Hydrogen Supply Chains for Mobility—Environmental and Economic Assessment
Abstract
:1. Introduction
2. Methods and Assumptions
2.1. Goal and Scope
- First, they must be recommended by the International Reference Life Cycle Data System (ILCD) [28].
- Second, the quality of the impact assessment methods should be at least “recommended but in need of some improvements” [29].
- Third, impact categories must have a high importance for the assessment of the mobility sector.
2.2. Inventory
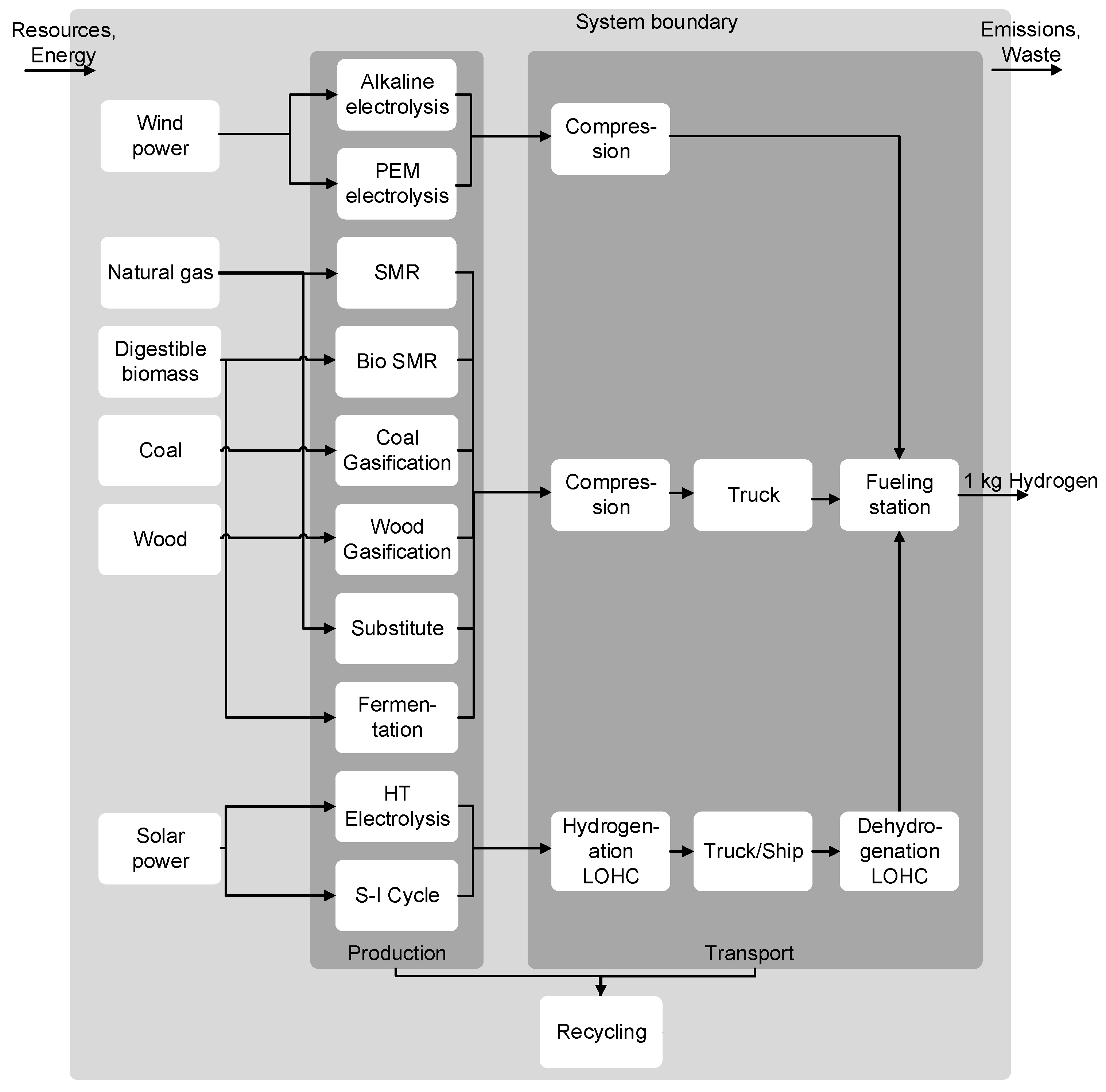
2.2.1. Hydrogen Production
2.2.2. Hydrogen Transport and Distribution
2.2.3. Background Data
3. Results
3.1. Enviromental Assessment
3.1.1. Impact Category Climate Change
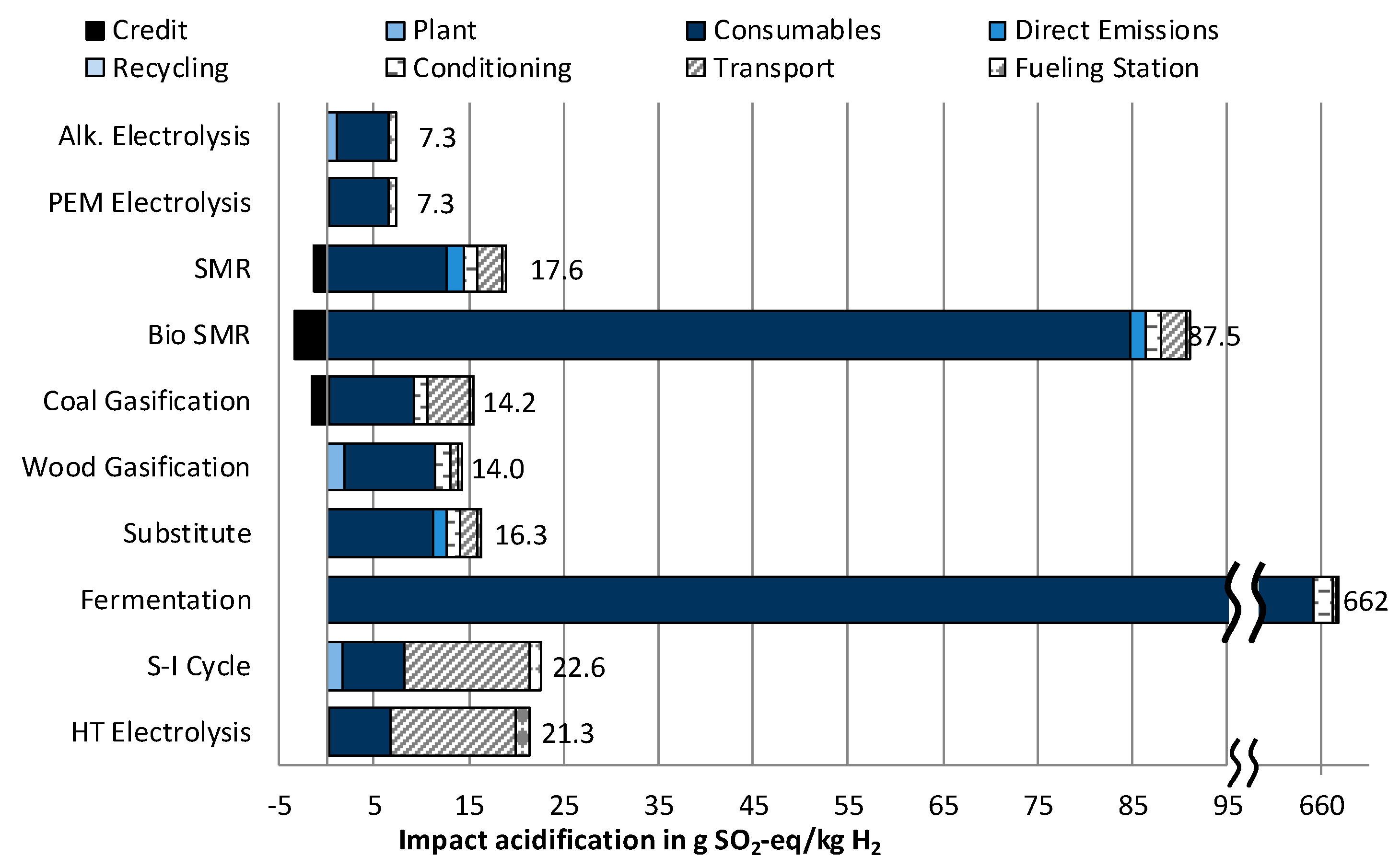
3.1.2. Impact Category Acidification
3.1.3. Impact Category Eutrophication
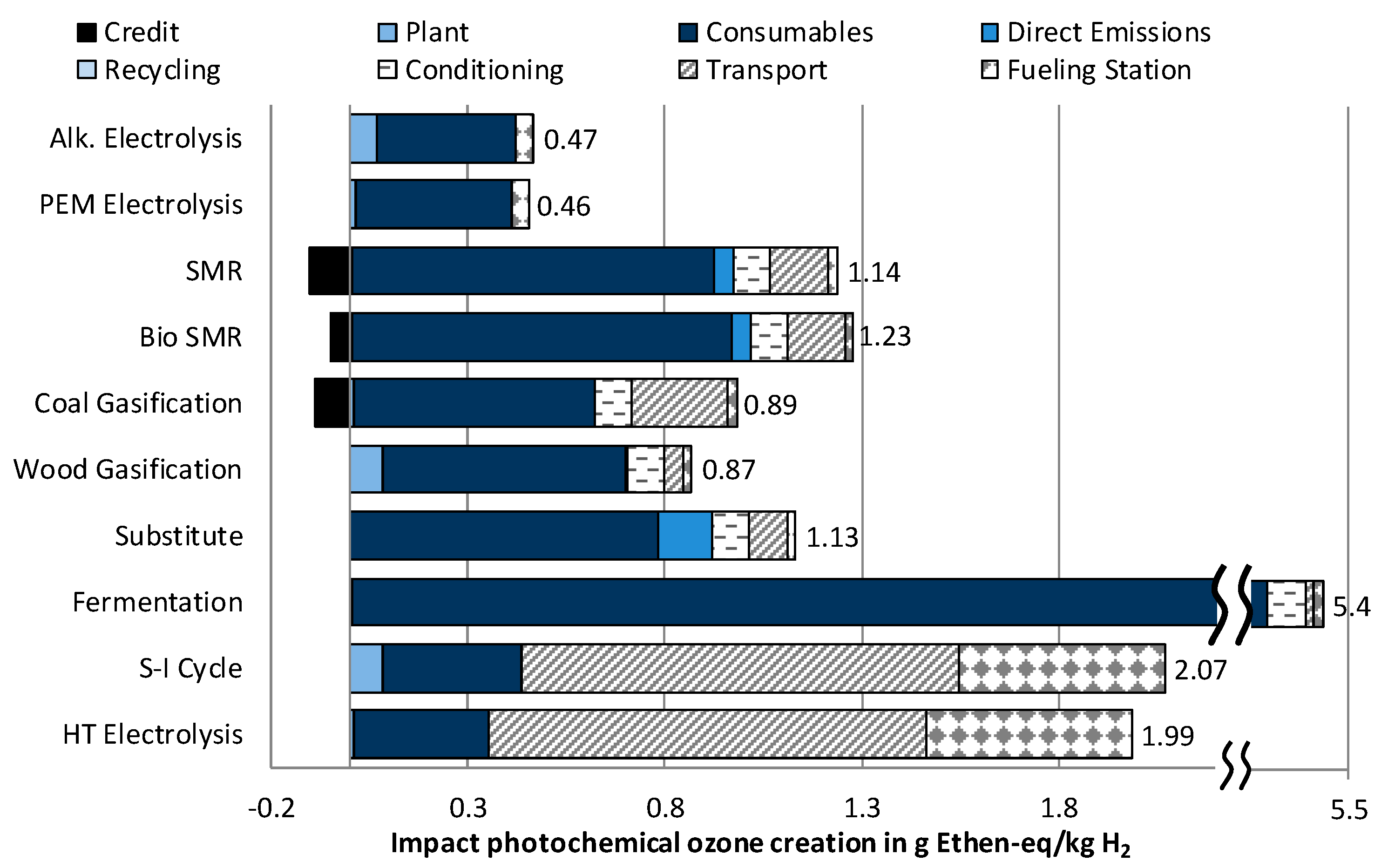
3.1.4. Impact Category Photochemical Ozone Creation
3.1.5. Impact Category Particulate Matter
3.1.6. Impact Category Human Toxicity
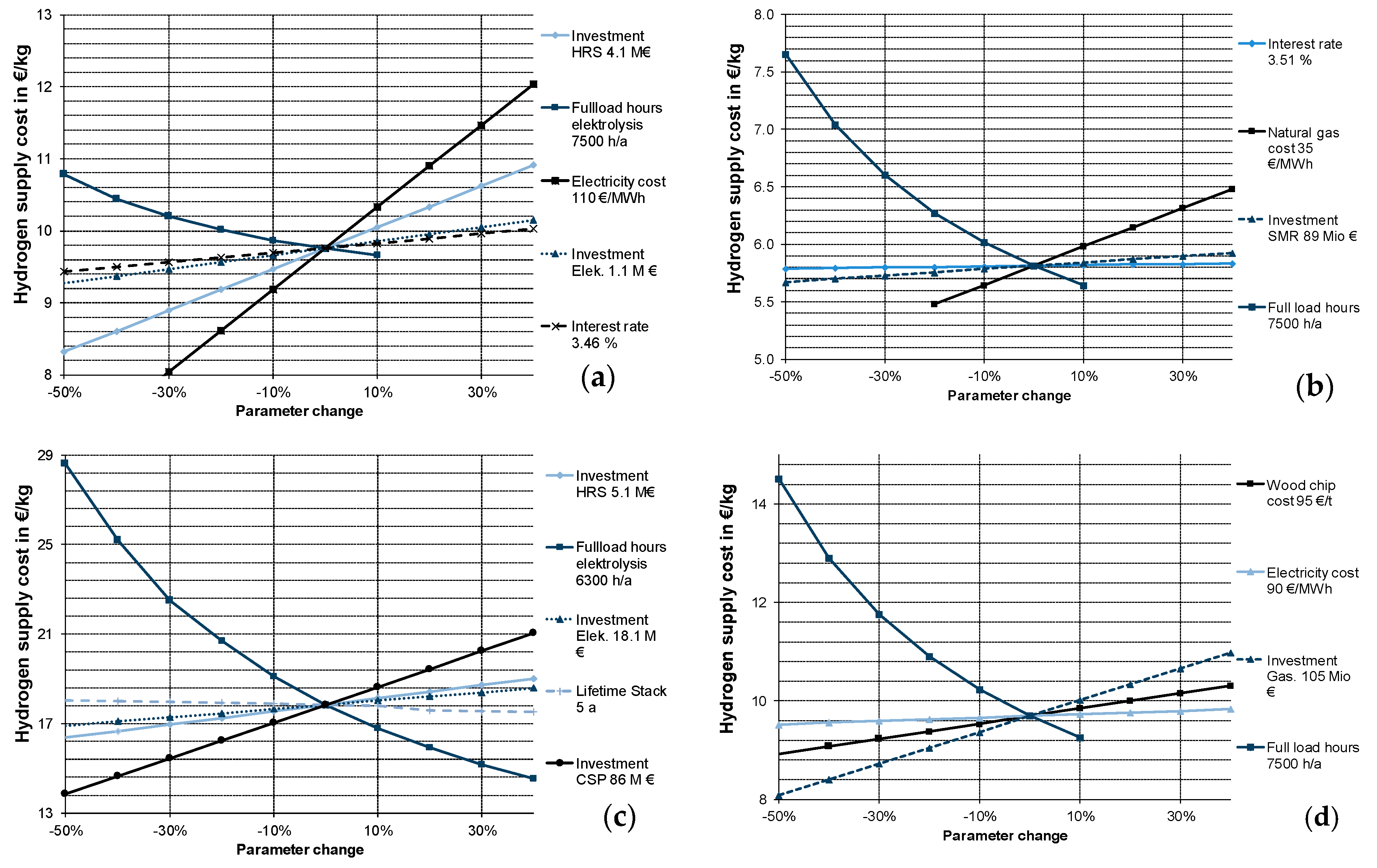
3.1.7. Sensitivity Analysis
3.2. Economic Assessment
3.2.1. Levelized Cost of Hydrogen Supply
3.2.2. Sensitivity Analysis
4. Final Considerations
- In five out of six environmental impact categories, wind powered electrolysis reaches the lowest results. However, these hydrogen supply chains have significantly higher costs than the fossil fuel and biomass-based supply chains. The differences between PEM and alkaline electrolysis are very small. If it were necessary to choose only from between these two technologies, a more detailed assessment would be necessary.
- The lowest costs can be achieved with the usage of substituted hydrogen from chemical industry, but with high impacts on climate change.
- The lowest cost of hydrogen supply from renewable sources can be achieved by steam reforming of biomethane, which would reduce the impact on Climate change by roughly 50% compared to conventional steam methane reforming (SMR). Simultaneously, high impacts regarding Acidification and Particulate matter occur.
- Hydrogen production from solar power shows, in itself, very low environmental impacts. When additionally considering, however, the long-distance transport to Germany, higher results are achieved in four out of six impact categories than from fossil fuel-based hydrogen. Furthermore, in the future, the costs of these technologies will also exceed further developed technologies by far.
- In both assessments, LCA and costs, always the biological hydrogen production showed the highest results due to a low efficiency, usage of crop grown biomass and high costs for investments.
- The two technologies used at the moment for hydrogen production—steam methane reforming (SMR) and coal gasification—stand out for their low costs. At the same time, they produce high impacts on Climate Change, and for coal gasification, also on Eutrophication.
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A
| AE | PEM | ||
|---|---|---|---|
| Capacity | kg/h | 26 | 48 |
| Life time stack | a/a | 10 | 8 |
| Full load hours | h | 7500 | 4000 |
| Operating resources | |||
| Electricity | kWh/kg H2 | 49 | 50 |
| Water | kg/kg H2 | 19 | 19 |
| Potassium hydroxide | g/kg H2 | 0.85 | - |
| Construction materials | |||
| Steel, low alloy | kg | 12,900 | 36,000 |
| Reinforcing steel | kg | - | 1600 |
| Aluminum | kg | 63 | 530 |
| Chrome | kg | 200 | - |
| Nickel | kg | 200 | - |
| Polyethylene granulate | kg | 50 | - |
| Platinum-group metals | g | - | 230 |
| Graphite | kg | - | 2.1 |
| Titan | g | - | 760 |
| Nafion | kg | - | 5.8 |
| Solvent | kg | - | 1.1 |
| Cast iron | kg | - | 260 |
| Copper | g | - | 650 |
| Silicon | kg | - | 1.3 |
| Cost | |||
| Investment | M€ | 1.0 | 2.5 |
| Cost stack replacement | M€/replacement | 0.35 | 0.98 |
| SMR | CG | WG | SUB | ||
|---|---|---|---|---|---|
| Capacity | kg/h | 5000 | 10,000 | 610 | 450 |
| Full load hours | h/a | 7500 | 7500 | 7500 | 7500 |
| Operating resources | |||||
| Electricity | kWh/kg H2 | 0.20 | - | 3.9 | 1.6 |
| Natural gas | MJ/kg H2 | 158 | - | - | 3.0 |
| Coal | kg/kg H2 | - | 7.3 | - | - |
| Wood chips | kg/kg H2 | - | - | - | 3.0 |
| Water | kg/kg H2 | 14.4 | 5.0 | 5.9 | - |
| Triethylene glycol | g/kg H2 | - | 60 | - | - |
| Olivin | g/kg H2 | - | - | 260 | - |
| N2 liquid | g/kg H2 | - | - | 360 | - |
| Ash | g/kg H2 | - | - | 320 | - |
| Steam, credit | kg/kg H2 | 5.3 | - | - | - |
| Electricity, credit | kWh/kg H2 | - | 1.0 | - | - |
| Sulfur, credit | g/kg H2 | - | 91 | - | - |
| Construction materials | |||||
| Steel, low alloy | t | 100 | - | - | - |
| Steel, high alloy | t | 60 | - | - | - |
| Steel, no alloy | t | 240 | - | - | - |
| Zinc | t | 0.3 | - | - | - |
| Aluminum | t | 3.5 | - | - | - |
| Nickel | t | 0.3 | - | - | - |
| Copper | t | 0.1 | - | - | - |
| Concrete | t | 1,300 | - | - | - |
| Plant 1 | Units | - | 87.5 | 4.6 | 1 × 10−5 |
| Direct emissions | |||||
| CO2 | kg/kg H2 | 8.55 | 19.3 | - | 6.7 |
| CH4 | g/kg H2 | 1.82 | - | 0.1 | 2.0 |
| CO | g/kg H2 | 1.1 | - | 0.01 | 0.17 |
| NMHC | g/kg H2 | 3.4 | - | 0.0007 | 0.32 |
| NOx | g/kg H2 | 2.3 | 0.010 | 0.03 | 1.9 |
| SO2 | mg/kg H2 | 110 | - | 43 | 65 |
| PM2.5 | mg/kg H2 | 22 | 390 | 3.4 | 12 |
| H2S | mg/kg H2 | - | 5.62 | 0.0009 | - |
| Further emissions | 2 | 2 | |||
| Cost | |||||
| Investment | M€ | 89 | 480 | 105 | 0.45 |
| Capacity | kg/h | 60 |
| Full load hours dark fermentation | h/a | 7500 |
| Full load hours photo fermentation | h/a | 3300 |
| Operating resources | ||
| Feedstock mix | kg/kg H2 | 262 |
| Electricity | kWh/kg H2 | 1.8 |
| Heat | kWh/kg H2 | 7.3 |
| Potassium hydroxide | kg/kg H2 | 1.5 |
| Dipotassium phosphate | kg/kg H2 | 0.30 |
| Water | kg/kg H2 | 240 |
| Waste water | m3/h | 1.3 |
| Construction materials | ||
| Plant 1 | Units | 1.0 |
| Cost | ||
| Investment photobioreactor | M€ | 96 |
| Investment balance of plant | M€ | 26 |
| S-I | HT | ||
|---|---|---|---|
| Capacity | kg/h | 414 | 208 |
| Full load hours | h/a | 6300 | 6300 |
| Maximum temperature | °C | 850 | 800 |
| Operating resources | |||
| Electricity | kWh/kg H2 | 20 | 36 |
| Heat | kWh/kg H2 | 78 | 2.5 |
| Water | kg/kg H2 | 9 | 9.9 |
| Construction materials | |||
| Sulfuric acid | t | 6.5 | - |
| Iodine | t | 120 | - |
| Helium | t | 2 | - |
| Inconel | t | 40 | - |
| Steel, low alloy | t | - | 6.7 |
| Nickel oxide | kg | - | 910 |
| Yttrium-stabilized zirconium oxide | kg | - | 800 |
| Lanthanum strontium manganite | kg | - | 160 |
| Solvent | t | - | 3.0 |
| Balance of plant 1 | t | 1000 | 50 |
| Direct emissions 2 | |||
| Sulfuric acid | kg | 320 | - |
| Iodine | kg | 5300 | - |
| SO2 | kg | 210 | - |
| Cost | |||
| Investment hydrogen production | M€ | 150 | 10 |
| Investment solar tower power plant | M€ | 220 | 89 |
| Fueling station | ||
| Capacity | kg/d | 700 |
| Capacity utilization | % | 75 |
| Number storage tank bundles | 1 | |
| Operating resources | ||
| Electricity (onsite H2 production, LOHC) 1 | kWh/kg H2 | 3.2 |
| Electricity (delivered H2) 1 | kWh/kg H2 | 1.0 |
| Construction materials | ||
| Gaseous fueling station 2 | unit | 0.8 |
| Cost | ||
| Investment | M€ | 4.3 |
| Storage tank bundle | M€/bundle | 0.25 |
| High pressure transport | ||
| Capacity per transport | kg | 1100 |
| Pressure level | bar | 500 |
| Operating resources | ||
| Electricity 3 | kWh/kg H2 | 2.2 |
| Capacity dehydrogenation | kW | 970 |
| Full load hours (de)hydrogenation | h | 6570 1 |
| Capacity hydrogenation | MW | 5.8/11.6 2 |
| Operating resources | ||
| Heat dehydrogenation 3 | kWh/kg H2 | 10 |
| Electricity for dehydrogenation | kWh/kg H2 | 1.6 |
| Cost | ||
| Investment dehydrogenation | M€ | 0.78 |
| Investment hydrogenation | M€ | 429/648 2 |
| Cost LOHC | €/kg | 4.0 |
| Demand LOHC | t/fueling station | 40 |
| Transport | ||
| Distance ship | km | 3600 |
| Distance truck (Germany + Algeria) | km | 465 + 650 |
| Storing capacity H2 in LOHC | % (weight) | 6.2 |
| Production LOHC | ||
| Heat | kWh/kg LOHC | 0.37 |
| Electricity | kWh/kg LOHC | 0.06 |
| Chlorine | kg/kg LOHC | 0.52 |
| Toluene | kg/kg LOHC | 1.12 |
| Disposal hydrochloric acid | kg/kg LOHC | 0.49 |
References
- Global Market Insights. Fuel Cell Electric Vehicle Market Size By Vehicle (PCV, LCV, HCV, E-Bikes, Forklifts), By Distance (Short Range, Long Range), Industry Analysis Report, Regional Outlook (U.S., Canada, Germany, UK, France, Italy, Spain, Sweden, Norway, Netherlands, China, Japan, India, Korea, Brazil, Mexico, Argentina, Saudi Arabia, UAE, South Africa), Growth Potential, Price Trends, Competitive Market Share & Forecast, 2017–2024; Global Market Insights: Selbyville, DE, USA, 2018. [Google Scholar]
- Da Silva Veras, T.; Mozer, T.S.; da Costa Rubim Messeder dos Santos, D.; da Silva César, A. Hydrogen: Trends, production and characterization of the main process worldwide. Int. J. Hydrogen Energy 2017, 42, 2018–2033. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Wahid, M.A. Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development. Renew. Sustain. Energy Rev. 2016, 57, 850–866. [Google Scholar] [CrossRef]
- Sinigaglia, T.; Lewiski, F.; Santos Martins, M.E.; Mairesse Siluk, J.C. Production, storage, fuel stations of hydrogen and its utilization in automotive applications-a review. Int. J. Hydrogen Energy 2017, 42, 24597–24611. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Review and evaluation of hydrogen production methods for better sustainability. Int. J. Hydrogen Energy 2015, 40, 11094–11111. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Gnanapragasam, N.V.; Reddy, B.V.; Rosen, M.A. A Methodology for Assessing the Sustainability of Hydrogen Production from Solid Fuels. Sustainability 2010, 2, 1472–1491. [Google Scholar] [CrossRef]
- Kuckshinrichs, W.; Ketelaer, T.; Koj, J.C. Economic Analysis of Improved Alkaline Water Electrolysis. Front. Energy Res. 2017, 5, 1. [Google Scholar] [CrossRef]
- Shaner, M.R.; Atwater, H.A.; Lewis, N.S.; McFarland, E.W. A comparative technoeconomic analysis of renewable hydrogen production using solar energy. Energy Environ. Sci. 2016, 9, 2354–2371. [Google Scholar] [CrossRef]
- Han, W.; Liu, Z.; Fang, J.; Huang, J.; Zhao, H.; Li, Y. Techno-economic analysis of dark fermentative hydrogen production from molasses in a continuous mixed immobilized sludge reactor. J. Clean. Prod. 2016, 127, 567–572. [Google Scholar] [CrossRef]
- Bhandari, R.; Trudewind, C.A.; Zapp, P. Life cycle assessment of hydrogen production via electrolysis—A review. J. Clean. Prod. 2014, 85, 151–163. [Google Scholar] [CrossRef]
- Valente, A.; Iribarren, D.; Dufour, J. Harmonised life-cycle global warming impact of renewable hydrogen. J. Clean. Prod. 2017, 149, 762–772. [Google Scholar] [CrossRef]
- Mehmeti, A.; Angelis-Dimakis, A.; Arampatzis, G.; McPhail, S.; Ulgiati, S. Life Cycle Assessment and Water Footprint of Hydrogen Production Methods: From Conventional to Emerging Technologies. Environments 2018, 5, 24. [Google Scholar] [CrossRef]
- Wulf, C.; Kaltschmitt, M. Life Cycle Assessment of hydrogen supply chain with special attention on hydrogen refuelling stations. Int. J. Hydrogen Energy 2012, 37, 16711–16721. [Google Scholar] [CrossRef]
- Ozawa, A.; Inoue, M.; Kitagawa, N.; Muramatsu, R.; Anzai, Y.; Genchi, Y.; Kudoh, Y. Assessing Uncertainties of Well-To-Tank Greenhouse Gas Emissions from Hydrogen Supply Chains. Sustainability 2017, 9, 1101. [Google Scholar] [CrossRef]
- Burkhardt, J.; Patyk, A.; Tanguy, P.; Retzke, C. Hydrogen mobility from wind energy—A Life Cycle Assessment focusing on the fuel supply. Appl. Energy 2016, 181, 54–64. [Google Scholar] [CrossRef]
- ISO 14040:2006 Environmental Management—Life Cycle Assessment—Principles and Framework; German and English Version; Beuth Verlag: Berlin, Germany, 2006.
- ISO 14044:2006 Environmental Management—Life Cycle Assessment—Requirements and Guidelines; German and English Version; Beuth Verlag: Berlin, Germany, 2006.
- VGB Powertech. Levelised Cost of Electricity; VGB PowerTech e.V.: Essen, Germany, 2015. [Google Scholar]
- Deutsche Bundesbank. Time Series BBK01.WZ3449: Term Structure of Interest Rates on Listed Federal securities (Method by Svensson)/Residual Maturity of 20.0 years/End of Month. Available online: http://www.bundesbank.de/Navigation/EN/Statistics/Time_series_databases/Macro_economic_time_series/its_details_value_node.html?listId=www_skms_it03a&tsId=BBK01.WZ3449 (accessed on 15 September 2017).
- Statista. Inflation Rate in Germany from 1992 to 2015 (Change of CPI Compared to the Previous Year). Available online: https://www.statista.com/statistics/262859/inflation-rate-in-germany-changes-of-the-cpi-compared-to-the-previous-year/ (accessed on 15 September 2017).
- Verband der Chemischen Industrie (VCI). Chemiewirtschaft in Zahlen; Verband der Chemischen Industrie: Frankfurt am Main, Germany, 2017. [Google Scholar]
- Schoots, K.; Ferioli, F.; Kramer, G.; Vanderzwaan, B. Learning curves for hydrogen production technology: An assessment of observed cost reductions. Int. J. Hydrogen Energy 2008, 33, 2630–2645. [Google Scholar] [CrossRef]
- EU (European Union); G. Technology Readiness Levels (TRL). Horizon 2020—Work Programme 2014–2015: General Annexes; European Commission: Brussels, Belgium, 2014. [Google Scholar]
- Reuß, M.; Grube, T.; Robinius, M.; Wasserscheid, P.; Stolten, D. Seasonal storage and alternative carriers: A flexible hydrogen supply chain model. Appl. Energy 2017, 200, 290–302. [Google Scholar] [CrossRef]
- Nitsch, J.; Gerhardt, J.; Wenzel, B. Langfristszenarien und Strategien für den Ausbau der Erneuerbaren Energien in Deutschland bei Berücksichtigung der Entwicklung in Europa und Global: Schlussbericht; Deutsches Zentrum für Luft- und Raumfahrt; Fraunhofer IWES; Ingenieurbüro für neue Energien: Teltow, Germany, 2012. [Google Scholar]
- Capros, P.; de Vita, A.; Tasios, N.; Siskos, P.; Kannavou, M.; Petropoulos, A.; Evangeloppoulau, S.; Zampara, M.; Papadopoulos, D.; Nakos, C.; et al. EU Reference Scenario 2016: Energy, Transport and GHG Emissions Trends to 2050; European Union: Luxembourg, 2016. [Google Scholar]
- European Commission-Joint Research Centre (EU-JRC). Recommendations for Life Cycle Impact Assessment in the European Context—Based on Existing Environmental Impact Assessment Models and Factors; European Commission-Joint Research Centre; Institute for Environment and Sustainability: Luxembourg, 2011. [Google Scholar]
- Hauschild, M.Z.; Goedkoop, M.; Guinée, J.; Heijungs, R.; Huijbregts, M.; Jolliet, O.; Margni, M.; De Schryver, A.; Humbert, S.; Laurent, A.; et al. Identifying best existing practice for characterization modeling in life cycle impact assessment. Int. J. Life Cycle Assess. 2013, 18, 683–697. [Google Scholar] [CrossRef]
- Guinée, J.B.; Gorrée, M.; Heijungs, R.; Huppes, G.; Kleijn, R.; Koning, A.D.; van Oers, L.; Wegener Sleeswijk, A.; Suh, S.; Haes, U.D.; et al. LCA—An Operational Guide to the ISO-Standards: Part 2b: Operational Annex; Universiteit Leiden Institute of Environmental Sciences: Leiden, The Netherlands, 2001; p. 276. [Google Scholar]
- Humbert, S.; Marshall, J.D.; Shaked, S.; Spadaro, J.V.; Nishioka, Y.; Preiss, P.; McKone, T.E.; Horvath, A.; Jolliet, O. Intake Fraction for Particulate Matter: Recommendations for Life Cycle Impact Assessment. Environ. Sci. Technol. 2011, 45, 4808–4816. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, R.K.; Bachmann, T.M.; Gold, L.S.; Huijbregts, M.A.J.; Jolliet, O.; Juraske, R.; Koehler, A.; Larsen, H.F.; MacLeod, M.; Margni, M.; et al. USEtox—The UNEP-SETAC toxicity model: Recommended characterisation factors for human toxicity and freshwater ecotoxicity in life cycle impact assessment. Int. J. Life Cycle Assess. 2008, 13, 532–546. [Google Scholar] [CrossRef]
- Swiss Centre for Live Cycle Inventories. Ecoinvent Database Version 3.1; Swiss Centre for Live Cycle Inventories: Zürich, Switzerland, 2014. [Google Scholar]
- Wulf, C.; Linssen, J.; Zapp, P. Power-to-Gas—Concepts, Demonstration and Prospects. In Hydrogen Supply Chains: Design, Deployment and Operation; Azzaro-Pantel, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; in press. [Google Scholar]
- Müller-Langer, F.; Tzimas, E.; Kaltschmitt, M.; Peteves, S.D. Techno-economic assessment of hydrogen production processes for the hydrogen economy for the short and medium term. Int. J. Hydrogen Energy 2007, 32, 3797–3810. [Google Scholar] [CrossRef]
- Doctor, R.D.; Molburg, J.C.; Brockmeier, N.F.; Manfredo, L.; Gorokhov, V.; Ramezan, M.; Stiegel, G.J. Life-Cycle Analysis of a Shell Gasification-Based Multi-Product System with CO2 Recovery. In Proceedings of the First National Conference on Carbon Sequestration, Washington, DC, USA, 15–17 May 2001. [Google Scholar]
- Scheftelowitz, M.; Schicketanz, S.; Reinicke, F.; Beil, M. Stromerzeugung aus Biomasse: Zwischenbericht; Deutsches Biomasseforschungszentrum (DBFZ): Leipzig, Germany, 2013. [Google Scholar]
- Kaltschmitt, M.; Weinberg, J.; Stegelmeier, M.; Bohnenschäfer, W.; Gansler, J.; Ebert, M. Teilbericht B: Analyse der Erneuerbaren Energien am Wärmemarkt. In Ökologische und Ökonomische Optimierung des Wärmemarktes; Schultz, R., Hochi, J., Personn, H., Eds.; Ibidem-Verlag: Stuttgart, Germany, 2012; pp. 109–176. [Google Scholar]
- Wurster, R.; Schaloske, M. Wasserstoff und Brennstoffzellen in der Energie- und Mobilitätswende. DWV-Mitteilungen 2015, 19–2, 3–4. [Google Scholar]
- International Maritime Organization (IMO). International Convention for the Prevention of Pollution from Ships (MARPOL): Annex VI Prevention of Air Pollution from Ships; International Maritime Organization IMO: London, UK, 2005. [Google Scholar]
- Wulf, C.; Zapp, P. Assessment of system variations for hydrogen transport by Liquid Organic Hydrogen Carriers. Int. J. Hydrogen Energy 2018. [Google Scholar] [CrossRef]
- Kiesel, F. Bruttostromerzeugung in Deutschland von 1990 bis 2012 nach Energieträgern; AG Energiebilanzen e.V.: Berlin, Germany, 2013. [Google Scholar]
- Müller-Langer, F. Analyse und Bewertung Ausgewählter Zukünftiger Biokraftstoffoptionen auf der Basis fester Biomasse. Ph.D. Thesis, Technische Universität Hamburg, Hamburg, Germany, 2011. [Google Scholar]
- DESTATIS. Imports of Hard Coal up 15.2% in 2013—Press Release 141. Available online: https://www.destatis.de/DE/PresseService/Presse/Pressemitteilungen/2014/04/PD14_141_51.html (accessed on 4 April 2018).
- Belau, T. Energiepflanzen: Daten für die Planung des Energiepflanzenanbaus, 2nd ed.; Kuratorium für Technik und Bauwesen in der Landwirtschaft: Darmstadt, Germany, 2012. [Google Scholar]
- Kaltschmitt, M.; Schlüter, M.; Schulz, D.; Skiba, M.; Özdirik, B. Stromerzeugung aus Windenergie. In Erneuerbare Energien, 5th ed.; Kaltschmitt, M., Streicher, W., Wiese, A., Eds.; Springer Vieweg: Berlin, Germany, 2013; pp. 453–554. [Google Scholar]
- Statista. Industriestrompreise in Deutschland in den Jahren 2000 bis 2016 (in Euro-Cent pro Kilowattstunde). Available online: https://de.statista.com/statistik/daten/studie/155964/umfrage/entwicklung-der-industriestrompreise-in-deutschland-seit-1995/ (accessed on 4 April 2018).
- Heimann, M. Entgelte der Amprion GmbH gültig ab 01.01.2015; Amprion GmbH: Dortmund, Germany, 2014. [Google Scholar]
- DESTATIS. Preise: Daten zur Energiepreisentwicklung; Statistisches Bundesamt: Wiesbaden, Germany, 2014. [Google Scholar]
- C.A.R.M.E.N. Energieholz-Index Grafiken. Available online: http://www.carmen-ev.de/infothek/preisindizes/hackschnitzel/graphiken (accessed on 4 April 2018).
- Deutsche Energie Agentur (DENA). Branchenkompass: Biomethan in KWK; Deutsche Energie Agentur DENA: Berlin, Germany, 2013. [Google Scholar]
- Hamburg Wasser. Gebühren, Abgaben und Preise. Available online: http://www.hamburgwasser.de/tarife-und-gebuehren.html (accessed on 18 August 2017).
- Alibaba.com. Available online: http://www.alibaba.com/ (accessed on 4 April 2018).
- Bundesverband der Energie- und Wasserwirtschaft (BDEW). BDEW-Strompreisanalyse Mai 2016: Haushalte und Industrie; Bundesverband der Energie- und Wasserwirtschaft: Berlin, Germany, 2016. [Google Scholar]
- Ritschel, W.; Schiffer, H.-W. Weltmarkt für Steinkohle; RWE Power: Essen, Germany, 2007. [Google Scholar]
- Schlesinger, M.; Hofer, P.; Kemmler, A.; Kirchner, A.; Koziel, S.; Ley, A.; Piégsa, A.; Seefeldt, S.; Weinert, K.; Lindenberger, D.; et al. Entwicklung der Energiemärkte—Energiereferenzprognose; EWI: Basel, Switzerland; GWS: Köln, Germany; Prognos: Osnabrück, Germany, 2014. [Google Scholar]
- Arndt, W.-H.; Döge, N.; Marker, S. Elektrifizierungspotential Kommerzieller Kraftfahrzeug-Flotten im Wirtschaftsverkehr als Dezentrale Energie-Ressource in Städtischen Verteilnetzen—komDRIVE; Universitätsverlag der TU Berlin: Berlin, Germany, 2017. [Google Scholar]
- Helmholtz Association. Helmholtz Alliance ENERGY-TRANS. Available online: https://www.energy-trans.de/english/24.php (accessed on 8 April 2018).
- Bernath, C.; Bossmann, T.; Deac, G.; Elsland, R.; Fleiter, T.; Kühn, A.; Pfluger, B.; Ragwitz, M.; Rehfeldt, M.; Sensfuß, F.; et al. Langfristszenarien für die Transformation des Energiesystems in Deutschland—Modul 3: Referenzszenario und Basisszenario Studie im Auftrag des Bundesministeriums für Wirtschaft und Energie; Fraunhofer ISI: Karlsruhe, Germany; Consentec GmbH: Aachen, Germany; IFEU: Heidelberg, Germany, 2017. [Google Scholar]
- Pehnt, M. Ganzheitliche Bilanzierung von Brennstoffzellen in der Energie- und Verkehrstechnik; VDI-Verlag: Düsseldorf, Germany, 2002. [Google Scholar]
- Millet, P.; Grigoriev, S. Water Electrolysis Technologies. In Renewable Hydrogen Technologies; Gandia, L.M., Arzamedi, G., Dieguez, P.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 19–41. [Google Scholar]
- Smolinka, T.; Günther, M.; Garche, J. Stand und Entwicklungspotenzial der Wasserelektrolyse zur Herstellung von Wasserstoff aus Regenerativen Energien: NOW-Studie; Fraunhofer ISE, FCBAT: Freiburg, Geramny, 2011. [Google Scholar]
- Melaina, M.; Penev, M. Hydrogen Station Cost Estimates: Comparing Hydrogen Station Cost Calculator Results with Other Recent Estimates; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2013. [Google Scholar]
- Wurster, R. HyWays—An Integrated Project to Develop the European Hydrogen Energy Roadmap—Deliverable 1.4: Technology Fact Sheets; European Commission: Brussels, Belgium, 2007. [Google Scholar]
- Qin, D.; Brooker, P.; Srinivasan, S. Hydrogen Fueling Stations Infrastructure; University of Central Florida: Cocoa, FL, USA, 2014. [Google Scholar]
- Geitmann, S. USA Investieren in Wasserstoff-Infrastruktur: HZwei Blog. Available online: http://www.hzwei.info/blog/2014/10/20/usa-investieren-in-wasserstoff-infrastruktur/ (accessed on 8 March 2018).
- Hydrogenics. HySTAT® Hydrogen Generators; Hydrogenics: Oevel, Belgium, 2011. [Google Scholar]
- Ernst and Young; Ludwig-Bölkow Systemtechnik; Becker Büttner Held. Fahrplan zur Realisierung einer Windwasserstoff-Wirtschaft in der Region Unterelbe: Kurzdarstellung; ChemCoast e. V.: Laatzen, Germany, 2013. [Google Scholar]
- Bernhart, W.; Riederle, S.; Yoon, M. Fuel Cells: A Realistic Alternative for Zero Emission? Roland Berger Strategy Consultants: Stuttgart, Germany, 2013. [Google Scholar]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Proton on Site. C Series: Hydrogen Generation Systems; Proton on Site: Wallingford, CT, USA, 2014. [Google Scholar]
- Krewitt, W.; Pehnt, M.; Fischedick, M.; Temming, H.V. Brennstoffzellen in der Kraft-Wärme-Kopplung: Ökobilanzen, Szenarien, Marktpotenziale; Erich Schmidt Verlag GmbH & Co.: Berlin, Germany, 2004. [Google Scholar]
- Katikaneni, S.P.; Al-Muhaish, F.; Harale, A.; Pham, T.V. On-site hydrogen production from transportation fuels: An overview and techno-economic assessment. Int. J. Hydrogen Energy 2014, 39, 4331–4350. [Google Scholar] [CrossRef]
- Simbeck, D.; Chang, E. Hydrogen Supply: Cost Estimate for Hydrogen Pathways: Scoping Analysis; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2002. [Google Scholar]
- Bressan, L.; Davis, C. Driving down costs in hydrogen production. Process. Shale Feedstocks 2013, 18, 23–27. [Google Scholar]
- Kabelac, S.; Gnielinski, V.; Kind, M.; Martin, H.; Mewes, D.; Schaber, K.S.P. VDI-Wärmeatlas: [Berechnungsunterlagen für Druckverlust, Wärme- und Stoffübergang]; Zehnte, B., Erweiterte, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- McKinsey. A Portfolio of Power-Trains for Europe: A Fact-Based Analysis: The Role of Battery Electric Vehicles, Plug-in Hybrids and Fuel Cell Electric Vehicles; McKinsey: Düsseldorf, Germany, 2011. [Google Scholar]
- Kreutz, T.; Williams, R.; Consonni, S.; Chiesa, P. Co-production of hydrogen, electricity and CO from coal with commercially ready technology. Part B: Economic analysis. Int. J. Hydrogen Energy 2005, 30, 769–784. [Google Scholar] [CrossRef]
- Gellert, S. Thermochemische Herstellung von Wasserstoff aus Biomasse unter Besonderer Berücksichtigung der Rohgasreformierung. Ph.D. Thesis, Hamburg University of Technology, Hamburg, Germany, 2013. [Google Scholar]
- Gunnarsson, I. GoBiGas-Projektet Omvandling av Skogsavfall till Färdigt Fordonsbränsle; Göteborg Energi: Göteborg, Sweden, 2014. [Google Scholar]
- HaasEngineering. Gastrocknungsanlage; HaasEngineering: Gundelfingen, Germany, 2009. [Google Scholar]
- Knörr, W.; Schacht, A.; Schmidt, P.R.; Weindorf, W.; Michaelis, J.; Wietschel, M.; Merten, F.; Viebahn, P.; Holdi, H. Überleitung der Ergebnisse aus GermanHy in das Emissionsberechnungsmodell TREMOD: Schlussbericht Teil II; Nationale Organisation Wasserstoff- und Brennstoffzellentechnologie NOW: Berlin, Germany, 2013. [Google Scholar]
- Claasen, P.A.M. HYVOLUTION: Non-Thermal Production of Pure Hydrogen from Biomass; Dienst Landbouwkundig Onderzoek, Food & Biobased Research: Wageningen, The Netherlands, 2011. [Google Scholar]
- Ochs, D.; Wukovits, W.; Ahrer, W. Life cycle inventory analysis of biological hydrogen production by thermophilic and photo fermentation of potato steam peels (PSP). J. Clean. Prod. 2010, 18, 88–94. [Google Scholar] [CrossRef]
- Djomo, S.N.; Blumberga, D. Comparative Life Cycle Assessment of three biohydrogen pathways. Bioresour. Technol. 2011, 102, 2684–2694. [Google Scholar] [CrossRef] [PubMed]
- Liberatore, R.; Lanchi, M.; Giaconia, A.; Tarquini, P. Energy and economic assessment of an industrial plant for the hydrogen production by water-splitting through the sulfur-iodine thermochemical cycle powered by concentrated solar energy. Int. J. Hydrogen Energy 2012, 37, 9550–9565. [Google Scholar] [CrossRef]
- Lattin, W.; Utgikar, V. Global warming potential of the sulfur–iodine process using Life Cycle Assessment methodology. Int. J. Hydrogen Energy 2009, 34, 737–744. [Google Scholar] [CrossRef]
- Kaltschmitt, M.; Weinrebe, G.; Wulf, C. Solarthermische Stromerzeugung. In Erneuerbare Energien: Systemtechnik, Wirtschaftlichkeit und Umweltaspekte, 5th ed.; Kaltschmitt, M., Streicher, W., Wiese, A., Eds.; Springer Vieweg: Berlin, Germany, 2013; pp. 263–352. [Google Scholar]
- Leybros, J.; Gilardi, T.; Saturnin, A.; Mansilla, C.; Carles, P. Plant sizing and evaluation of hydrogen production costs from advanced processes coupled to a nuclear heat source. Part I: Sulphur–iodine cycle. Int. J. Hydrogen Energy 2010, 35, 1008–1018. [Google Scholar] [CrossRef]
- Giraldi, M.R.; François, J.-L.; Castro-Uriegas, D. Life cycle greenhouse gases emission analysis of hydrogen production from S–I thermochemical process coupled to a high temperature nuclear reactor. Int. J. Hydrogen Energy 2012, 37, 13933–13942. [Google Scholar] [CrossRef]
- Patyk, A.; Bachmann, T.M.; Brisse, A. Life Cycle Assessment of H2 generation with high temperature electrolysis. Int. J. Hydrogen Energy 2013, 38, 3865–3880. [Google Scholar] [CrossRef]
- Mathiesen, B.V.; Ridjan, I.; Connolly, D.; Nielsen, M.P.; Vang Hendriksen, P.; Bjerg Mogensen, M.; Højgaard Jensen, S.; Dalgaard Ebbesen, S. Technology Data for High Temperature Solid Oxide Electrolyser Cells, Alkali and PEM Electrolysers; Department of Development and Planning, Aalborg University: Aalborg, Denmark, 2013. [Google Scholar]
- Harvego, E.A.; McKellar, M.G.; Sohal, M.S.; O’Brien, J.E.; Herring, J.S. Economic Analysis of the Reference Design for a Nuclear-Driven High-Temperature-Electrolysis Hydrogen Production Plant; Idaho National Laboratory (INL): Idaho Falls, ID, USA, 2008. [Google Scholar]
- Guan, J.; Minh, N.; Ramamurthi, B.; Ruud, J.; Hong, J.-K.; Riley, P.; Weng, D. High Performance Flexible Reversible Solid Oxide Fuel Cell; GE Global Research Center: Torrance, CA, USA, 2004. [Google Scholar]
- The Linde Group; Daimler AG. Linde und Daimler bauen. DWV-Mitteilungen 2014, 18–2, 20. [Google Scholar]
- Hampel, B.; Bauer, S.; Heublein, N.; Hirsch, C.; Sattelmayer, T. Feasibility Study on Dehydrogenation of LOHC Using Excess Exhaust Heat From a Hydrogen Fueled Micro Gas Turbine. In Proceedings of the ASME Turbo Expo 2015: Turbine Technical Conference and Exposition, Monteral, QC, Canada, 15–19 June 2015. [Google Scholar]
- Teichmann, D. Konzeption und Bewertung einer Nachhaltigen Energieversorgung auf Basis Flüssiger Wasserstoffträger (LOHC); Friedrich-Alexander-Universität, Erlangen-Nürnberg: Erlangen, Germany, 2015. [Google Scholar]
- Brückner, N.; Obesser, K.; Bösmann, A.; Teichmann, D.; Arlt, W.; Dungs, J.; Wasserscheid, P. Evaluation of Industrially Applied Heat-Transfer Fluids as Liquid Organic Hydrogen Carrier Systems. ChemSusChem 2014, 7, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Adametz, P.; Müller, K.; Lechner, R.; Müller, S.; Brautsch, M.; Arlt, W. Energy and Carbon Foot Print Analysis of the Production of a Liquid Organic Hydrogen Carrier. In Proceedings of the AIChE Annual Meeting, Salt Lake City, UT, USA, 8–13 November 2015. [Google Scholar]




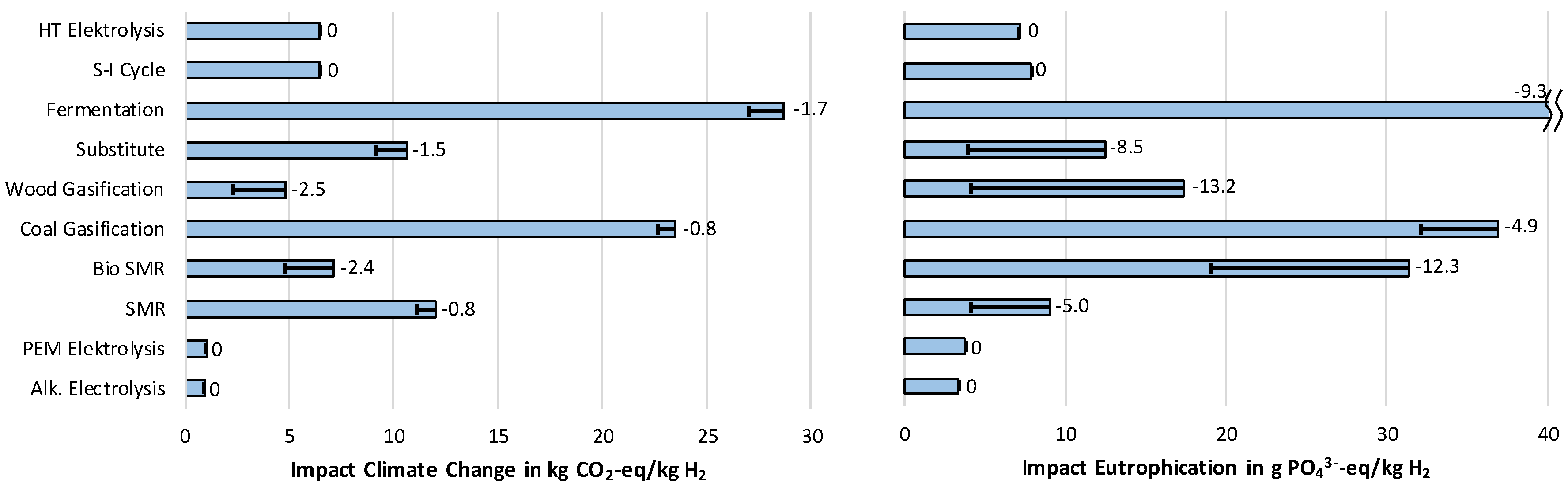
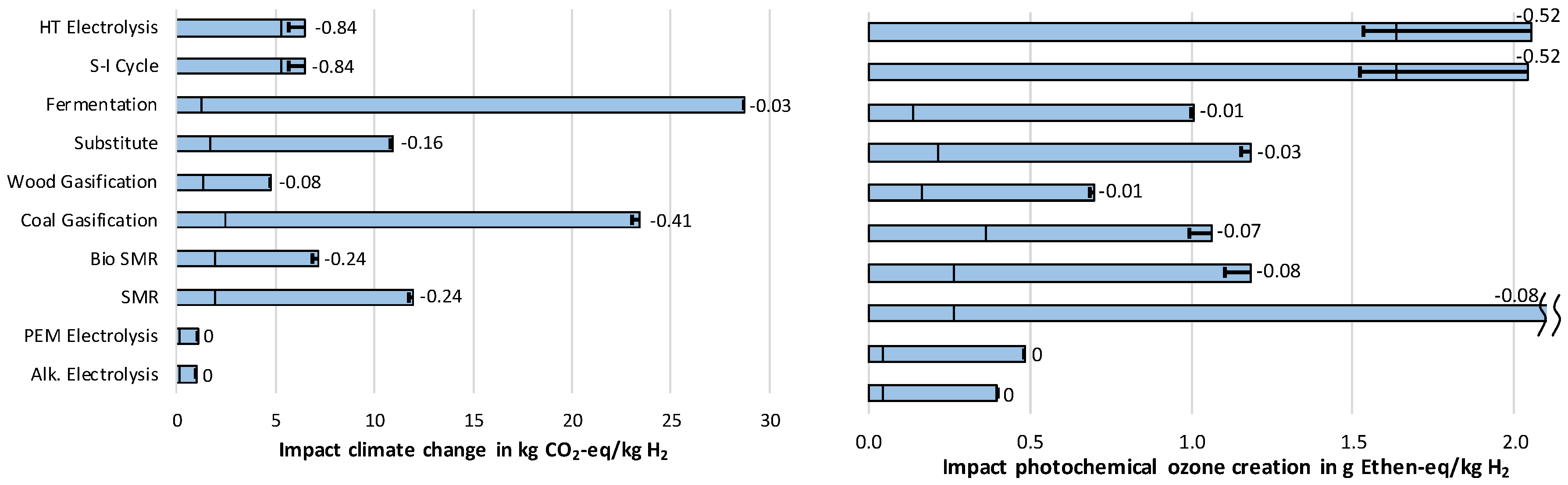

| Technology | Production Capacity (kg/h) | Number Plants | Transport Distance (km) |
|---|---|---|---|
| Steam reforming | 5000 | 5 | 150 |
| Coal gasification | 10,000 | 1 | 250 |
| Wood gasification | 610 | 43 | 50 |
| Combined fermentation | 60 | 430 | 20 |
| Substitution | 450 | 58 | 100 |
| Lignite | % | 25.0 |
| Hard coal | % | 18.0 |
| Solid biomass | % | 1.9 |
| Biogas | % | 4.5 |
| Hydropower | % | 3.6 |
| Photovoltaics | % | 4.4 |
| Wind power | % | 8.4 |
| Natural gas | % | 12.4 |
| Nuclear power | % | 15.7 |
| Oil | % | 1.1 |
| Energy | ||
| Electricity grid mix Germany | ct€/kWh | 8.95 |
| Electricity from wind power 1 | ct€/kWh | 10.95 |
| “Surplus” wind power electricity 2 | ct€/kWh | 9.18 |
| Natural gas | ct€/kWh | 3.47 |
| Hard coal | €/t | 105 |
| Forest chips | €/t | 95.0 |
| Digestible biomass | €/t | 17.2 |
| Biomethane | ct€/kWh | 7.0 |
| Auxiliary supplies | ||
| Water/sewage water | €/m³ | 3.83 |
| Triethylene glycol | €/t | 985 |
| Potassium hydroxide | €/t | 606 |
| Olivine | €/t | 76.0 |
| Nitrogen | €/t | 183 |
| Dipotassium phosphate | €/t | 1360 |
| Waste | ||
| Ash | €/t | 60 |
| Lignite | % | 0.0 |
| Hard coal | % | 2.1 |
| Solid biomass | % | 4.4 |
| Biogas | % | 4.9 |
| Hydropower | % | 4.4 |
| Photovoltaics | % | 16.5 |
| Wind power | % | 51.8 |
| Natural gas | % | 11.5 |
| Nuclear power | % | 0.0 |
| Oil | % | 0.0 |
| Geothermal power | % | 3.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wulf, C.; Kaltschmitt, M. Hydrogen Supply Chains for Mobility—Environmental and Economic Assessment. Sustainability 2018, 10, 1699. https://doi.org/10.3390/su10061699
Wulf C, Kaltschmitt M. Hydrogen Supply Chains for Mobility—Environmental and Economic Assessment. Sustainability. 2018; 10(6):1699. https://doi.org/10.3390/su10061699
Chicago/Turabian StyleWulf, Christina, and Martin Kaltschmitt. 2018. "Hydrogen Supply Chains for Mobility—Environmental and Economic Assessment" Sustainability 10, no. 6: 1699. https://doi.org/10.3390/su10061699





