Effects of Near Natural Forest Management on Soil Greenhouse Gas Flux in Pinus massoniana (Lamb.) and Cunninghamia lanceolata (Lamb.) Hook. Plantations
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site Description
2.2. Experimental Design
2.3. Measurement and Statistical Analysis
2.3.1. Soil CO2, N2O, and CH4 Measurement
2.3.2. Micro-Environmental Data Measurement
2.3.3. Soil and Litterfall Sampling and Measurements
2.3.4. Fine Root Sampling and Measurements
2.3.5. Biogeochemical Properties Analysis of Plant and Soil Samples
2.3.6. Statistical Analysis
3. Results
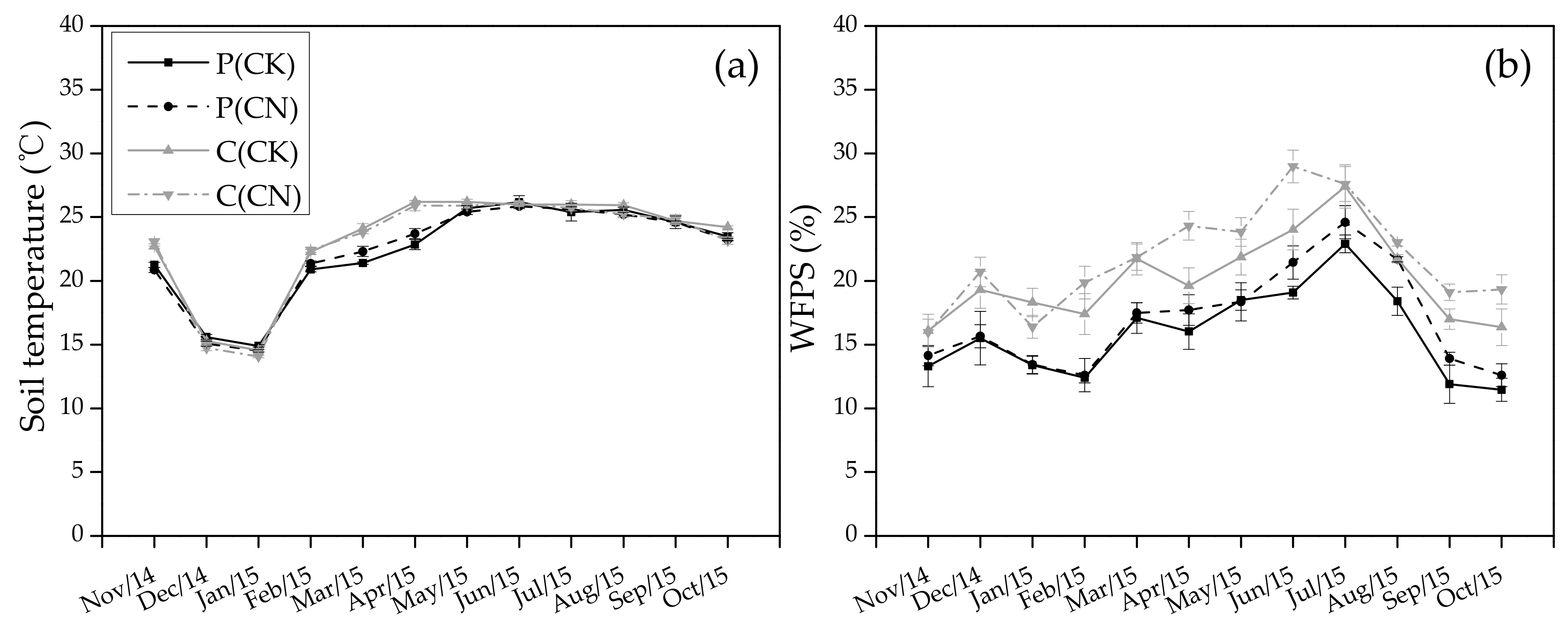
3.1. Soil Temperature and Moisture
3.2. Seasonal Variation in Soil Greenhouse Gas Flux
3.3. The Effects of Plantation Type on Soil Greenhouse Gas Flux
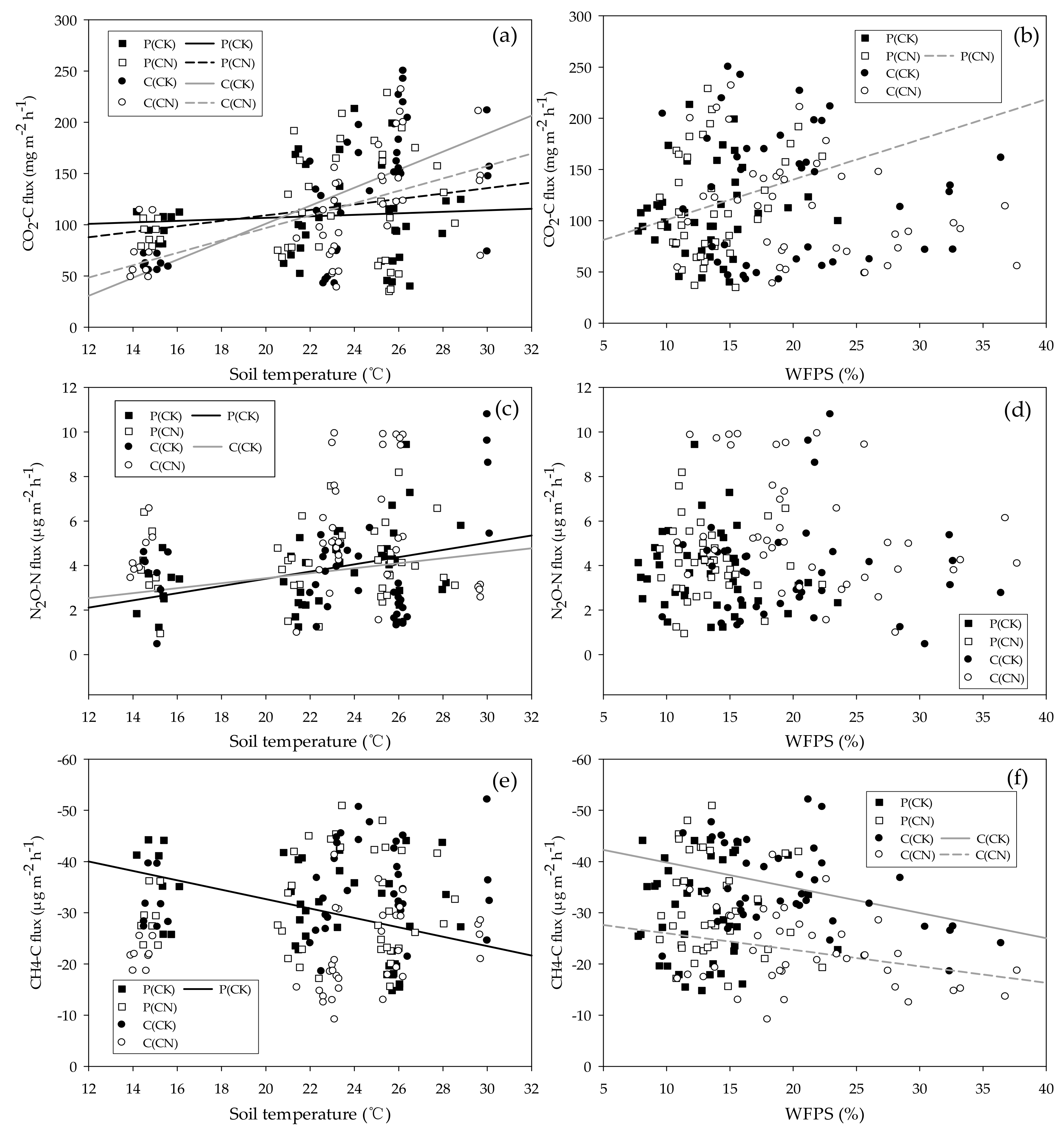
3.4. Main Influencing Factors on Soil Greenhouse Gas Flux
4. Discussion
4.1. CO2 Flux and Main Influencing Factors
4.2. N2O Flux and Main Influencing Factors
4.3. CH4 Flux and Main Influencing Factors
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II, and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Tang, X.; Liu, S.; Zhou, G.; Zhang, D.; Zhou, C. Soil-atmospheric exchange of CO2, CH4, and N2O in three subtropical forest ecosystems in southern China. Glob. Chang. Biol. 2006, 12, 546–560. [Google Scholar] [CrossRef]
- Lal, R. Forest soils and carbon sequestration. For. Ecol. Manag. 2005, 220, 242–258. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Gasche, R.; Breuer, L.; Papen, H. Fluxes of NO and N2O from temperate forest soils: Impact of forest type, N deposition and of liming on the NO and N2O emissions. Nutr. Cycl. Agroecosyst. 1997, 48, 79–90. [Google Scholar] [CrossRef]
- Pitz, S.; Megonigal, J.P. Temperate forest methane sink diminished by tree emissions. New Phytol. 2017, 214, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Mer, J.L.; Roger, P. Production, oxidation, emission and consumption of methane by soils: A review. Eur. J. Soil Biol. 2001, 37, 25–50. [Google Scholar] [CrossRef]
- Bodelier, P.L.E.; Laanbroek, H.J. Nitrogen as a regulatory factor of methane oxidation in soils and sediments. FEMS Microbiol. Ecol. 2004, 47, 265–277. [Google Scholar] [CrossRef]
- Solomon, S.; Qin, D.; Manning, M.; Chen, Z. Changes in atmospheric constituents and in radiative forcing. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Butterbach-Bahl, K.; Gasche, R.; Willibald, G.; Papen, H. Exchange of N-gases at the Höglwald Forest—A summary. Plant Soil 2002, 240, 117–123. [Google Scholar] [CrossRef]
- Guo, L.B.; Gifford, R.M. Soil carbon stocks and land use change: A meta analysis. Glob. Chang. Biol. 2002, 8, 345–360. [Google Scholar] [CrossRef]
- Wu, C.; Wei, X.; Mo, Q.; Li, Q.; Li, X.; Shu, C.; Liu, L.; Liu, Y. Effects of stand origin and near-natural restoration on the stock and structural composition of fallen trees in mid-subtropical forests. Forests 2015, 6, 4439–4450. [Google Scholar] [CrossRef]
- Emborg, J.; Christensen, M.; Heilmannclausen, J. The structural dynamics of Suserup Skov, a near-natural temperate deciduous forest in Denmark. For. Ecol. Manag. 2000, 126, 173–189. [Google Scholar] [CrossRef]
- Wang, G.; Liu, F. The influence of gap creation on the regeneration of Pinus tabuliformis planted forest and its role in the near-natural cultivation strategy for planted forest management. For. Ecol. Manag. 2011, 262, 413–423. [Google Scholar] [CrossRef]
- Jonard, M.; André, F.; Jonard, F.; Mouton, N.; Procès, P.; Ponette, Q. Soil carbon dioxide efflux in pure and mixed stands of oak and beech. Ann. For. Sci. 2007, 64, 141–150. [Google Scholar] [CrossRef]
- Ullah, S.; Frasier, R.; King, L.; Picotteanderson, N.; Moore, T. Potential fluxes of N2O and CH4 from soils of three forest types in Eastern Canada. Soil Biol. Biochem. 2008, 40, 986–994. [Google Scholar] [CrossRef]
- Amanda, M.; Dan, P.; Angela, B.H. Methane and nitrous oxide emissions from mature forest stands in the boreal forest, Saskatchewan, Canada. For. Ecol. Manag. 2009, 258, 1073–1083. [Google Scholar]
- Menyailo, O.V.; Hungate, B.A. Interactive effects of tree species and soil moisture on methane consumption. Soil Biol. Biochem. 2003, 35, 625–628. [Google Scholar] [CrossRef]
- Borken, W.; Beese, F. Methane and nitrous oxide fluxes of soils in pure and mixed stands of European beech and Norway spruce. Eur. J. Soil Sci. 2006, 57, 617–625. [Google Scholar] [CrossRef]
- Shi, Z.; Li, Y.; Wang, S.; Wang, G.; Ruan, H.; He, R.; Tang, Y.; Zhang, Z. Accelerated soil CO2 efflux after conversion from secondary oak forest to pine plantation in southeastern China. Ecol. Res. 2009, 24, 1257–1265. [Google Scholar] [CrossRef]
- Barrena, I.; Menéndez, S.; Duñabeitia, M.; Merino, P.; Florian Stange, C.; Spott, O.; González-Murua, C.; Estavillo, J.M. Greenhouse gas fluxes (CO2, N2O and CH4) from forest soils in the Basque Country: Comparison of different tree species and growth stages. For. Ecol. Manag. 2013, 310, 600–611. [Google Scholar] [CrossRef]
- Bréchet, L.; Ponton, S.; Roy, J.; Freycon, V.; Coûteaux, M.; Bonal, D.; Epron, D. Do tree species characteristics influence soil respiration in tropical forests? A test based on 16 tree species planted in monospecific plots. Plant Soil 2009, 319, 235–246. [Google Scholar] [CrossRef]
- Leitner, S.; Sae-Tun, O.; Kranzinger, L.; Zechmeister-Boltenstern, S.; Zimmermann, M. Contribution of litter layer to soil greenhouse gas emissions in a temperate beech forest. Plant Soil 2016, 403, 455–469. [Google Scholar] [CrossRef]
- Yamulki, S.; Morison, J.I.L. Annual greenhouse gas fluxes from a temperate deciduous oak forest floor. Forestry 2017, 90, 541–552. [Google Scholar] [CrossRef]
- Daniel, L.; Whendeel, S.; Matteo, D. Temporal dynamics in soil oxygen and greenhouse gases in two humid tropical forests. Ecosystems 2011, 14, 171–182. [Google Scholar]
- Wang, H.; Liu, S.; Wang, J.; Shi, Z.; Lu, L.; Zeng, J.; Ming, A.; Tang, J.; Yu, H. Effects of tree species mixture on soil organic carbon stocks and greenhouse gas fluxes in subtropical plantations in China. For. Ecol. Manag. 2013, 300, 4–13. [Google Scholar] [CrossRef]
- Janssens, I.A.; Sampson, D.A.; Curielyuste, J.; Carrara, A.; Ceulemans, R. The carbon cost of fine root turnover in a Scots pine forest. For. Ecol. Manag. 2002, 168, 231–240. [Google Scholar] [CrossRef]
- Shen, J.; Li, R.; Zhang, F.; Fan, J.; Tang, C.; Rengel, Z. Crop yields, soil fertility and phosphorus fractions in response to long-term fertilization under the rice monoculture system on a calcareous soil. Field Crop Res. 2004, 86, 225–238. [Google Scholar] [CrossRef]
- Huang, X.; Liu, S.; Wang, H.; Hu, Z.; Li, Z.; You, Y. Changes of soil microbial biomass carbon and community composition through mixing nitrogen-fixing species with Eucalyptus urophylla in subtropical China. Soil Biol. Biochem. 2014, 73, 42–48. [Google Scholar] [CrossRef]
- Janssens, I.A.; Lankreijer, H.; Matteucci, G.; Kowalski, A.S.; Buchmann, N.; Epron, D.; Pilegaard, K.; Kutsch, W.; Longdoz, B.; Grünwald, T. Productivity overshadows temperature in determining soil and ecosystem respiration across European forests. Glob. Chang. Biol. 2001, 7, 269–278. [Google Scholar] [CrossRef]
- Epron, D.; Bosc, A.; Bonal, D.; Freycon, V. Spatial variation of soil respiration across a topographic gradient in a tropical rain forest in French Guiana. J. Trop. Ecol. 2006, 22, 565–574. [Google Scholar] [CrossRef]
- Livesley, S.J.; Kiese, R.; Miehle, P.; Weston, C.J.; Butterbachbahl, K.; Arndt, S.K. Soil-atmosphere exchange of greenhouse gases in a Eucalyptus marginata woodland, a clover-grass pasture, and Pinus radiata and Eucalyptus globulus plantations. Glob. Chang. Biol. 2009, 15, 425–440. [Google Scholar] [CrossRef]
- Xu, X.; Hirata, E. Decomposition patterns of leaf litter of seven common canopy species in a subtropical forest: N and P dynamics. Plant Soil 2005, 273, 279–289. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, S.; Liu, Q.; Jiang, J.; Wang, R.; Li, N. Responses of soil respiration to land use conversions in degraded ecosystem of the semi-arid Loess Plateau. Ecol. Eng. 2015, 74, 196–205. [Google Scholar] [CrossRef]
- Hu, X.; Liu, L.; Zhu, B.; Du, E.; Hu, X.; Li, P.; Zhou, Z.; Ji, C.; Zhu, J.; Shen, H. Asynchronous responses of soil carbon dioxide, nitrous oxide emissions and net nitrogen mineralization to enhanced fine root input. Soil Biol. Biochem. 2016, 92, 67–78. [Google Scholar] [CrossRef]
- Rosenkranz, P.; Brüggemann, N. Soil N and C trace gas fluxes and microbial soil N turnover in a sessile oak (Quercus petraea (Matt.) Liebl.) forest in Hungary. Plant Soil 2006, 286, 301–322. [Google Scholar] [CrossRef]
- Gundersen, P.; Christiansen, J.R.; Alberti, G.; Brüggemann, N.; Castaldi, S.; Gasche, R.; Kitzler, B.; Klemedtsson, L.; Lobo-do-Vale, R.; Moldan, F.; et al. The response of methane and nitrous oxide fluxes to forest change in Europe. Biogeosciences 2012, 9, 3999–4012. [Google Scholar] [CrossRef]
- Pilegaard, K.; Skiba, U.; Ambus, P.; Beier, C.; Brüggemann, N.; Butterbachbahl, K.; Dick, J.; Dorsey, J.; Duyzer, J.; Gallagher, M. Factors controlling regional differences in forest soil emission of nitrogen oxides (NO and N2O). Biogeosciences 2006, 3, 651–661. [Google Scholar] [CrossRef]
- Morishita, T.; Sakata, T.; Takahashi, M.; Ishizuka, S.; Mizoguchi, T.; Inagaki, Y.; Terazawa, K.; Sawata, S.; Igarashi, M.; Yasuda, H.; et al. Methane uptake and nitrous oxide emission in Japanese forest soils and their relationship to soil and vegetation types. Soil Sci. Plant Nutr. 2007, 53, 678–691. [Google Scholar] [CrossRef]
- Weslien, P.; Klemedtsson, A.Å.K.; Börjesson, A.G.; Klemedtsson, L. Strong pH influence on N2O and CH4 fluxes from forested organic soils. Eur. J. Soil Sci. 2009, 60, 311–320. [Google Scholar] [CrossRef]
- Rowlings, D.W.; Grace, P.R.; Kiese, R.; Weier, K.L. Environmental factors controlling temporal and spatial variability in the soil-atmosphere exchange of CO2, CH4 and N2O from an Australian subtropical rainforest. Glob. Chang. Biol. 2012, 18, 726–738. [Google Scholar] [CrossRef]
- Gütlein, A.; Gerschlauer, F.; Kikoti, I.; Kiese, R. Impacts of climate and land use on N2O and CH4 fluxes from tropical ecosystems in the Mt. Kilimanjaro region, Tanzania. Glob. Chang. Biol. 2018, 24, 1239–1255. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Corre, M.D.; Schrell, W.; Veldkamp, E. Gross N2O emission and gross N2O uptake in soils under temperate spruce and beech forests. Soil Biol. Biochem. 2017, 112, 228–236. [Google Scholar] [CrossRef]
- Werner, C.; Kiese, R.; Butterbach-Bahl, K. Soil-atmosphere exchange of N2O, CH4, and CO2 and controlling environmental factors for tropical rain forest sites in western Kenya. J. Geophys. Res. 2007, 112, D3308. [Google Scholar] [CrossRef]
- Verchot, L.V.; Davidson, E.A.; Cattânio, J.H.; Ackerman, I.L. Land-use change and biogeochemical controls of methane fluxes in soils of eastern Amazonia. Ecosystems 2000, 3, 41–56. [Google Scholar] [CrossRef]
- Bárcena, T.G.; D’Imperio, L.; Gundersen, P.; Vesterdal, L.; Priemé, A.; Christiansen, J.R. Conversion of cropland to forest increases soil CH4 oxidation and abundance of CH4 oxidizing bacteria with stand age. Appl. Soil Ecol. 2014, 79, 49–58. [Google Scholar] [CrossRef]


| Year | Management | Plantation Type | |||
|---|---|---|---|---|---|
| P(CK) | P(CN) | C(CK) | C(CN) | ||
| 1993 | Afforestation | 2500 trees ha−1 | 2500 trees ha−1 | 2500 trees ha−1 | 2500 trees ha−1 |
| 1993–1995 | Tending for new plantations | 6 times | 6 times | 6 times | 6 times |
| 2000 | Released thinning | 1600 trees ha−1 | 1600 trees ha−1 | 1600 trees ha−1 | 1600 trees ha−1 |
| 2004 | Increment felling | 1200 trees ha−1 | 1200 trees ha−1 | 1200 trees ha−1 | 1200 trees ha−1 |
| 2007 | Intensity thinning | No 1200 trees ha−1 | Yes 600 trees ha−1 | No 1200 trees ha−1 | Yes 600 trees ha−1 |
| 2008 | Complementary planting | No | Planting Q. griffithii and E. fordii with 300 trees ha−1 respectively | No | Planting Q. griffithii and E. fordii with 300 trees ha−1 respectively |
| 2009 | Tending | No | 2 times | No | 2 times |
| 2016 | Average DBH | 22.2 ± 1.3 cm for P. massoniana | 32.2 ± 1.6 cm for P. massoniana | 17.1 ± 2.1 cm for C. lanceolata | 22.3 ± 0.8 cm for C. lanceolata |
| 2016 | Average height | 16.7 ± 0.5 m for P. massoniana | 17.3 ± 0.7 m for P. massoniana | 17.1 ± 0.4 m for C. lanceolata | 17.2 ± 0.4 m for C. lanceolata |
| Plantation Type | P(CK) | P(CN) | C(CK) | C(CN) |
|---|---|---|---|---|
| CO2-C flux (mg m−2 h−1) | ||||
| T(°C) | CO2 = 0.71T + 92.31 | CO2 = 2.67T + 55.83 | CO2 = 9.14T + 80.44 | CO2 = 6.05T + 24.15 |
| R2 = 0.11, p < 0.05 | R2 = 0.15, p < 0.05 | R2 = 0.37, p < 0.001 | R2 = 0.30, p < 0.001 | |
| W(%) | R2 = 0.01, p = 0.47 | CO2 = 3.92W + 61.71 | R2 = 0.06, p = 0.61 | R2 = 0.04, p = 0.28 |
| R2 = 0.13, p < 0.05 | ||||
| T(°C) + W(%) | R2 = 0.01, p = 0.80 | R2 = 0.09, p = 0.17 | CO2 = 9.19T + 0.98W − 103.51 | CO2 = 5.34T − 1.28W + 19.03 |
| R2 = 0.41, p < 0.001 | R2 = 0.33, p < 0.01 | |||
| N2O-N flux (μg m−2 h−1) | ||||
| T(°C) | N2O = 0.16T + 0.17 | R2 = 0.02, p = 0.43 | N2O = 0.11T + 1.19 | R2 = 0.00, p = 0.77 |
| R2 = 0.16, p < 0.01 | R2 = 0.16, p < 0.05 | |||
| W(%) | R2 = 0.03, p = 0.297 | R2 = 0.01, p = 0.54 | R2 = 0.01, p = 0.90 | R2 = 0.00, p = 0.77 |
| T(°C) + W(%) | N2O = 0.19T − 0.13W + 1.32 | R2 = 0.03, p = 0.54 | R2 = 0.06, p = 0.30 | R2 = 0.15, p = 0.05 |
| R2 = 0.22, p < 0.01 | ||||
| CH4-C flux (μg m−2 h−1) | ||||
| T(°C) | CH4 = 0.92T − 51.07 | R2 = 0.01, p = 0.13 | R2 = 0.05, p = 0.15 | R2 = 0.04, p = 0.25 |
| R2 = 0.17, p < 0.01 | ||||
| W(%) | R2 = 0.00, p = 0.998 | R2 = 0.01, p = 0.454 | CH4 = 0.49W − 44.75 | CH4 = 0.24W − 29.21 |
| R2 = 0.15, p < 0.01 | R2 = 0.10, p < 0.05 | |||
| T(°C) + W(%) | CH4 = 0.96T − 0.23W − 48.95 | R2 = 0.03, p = 0.56 | CH4 = −0.23T + 0.44W − 38.50 | R2 = 0.09, p = 0.16 |
| R2 = 0.18, p < 0.05 | R2 = 0.16, p < 0.05 | |||
| Plantation Type | P(CK) | P(CN) | C(CK) | C(CN) |
|---|---|---|---|---|
| CO2-C flux (mg m−2 h−1) | 103.3 ± 9.7cd | 121.6 ± 4.8ab | 112.4 ± 8.9bc | 128.7 ± 5.0a |
| N2O-N flux (μg m−2 h−1) | 3.6 ± 0.1cd | 4.3 ± 0.5b | 3.8 ± 0.2bc | 5.6 ± 1.1a |
| CH4-C flux (μg m−2 h−1) | −34.7 ± 1.7c | −27.2 ± 1.6b | −34.9 ± 2.8c | −22.4 ± 1.8a |
| Properties | P(CK) | P(CN) | C(CK) | C(CN) |
|---|---|---|---|---|
| Litterfall quantity (t hm−2 r−1) | 10.23 ± 0.94a | 10.84 ± 0.49a | 9.02 ± 0.19b | 9.54 ± 0.34b |
| Fine root biomass (t hm−2) | 0.81 ± 0.07b | 1.36 ± 0.22a | 0.64 ± 0.26b | 1.33 ± 0.28a |
| C:N of leaf litter | 48.07 ± 4.82c | 37.49 ± 4.77d | 68.13 ± 8.12a | 52.70 ± 6.92b |
| C:N of fine root | 57.53 ± 10.7a | 39.70 ± 5.70c | 55.38 ± 3.30a | 45.70 ± 4.40b |
| Soil porosity (%) | 56.80 ± 2.83a | 56.04 ± 2.58a | 49.05 ± 4.99b | 45.17 ± 4.86b |
| Soil temperature (°C) | 22.15 ± 0.12d | 22.47 ± 0.17c | 22.73 ± 0.04b | 23.04 ± 0.03a |
| Soil WFPS (%) | 13.06 ± 0.56b | 13.67 ± 0.49b | 19.91 ± 1.00a | 21.28 ± 1.06a |
| Soil pH | 4.18 ± 0.04d | 4.31 ± 0.08c | 4.67 ± 0.07b | 4.91 ± 0.20a |
| Soil organic C(g kg−1) | 25.99 ± 1.32b | 29.15 ± 2.42a | 17.24 ± 1.85d | 21.61 ± 2.58c |
| Soil total N(g kg−1) | 2.58 ± 0.04 | 3.28 ± 0.12 | 2.29 ± 0.15 | 3.32 ± 0.13 |
| Soil available N (mg kg−1) | 94.37 ± 3.94b | 103.32 ± 5.62a | 77.0 ± 9.07c | 96.25 ± 7.27ab |
| Soil total P (g kg−1) | 0.28 ± 0.01a | 0.25 ± 0.02b | 0.24 ± 0.03b | 0.21 ± 0.01c |
| Soil C:N | 17.06 ± 0.50a | 15.34 ± 0.72c | 16.42 ± 0.14b | 15.16 ± 0.46c |
| Soil NH4+-N content (mg kg−1) | 20.30 ± 2.07 b | 26.67 ± 3.35a | 18.44 ± 2.17b | 24.56 ± 4.02a |
| Soil NO3−-N content (mg kg−1) | 21.97 ± 1.83b | 25.00 ± 2.21a | 18.36 ± 2.28b | 24.65 ± 4.19a |
| Soil microbial biomass C (mg kg−1) | 301.12 ± 24.54b | 388.12 ± 11.76a | 234.44 ± 29.49c | 312.50 ± 32.51b |
| Soil microbial biomass N (mg kg−1) | 39.07 ± 6.59bc | 53.30 ± 8.11a | 36.40 ± 6.45c | 46.51 ± 4.21ab |
| Parameters | Models |
|---|---|
| CO2-C flux (mg m−2 h−1) (Y1) | |
| C:N ratio of fine root (X1) Soil temperature (°C) (X2) | Y1 = −0.707X1 + 16.2X2 − 217.0, R2 = 0.774, p < 0.001 |
| N2O-N flux (μg m−2 h−1) (Y2) | |
| C:N ratio of leaf litter (X3) Soil available N (mg kg−1) (X4) | Y2 = −0.044X3 + 0.16X4 + 5.886, R2 = 0.693, p < 0.001 |
| CH4-C flux (μg m−2 h−1) (Y3) | |
| C:N ratio of leaf litter (X5) | Y3 = 0.343X5 − 6.026, R2 = 0.624, p < 0.001 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ming, A.; Yang, Y.; Liu, S.; Wang, H.; Li, Y.; Li, H.; Nong, Y.; Cai, D.; Jia, H.; Tao, Y.; et al. Effects of Near Natural Forest Management on Soil Greenhouse Gas Flux in Pinus massoniana (Lamb.) and Cunninghamia lanceolata (Lamb.) Hook. Plantations. Forests 2018, 9, 229. https://doi.org/10.3390/f9050229
Ming A, Yang Y, Liu S, Wang H, Li Y, Li H, Nong Y, Cai D, Jia H, Tao Y, et al. Effects of Near Natural Forest Management on Soil Greenhouse Gas Flux in Pinus massoniana (Lamb.) and Cunninghamia lanceolata (Lamb.) Hook. Plantations. Forests. 2018; 9(5):229. https://doi.org/10.3390/f9050229
Chicago/Turabian StyleMing, Angang, Yujing Yang, Shirong Liu, Hui Wang, Yuanfa Li, Hua Li, You Nong, Daoxiong Cai, Hongyan Jia, Yi Tao, and et al. 2018. "Effects of Near Natural Forest Management on Soil Greenhouse Gas Flux in Pinus massoniana (Lamb.) and Cunninghamia lanceolata (Lamb.) Hook. Plantations" Forests 9, no. 5: 229. https://doi.org/10.3390/f9050229
APA StyleMing, A., Yang, Y., Liu, S., Wang, H., Li, Y., Li, H., Nong, Y., Cai, D., Jia, H., Tao, Y., & Sun, D. (2018). Effects of Near Natural Forest Management on Soil Greenhouse Gas Flux in Pinus massoniana (Lamb.) and Cunninghamia lanceolata (Lamb.) Hook. Plantations. Forests, 9(5), 229. https://doi.org/10.3390/f9050229






