Dispersal and Propagule Pressure of Botryosphaeriaceae Species in a Declining Oak Stand is Affected by Insect Vectors
Abstract
:1. Introduction
2. Materials and Methods
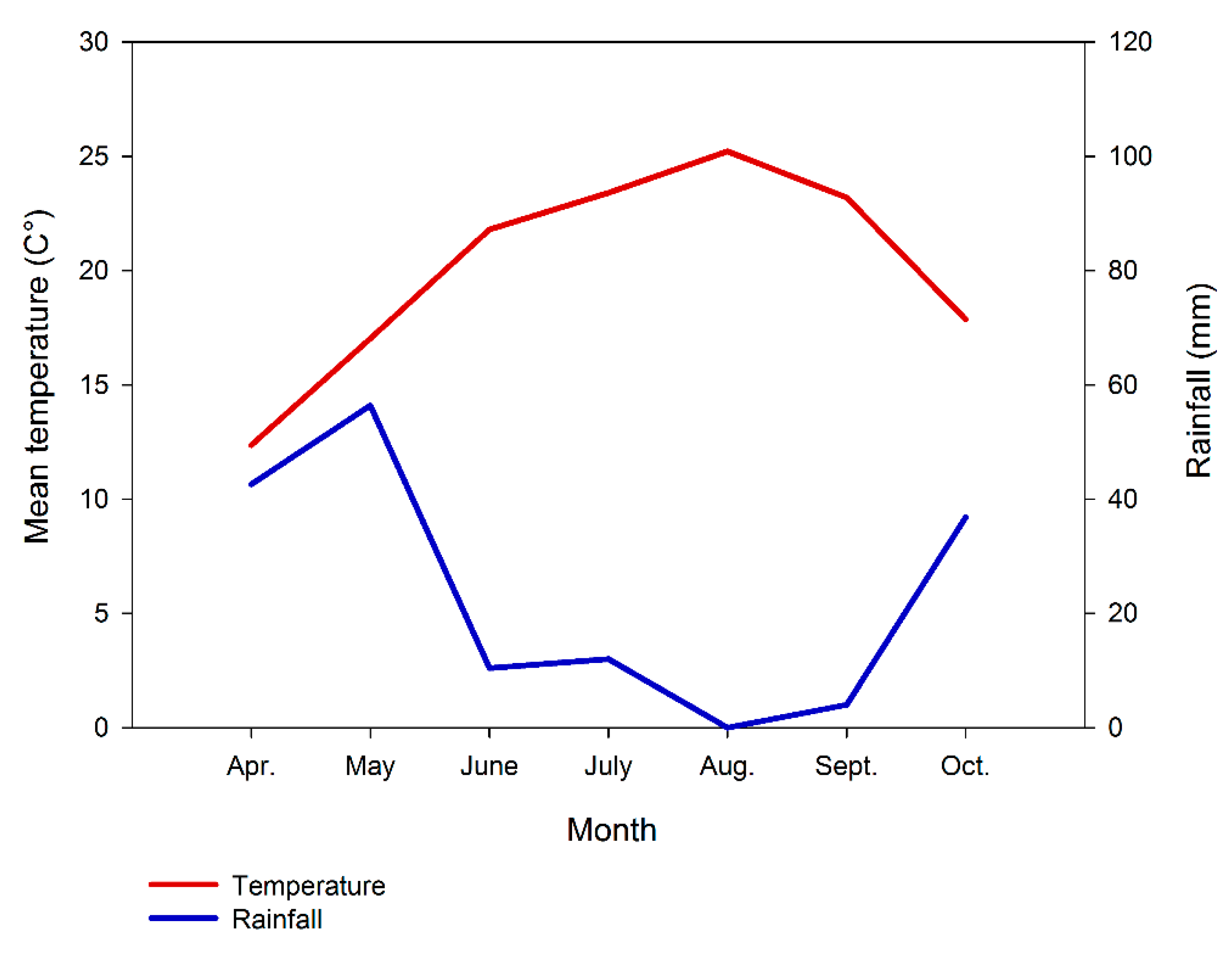
2.1. Study Area
2.2. Plant Sample Collection
2.3. Fungal Isolation from Plants
2.4. Molecular Identification of Isolates
2.5. Insect Collection
2.6. Fungal Isolation from Insects
2.7. Data Analysis
3. Results
3.1. Recovered Fungal Taxa
3.2. Recovered Insect Species
3.3. Fungus-Insect Associations
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Brasier, C.M.; Scott, J.K. European oak declines and global warming: A theoretical assessment with special reference to the activity of Phytophthora cinnamomi. EPPO Bull. 1994, 24, 221–232. [Google Scholar] [CrossRef]
- Ragazzi, A.; Vagniluca, S.; Moricca, S. European expansion of oak decline: Involved microorganisms and methodological approaches. Phytopathol. Mediterr. 1995, 34, 207–226. [Google Scholar]
- Thomas, F.M.; Blanck, R.; Hartmann, G. Abiotic and biotic factors and their interactions as causes of oak decline in Central Europe. For. Pathol. 2002, 32, 277–307. [Google Scholar] [CrossRef]
- Sturrock, R.N.; Frankel, S.J.; Brown, A.V.; Hennon, P.E.; Kliejunas, J.T.; Lewise, K.J.; Worrallf, J.J.; Woods, A.J. Climate change and forest diseases. Plant Pathol. 2011, 60, 49–133. [Google Scholar] [CrossRef]
- Diffenbaugh, N.S.; Pal, J.S.; Giorgi, F.; Gao, X. Heat stress intensification in the Mediterranean climate change hotspot. Geophys. Res. Lett. 2007, 34, 1–6. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.T.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Moricca, S.; Linaldeddu, B.T.; Ginetti, B.; Scanu, B.; Franceschini, A.; Ragazzi, A. Endemic and emerging pathogens threatening cork oak trees: Management options for conserving a unique forest ecosystem. Plant Dis. 2016, 100, 2184–2193. [Google Scholar] [CrossRef]
- Tiberi, R.; Branco, M.; Bracalini, M.; Croci, F.; Panzavolta, T. Cork oak pests: A review of insect damage and management. Ann. For. Sci. 2016, 73, 219–232. [Google Scholar] [CrossRef]
- Manion, P.D. Tree Disease Concepts, 2nd ed.; Prentice-Hall: Englewood Cliffs, NJ, USA, 1991. [Google Scholar]
- Halmschlager, E. Endophytic fungi and oak decline. In Recent Advances in Studies on Oak Decline, Proceedings of An International Congress, Selva di Fasano, Brindisi, Italy, 13–18 September 1992; Luisi, N., Lerario, P., Vannini, A., Eds.; Dipartimento di Patologia Vegetale, Università Degli Studi: Bari, Italy, 1993; pp. 77–83. [Google Scholar]
- Kowalski, T.; Kehr, R.D. Endophytic fungi colonization of branch bases in several forest tree species. Sydowia 1992, 44, 137–168. [Google Scholar]
- Halmschlager, E.; Butin, H.; Donaubauer, E. Endophytische pilze in blättern und zweigen von Quercus petraea. Eur. J. For. Pathol. 1993, 23, 51–63. [Google Scholar] [CrossRef]
- Ragazzi, A.; Moricca, S.; Capretti, P.; Dellavalle, I.; Mancini, F.; Turco, E. Endophytic fungi in Quercus cerris: Isolation frequency in relation to phenological phase, tree health and the organ affected. Phytopathol. Mediterr. 2001, 40, 165–171. [Google Scholar] [CrossRef]
- Ragazzi, A.; Moricca, S.; Capretti, P.; Dellavalle, I.; Turco, E. Differences in composition of endophytic mycobiota in twigs and leaves of healthy and declining Quercus species in Italy. For. Pathol. 2003, 33, 31–38. [Google Scholar] [CrossRef]
- Moricca, S.; Ginetti, B.; Ragazzi, A. Species- and organ-specificity in endophytes colonizing healthy and declining Mediterranean oaks. Phytopathol. Mediterr. 2012, 51, 587–598. [Google Scholar] [CrossRef]
- Cohen, S.D. Technique for large scale isolation of Discula umbrinella and other foliar endophytic fungi from Quercus species. Mycologia 1999, 91, 917–922. [Google Scholar] [CrossRef]
- Moricca, S.; Ragazzi, A. The holomorph Apiognomonia quercina/Discula quercina as a pathogen/endophyte in oak. In Endophytes of Forest Trees; Pirttilä, A.M., Frank, A.C., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 47–66. [Google Scholar]
- Booth, C. The Genus Fusarium; Commonwealth Mycological Institute: Kew, UK, 1971. [Google Scholar]
- Gams, W. Cephalosporium-Artige Schimmelpilze (Hyphomycetes); Gustav Fischer Verlag: Stuttgart, Germany, 1971. [Google Scholar]
- Charmichael, J.W.; Kendrich, W.B.; Conner, I.L.; Sigler, L. Genera of Hyphomycetes; The University of Alberta Press: Edmonton, AB, Canada, 1980. [Google Scholar]
- Sutton, B.C. The Coelomycetes Fungi Imperfecti with Pycnidia, Acervuli and Stromata; Commonwealth Mycological Institute: Kew, UK, 1980. [Google Scholar]
- Von Arx, J.A. Plant Pathogenic Fungi; Cramer, J., Ed.; Beihefte zur Nova Hedwigia: Berlin, Germany, 1987. [Google Scholar]
- Moricca, S.; Raddi, P.; Borja, I.; Vendramin, G.G. Differentiation of Seiridium species associated with virulent cankers on cypress in the Mediterranean region by PCR-SSCP. Plant Pathol. 2000, 49, 774–781. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplified and direct sequencing of fungal ribosomal RNA genes for phylogenies. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Benson, D.A.; Cavanaugh, M.; Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2013, 41, D36–D42. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Márquez, S.; Bills, G.F.; Zabalgogeazcoa, I. Diversity and structure of the fungal endophytic assemblages from two sympatric coastal grasses. Fungal Divers. 2008, 33, 87–100. [Google Scholar]
- Sabbatini Peverieri, G.; Villari, C.; Tiberi, R.; Capretti, P. Occurrence of fungal root rot diseases on pine trees in Tuscany and its relationship with Tomicus destruens. J. Plant Pathol. 2005, 87, 304. [Google Scholar]
- Slippers, B.; Wingfield, M.J. Botryosphaeriaceae as endophytes and latent pathogens of woody plants: Diversity, ecology and impact. Fungal Biol. Rev. 2007, 21, 90–106. [Google Scholar] [CrossRef]
- Jurc, M.; Bojović, S.; Komjanc, B.; Krč, J. Xylophagous entomofauna in branches of oaks (Quercus spp.) and its significance for oak health in the Karst region of Slovenia. Biologia 2009, 64, 130–138. [Google Scholar] [CrossRef]
- Torres-Vila, L.M.; Sanchez-González, Á.; Ponce-Escudero, F.; Martín-Vertedor, D.; Ferrero-García, J.J. Assessing mass trapping efficiency and population density of Cerambyx welensii Küster by mark-recapture in dehesa open woodlands. Eur. J. For. Res. 2012, 131, 1103–1116. [Google Scholar] [CrossRef]
- Sallé, A.; Nageleisen, L.; Lieutier, F. Bark and wood boring insects involved in oak declines in Europe: Current knowledge and future prospects in a context of climate change. For. Ecol. Manag. 2014, 328, 79–93. [Google Scholar] [CrossRef]
- Curletti, G. I Buprestidi d’Italia; Museo Civico di Scienze Naturali: Brescia, Italy, 1994. [Google Scholar]
- Contarini, E. Elenco faunistico commentato (check-list) dei Cerambicidi (Coleoptera Xylophytophaga) del Parco Naturale della Vena del Gesso romagnola. Quad. Stud. Nat. Romagna 2014, 40, 39–65. [Google Scholar]
- Tiberi, R.; Panzavolta, T.; Bracalini, M.; Ragazzi, A.; Ginetti, B.; Moricca, S. Interactions between insects and fungal pathogens of forest and ornamental trees. Ital. J. Mycol. 2016, 45, 54–65. [Google Scholar] [CrossRef]
- Martín, J.; Cabezas, J.; Buyolo, T.; Patón, D. The relationship between Cerambyx spp. damage and subsequent Biscogniauxia mediterranum infection on Quercus suber forests. For. Ecol. Manag. 2005, 216, 166–174. [Google Scholar] [CrossRef]
- Tiberi, R.; Ragazzi, A.; Marianelli, L.; Peverieri Sabbatini, P.; Roversi, P.F. Insects and fungi involved in oak decline in Italy. In IOBC/WPRS Bulletin, Working Group “Integrated Protection in Oak Forests”, Proceedings of the Meeting at Oeiras, Lisbonne, Portugal, 1–4 October 2001; Villemant, C., Sousa, E., Eds.; IOBC: Zürich, Switzerland, 2002; Volume 25, pp. 67–74. [Google Scholar]
- Torres-Vila, L.M.; Mendiola-Diaz, F.J.; Conejo-Rodríguez, Y.; Sánchez-González, Á. Reproductive traits and number of matings in males and females of Cerambyx welensii (Coleoptera: Cerambycidae) an emergent pest of oaks. Butt. Entomol. Res. 2016, 106, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Belhoucine, L.; Bouhraoua, R.T.; Meijer, M.; Houbraken, J.; Harrak, M.J.; Samson, R.A.; Equihua-Martinez, A.; Pujade-Villar, J. Mycobiota associated with Platypus cylindrus (Coleoptera: Curculionidae, Platypodidae) in cork oak stands of North West Algeria, Africa. Afr. J. Microbiol. Res. 2011, 5, 4411–4423. [Google Scholar] [CrossRef]
- Schumacher, J.; Heydeck, P.; Dahms, C. Increasing endangerment of forests by pathogenic thermophilic fungi-demonstrated by the example of the microfungus Diplodia pinea (DESM.) Kickx on Pinus. In Julius-Kühn-Archiv, Proceedings of the 57th German Plant Protection Conference, Berlin, Germany, 6–9 September 2010; Julius Kühn Institut, Bundesforschungsinstitut für Kulturpflanzen: Quedlinburg, Germany, 2010; Volume 428, p. 372. [Google Scholar]
- Cárdenas, A.M.; Gallardo, P. The effect of temperature on the preimaginal development of the jewel beetle, Coraebus florentinus (Coleoptera: Buprestidae). Eur. J. Entomol. 2012, 109, 21–28. [Google Scholar] [CrossRef]
- Brown, N.; Jeger, M.; Kirk, S.; Williams, D.; Xu, X.; Pautasso, M.; Denman, S. Acute Oak Decline and Agrilus biguttatus: The co-occurrence of stem bleeding and D-shaped emergence holes in Great Britain. Forests 2017, 8, 87. [Google Scholar] [CrossRef]
- Ragazzi, A.; Moricca, S.; Capretti, P.; Dellavalle, I. Endophytic presence of Discula quercina on declining Quercus cerris. J. Phytopathol. 1999, 147, 437–440. [Google Scholar] [CrossRef]
- Ragazzi, A.; Moricca, S.; Dellavalle, I. Water Stress and the Development of Cankers by Diplodia mutila on Quercus robur. J. Phytopathol. 1999, 147, 425–428. [Google Scholar] [CrossRef]
- Desprez-Loustau, M.L.; Marcais, B.; Nageleisen, L.M.; Piou, D.; Vannini, A. Interactive effects of drought and pathogens in forest trees. Ann. For. Sci. 2006, 63, 597–612. [Google Scholar] [CrossRef]
- Haavik, L.J.; Billings, S.A.; Guldin, J.M.; Stephen, F.M. Emergent insects, pathogens and drought shape changing patterns in oak decline in North America and Europe. For. Ecol. Manag. 2015, 354, 190–205. [Google Scholar] [CrossRef]
- Jactel, H.; Petit, J.; Desprez-Loustau, M.L.; Delzon, S.; Piou, D.; Battisti, A.; Koricheva, J. Drought effects on damage by forest insects and pathogens: A meta-analysis. Glob. Chang. Biol. 2012, 18, 267–276. [Google Scholar] [CrossRef]
- Sieber, T.N. Endophytic fungi in forest trees: Are they mutualists? Fungal Biol. Rev. 2007, 21, 75–89. [Google Scholar] [CrossRef]
- Sakalidis, M.L.; Slippers, B.; Wingfield, B.D.; Hardy, G.E.St.J.; Burgess, T.I. The challenge of understanding the origin, pathways and extent of fungal invasions: Global populations of the Neofusicoccum parvum-N. ribis species complex. Divers. Distrib. 2013, 19, 873–883. [Google Scholar] [CrossRef]
- Dreaden, T.J.; Shin, K.; Smith, J.A. First report of Diplodia corticola causing branch cankers on live oak (Quercus virginiana) in Florida. Plant Dis. 2011, 95, 1027. [Google Scholar] [CrossRef]
- Lynch, S.C.; Eskalen, A.; Zambino, P.; Scott, T. First report of bot canker caused by Diplodia corticola on coast live oak (Quercus agrifolia) in California. Plant Dis. 2010, 94, 1510. [Google Scholar] [CrossRef]
- Alves, A.; Correia, A.; Luque, J.; Phillips, A.J.L. Botryosphaeria corticola sp. nov. on Quercus species, with notes and description of Botryosphaeria stevensii and its anamorph Diplodia mutila. Mycologia 2004, 96, 598–613. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Linaldeddu, B.T.; Deidda, A.; Scanu, B.; Phillips, A.J.L. The complex of Diplodia species associated with Fraxinus and some other woody hosts in Italy and Portugal. Fungal Divers. 2014, 67, 143–156. [Google Scholar] [CrossRef]
- Hanso, M.; Drenkhan, R. Diplodia pinea is a new pathogen on Austrian pine (Pinus nigra) in Estonia. Plant Pathol. 2009, 58, 797. [Google Scholar] [CrossRef]
- Denman, S.; Plummer, S.; Kirk, S.; Peace, A.; McDonald, J.E. Isolation studies reveal a shift in the cultivable microbiome of oak affected with Acute Oak Decline. Syst. Appl. Microbiol. 2016, 39, 484–490. [Google Scholar] [CrossRef] [PubMed]
| Month of Collection | Frequency of Isolation (%) (600 Monthly Samplings) | ||||
|---|---|---|---|---|---|
| Fungus Species | |||||
| Botryosphaeria dothidea | Diplodia corticola | Diplodia seriata | Dothiorella sarmentorum | Neofusicoccum parvum | |
| April | 1.75 (0.65) | 3.37 a (3.24) | 3.25 a (1.35) | 1.37 a (0.20) | 1.25 a (0) |
| May | 3.75 b (2.25) | 4.50 a (3.59) | 3.50 a (1.80) | 1.89 a (0.33) | 1.50 a (1.10) |
| June | 6.90 c (1.12) | 4.75 a (1.98) | 3.78 a (1.00) | 3.12 b (0.78) | 1.87 a (0.90) |
| July | 8.78 d (0.72) | 4.90 a (3.59) | 4.25 a (2.10) | 3.12 b (1.23) | 2.00 a (0.20) |
| August | 9.00 d (2.20) | 13.89 b (0.65) | 6.75 b (3.08) | 3.25 b (0.50) | 3.00 b (1.25) |
| September | 10.25 d (0.12) | 15.25 bc (5.82) | 7.65 b (1.80) | 3.50 b (1.30) | 3.25 b (2.00) |
| October | 12.50 e (0.10) | 16.00 c (1.67) | 11.97 c (0.89) | 8.97 c (5.95) | 3.50 b (1.35) |
| Variability | df | Deviation | Variance | F |
|---|---|---|---|---|
| Total | 13 | 1575.21 | ||
| Between pathogens | 4 | 915.07 | 228.76 | 57.04 ** |
| Between months | 6 | 648.11 | 108.01 | 26.93 ** |
| Error | 3 | 12.03 | 4.01 |
| Coleoptera Species Collected | Botryosphaeriaceae Species Isolated | Number of Insects Tested | Isolation Frequency (%) |
|---|---|---|---|
| Buprestidae | |||
| Acmaeoderella adspersula (Illiger, 1803) | 3 | 0 | |
| Acmaeoderella flavofasciata (Piller and Mitterpacher, 1783) | 8 | 0 | |
| Anthaxia millefolii polychloros Abeille de Perrin, 1894 | 15 | 0 | |
| Anthaxia scutellaris Gene, 1839 | 15 | 0 | |
| Anthaxia thalassophila Abeille de Perrin, 1900 | Botryosphaeria dothidea | 6 | 33.33 |
| Anthaxia umbellatarum (Fabricius, 1787) | 11 | 0 | |
| Coraebus fasciatus (Villers, 1789) | Diplodia seriata | 12 | 33.33 |
| Latipalpis plana (Olivier, 1790) | 3 | 0 | |
| Cerambycidae | |||
| Callimus abdominalis (Olivier, 1795) * | 0 | NA | |
| Cerambyx welensii (Küster, 1845) | Diplodia corticola | 8 | 37.50 |
| Chlorophorus sartor (Müller, 1766) | Diplodia seriata | 4 | 25.00 |
| Chlorophorus glabromaculatus (Goeze, 1777) * | 0 | NA | |
| Deilus fugax (Olivier, 1790) | 1 | 0 | |
| Deroplia genei (Aragona, 1830) * | 0 | NA | |
| Niphona picticornis Mulsant, 1839 * | 0 | NA | |
| Phymatodes testaceus (Linnaeus, 1758) | 13 | 0 | |
| Pseudosphegesthes cinerea (Laporte and Gory, 1836) * | 0 | NA | |
| Purpuricenus kaehleri (Linnaeus, 1758) | Diplodia seriata | 23 | 8.69 |
| Stenopterus rufus (Linnaeus, 1767) | 9 | 0 | |
| Stictoleptura cordigera (Fuessly, 1775) | 2 | 0 | |
| Stictoleptura rufa (Brulle, 1832) * | 0 | NA | |
| Trichoferus holosericeus (Rossi, 1790) | 12 | 0 | |
| Curculionidae: Scolytinae | |||
| Xyleborus dispar (Fabricius, 1792) * | 0 | NA | |
| Scolytus intricatus (Ratzeburg, 1837) * | 0 | NA | |
| Xyleborinus saxesenii Ratzeburg, 1837 * | 0 | NA | |
| Xyleborus monographus (Fabricius, 1792) * | 0 | NA |
| Insect Species | Month of Collection | |||||||
|---|---|---|---|---|---|---|---|---|
| April | May | June | July | August | September | October | Total | |
| Xylophagous Beetles Associated with Fungi | ||||||||
| Anthaxia thalassophila | 0 | 0 | 13.04 | 0 | 0 | 0 | 0 | 4.83 |
| Coraebus fasciatus | 0 | 0 | 0 | 18.99 | 0 | 0 | 0 | 4.53 |
| Cerambyx welensii | 0 | 0 | 0 | 6.33 | 14.71 | 0 | 0 | 4.53 |
| Chlorophorus sartor | 0 | 0 | 0 | 2.53 | 5.88 | 0 | 0 | 1.81 |
| Purpuricenus kaehleri | 0 | 0 | 5.80 | 15.19 | 23.53 | 0 | 0 | 10.88 |
| Total frequency | 0 | 0 | 17.39 | 43.04 | 44.12 | 0 | 0 | 26.59 |
| Bark-beetles | 0 | 0 | 0 | 27.85 | 27.94 | 62.50 | 0 | 15.41 |
| Total number of xylophagous beetles | 4 | 26 | 138 | 79 | 68 | 16 | 0 | 331 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panzavolta, T.; Panichi, A.; Bracalini, M.; Croci, F.; Ginetti, B.; Ragazzi, A.; Tiberi, R.; Moricca, S. Dispersal and Propagule Pressure of Botryosphaeriaceae Species in a Declining Oak Stand is Affected by Insect Vectors. Forests 2017, 8, 228. https://doi.org/10.3390/f8070228
Panzavolta T, Panichi A, Bracalini M, Croci F, Ginetti B, Ragazzi A, Tiberi R, Moricca S. Dispersal and Propagule Pressure of Botryosphaeriaceae Species in a Declining Oak Stand is Affected by Insect Vectors. Forests. 2017; 8(7):228. https://doi.org/10.3390/f8070228
Chicago/Turabian StylePanzavolta, Tiziana, Andrea Panichi, Matteo Bracalini, Francesco Croci, Beatrice Ginetti, Alessandro Ragazzi, Riziero Tiberi, and Salvatore Moricca. 2017. "Dispersal and Propagule Pressure of Botryosphaeriaceae Species in a Declining Oak Stand is Affected by Insect Vectors" Forests 8, no. 7: 228. https://doi.org/10.3390/f8070228







