Austropuccinia psidii in the Epidermis of Eucalyptus Hybrid Leaves with Different Resistance Levels
Abstract
:1. Introduction
2. Materials and Methods
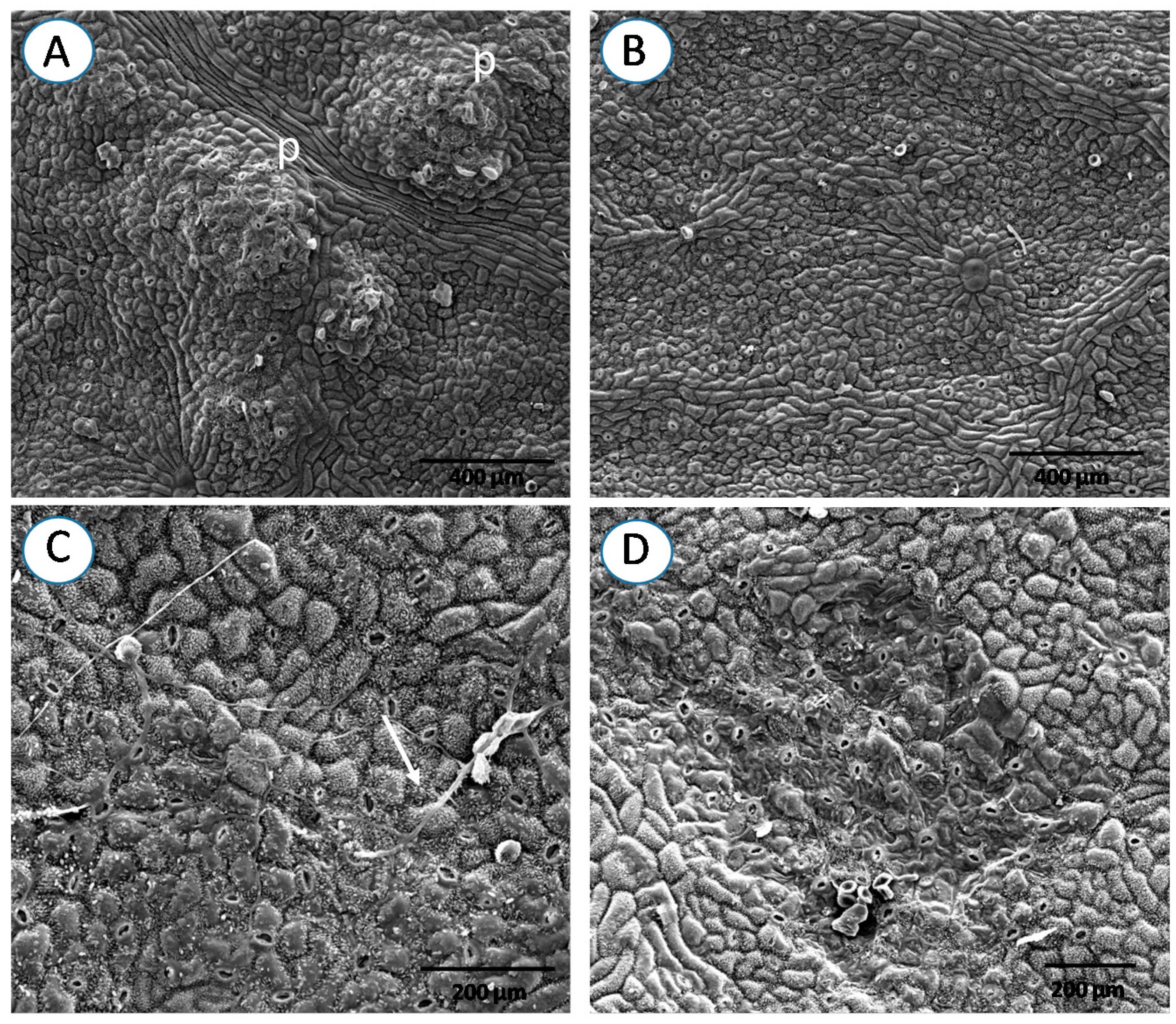
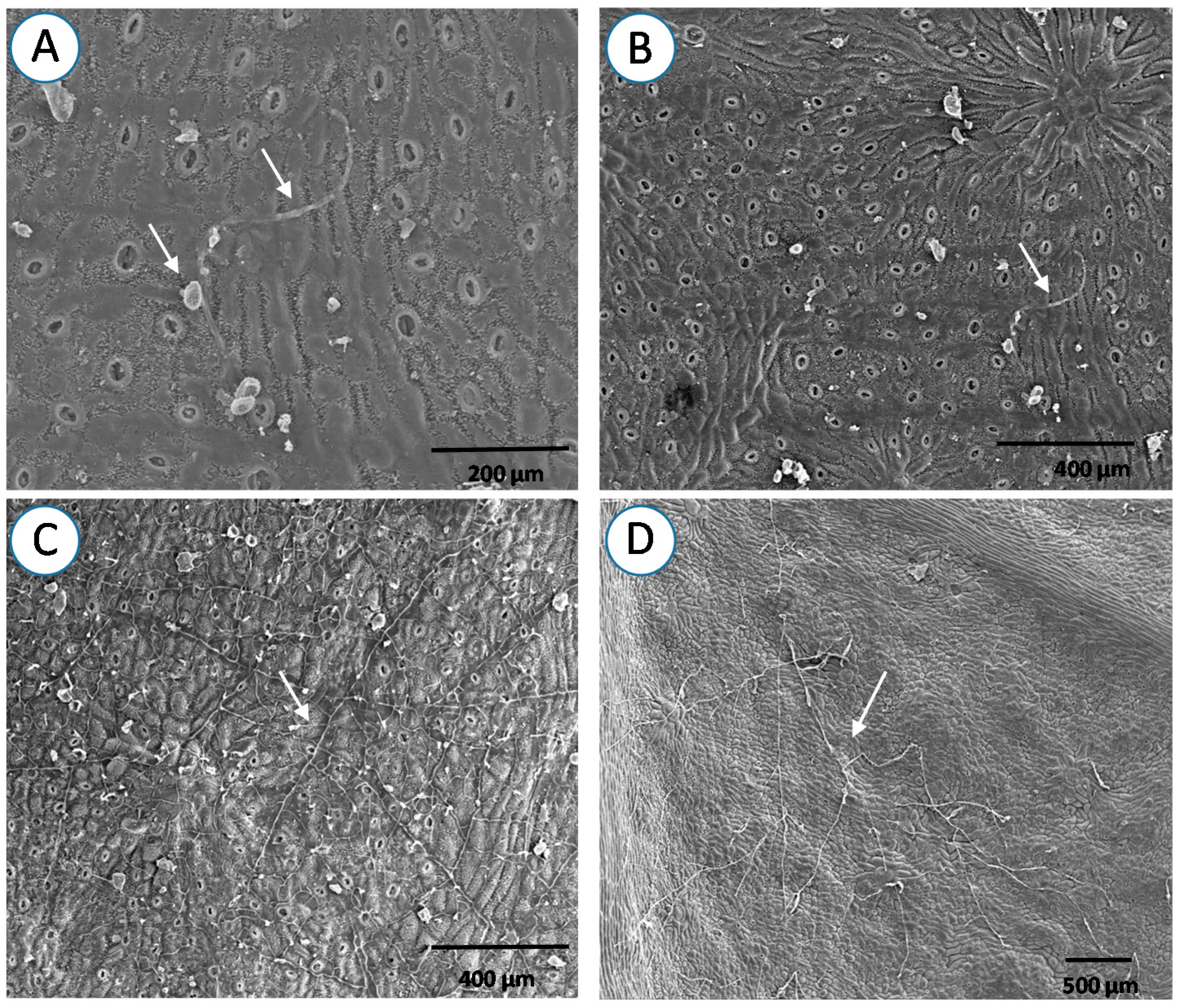
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alfenas, A.C.; Zauza, E.A.V.; Mafia, R.G.; Assis, T.F. Clonagem e Doenças do Eucalipto, 2nd ed.; Editora UFV: Viçosa, Brazil, 2009. [Google Scholar]
- Miranda, A.C.; Moraes, M.L.T.; Tambarussi, E.V.; Furtado, E.L.; Mori, E.S.; Silva, P.H.M.; Sebbenn, A.M. Heritability for resistance to Puccinia psidii Winter rust in Eucalyptus grandis Hill ex Maiden in Southwestern Brazil. Tree Genet. Genomes 2013, 9, 321–329. [Google Scholar] [CrossRef]
- Beenken, L. Austropuccinia: A new genus name for the myrtle rust Puccinia psidii placed within the redefined family Sphaerophragmiaceae (Pucciniales). Phytotaxa 2017, 297, 53–61. [Google Scholar] [CrossRef]
- Figueiredo, M.B. Doenças fúngicas emergentes em grandes culturas. Biológico 2001, 63, 29–32. [Google Scholar]
- Morin, L.; Aveyard, R.; Lidbetter, J.R.; Wilson, P.G. Investigating the host-range of the rust fungus Puccinia psidii sensu lato across tribes of the family Myrtaceae present in Australia. PLoS ONE 2012, 7, e35434. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, Y. Recent outbreaks of rust diseases and the importance of basic biological research for controlling rusts. J. Gen. Plant Pathol. 2014, 80, 375–388. [Google Scholar] [CrossRef]
- Ferreira, F.A. Patologia Florestal–Principais Doenças Florestais do Brasil; Sociedade de Investigações Florestais: Viçosa, Brazil, 1989. [Google Scholar]
- Demuner, N.L.; Alfenas, A.C. Fungicidas sistêmicos para o controle da ferrugem causada por Puccinia psidii em Eucalyptus cloeziana. Fitopatol. Bras. 1991, 16, 174–177. [Google Scholar]
- Coutinho, T.A.; Wingfield, M.J.; Alfenas, A.C.; Crous, P.W. Eucalyptus rust: A disease with the potential for serious international implications. Plant Dis. 1998, 82, 819–825. [Google Scholar] [CrossRef]
- Mendgen, K.; Hahn, M.; Deising, H. Morphogenesis and mechanisms of penetration by plant pathogenic fungi. Annu. Rev. Phytopathol. 1996, 34, 367–386. [Google Scholar] [CrossRef] [PubMed]
- Glen, M.; Alfenas, A.C.; Zauza, E.A.V.; Wingfield, M.J.; Mohammed, C. Puccinia psidii: A threat to the Australian environment and economy—A review. Australas. Plant Pathol. 2007, 36, 1–16. [Google Scholar] [CrossRef]
- Bedendo, I.P. Ferrugens. In Manual de Fitopatologia: Princípios e Conceitos; Bergamin Filho, A., Kimati, H., Amorim, L., Eds.; Agronômica Ceres: São Paulo, Brazil, 1995; pp. 872–880. [Google Scholar]
- Terhune, B.T.; Hoch, H. Substrate hydrophobicity and adhesion of Uromyces urediospores and germlings. Exp. Mycol. 1993, 17, 241–252. [Google Scholar] [CrossRef]
- Bailey, J.A.; O’Connell, R.J.; Pring, R.J.; Nash, C. Infection strategies of Colletotrichum species. In Colletotrichum: Biology, Pathology and Control; Bailey, J.A., Jeger, M.J., Eds.; CAB International: Wallingford, UK, 1992; pp. 88–120. [Google Scholar]
- Xavier, A.A.; da Silva, A.C.; Guimarães, L.M.S.; Matsuoka, K.; Hodges, C.S.; Alfenas, A.C. Infection process of Puccinia psidii in Eucalyptus grandis leaves of different ages. Trop. Plant Pathol. 2015, 40, 318–325. [Google Scholar] [CrossRef]
- Ruiz, R.A.R.; Alfenas, A.C.; Ferreira, F.A. Influência da temperatura, do tempo de molhamento foliar, fotoperíodo e intensidade de luz sobre a infecção de Puccinia psidii em eucalipto. Fitopatol. Bras. 1989, 14, 55–61. [Google Scholar]
- Pinto, C.S.; Costa, R.M.L.; Moraes, C.B.; Pieri, C.; Tambarussi, E.V.; Furtado, E.L.; Mori, E.S. Genetic variability in progenies of Eucalyptus dunnii Maiden for resistance to Puccinia psidii. Crop Breed. Appl. Biotechnol. 2014, 14, 187–193. [Google Scholar] [CrossRef]
- Medice, R.; Alves, E.; Assis, R.T.; Magno, R.G., Jr.; Lopes, E.A.G.L. Óleos essenciais no controle da ferrugem asiática da soja Phakopsora pachyrhizi Syd. & P. Syd. Cienc. Agrotec. 2007, 31, 83–90. [Google Scholar]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Automat. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Magnani, E.B.Z.; Alves, E.; Araújo, D.V. Eventos dos processos de pré-penetração, penetração e colonização de Phakopsora pachyrhizi em folíolos de soja. Fitopatol. Bras. 2007, 32, 156–160. [Google Scholar] [CrossRef]
- Hughes, F.L. Scanning electron microscopy of early infection in the uredial stage of Puccinia sorghi in Zea mays. Plant Pathol. 1985, 34, 61–68. [Google Scholar] [CrossRef]
- Lennox, C.L.; Rijkenberg, F.H.J. Scanning electron microscopy study of infection structure formation of Puccinia graminis f. sp. tritici in host and non-host cereal species. Plant Pathol. 1989, 38, 547–556. [Google Scholar]
- Hu, G.; Rijkenberg, F.H.J. Scanning electron microscopy of early infection structure formation by Puccinia recondita f. sp. tritici on and in susceptible and resistant wheat lines. Mycol. Res. 1998, 102, 391–399. [Google Scholar]
- Hunt, P. Cuticular penetration by germinating uredospores. Trans. Br. Mycol. Soc. 1983, 51, 103–112. [Google Scholar] [CrossRef]
- Pryce-Jones, E.; Carver, T.; Gurr, S.J. The roles of cellulase enzymes and mechanical force in host penetration by Erysiphe graminis f. sp. hordei. Physiol. Mol. Plant Pathol. 1999, 55, 175–182. [Google Scholar] [CrossRef]
- Boava, L.P.; Kuhn, O.J.; Pascholati, S.F.; Di Piero, R.M.; Furtado, E.L. Atividade de quitinases e peroxidases em folhas de eucalipto em diferentes estágios de desenvolvimento após tratamento com acibenzolar-S-metil (ASM) e inoculação com Puccinia psidii. Trop. Plant Pathol. 2010, 35, 124–128. [Google Scholar] [CrossRef]
- Tucker, S.L.; Talbot, N.J. Surface attachment and pre-penetration stage development by plant pathogenic fungi. Annu. Rev. Phytopathol. 2001, 39, 385–417. [Google Scholar] [CrossRef] [PubMed]
- Adendorff, R.; Rijkenberg, F.H.J. Direct penetration from urediospores of Phakopsora apoda. Mycol. Res. 2000, 104, 317–324. [Google Scholar] [CrossRef]
- Martin, J.T. Role of cuticle in the defense against plant disease. Annu. Rev. Phytopathol. 1964, 2, 81–100. [Google Scholar] [CrossRef]
- Wynn, W.R.; Staples, R.C. Tropism of fungi in host recognition. In Plant Disease Control: Resistance and Susceptibility; Staples, R.C., Toenniessen, G.H., Eds.; Wiley: New York, NY, USA, 1981; pp. 45–69. [Google Scholar]
- Vaz Patto, M.C.; Niks, R.E. Leaf wax layer may prevent appressorium differentiation but does not influence orientation of the leaf rust fungus Puccinia hordei on Hordeum chilense leaves. Eur. J. Plant Pathol. 2001, 107, 795–803. [Google Scholar] [CrossRef]
- Jelitto, T.C.; Page, H.A.; Read, N.D. Role of external signals in regulating the pre-penetration phase of infection by the rice blast fungus, Magnaporthe grisea. Planta 1994, 194, 471–477. [Google Scholar] [CrossRef]
- Goodman, R.N.; Kiraly, Z.; Wood, K.R. The infection process. In The Biochemistry and Physiology of Plant Disease; Goodman, R.N., Kiraly, Z., Wood, K.R., Eds.; University of Missouri Press: Colombia, MO, USA, 1986; pp. 1–45. [Google Scholar]
- Araújo, J.C.A.; Matsuoka, K. Histopatologia da interação Alternaria solani em tomateiros resistente e suscetível. Fitopatol. Bras. 2004, 29, 268–275. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Rep, M.; Pieterse, C.M.J. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef] [PubMed]
- Whetten, R.W.; MacKay, J.J.; Sederoff, R.R. Recent advances in understanding lignin biosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 585–609. [Google Scholar] [CrossRef] [PubMed]
- Vidhyasekaran, P.; Ponmalar, T.R.; Samiyappan, R.; Velazhahan, R.; Vimala, R.; Ramanathan, A. Host-specific toxin production by Rhizoctonia solani, the rice sheath blight pathogen. Phytopathology 1997, 87, 1258–1263. [Google Scholar] [CrossRef] [PubMed]
- Montealegre, J.R.; Lopez, C.; Stadnik, M.J.; Henrıquez, J.L.; Herrera, R.; Polanco, R.; Di Piero, R.M.; Perez, L.M. Control of grey rot of apple fruits by biologically active natural products. Trop. Plant Pathol. 2010, 35, 271–276. [Google Scholar]
- Godard, S.; Slacanin, I.; Viret, O.; Gindro, K. Induction of defense mechanisms in grapevine leaves by emodin- and anthraquinone-rich plant extracts and their conferred resistance to downy mildew. Plant Physiol. Biochem. 2009, 47, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Delaquis, P.J.; Stanich, K.; Girard, B.; Mazza, G. Antimicrobial activity of individual and mixed fractions of dill, cilantro, coriander and eucalyptus essential oils. Int. J. Food Microbiol. 2002, 74, 101–109. [Google Scholar] [CrossRef]
- Vilela, G.R.; Almeida, G.S.; D’Arce, M.A.B.R.; Moraes, M.H.D.; Brito, J.O.; Silva, M.F.G.F.; Silva, S.C.; Piedade, S.M.S.; Calori-Domingues, M.A.; Gloria, E.M. Activity of essential oil and its major compound, 1,8-cineole, from Eucalyptus globulus Labill., against the storage fungi Aspergillus flavus Link and Aspergillus parasiticus Speare. J. Stored Prod. Res. 2009, 45, 108–111. [Google Scholar] [CrossRef]
- Amorim, E.P.D.R.; Andrade, F.W.R.D.; Moraes, E.M.D.S.; Silva, J.C.D.; Lima, R.D.S.; Lemos, E.E.P.D. Antibacterial activity of essential oils and extracts on the development of Ralstonia solanacearum in banana seedlings. Rev. Bras. Frutic. 2011, 33, 392–398. [Google Scholar] [CrossRef]
- Pereira, R.B.; Lucas, G.C.; Perina, F.J.; Resende, M.L.V.; Alves, E. Potential of essential oils for the control of brown eye spot in coffee plants. Ciênc. Agrotec. 2011, 35, 115–123. [Google Scholar] [CrossRef]
- Bigirimana, J.; Höfte, M. Bean anthracnose: inoculation methods and influence of plant stage on resistance of Phaseolus vulgaris cultivars. J. Phytopathol. 2001, 149, 403–408. [Google Scholar] [CrossRef]
- Rios, G.P.; Andrade, E.M.; Costa, J.L.S. Avaliação da resistência de cultivares e linhagens do feijoeiro comum a diferentes populações de Uromyces appendiculatus. Fitopatol. Bras. 2001, 26, 128–133. [Google Scholar] [CrossRef]
- Jerba, V.F.; Rodella, R.A.; Furtado, E.L. Relação entre a estrutura foliar de feijoeiro e a pré-infecção por Glomerella cingulata f.sp. phaseoli. Pesqui. Agropecu. Bras. 2005, 40, 217–223. [Google Scholar] [CrossRef]
- Gonçalves, E.C.P.; Centurion, M.A.P.C.; Di Mauro, A.O. Avaliação da reação de genótipos de soja ao oídio em diferentes condições. Summa Phytopathol. 2009, 35, 151–153. [Google Scholar] [CrossRef]

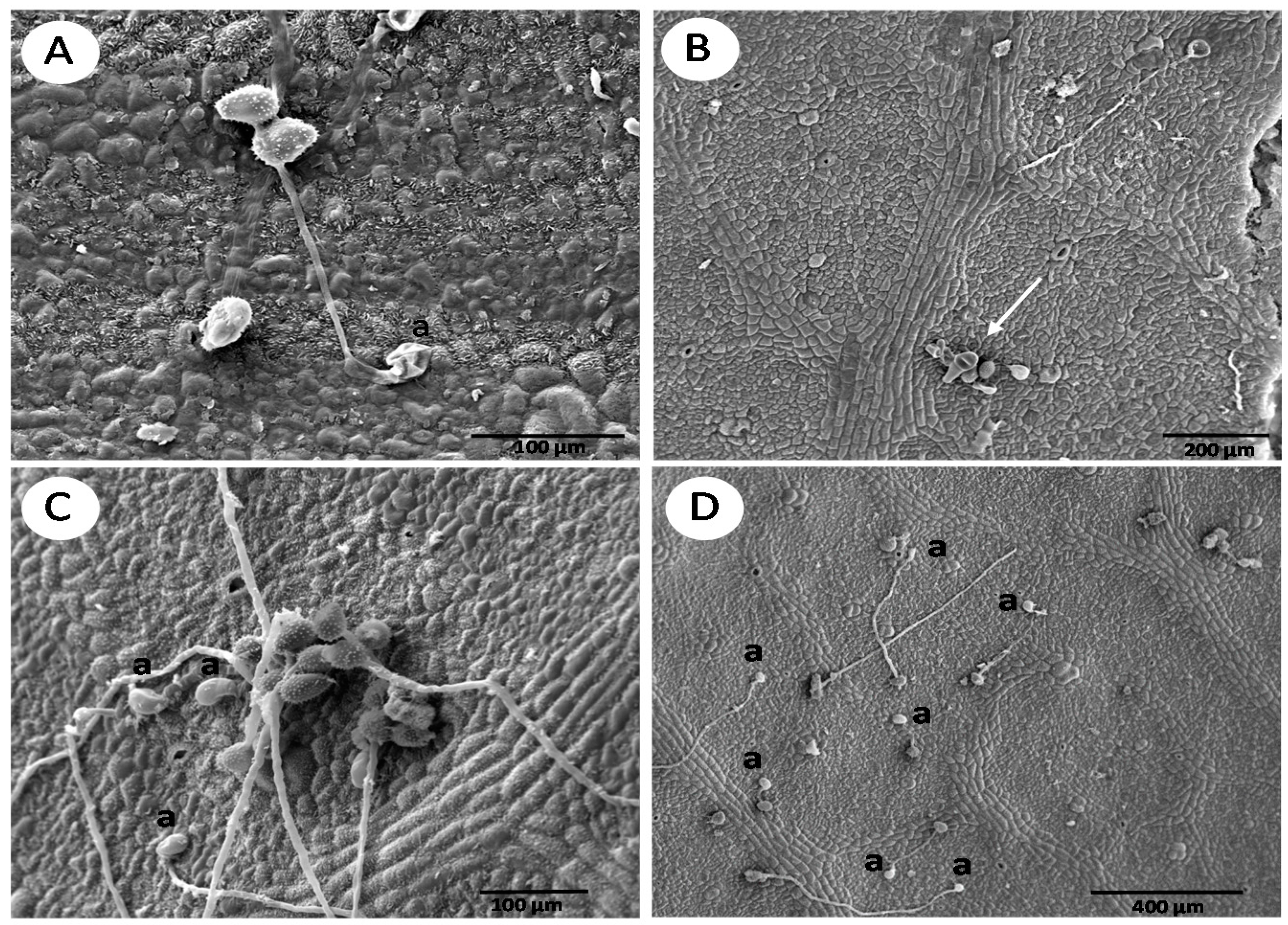
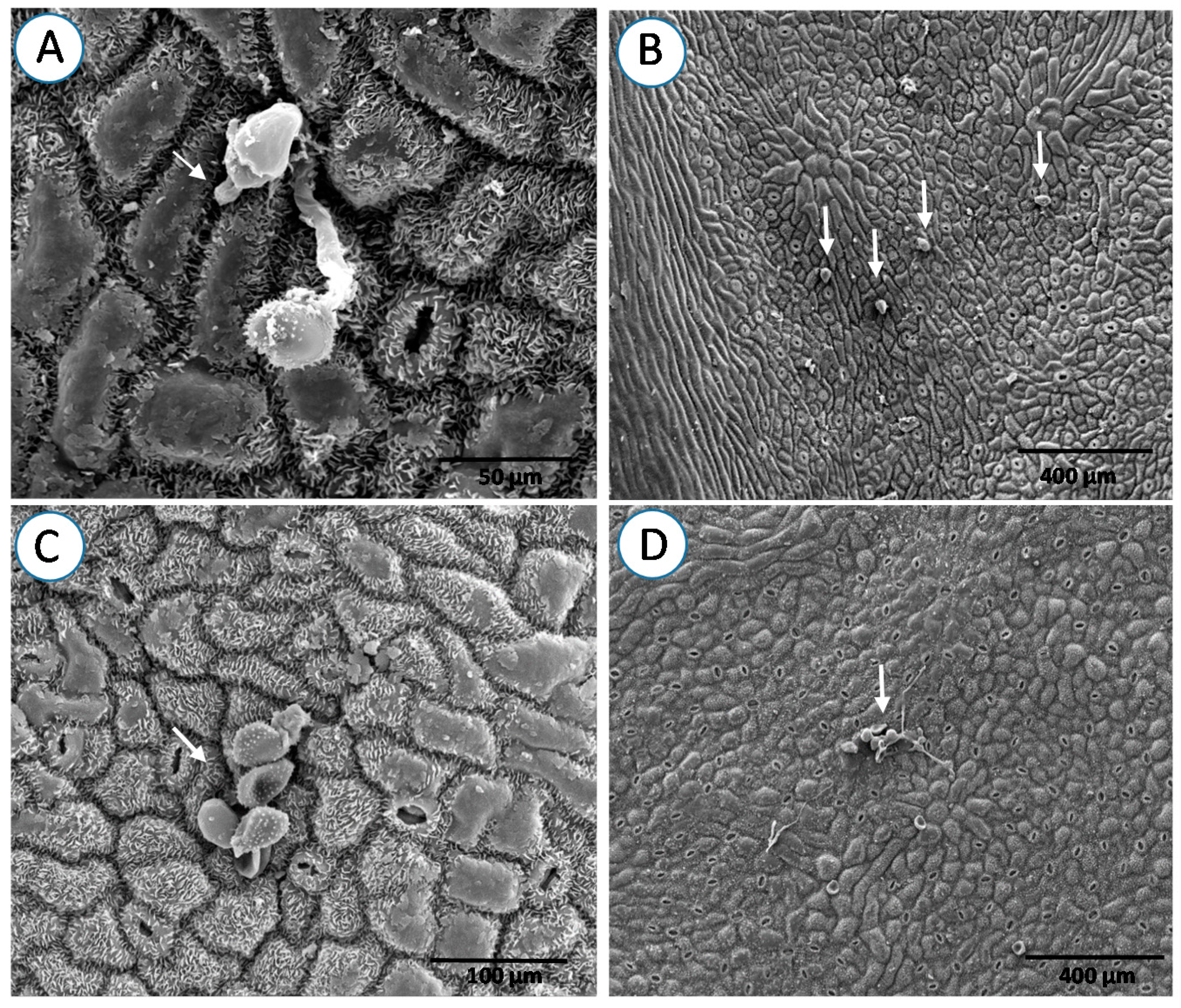
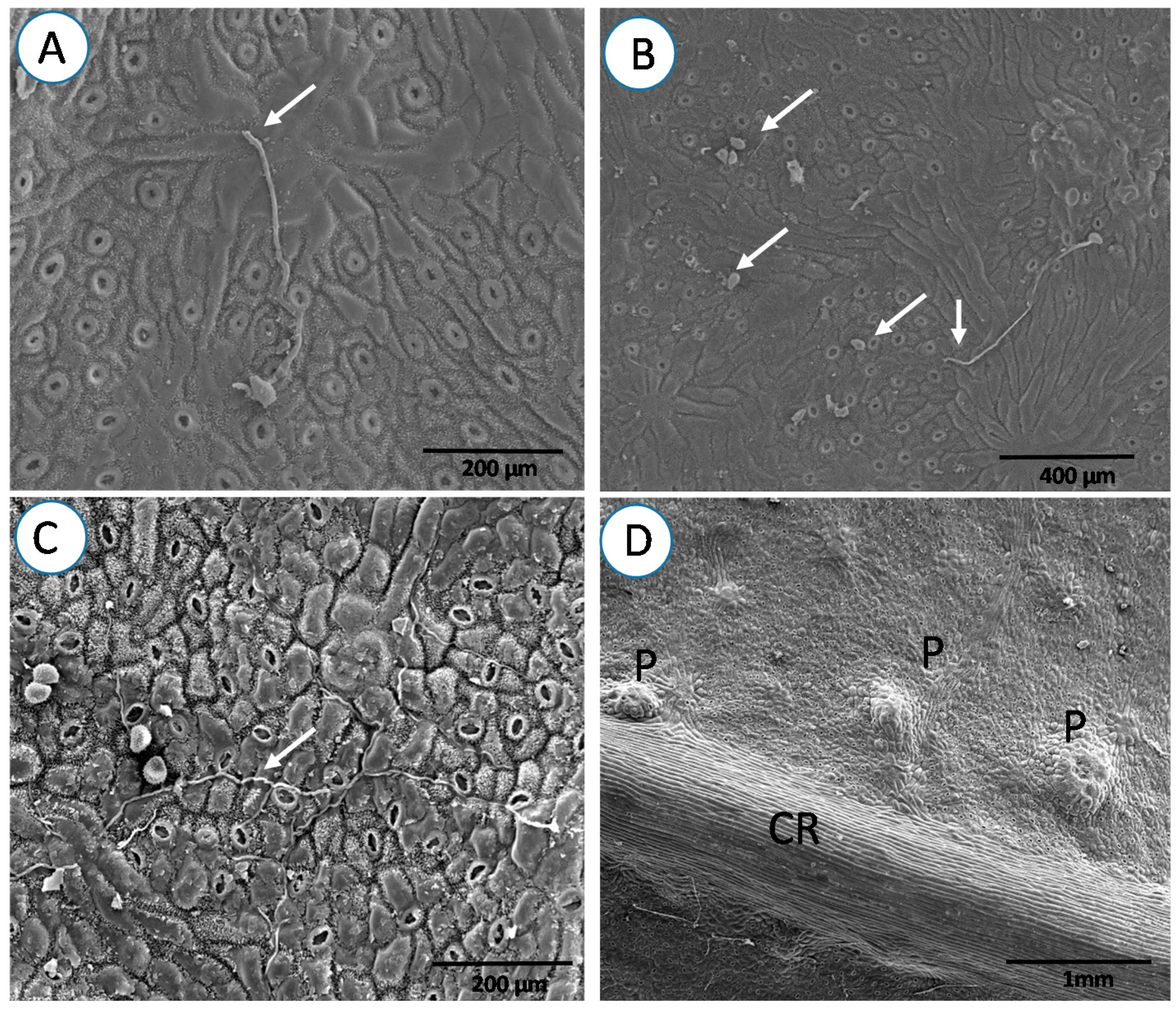
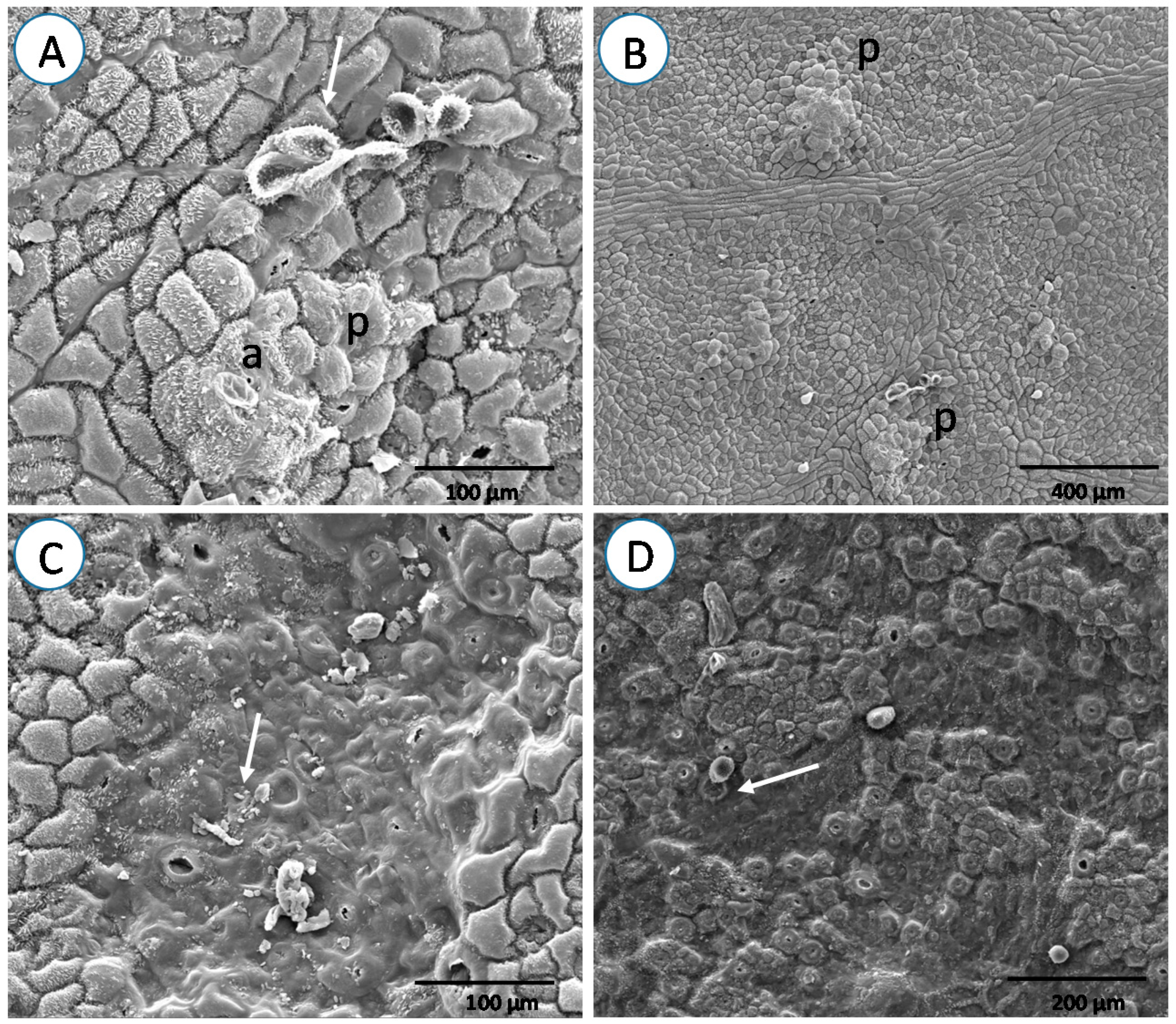
| Sources of Variation | Degrees of Freedom | Germination | Appressorium |
|---|---|---|---|
| Fcalc | Fcalc | ||
| Clone | 1 | 554.2391 *** | 336.3952 *** |
| Leaf stage | 2 | 190.0900 *** | 315.4363 *** |
| Clone × leaf stage | 2 | 181.7854 *** | 304.0159 *** |
| Error A | 18 | ||
| Hours after inoculation | 1 | 4.3235 ns | 2.5946 ns |
| Clone × hours after inoculation | 1 | 1.4118 ns | 0.6486 ns |
| Leaf stage × hours after inoculation | 2 | 0.0221 ns | 0.7703 ns |
| Clone × leaf stage × hours after inoculation | 2 | 0.2868 ns | 0.2838 ns |
| Error B | 18 |
| Leaf Stage | Germination (%) | |
|---|---|---|
| Resistant Clone | Susceptible Clone | |
| 1st | 2 bA | 65.6 aA |
| 3rd | 1.6 bA | 15.4 aB |
| 5th | 1.3 bA | 12.9 aB |
| Appressorium (%) | ||
| Leaf stage | ||
| 1st | 0.5 bA | 55.1 aA |
| 3rd | 0.1 aA | 1.9 aB |
| 5th | 0 aA | 0 aB |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, R.R.; Silva, A.C.d.; Rodella, R.A.; Serrão, J.E.; Zanuncio, J.C.; Furtado, E.L. Pre-Infection Stages of Austropuccinia psidii in the Epidermis of Eucalyptus Hybrid Leaves with Different Resistance Levels. Forests 2017, 8, 362. https://doi.org/10.3390/f8100362
Silva RR, Silva ACd, Rodella RA, Serrão JE, Zanuncio JC, Furtado EL. Pre-Infection Stages of Austropuccinia psidii in the Epidermis of Eucalyptus Hybrid Leaves with Different Resistance Levels. Forests. 2017; 8(10):362. https://doi.org/10.3390/f8100362
Chicago/Turabian StyleSilva, Renata Ruiz, André Costa da Silva, Roberto Antônio Rodella, José Eduardo Serrão, José Cola Zanuncio, and Edson Luiz Furtado. 2017. "Pre-Infection Stages of Austropuccinia psidii in the Epidermis of Eucalyptus Hybrid Leaves with Different Resistance Levels" Forests 8, no. 10: 362. https://doi.org/10.3390/f8100362





