Salvage-Logging after Windstorm Leads to Structural and Functional Homogenization of Understory Layer and Delayed Spruce Tree Recovery in Tatra Mts., Slovakia
Abstract
:1. Introduction
- (i)
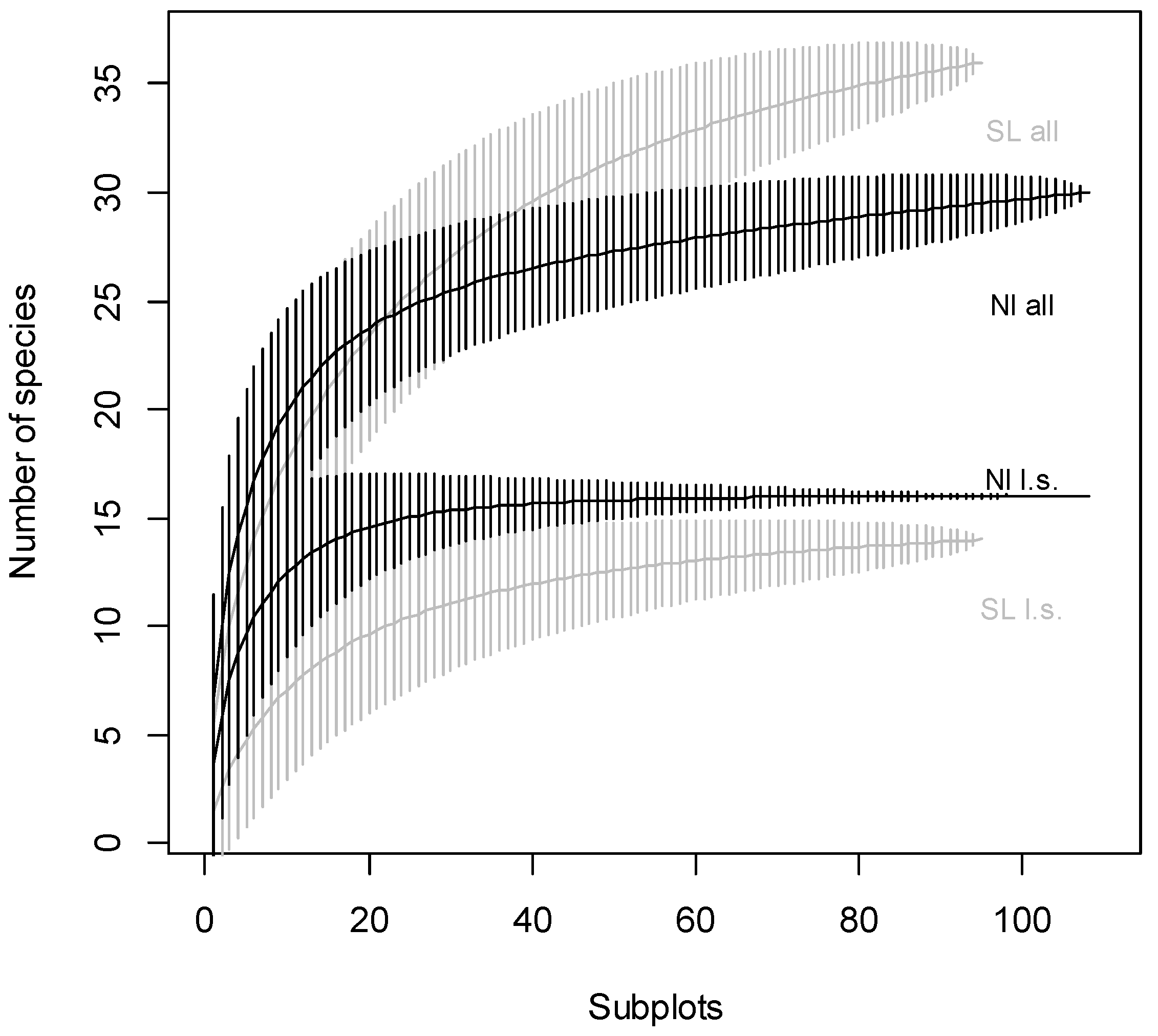
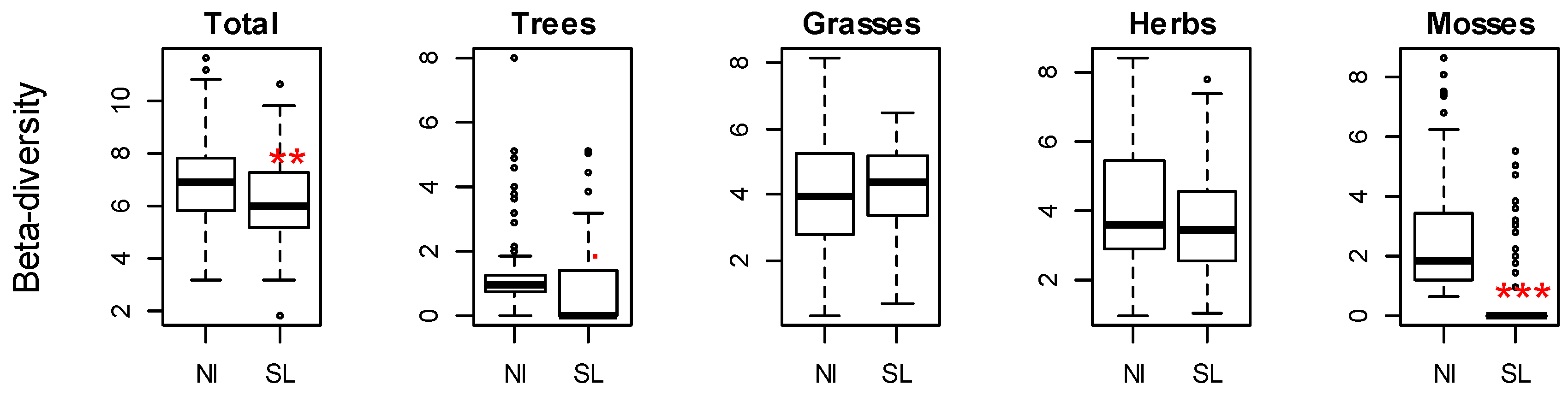
- Will patterns of alpha and beta-diversity differ between non-intervention and salvage-logged sites?
- (ii)
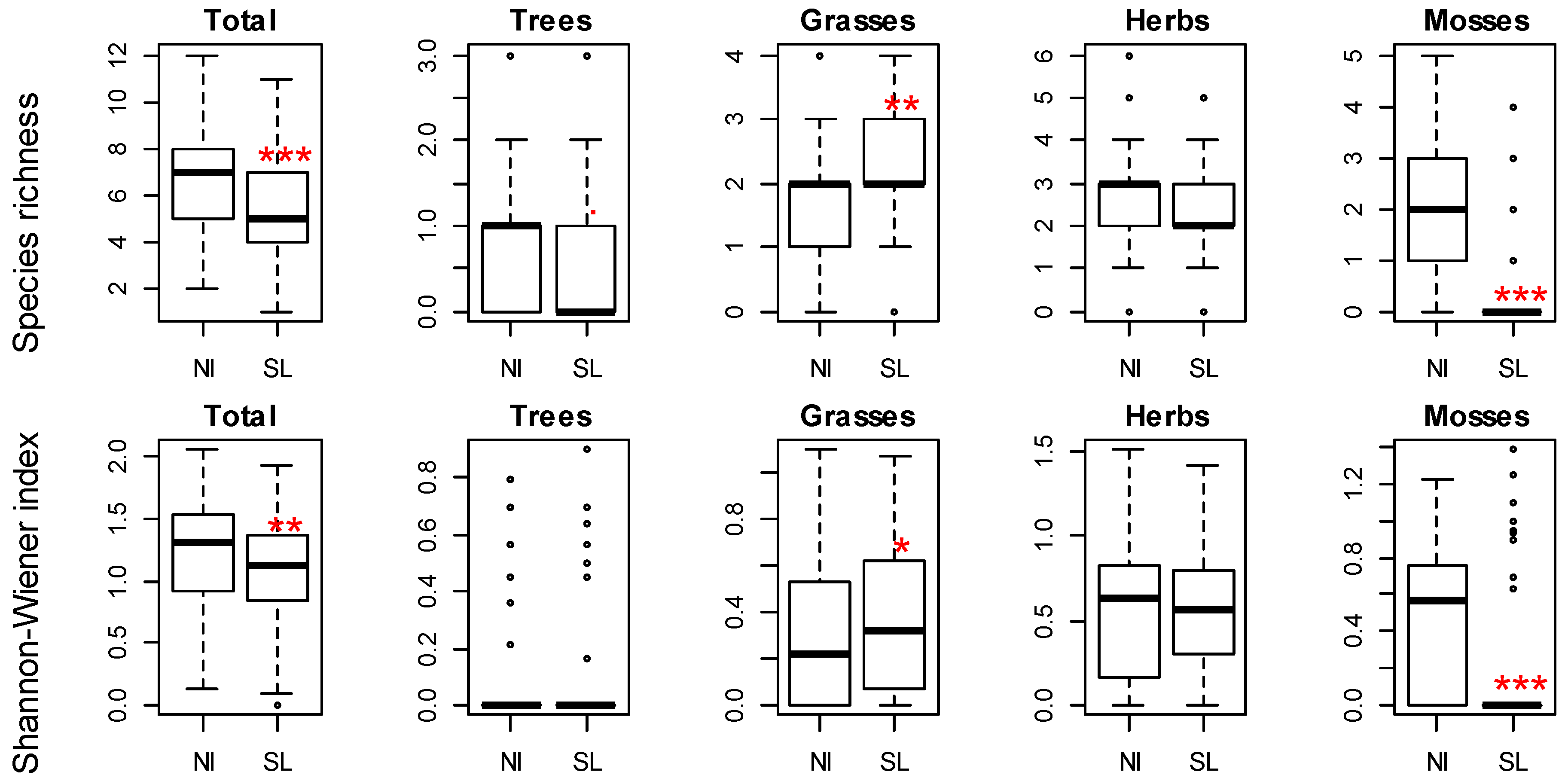
- Will the late successional species be more prominent on the non-intervention site in comparison to the salvage-logged site?
2. Materials and Methods
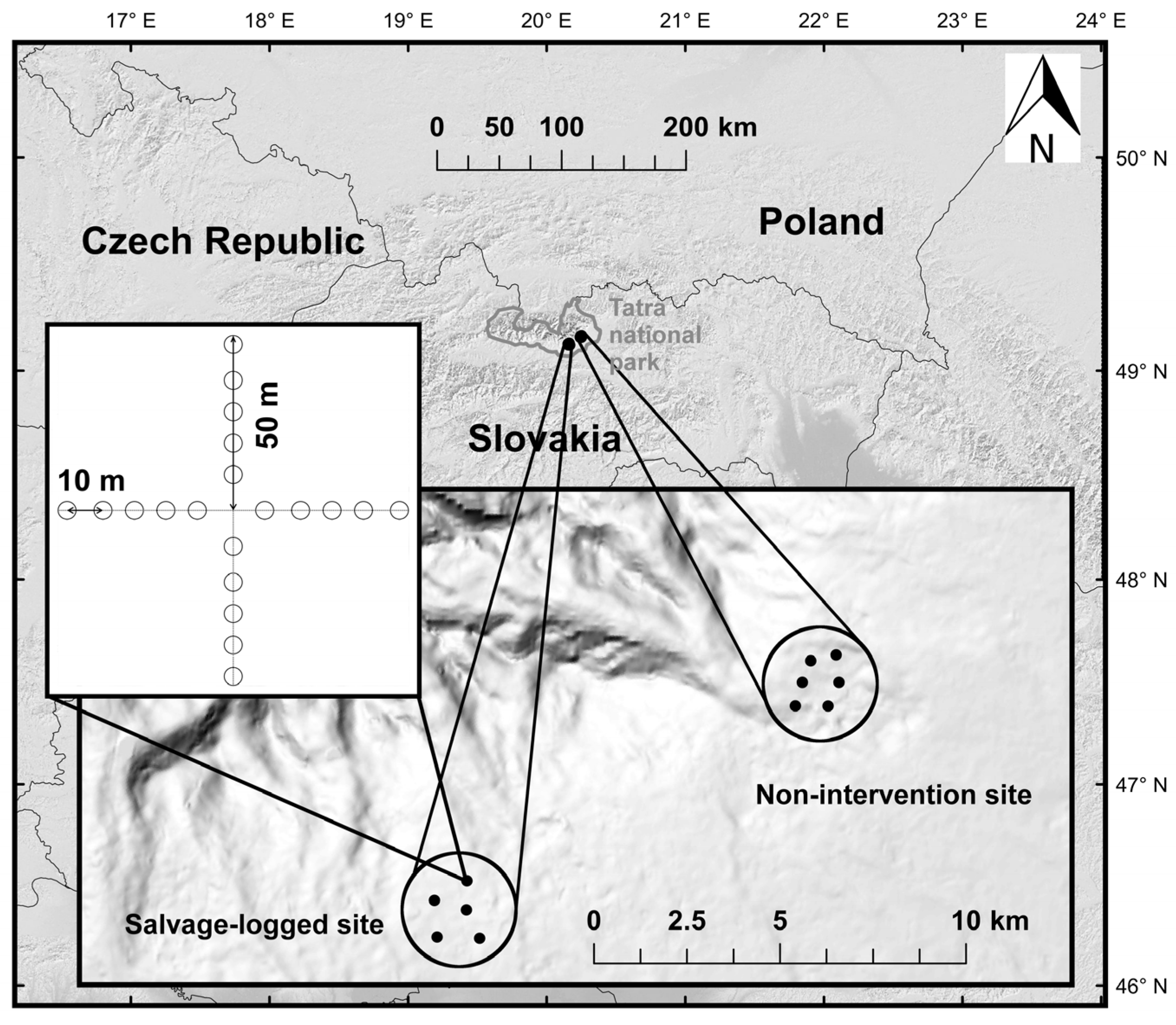
2.1. Study Area
2.2. Sampling Design
2.3. Statistical Analyses
3. Results
4. Discussion
Management Implications
5. Conclusions
Nomenclature
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| NI | non-intervention |
| SL | salvage-logged |
References
- Čada, V.; Morrissey, R.C.; Michalová, Z.; Bače, R.; Janda, P.; Svoboda, M. Frequent Severe Natural Disturbances and Non-Equilibrium Landscape Dynamics Shaped the Mountain Spruce Forest in Central Europe. For. Ecol. Manag. 2016, 363, 169–178. [Google Scholar] [CrossRef]
- Rumbaitis del Rio, C.M. Changes in Understory Composition Following Catastrophic Windthrow and Salvage Logging in a Subalpine Forest Ecosystem. Can. J. For. Res. 2006, 36, 2943–2954. [Google Scholar] [CrossRef]
- Seidl, R.; Schelhaas, M.; Lexer, M.J. Unraveling the Drivers of Intensifying Forest Disturbance Regimes in Europe. Glob. Chang. Biol. 2011, 17, 2842–2852. [Google Scholar] [CrossRef]
- Usbeck, T.; Wohlgemuth, T.; Dobbertin, M.; Pfister, C.; Bürgi, A.; Rebetez, M. Increasing Storm Damage to Forests in Switzerland from 1858 to 2007. Agric. For. Meteorol. 2010, 150, 47–55. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate Change Impacts, Adaptive Capacity, and Vulnerability of European Forest Ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Kramer, K.; Brang, P.; Bachofen, H.; Bugmann, H.; Wohlgemuth, T. Site Factors are More Important than Salvage Logging for Tree Regeneration After Wind Disturbance in Central European Forests. For. Ecol. Manag. 2014, 331, 116–128. [Google Scholar] [CrossRef]
- Bače, R.; Svoboda, M.; Janda, P.; Morrissey, R.C.; Wild, J.; Clear, J.L.; Čada, V.; Donato, D.C. Legacy of Pre-Disturbance Spatial Pattern Determines Early Structural Diversity Following Severe Disturbance in Montane Spruce Forests. PLoS ONE 2015, 10, e0139214. [Google Scholar] [CrossRef] [PubMed]
- Kulakowski, D.; Seidl, R.; Holeksa, J.; Kuuluvainen, T.; Nagel, T.A.; Panayotov, M.; Svoboda, M.; Thorn, S.; Vacchiano, G.; Whitlock, C.; et al. A Walk on the Wild Side: Disturbance Dynamics and the Conservation and Management of European Mountain Forest Ecosystems. For. Ecol. Manag. 2016. [Google Scholar] [CrossRef]
- Donato, D.C.; Campbell, J.L.; Franklin, J.F. Multiple Successional Pathways and Precocity in Forest Development: Can some Forests be Born Complex? J. Veg. Sci. 2012, 23, 576–584. [Google Scholar] [CrossRef]
- Franklin, J.F. Preserving Biodiversity: Species, Ecosystems, Or Landscapes? Ecol. Appl. 1993, 3, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Ulanova, N.G. The Effects of Windthrow on Forests at Different Spatial Scales: A Review. For. Ecol. Manag. 2000, 135, 155–167. [Google Scholar] [CrossRef]
- Kupferschmid, A.D.; Brang, P.; Schönenberger, W.; Bugmann, H. Predicting Tree Regeneration in Picea Abies Snag Stands. Eur. J. For. Res. 2006, 125, 163–179. [Google Scholar] [CrossRef]
- Wild, J.; Kopecký, M.; Svoboda, M.; Zenáhlíková, J.; Edwards-Jonášová, M.; Herben, T. Spatial Patterns with Memory: Tree Regeneration After stand-replacing Disturbance in Picea Abies Mountain Forests. J. Veg. Sci. 2014, 25, 1327–1340. [Google Scholar] [CrossRef]
- Baier, R.; Meyer, J.; Göttlein, A. Regeneration Niches of Norway Spruce (Picea Abies [L.] Karst.) Saplings in Small Canopy Gaps in Mixed Mountain Forests of the Bavarian Limestone Alps. Eur. J. For. Res. 2007, 126, 11–22. [Google Scholar] [CrossRef]
- Bütler, R.; Lachat, T.; Schlaepfer, R. Grundlagen Für Eine Alt-Und Totholzstrategie Der Schweiz.; Laboratorium für Ökosystemmanagement (GECOS): Lausanne, Switzerland, 2005. [Google Scholar]
- Holeksa, J.; Zywiec, M.; Parusel, J.; Szewczyk, J.; Zielonka, T. Subalpine Spruce Forests in the Babia Góra National Park. Structure, Production, Coarse Woody Debris and Regeneration Processes of Norway Spruce Natural Forest in National Nature Reserves Babia Hora and Pilsko; Technical University in Zwolen: Zwolen, Poland, 2008; pp. 49–96. [Google Scholar]
- Stevens, V. The Ecological Role of Coarse Woody Debris: An Overview of the Ecological Importance of CWD in BC Forests; Ministry of Forests, Research Program: Victoria, BC, Canada, 1997.
- Bottero, A.; Garbarino, M.; Long, J.N.; Motta, R. The Interacting Ecological Effects of Large-Scale Disturbances and Salvage Logging on Montane Spruce Forest Regeneration in the Western European Alps. For. Ecol. Manag. 2013, 292, 19–28. [Google Scholar] [CrossRef]
- Marzano, R.; Garbarino, M.; Marcolin, E.; Pividori, M.; Lingua, E. Deadwood Anisotropic Facilitation on Seedling Establishment After a Stand-Replacing Wildfire in Aosta Valley (NW Italy). Ecol. Eng. 2013, 51, 117–122. [Google Scholar] [CrossRef]
- Jonášová, M.; Prach, K. The Influence of Bark Beetles Outbreak vs. Salvage Logging on Ground Layer Vegetation in Central European Mountain Spruce Forests. Biol. Conserv. 2008, 141, 1525–1535. [Google Scholar] [CrossRef]
- Keeton, W.S. Managing for Late-successional/old-Growth Characteristics in Northern Hardwood-Conifer Forests. For. Ecol. Manag. 2006, 235, 129–142. [Google Scholar] [CrossRef]
- Keeton, W.; Chernyavskyy, M.; Gratzer, G.; Main-Knorn, M.; Shpylchak, M.; Bihun, Y. Structural Characteristics and Aboveground Biomass of old-growth spruce–fir Stands in the Eastern Carpathian Mountains, Ukraine. Plant Biosyst. 2010, 144, 148–159. [Google Scholar] [CrossRef]
- Lehnert, L.W.; Bässler, C.; Brandl, R.; Burton, P.J.; Müller, J. Conservation Value of Forests Attacked by Bark Beetles: Highest Number of Indicator Species is found in Early Successional Stages. J. Nat. Conserv. 2013, 21, 97–104. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Burton, P.J.; Franklin, J.F. Salvage Logging and Its Ecological Consequences; Island Press: Washington, DC, USA, 2012. [Google Scholar]
- Hewitt, J.; Thrush, S.; Lohrer, A.; Townsend, M. A Latent Threat to Biodiversity: Consequences of Small-Scale Heterogeneity Loss. Biodivers. Conserv. 2010, 19, 1315–1323. [Google Scholar] [CrossRef]
- Clavel, J.; Julliard, R.; Devictor, V. Worldwide Decline of Specialist Species: Toward a Global Functional Homogenization? Front. Ecol. Environ. 2011, 9, 222–228. [Google Scholar] [CrossRef]
- Lockwood, J.L.; Pimm, S.L. Biological Diversity: Species: Would any of them be Missed? Curr. Biol. 1994, 4, 455–457. [Google Scholar] [CrossRef]
- Peterson, G.; Allen, C.R.; Holling, C.S. Ecological Resilience, Biodiversity, and Scale. Ecosystems 1998, 1, 6–18. [Google Scholar] [CrossRef]
- Bengtsson, J.; Nilsson, S.G.; Franc, A.; Menozzi, P. Biodiversity, Disturbances, Ecosystem Function and Management of European Forests. For. Ecol. Manag. 2000, 132, 39–50. [Google Scholar] [CrossRef]
- Mikoláš, M.; Tejkal, M.; Kuemmerle, T.; Griffiths, P.; Svoboda, M.; Hlásny, T.; Leitão, P.J.; Morrissey, R.C. Forest Management Impacts on Capercaillie (Tetrao Urogallus) Habitat Distribution and Connectivity in the Carpathians. Landsc. Ecol. 2017, 32, 163–179. [Google Scholar] [CrossRef]
- White, P.S.; Jentsch, A. The search for generality in studies of disturbance and ecosystem dynamics. In Progress in Botany: Genetics Physiology Systematics Ecology; Springer: Berlin/Heidelberg, Germany, 2001; pp. 399–450. [Google Scholar]
- Lindenmayer, D.; Noss, R. Salvage Logging, Ecosystem Processes, and Biodiversity Conservation. Conserv. Biol. 2006, 20, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, U.; Örlander, G. Water Uptake by Planted Picea Abies in Relation to Competing Field Vegetation and Seedling Rooting Depth on Two Grass-Dominated Sites in Southern Sweden. Scand. J. For. Res. 1999, 14, 312–319. [Google Scholar] [CrossRef]
- Karlsson, M.; Nilsson, U.; Örlander, G. Natural Regeneration in Clear-Cuts: Effects of Scarification, Slash Removal and Clear-Cut Age. Scand. J. For. Res. 2002, 17, 131–138. [Google Scholar] [CrossRef]
- Hanssen, K.H. Effects of Seedbed Substrates on Regeneration of Picea Abies from Seeds. Scand. J. For. Res. 2002, 17, 511–521. [Google Scholar] [CrossRef]
- Dovčiak, M.; Hrivnák, R.; Ujházy, K.; Gömöry, D. Seed Rain and Environmental Controls on Invasion of Picea Abies into Grassland. Plant Ecol. 2008, 194, 135–148. [Google Scholar] [CrossRef]
- Jankovič, J.; Celer, S.; Caboun, V.; Fleischer, P.; Gubka, K.; Hlavac, P.; Chromek, I.; Juleny, J.; Kamensky, M.; Koren, M. Projekt Revitalizácie Lesných Ekosystémov Na Území Vysokých Tatier Postihnutom Veternou Kalamitou Dňa 19. 11. 2004; Národné Lesnícke Centrum: Zvolen, Slovakia, 2007. [Google Scholar]
- Vakula, J.; Zúbrik, M.; Brutovský, D.; Gubka, A.; Ferenčík, J.; Kaštier, P. Forest Conservation Project of High Tatra National Park After Windstorm of November 2004; National Forest Centre, Forest Research Institute: Zvolen, Slovakia, 2007. [Google Scholar]
- Zielonka, T.; Holeksa, J.; Fleischer, P.; Kapusta, P. A tree-ring Reconstruction of Wind Disturbances in a Forest of the Slovakian Tatra Mountains, Western Carpathians. J. Veg. Sci. 2010, 21, 31–42. [Google Scholar] [CrossRef]
- Janda, P.; Trotsiuk, V.; Mikoláš, M.; Bače, R.; Nagel, T.A.; Seidl, R.; Seedre, M.; Morrissey, R.C.; Kucbel, S.; Jaloviar, P.; et al. The Historical Disturbance Regime of Mountain Norway Spruce Forests in the Western Carpathians and its Influence on Current Forest Structure and Composition. For. Ecol. Manag. 2016. [Google Scholar] [CrossRef]
- Fleischer, P.; Homolová, Z. Long-Term Research on Ecological Condition in the Larch-Spruce Forests in High Tatras After Natural Disturbances. Lesnícky Časopis For. J. 2011, 57, 237–250. [Google Scholar]
- GömöRyOvá, E.; StřElcOvá, K.; ŠKvaREnIna, J.; Bebej, J.; GömöRy, D. The Impact of Windthrow and Fire Disturbances on Selected Soil Properties in the Tatra National Park. Soil Water Res. 2008, 3, 574–580. [Google Scholar]
- Budzáková, M.; Galvánek, D.; Littera, P.; Sibik, J. The Wind and Fire Distrurbance in Central European Mountain Spruce Forests: The Regeneration After Four Years. Acta Soc. Bot. Pol. 2013, 82. [Google Scholar] [CrossRef]
- Koreň, M. Vetrová Kalamita 19 Novembra 2004–Nové Pohľady a Konsekvencie. Tatry 2005, 44, 6–29. [Google Scholar]
- Krebs, C. Ecological Methodology, 2nd ed.; A. Wesley Longman: New York, NY, USA, 1999. [Google Scholar]
- Lepš, J.; Šmilauer, P. Mnohorozměrná Analýza Ekologických Dat; Biologická Fakulta Jihočeské Univerzity v Českých Budějovicích: České Budějovice, Czech Republic, 2000. [Google Scholar]
- Kindt, R.; van Damme, P.; Simons, A. Patterns of Species Richness at Varying Scales in Western Kenya: Planning for Agroecosystem Diversification. Biodivers. Conserv. 2006, 15, 3235–3249. [Google Scholar] [CrossRef]
- Ellenberg, H.; Weber, H.E.; Düll, R.; Wirth, V.; Werner, W.; Paulißen, D. Zeigerwerte von Pflanzen in Mitteleuropa; Verlag Erich Goltze KG: Göttingen, Germany, 1992. [Google Scholar]
- Chytrý, M.; Kučera, T.; Kočí, M.; Grulich, V.; Lustyk, P. Katalog Biotopů České Republiky; Agentura Ochrany Přírody a Krajiny ČR: Praha, Czech Republic, 2001. [Google Scholar]
- Tichý, L.; Bruelheide, H. JUICE, Software for Vegetation Classification. J. Veg. Sci. 2002, 13, 451–453. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Package vegan: Community ecology package. R package version 2.2-0. 2014. Available online: http://cran.rproject.org/web/packages/vegan/ (accessed on 14 January 2014).
- Peterson, C.J.; Leach, A.D. Limited Salvage Logging Effects on Forest Regeneration After moderate-severity Windthrow. Ecol. Appl. 2008, 18, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Fischer, H.S.; Kopecký, M.; Macek, M.; Wild, J. Small Changes in Species Composition Despite Stand-Replacing Bark Beetle Outbreak in Picea Abies Mountain Forests 1. Can. J. For. Res. 2015, 45, 1164–1171. [Google Scholar] [CrossRef]
- Zeppenfeld, T.; Svoboda, M.; DeRose, R.J.; Heurich, M.; Müller, J.; Čížková, P.; Starý, M.; Bače, R.; Donato, D.C. Response of Mountain Picea Abies Forests to stand-replacing Bark Beetle Outbreaks: Neighbourhood Effects Lead to self-replacement. J. Appl. Ecol. 2015, 52, 1402–1411. [Google Scholar] [CrossRef]
- Pyšek, P. Dominant Species Exchange during Succession in Reclaimed Habitats: A Case Study from Areas Deforested by Air Pollution. For. Ecol. Manag. 1992, 54, 27–44. [Google Scholar] [CrossRef]
- Jonášová, M.; Vávrová, E.; Cudlín, P. Western Carpathian Mountain Spruce Forest After a Windthrow: Natural Regeneration in Cleared and Uncleared Areas. For. Ecol. Manag. 2010, 259, 1127–1134. [Google Scholar] [CrossRef]
- Zielonka, T.; Piątek, G. The Herb and Dwarf Shrubs Colonization of Decaying Logs in Subalpine Forest in the Polish Tatra Mountains. Plant Ecol. 2004, 172, 63–72. [Google Scholar] [CrossRef]
- Valkonen, S.; Maguire, D.A. Relationship between Seedbed Properties and the Emergence of Spruce Germinants in Recently Cut Norway Spruce Selection Stands in Southern Finland. For. Ecol. Manag. 2005, 210, 255–266. [Google Scholar] [CrossRef]
- Brang, V.P. Ansainungsgunst Und Verteilung Der Direktstrahlnng in Schlitzförmigen Bestandesöffnungen Zwischenalpiner Fichtenwälder. Schweiz. Z. Forstwes. 1996, 147, 761–784. [Google Scholar]
- Hanssen, K.H. Natural Regeneration of Picea Abies on Small Clear-Cuts in SE Norway. For. Ecol. Manag. 2003, 180, 199–213. [Google Scholar] [CrossRef]
- Mori, A.; Mizumachi, E. Season and Substrate Effects on the First-Year Establishment of Current-Year Seedlings of Major Conifer Species in an Old-Growth Subalpine Forest in Central Japan. For. Ecol. Manag. 2005, 210, 461–467. [Google Scholar] [CrossRef]
- Macek, M.; Wild, J.; Kopecký, M.; Červenka, J.; Svoboda, M.; Zenáhlíková, J.; Brůna, J.; Mosandl, R.; Fischer, A. Life and Death of Picea Abies After bark-beetle Outbreak: Ecological Processes Driving Seedling Recruitment. Ecol. Appl. 2016. [Google Scholar] [CrossRef] [PubMed]
- Thorn, S.; Bässler, C.; Bernhardt-Römermann, M.; Cadotte, M.; Heibl, C.; Schäfer, H.; Seibold, S.; Müller, J. Changes in the Dominant Assembly Mechanism Drive Species Loss Caused by Declining Resources. Ecol. Lett. 2016, 19, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Wohlgemuth, T.; Kull, P.; Wüthrich, H. Disturbance of Microsites and Early Tree Regeneration After Windthrow in Swiss Mountain Forests due to the Winter Storm Vivian 1990. For. Snow Landsc. Res. 2002, 77, 2. [Google Scholar]
- Waldron, K.; Ruel, J.; Gauthier, S.; De Grandpré, L.; Peterson, C.J. Effects of post-windthrow Salvage Logging on Microsites, Plant Composition and Regeneration. Appl. Veg. Sci. 2014, 17, 323–337. [Google Scholar] [CrossRef]
- Newton, M.; Fitzgerald, S.; Rose, R.R.; Adams, P.W.; Tesch, S.D.; Atzet, T.; Powers, R.F.; Skinner, C. Comment on “Post-wildfire logging hinders regeneration and increases fire risk”. Science 2006, 313, 615a. [Google Scholar] [CrossRef] [PubMed]
- Kubát, K. Klíc Ke Kvetene Ceské Republiky; Česká Geografická Společnost: Praha, Czech Republic, 2002; Volume 1, 927p. [Google Scholar]
- Kremer, P.; Muhle, H. Lišejníky, Mechorosty, Kapraďorosty; Ikar: Praha, Czech Republic, 1998. [Google Scholar]
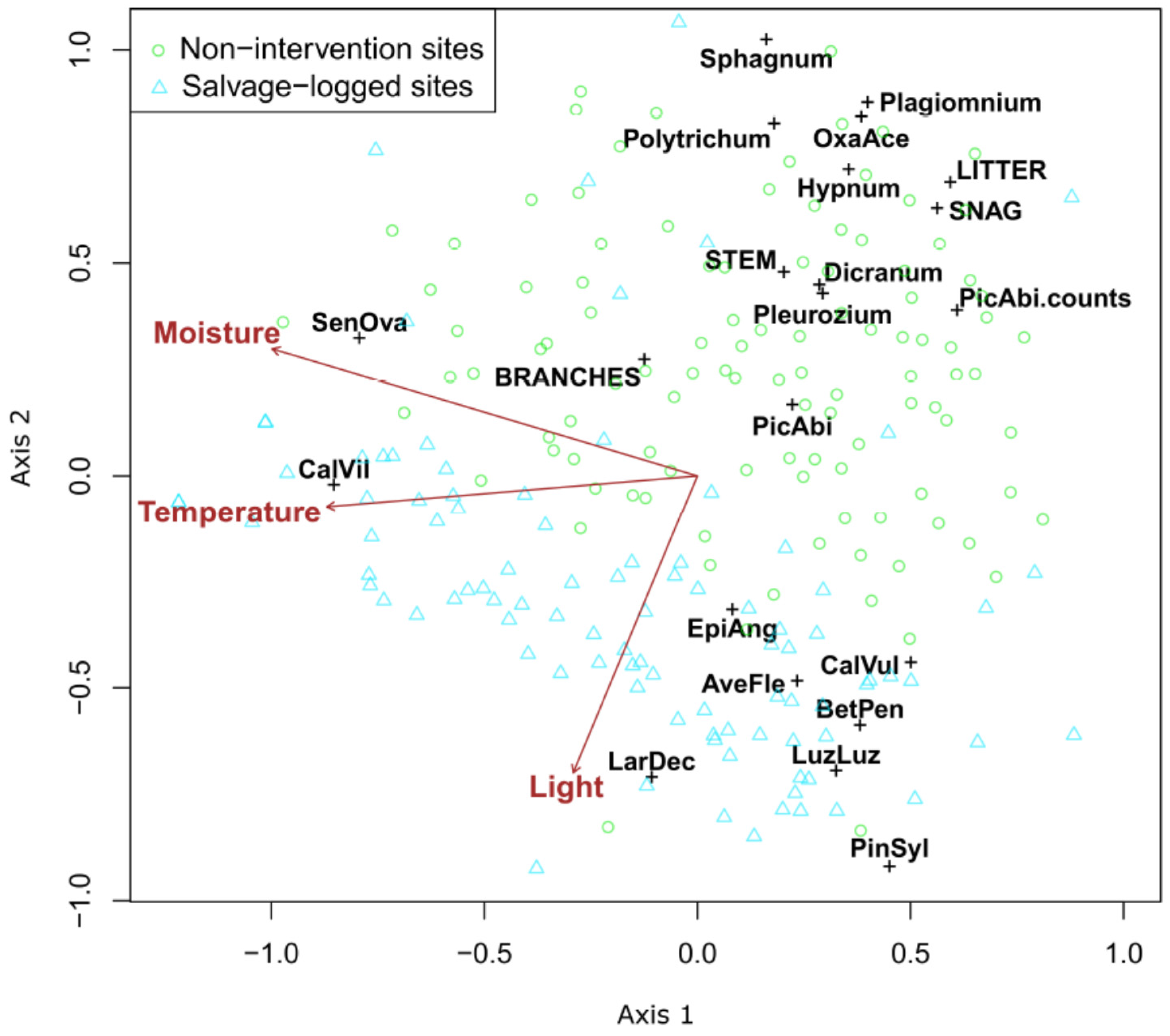
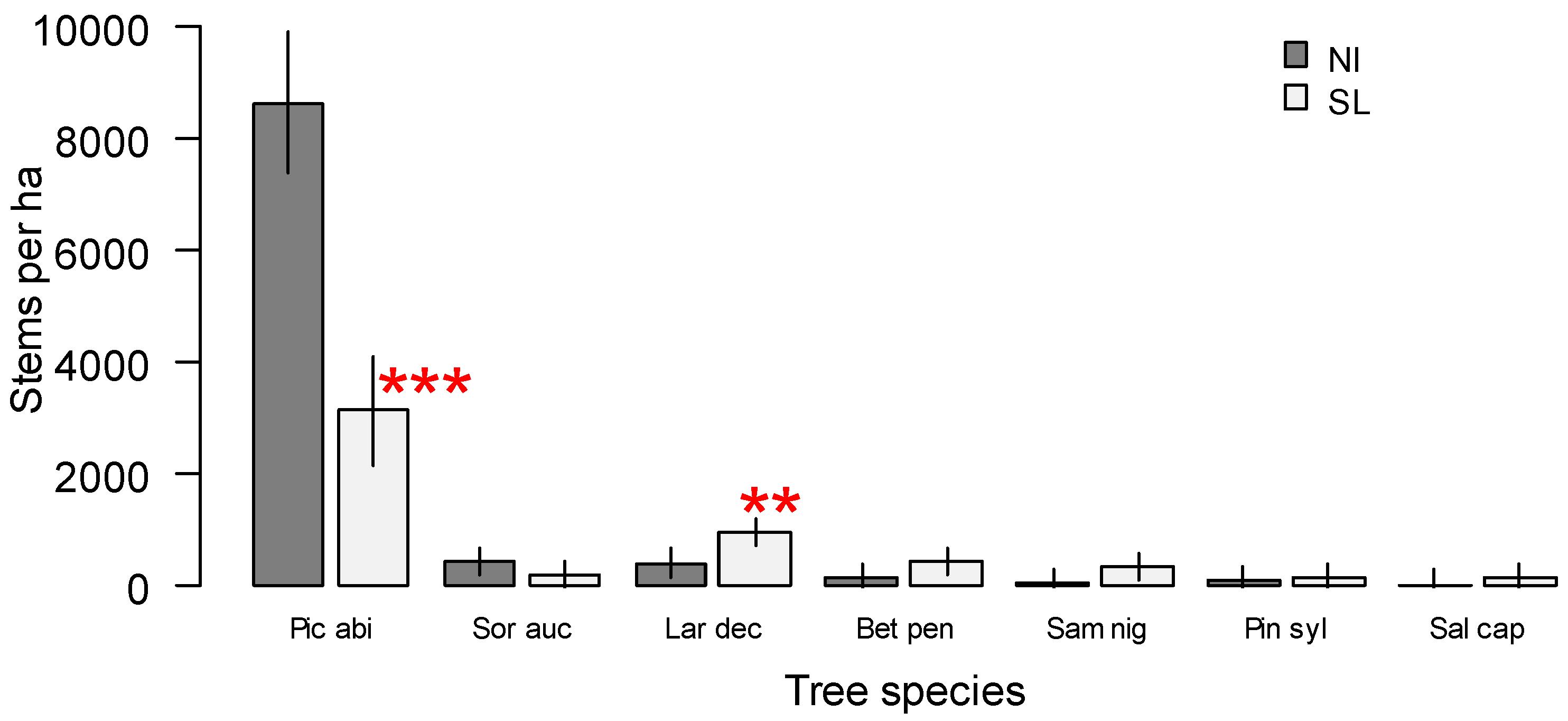
| Non-Intervention Site | Salvage-Logged Site | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Functional Group | Successional Stage | Frequency | Median Cover (%) | Maximum Cover (%) | Frequency per Subplot | Frequency | Median Cover (%) | Maximum Cover (%) | Frequency per Subplot | |
| Species | ||||||||||
| Avenella flexuosa | Grass | Early | 89 | 20 | 85 | 10 | 75 | 25 | 85 | 6 |
| Epilobium angustifolium | Herb | Early | 78 | 10 | 95 | 9 | 82 | 15 | 70 | 6 |
| Calamagrostis villosa | Grass | Early | 73 | 29 | 100 | 9 | 79 | 34 | 100 | 6 |
| Vaccinium myrtillus | Herb | Late | 63 | 11 | 60 | 10 | 46 | 10 | 75 | 7 |
| Picea abies | Tree | Late | 56 | 3 | 80 | 10 | 20 | 5 | 25 | 7 |
| Sphagnum sp. | Moss | Late | 54 | 5 | 70 | 10 | 3 | 3 | 5 | 9 |
| Hylocomium sp. | Moss | Late | 50 | 2 | 20 | 10 | 3 | 3 | 5 | 9 |
| Rubus idaeus | Herb | Early | 35 | 10 | 55 | 9 | 40 | 5 | 55 | 7 |
| Pleurozium sp. | Moss | Late | 32 | 2 | 60 | 10 | 19 | 3 | 30 | 8 |
| Dryopteris dilatata | Herb | Late | 30 | 3 | 25 | 11 | 6 | 5 | 5 | 9 |
| Deadwood | ||||||||||
| Stems | 95 | 10 | 60 | 9 | 14 | 5 | 20 | 7 | ||
| Branches | 85 | 5 | 55 | 9 | 30 | 5 | 95 | 6 | ||
| Litter | 32 | 2 | 9 | 10 | 3 | 3 | 3 | 8 | ||
| Stumps | 14 | 10 | 60 | 10 | 19 | 5 | 50 | 7 | ||
| Snags | 14 | 2 | 3 | 10 | 0 | |||||
| Species | Site | Indicator Value | p-Value | Frequency |
|---|---|---|---|---|
| Calamagrostis villosa | SL | 0.50 | 0.004 | 150 |
| Luzula luzuloides | SL | 0.32 | 0.001 | 50 |
| Larix decidua | SL | 0.20 | 0.001 | 26 |
| Melampyrum pratense | SL | 0.06 | 0.046 | 8 |
| Carex canescens | SL | 0.04 | 0.05 | 4 |
| Picea abies | NI | 0.42 | 0.001 | 83 |
| Pleurozium sp. | NI | 0.35 | 0.001 | 63 |
| Dicranum sp. | NI | 0.29 | 0.002 | 53 |
| Polytrichum sp. | NI | 0.21 | 0.001 | 23 |
| Hypnum sp. | NI | 0.21 | 0.003 | 38 |
| Oxalis acetosella | NI | 0.19 | 0.001 | 20 |
| Sphagnum sp. | NI | 0.13 | 0.003 | 18 |
| Hylocomium sp. | NI | 0.13 | 0.003 | 16 |
| Plagiomnium sp. | NI | 0.09 | 0.013 | 11 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalová, Z.; Morrissey, R.C.; Wohlgemuth, T.; Bače, R.; Fleischer, P.; Svoboda, M. Salvage-Logging after Windstorm Leads to Structural and Functional Homogenization of Understory Layer and Delayed Spruce Tree Recovery in Tatra Mts., Slovakia. Forests 2017, 8, 88. https://doi.org/10.3390/f8030088
Michalová Z, Morrissey RC, Wohlgemuth T, Bače R, Fleischer P, Svoboda M. Salvage-Logging after Windstorm Leads to Structural and Functional Homogenization of Understory Layer and Delayed Spruce Tree Recovery in Tatra Mts., Slovakia. Forests. 2017; 8(3):88. https://doi.org/10.3390/f8030088
Chicago/Turabian StyleMichalová, Zuzana, Robert C. Morrissey, Thomas Wohlgemuth, Radek Bače, Peter Fleischer, and Miroslav Svoboda. 2017. "Salvage-Logging after Windstorm Leads to Structural and Functional Homogenization of Understory Layer and Delayed Spruce Tree Recovery in Tatra Mts., Slovakia" Forests 8, no. 3: 88. https://doi.org/10.3390/f8030088





