Chl Fluorescence Parameters and Leaf Reflectance Indices Allow Monitoring Changes in the Physiological Status of Quercus ilex L. under Progressive Water Deficit
Abstract
:1. Introduction
2. Materials and Methods
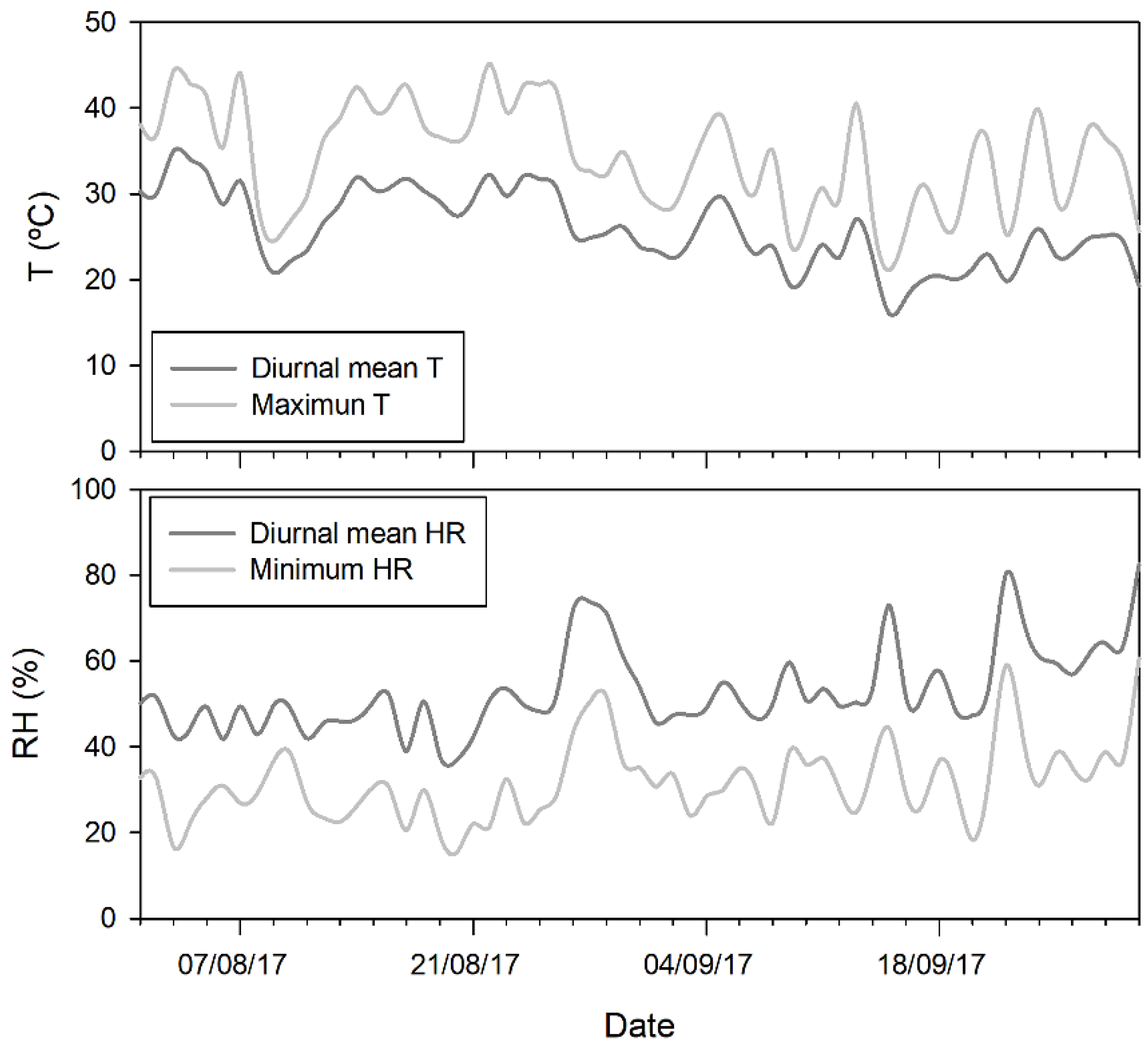
2.1. Plant Material and Experimental Conditions
2.2. Water Potential Measurements
2.3. Chlorophyll Fluorescence
2.4. Spectral Reflectance
2.5. Statistical Analysis
3. Results
3.1. Water Potential
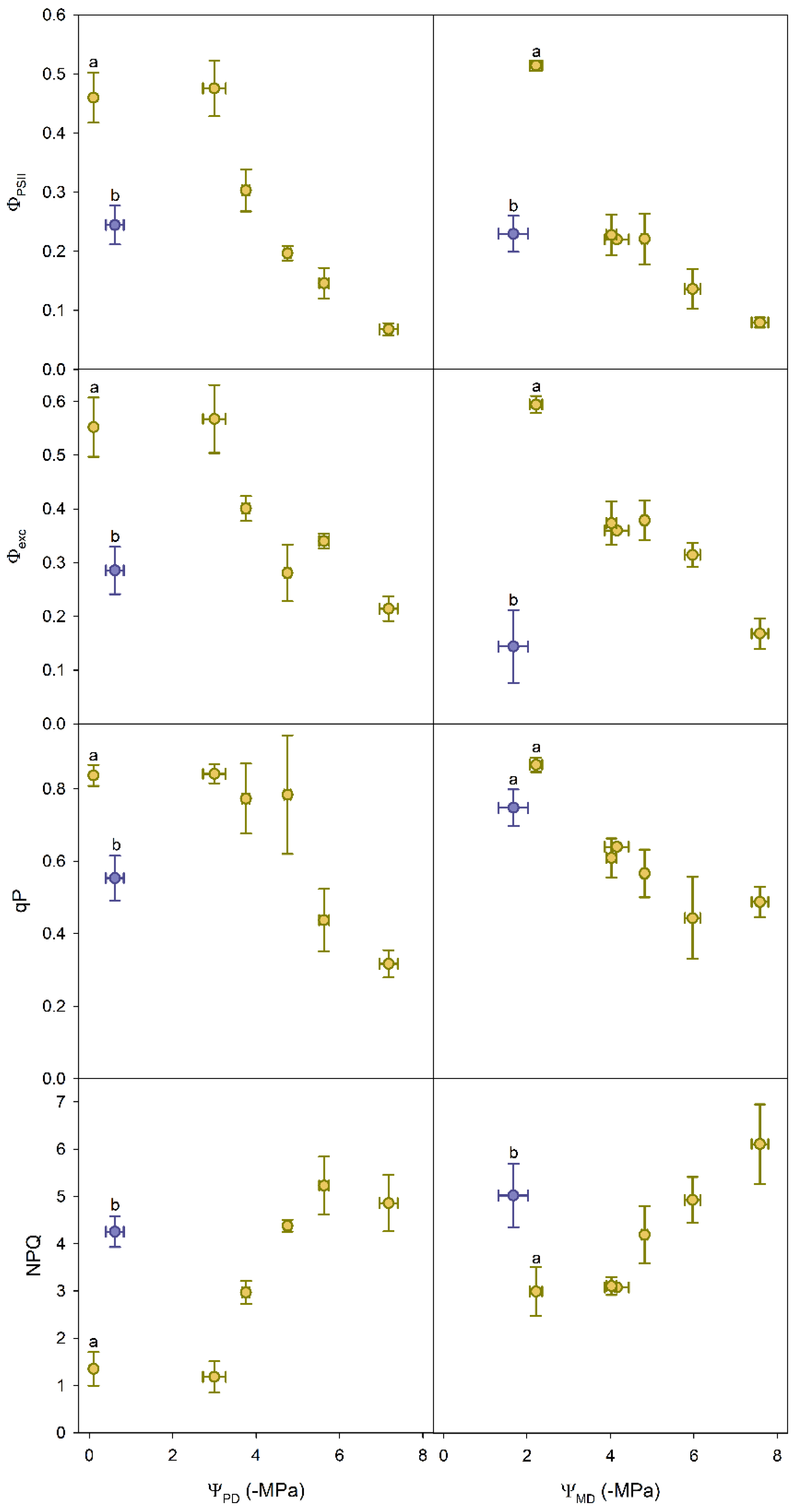
3.2. Chlorophyll Fluorescence Parameters
3.3. Reflectance Indices
4. Discussion
4.1. Response to Water Scarcity
4.2. Recovery from Water Scarcity
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reichstein, M.; Bahn, M.; Ciais, P.; Frank, D.; Mahecha, M.D.; Seneviratne, S.I.; Zscheischler, J.; Beer, C.; Buchmann, N.; Frank, D.C.; et al. Climate extremes and the carbon cycle. Nature 2013, 500, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.; Reichstein, M.; Bahn, M.; Frank, D.; Mahecha, M.D.; Smith, P.; Thonicke, K.; van der Velde, M.; Vicca, S.; Babst, F.; et al. Effects of climate extremes on the terrestrial carbon cycle: concepts, processes and potential future impacts. Glob. Change Biol. 2015, 2, 2861–2880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Buttlar, J.; Zscheischler, J.; Rammig, A.; Sippel, S.; Reichstein, M.; Knohl, A.; Jung, M.; Menzer, O.; Altaf Arain, M.; Buchmann, N.; et al. Impacts of droughts and extreme-temperature events on gross primary production and ecosystem respiration: A systematic assessment across ecosystems and climate zones. Biogeosciences 2018, 15, 1293–1318. [Google Scholar] [CrossRef]
- Gil-Pelegrín, E.; Saz, M.A.; Cuadrat, J.M.; Peguero-Pina, J.J.; Sancho-Knapik, D. Oaks under mediterranean-type climates: functional response to summer aridity. In Oaks Physiological Ecology. Exploring the Functional Diversity of Genus Quercus L., 1st ed.; Gil-Pelegrín, E., Peguero-Pina, J.J., Sancho-Knapik, D., Eds.; Springer: Cham, Switzerland, 2017; pp. 137–194. ISBN 978-3-319-69098-8. [Google Scholar]
- García-Mozo, H.; Mestre, A.; Galán, C. Phenological trends in southern Spain: A response to climate change. Agric. For. Meteorol. 2010, 150, 575–580. [Google Scholar] [CrossRef]
- Iglesias, A.; Garrote, L.; Flores, F.; Moneo, M. Challenges to manage the risk of water scarcity and climate change in the Mediterranean. Water Resour. Manag. 2007, 21, 775–788. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Sharkey, T.D. Stomatal conductance and photosynthesis. Annu. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Ciais, P.; Reichstein, M.; Viovy, N.; Granier, A.; Ogee, J.; Allard, V.; Aubinet, M.; Buchmann, N.; Bernhofer, C.; Carrara, A.; et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 2005, 437, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Calcerrada, J.; Sancho-Knapik, D.; Martin-StPaul, N.K.; Limousin, J.M.; McDowell, N.G.; Gil-Pelegrín, E. Drought-induced oak decline-Factors involved, physiological dysfunctions, and potential attenuation by forestry practices. In Oaks Physiological Ecology. Exploring the Functional Diversity of Genus Quercus L., 1st ed.; Gil-Pelegrín, E., Peguero-Pina, J.J., Sancho-Knapik, D., Eds.; Springer: Cham, Switzerland, 2017; pp. 419–451. ISBN 978-3-319-69098-8. [Google Scholar]
- Chaves, M.M.; Santos, T.P.; Souza, C.R.; Ortuño, M.F.; Rodrigues, M.L.; Lopes, C.M.; Maroco, J.P.; Pereira, J.S. Deficit irrigation in grapevine improves water-use efficiency while controlling vigour and production quality. Ann. Appl. Biol. 2007, 150, 237–252. [Google Scholar] [CrossRef] [Green Version]
- De Rigo, D.; Caudullo, G. Quercus ilex in Europe: distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publication Office of the European Union: Luxembourg, 2016; pp. 130–131. ISBN 978-92-79-36740-3. [Google Scholar]
- Rodriguez-Estevez, V.; Sanchez-Rodriguez, M.; Arce, C.; García, A.R.; Perea, J.M.; Gomez-Castro, A.G. Consumption of acorns by finishing iberian pigs and their function in the conservation of the Dehesa Agroecosystem. In Agroforestry for Biodiversity and Ecosystem Services—Science and Practice; Kaonga, M., Ed.; IntechOpen: London, UK, 2012; pp. 1–22. ISBN 978-953-51-0493-3. [Google Scholar]
- Reyna, S.; García-Barreda, S. Black truffle cultivation: A global reality. For. Syst. 2014, 23, 317–328. [Google Scholar] [CrossRef]
- Gil-Pelegrín, E.; Peguero-Pina, J.J.; Sancho-Knapik, D. Oaks and people: a long journey together. In Oaks Physiological Ecology. Exploring the Functional Diversity of Genus Quercus L., 1st ed.; Gil-Pelegrín, E., Peguero-Pina, J.J., Sancho-Knapik, D., Eds.; Springer: Cham, Switzerland, 2017; pp. 1–11. ISBN 978-3-319-69098-8. [Google Scholar]
- Sancho-Knapik, D.; Gómez Álvarez-Arenas, T.; Peguero-Pina, J.J.; Gil-Pelegrín, E. Air-coupled broadband ultrasonic spectroscopy as a new non-invasive and non-contact method for the determination of leaf water status. J. Exp. Bot. 2010, 61, 1385–1391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrs, H.D. Determination of water deficits in plant tissues. In Water Deficits and Plant Growth; Kozlowski, T.T., Ed.; Academic Press: New York, NY, USA, 1968; Volume 1, pp. 235–368. [Google Scholar]
- Slavík, B. Methods of Studying Plant Water Relations; Springer: Berlin, Germany, 1974; ISBN 3-540-06686-1. [Google Scholar]
- Peguero-Pina, J.J.; Sancho-Knapik, D.; Martín, P.; Saz, M.A.; Gea-Izquierdo, G.; Cañellas, I.; Gil-Pelegrín, E. Evidence of vulnerability segmentation in a deciduous Mediterranean oak (Quercus subpyrenaica E. H. del Villar). Trees 2015, 29, 1917–1927. [Google Scholar] [CrossRef]
- Sancho-Knapik, D.; Medrano, H.; Peguero-Pina, J.J.; Mencuccini, M.; Fariñas, M.D.; Gómez Álvarez-Arenas, T.; Gil-Pelegrín, E. The application of leaf ultrasonic resonance to Vitis vinifera L. suggests the existence of a diurnal osmotic adjustment subjected to photosynthesis. Front. Plant Sci. 2016, 7, 1601. [Google Scholar] [CrossRef] [PubMed]
- Sancho-Knapik, D.; Sanz, M.A.; Peguero-Pina, J.J.; Niinemets, Ü.; Gil-Pelegrín, E. Changes of secondary metabolites in Pinus sylvestris L. needles under increasing soil water déficit. Ann. For. Sci. 2017, 74, 24. [Google Scholar] [CrossRef]
- Peñuelas, J.; Llusia, J.; Pinol, J.; Filella, I. Photochemical reflectance index and leaf photosynthetic radiation-use-efficiency assessment in Mediterranean trees. Int. J. Remote Sens. 1997, 18, 2863–2868. [Google Scholar] [CrossRef]
- Gitelson, A.; Merzlyak, M.N. Signature analysis of leaf reflectance spectra: algorithm development for remote sensing of chlorophyll. J. Plant. Physiol. 1996, 148, 494–500. [Google Scholar] [CrossRef]
- Huang, Z.; Turner, B.J.; Dury, S.J.; Wallis, I.R.; Foley, W.J. Estimating foliage nitrogen concentration from HYMAP data using continuum removal analysis. Remote. Sens. Environ. 2004, 93, 18–29. [Google Scholar] [CrossRef]
- Asner, G.P.; Carlson, K.M.; Martin, R.E. Substrate age and precipitation effects on Hawaiian forest canopies from spaceborne imaging spectroscopy. Remote. Sens. Environ. 2005, 98, 457–467. [Google Scholar] [CrossRef]
- Morales, F.; Abadía, A.; Abadía, J. Characterization of the xanthophyll cycle and other photosynthetic pigment changes induced by iron deficiency in sugar beet (Beta vulgaris L.). Plant Physiol. 1990, 94, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A.V.; Young, A.J.; Horton, P. Induction of nonphotochemical energy dissipation and absorbance changes in leaves. Plant Physiol. 1993, 102, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Vicca, S.; Balzarolo, M.; Filella, I.; Granier, A.; Herbst, M.; Knohl, A.; Longdoz, B.; Mund, M.; Nagy, Z.; Pintér, K.; et al. Remotely-sensed detection of effects of extreme droughts on gross primary production. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dobrowski, S.Z.; Pushnik, J.C.; Zarco-Tejada, P.J.; Ustin, S.L. Simple reflectance indices track heat and wáter stress-induced changes in steady-state chlorophyll fluorescence at the canopy scale. Remote Sens. Environ. 2005, 97, 403–414. [Google Scholar] [CrossRef]
- Sun, P.; Wahbi, S.; Tsonev, T.; Haworth, M.; Liu, S.; Centritto, M. On the use of leaf spectral indices to assess water status and photosynthetic limitations in Olea europaea L. during water-stress and recovery. PLoS ONE 2014, 9, e105165. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.; Maselli, F.; Gilabert, M.A.; Chiesi, M.; Martínez, B.; Seufert, G. Assessment of MODIS imagery to track light-use efficiency in a water-limited Mediterranean pine forest. Remote Sens. Environ. 2012, 123, 359–367. [Google Scholar] [CrossRef]
- Peguero-Pina, J.J.; Morales, F.; Flexas, J.; Gil-Pelegrín, E.; Moya, I. Photochemistry, remotely sensed physiological reflectance index and de-epoxidation state of the xanthophyll cycle in Quercus coccifera under intense drought. Oecologia 2008, 156, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Preece, C.; Filella, I.; Farré-Armengol, G.; Peñuelas, J. Assessment of the response of photosynthetic activity of mediterranean evergreen oaks to enhanced drought stress and recovery by using PRI and R690/R630. Forests 2017, 8, 386. [Google Scholar] [CrossRef]
- Horton, P.; Ruban, A.V.; Walters, R.G. Regulation of light harvesting in green plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 655–684. [Google Scholar] [CrossRef] [PubMed]
- Krause, G.; Weis, E. Chlorophyll fluorescence and photosynthesis: The basics. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 313–349. [Google Scholar] [CrossRef]
- Briantais, J.M.; Vernotte, C.; Picaud, M.; Krause, G.H. A quantitative study of the slow decline of chlorophyll a fluorescence in isolated chloroplasts. Biochim. Biophys. Acta 1979, 548, 128–138. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W. Photoprotection in an ecological context: The remarkable complexity of thermal energy dissipation. New Phytol. 2006, 172, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Porcar-Castell, A. A high-resolution portrait of the annual dynamics of photochemical and non-photochemical quenching in needles of Pinus sylvestris. Physiol. Plant. 2011, 143, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, A. Sustained energy dissipation in winter evergreens. New Phytol. 2014, 201, 57–65. [Google Scholar] [CrossRef]
- Turner, N.C. Measurement of plant water status by pressure chamber technique. Irrigation Sci. 1988, 9, 289–308. [Google Scholar] [CrossRef]
- Belkhodja, R.; Morales, F.; Quílez, R.; López-Millán, A.F.; Abadía, A.; Abadía, J. Iron deficiency causes changes in chlorophyll fluorescence due to the reduction in the dark of the photosystem II acceptor side. Photosynth. Res. 1998, 56, 265–276. [Google Scholar] [CrossRef]
- Morales, F.; Abadía, A.; Abadía, J. Photosynthesis, quenching of chlorophyll fluorescence and thermal energy dissipation in iron deficient sugar beet leaves. Aust. J. Plant Physiol. 1998, 25, 403–412. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Morales, F.; Abadía, A.; Abadía, J. Chlorophyll fluorescence and photon yield of oxygen evolution in iron-deficient sugar beet (Beta vulgaris L.) leaves. Plant Physiol. 1991, 97, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Abadía, J.; Morales, F.; Abadía, A. Photosystem II efficiency in low chlorophyll, iron-deficient leaves. Plant Soil 1999, 215, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Harbinson, J.; Genty, B.; Baker, N.R. Relationship between the quantum efficiencies of Photosystems I and II in pea leaves. Plant Physiol. 1989, 90, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Van Kooten, O.; Snel, J.H.F. The use of chlorophyll fluorescence in plant stress physiology. Photosynth. Res. 1990, 25, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Bilger, W.; Björkman, O. Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth. Res. 1990, 25, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Gamon, J.A.; Serrano, L.; Surfus, J.S. The photochemical reflectance index: an optical indicator of photosynthetic radiation use efficiency across species, functional types, and nutrient levels. Oecologia 1997, 112, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Peguero-Pina, J.J.; Gil-Pelegrín, E.; Morales, F. Three pools of zeaxanthin in Quercus coccifera leaves during light transitions with different roles in rapidly reversible photoprotective energy dissipation and photoprotection. J. Exp. Bot. 2013, 64, 1649–1661. [Google Scholar] [CrossRef] [PubMed]
- Gitelson, A.; Merzlyak, M.N. Spectral reflectance changes associated with autumn senescence of Aesculus hippocastanum L. and Acer platanoides L. leaves: spectral features and relation to chlorophyll estimation. J. Plant Physiol. 1994, 143, 286–292. [Google Scholar] [CrossRef]
- Richardson, A.D.; Berlyn, G.P. Spectral reflectance and photosynthetic properties of Betula papyrifera (Betulaceae) leaves along an elevational gradient on Mt. Mansfield, Vermont, USA. Am. J. Bot. 2002, 89, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J.; Filella, I.; Biel, C.; Serrano, L.; Savé, R. The reflectance at the 950–970 nm region as an indicator of plant water status. Int. J. Remote Sen. 1993, 14, 1887–1905. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I.; Serrano, L.; Savé, R. Cell wall elasticity and Water Index (R970 nm/R900 nm) in wheat under different nitrogen availabilities. Int. J. Remote Sen. 1996, 17, 373–382. [Google Scholar] [CrossRef]
- Filella, I.; Peñuelas, J. The red edge position and shape as indicators of plant chlorophyll content, biomass and hydric status. Int. J. Remote Sens. 1994, 15, 1459–1470. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N.; Lichtenthaler, H.K. Detection of red edge position and chlorophyll content by reflectance measurements near 700 nm. J. Plant Physiol. 1996, 148, 501–508. [Google Scholar] [CrossRef]
- Limousin, J.M.; Longepierre, D.; Huc, R.; Rambal, S. Change in hydraulic traits of Mediterranean Quercus ilex subjected to long-term throughfall exclusion. Tree Physiol. 2010, 30, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Zheng, S.; Zhong, Y.; Shangguan, Z. Contrasting dynamics of leaf potential and gas exchange during progressive drought cycles and recovery in Amorpha fruticosa and Robinia pseudoacacia. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Peguero-Pina, J.J.; Sancho-Knapik, D.; Morales, F.; Flexas, J.; Gil-Pelegrin, E. Differential photosynthetic performance and photoprotection mechanisms of three Mediterranean evergreen oaks under severe drought stress. Funct. Plant Biol. 2009, 36, 453–462. [Google Scholar] [CrossRef]
- Peñuelas, J.; Piñol, J.; Ogaya, R.; Filella, I. Estimation of plant water concentration by the reflectance Water Index WI (R900/R970). Int. J. Remote Sens. 1997, 18, 2869–2875. [Google Scholar] [CrossRef]
- Méthy, M. Analysis of photosynthetic activity at the leaf and canopy levels from reflectance measurements: A case study. Photosynthetica 2000, 38, 505–512. [Google Scholar] [CrossRef]
- Evain, S.; Flexas, J.; Moya, I. A new instrument for passive remote sensing: 2. Measurement of leaf and canopy reflectance changes at 531 nm and their relationship with photosynthesis and chlorophyll fluorescence. Remote Sens. Environ. 2004, 91, 175–185. [Google Scholar] [CrossRef]
- Goerner, A.; Reichstein, M.; Rambal, S. Estimation of photosynthetic light use efficiency in semi-arid ecosystems with the MODIS-derived photochemical reflectance index. In Proceedings of the 2008 IEEE International Geoscience and Remote Sensing Symposium, Boston, MA, USA, 7–11 July 2008; Volume 3, pp. 756–758. [Google Scholar]
- Garbulsky, M.F.; Peñuelas, J.; Gamon, J.A.; Inoue, Y.; Filella, I. The photochemical reflectance index (PRI) and the remote sensing of leaf, canopy and ecosystem radiation use efficiencies: A review and meta-analysis. Remote Sens. Environ. 2011, 115, 281–297. [Google Scholar] [CrossRef]
- Tsonev, T.; Wahbi, S.; Sun, P.; Sorrentino, G.; Centritto, M. Gas exchange, water relations and their relationships with photochemical reflectance index in Quercus ilex plants during water stress and recovery. Int. J. Agric. Biol. 2014, 16, 335–341. [Google Scholar]
- Garbulsky, M.F.; Peñuelas, J.; Ogaya, R.; Filella, I. Leaf and stand-level carbon uptake of a Mediterranean forest estimated using the satellite-derived reflectance indices EVI and PRI. Int. J. Remote Sens. 2013, 34, 1282–1296. [Google Scholar] [CrossRef]
- Curran, P.J.; Dungan, J.L.; Gholz, H.L. Exploring the relationship between reflectance red edge and chlorophyll concentration in slash pine leaves. Tree Physiol. 1995, 15, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Zarco-Tejada, P.J.; Hornero, A.; Hernández-Clemente, R.; Beck, P.S.A. Understanding the temporal dimension of the red-edge spectral region for forest decline detection using high-resolution hyperspectral and Sentinel-2a imagery. J. Photogramm. Remote Sens. 2018, 137, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Niu, Z.; Tang, Q.; Huang, W. Estimating chlorophyll content from hyperspectral vegetation indices: Modeling and validation. Agric. For. Meteorol. 2008, 148, 1230–1241. [Google Scholar] [CrossRef]
- Peguero-Pina, J.J.; Sancho-Knapik, D.; Barrón, E.; Camarero, J.J.; Vilagrosa, A.; Gil-Pelegrín, E. Morphological and physiological divergences within Quercus ilex support the existence of different ecotypes depending on climatic dryness. Ann. Bot. 2014, 114, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Gallé, A.; Haldimann, P.; Feller, U. Photosynthetic performance and water relations in young pubescent oak (Quercus pubescens) trees during drought stress and recovery. New Phytol. 2007, 174, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Martorell, S.; Diaz-Espejo, A.; Medrano, H.; Ball, M.C.; Choat, B. Rapid hydraulic recovery in Eucalyptus pauciflora after drought: linkages between stem hydraulics and leaf gas exchange. Plant Cell Environ. 2014, 37, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Galmés, J.; Medrano, H.; Flexas, J. Photosynthetic limitations in response to water stress and recovery in Mediterranean plants with different growth forms. New Phytol. 2007, 175, 81–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sancho-Knapik, D.; Mendoza-Herrer, Ó.; Gil-Pelegrín, E.; Peguero-Pina, J.J. Chl Fluorescence Parameters and Leaf Reflectance Indices Allow Monitoring Changes in the Physiological Status of Quercus ilex L. under Progressive Water Deficit. Forests 2018, 9, 400. https://doi.org/10.3390/f9070400
Sancho-Knapik D, Mendoza-Herrer Ó, Gil-Pelegrín E, Peguero-Pina JJ. Chl Fluorescence Parameters and Leaf Reflectance Indices Allow Monitoring Changes in the Physiological Status of Quercus ilex L. under Progressive Water Deficit. Forests. 2018; 9(7):400. https://doi.org/10.3390/f9070400
Chicago/Turabian StyleSancho-Knapik, Domingo, Óscar Mendoza-Herrer, Eustaquio Gil-Pelegrín, and José Javier Peguero-Pina. 2018. "Chl Fluorescence Parameters and Leaf Reflectance Indices Allow Monitoring Changes in the Physiological Status of Quercus ilex L. under Progressive Water Deficit" Forests 9, no. 7: 400. https://doi.org/10.3390/f9070400
APA StyleSancho-Knapik, D., Mendoza-Herrer, Ó., Gil-Pelegrín, E., & Peguero-Pina, J. J. (2018). Chl Fluorescence Parameters and Leaf Reflectance Indices Allow Monitoring Changes in the Physiological Status of Quercus ilex L. under Progressive Water Deficit. Forests, 9(7), 400. https://doi.org/10.3390/f9070400





