Suitability of Different Agricultural and Urban Organic Wastes as Feedstocks for the Production of Biochar—Part 2: Agronomical Evaluation as Soil Amendment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Description
2.2. Biochars Description
2.3. Soil Incubation Experiments
- S: Control soil, unfertilised.
- S + B (1%): Soil amended with biochar at 1%, expressed as dry weight (d.w.).
- S + B (2%): Soil amended with biochar at 1% (d.w.).
- S + M (1%): Soil amended with manure at 1% (d.w.).
- S + B + M (1%): Soil amended with biochar at 1% (d.w.) and manure at 1% (d.w.).
- S + F (1%): Soil fertilised with DAP at 0.2 gN kg−1 soil, the same amount of N provided by 1% M.
- S + B + F (1%): Soil amended with biochar at 1% (d.w.) and DAP at 0.2 gN kg−1 soil.
2.4. Pot Trials
2.5. Chemical Analyses
2.6. Calculations and Statistical Analyses
3. Results and Discussion
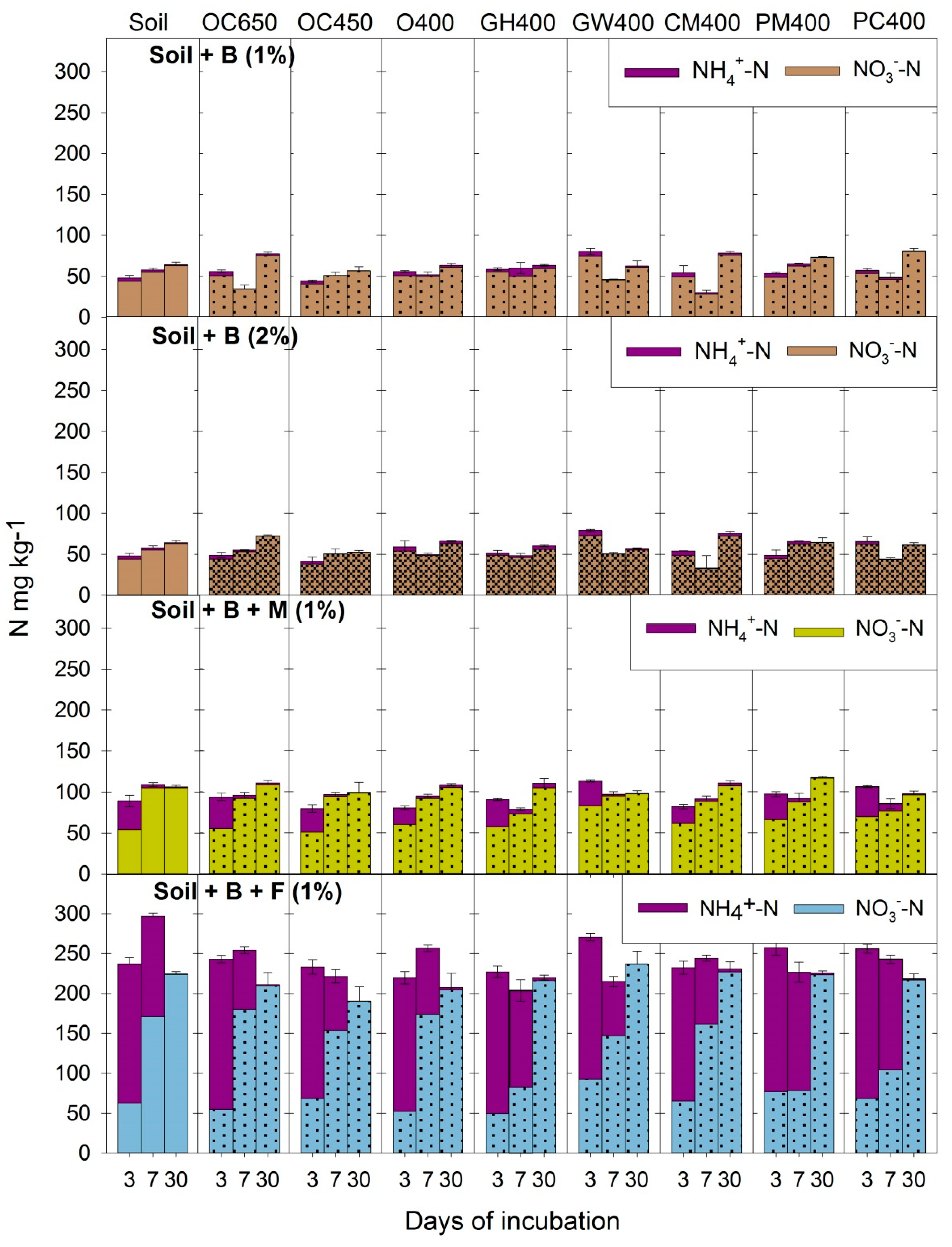
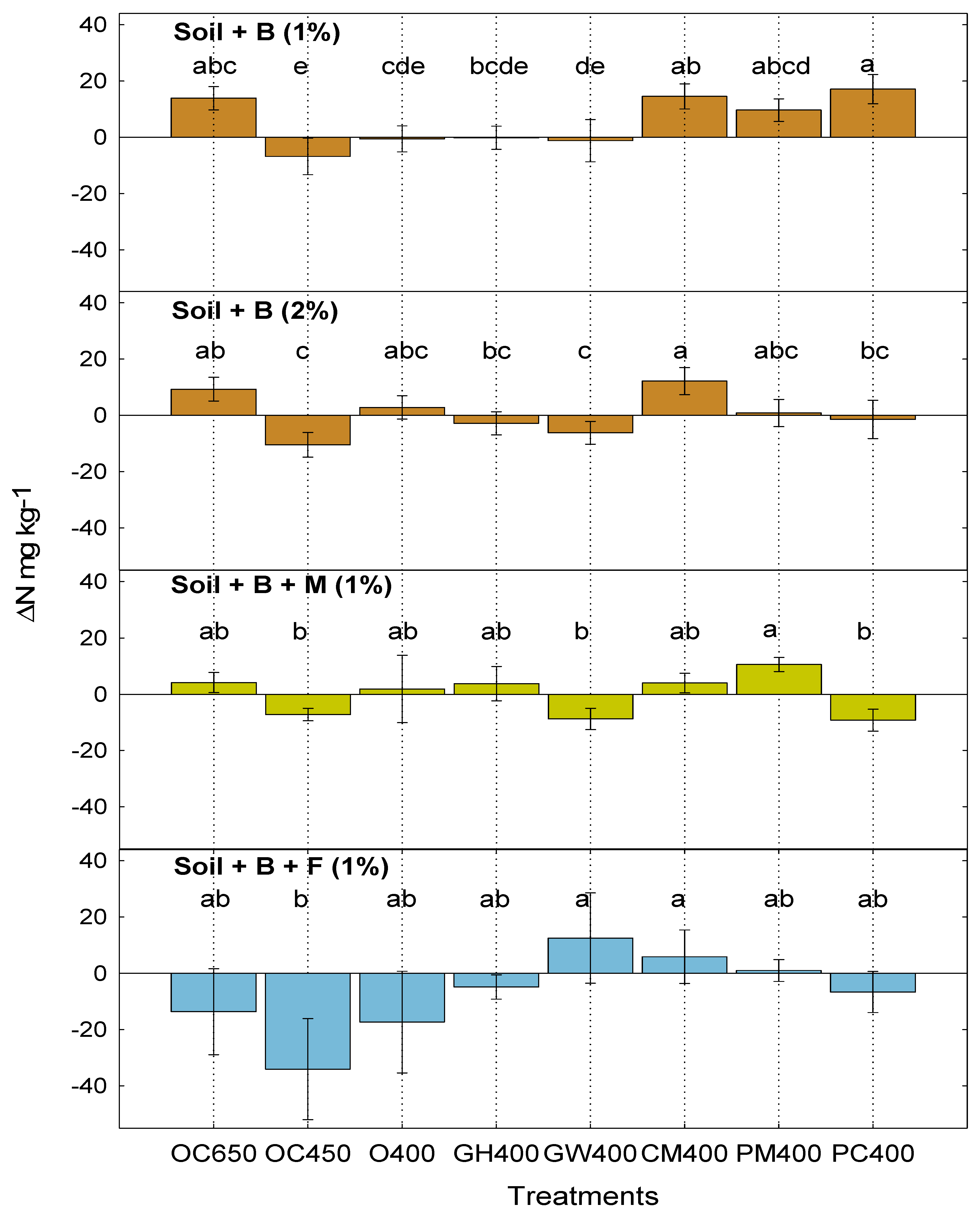
3.1. Dynamics of N in Soil: Ammonification and Nitrification Processes
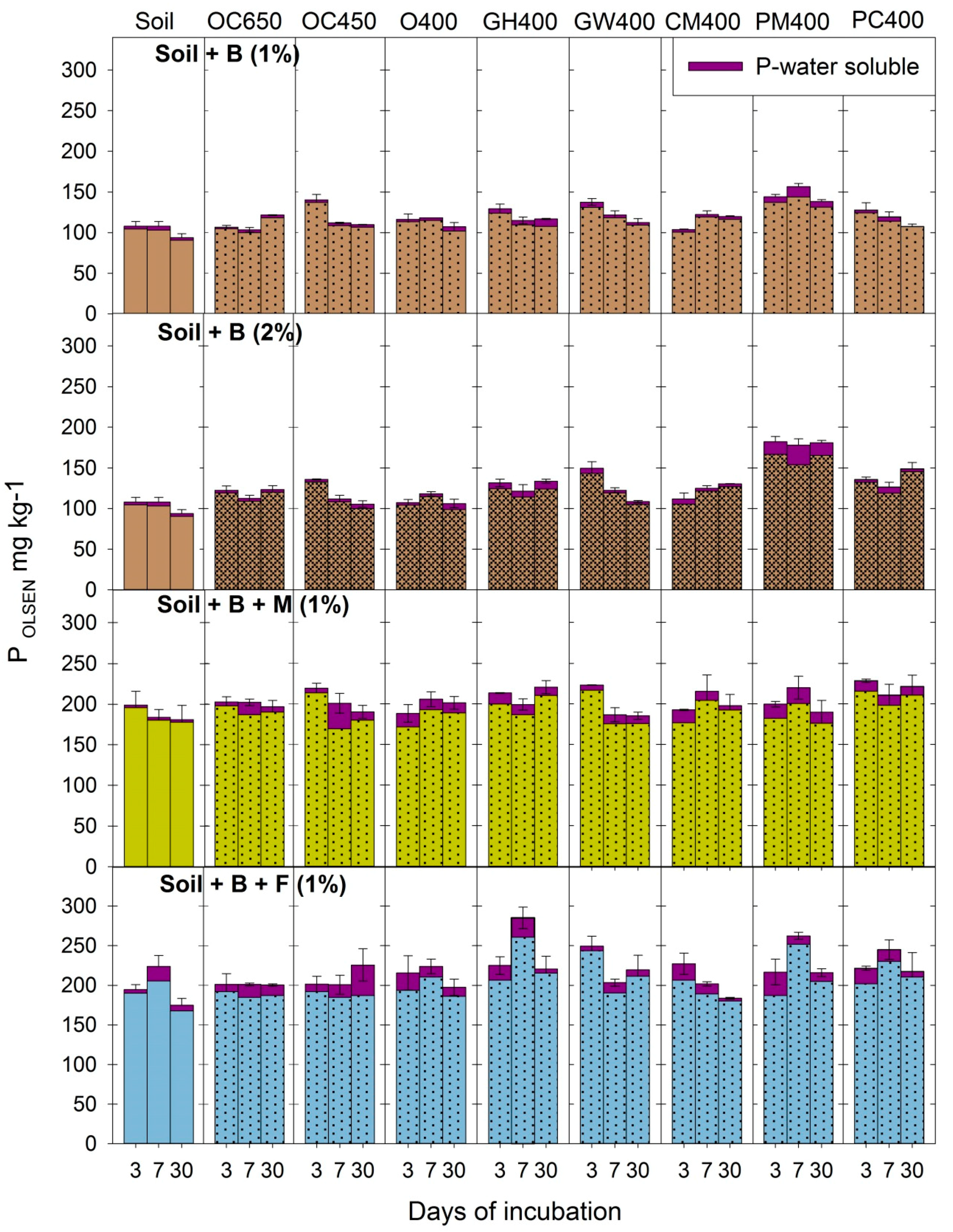
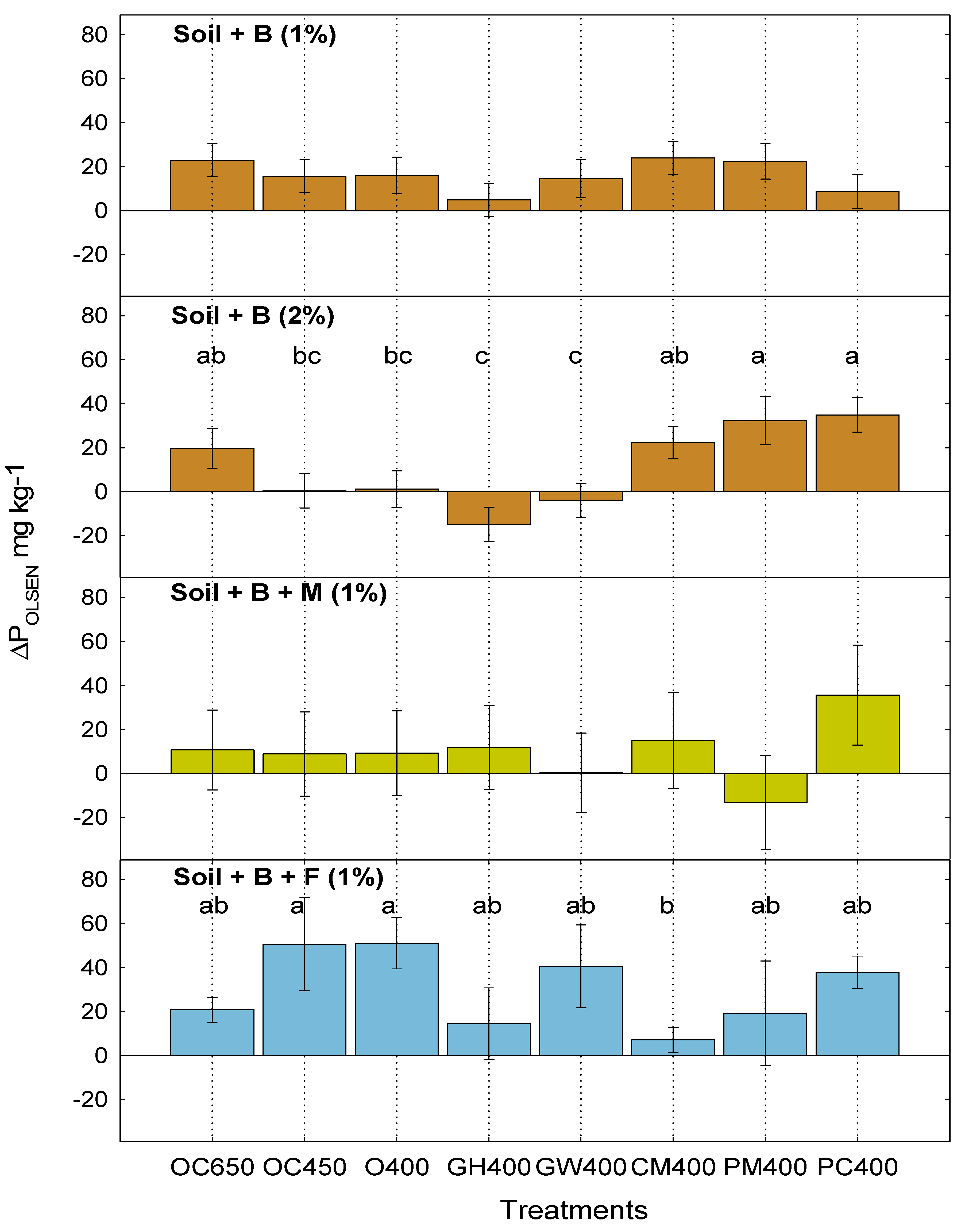
3.2. Dynamics of Available P in Soil
3.3. Dynamics of Available Micronutrients in Soil
3.4. Dynamics of DTPA-Extractable Potentially Toxic Metals in Soil
3.5. Effect of Biochar on Plant Growth
3.5.1. Phytotoxicity
3.5.2. Plant Growth and Nutritional Status
3.5.3. Effect of Biochar Types on N Use Efficiency
3.5.4. Potentially Toxic Metals in the Plant Shoot System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Commission. Proposal for a Directive of the European Parliament and of the Council amending Directive 2008/98/EC on Waste Brussels; European Commission: Brussels, Belgium, 2015; p. 25. [Google Scholar]
- Spokas, K.A. Review of the stability of biochar in soils: Predictability of O:C molar ratios. Carbon Manag. 2010, 1, 289–303. [Google Scholar] [CrossRef]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—A review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Stromberger, M.E.; Lentz, R.D.; Dungan, R.S. Hardwood biochar influences calcareous soil physicochemical and microbiological status. J. Environ. Qual. 2014, 43, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, J.A.; Stromberger, M.E.; Lentz, R.D.; Dungan, R.S. Hardwood biochar and manure co-application to a calcareous soil. Chemosphere 2016, 142, 84–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeLuca, T.H.; Gundale, M.J.; MacKenzie, M.D.; Jones, D.L. Biochar effects on soil nutrient transformations. In Biochar for Environmental Management: Science, Technology and Implementation, 2nd ed.; Lehmann, J., Joseph, S., Eds.; EartScan: New York, NY, USA, 2015; pp. 543–562. ISBN 978-0-415-70415-1. [Google Scholar]
- Taghizadeh-Toosi, A.; Clough, T.J.; Sherlock, R.R.; Condron, L.M. Biochar adsorbed ammonia is bioavailable. Plant Soil 2012, 350, 57–69. [Google Scholar] [CrossRef]
- Park, J.H.; Choppala, G.K.; Bolan, N.S.; Chung, J.W.; Chuasavathi, T. Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil 2011, 348, 439–451. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.G.A.; van der Velde, M.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Biederman, L.A.; Harpole, W.S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Enders, A.; Hanley, K.; Whitman, T.; Joseph, S.; Lehmann, J. Characterization of biochars to evaluate recalcitrance and agronomic performance. Bioresour. Technol. 2012, 114, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Gunes, A.; Inal, A.; Sahin, O.; Taskin, M.B.; Atakol, O.; Yilmaz, N. Variations in mineral element concentrations of poultry manure biochar obtained at different pyrolysis temperatures, and their effects on crop growth and mineral nutrition. Soil Use Manag. 2015, 31, 429–437. [Google Scholar] [CrossRef]
- Fornes, F.; Belda, R.M.; Lidón, A. Analysis of two biochars and one hydrochar from different feedstock: Focus set on environmental, nutritional and horticultural considerations. J. Clean. Prod. 2015, 86, 40–48. [Google Scholar] [CrossRef]
- Meyer-Kohlstock, D.; Schmitz, T.; Kraft, E. Organic waste for compost and biochar in the EU: Mobilizing the potential. Resources 2015, 4, 457–475. [Google Scholar] [CrossRef]
- Mandal, S.; Thangarajan, R.; Bolan, N.S.; Sarkar, B.; Khan, N.; Ok, Y.S.; Naidu, R. Biochar-induced concomitant decrease in ammonia volatilization and increase in nitrogen use efficiency by wheat. Chemosphere 2016, 142, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Olmo, M.; Villar, R.; Salazar, P.; Alburquerque, J.A. Changes in soil nutrient availability explain biochar’s impact on wheat root development. Plant Soil 2016, 399, 333–343. [Google Scholar] [CrossRef]
- Wrb, I.W.G. World Reference Base for Soil Resources, Update 2015, No. 106 ed.; World Soil Resources Reports; FAO: Rome, Italy, 2014; p. 203. ISBN 978-9-25-108369-7. [Google Scholar]
- López-Cano, I.; Cayuela, M.L.; Sánchez-Monedero, M.A. Suitability of different agricultural and urban organic wastes as feedstocks for the production of biochar—Part 1. Physicochemical characterisation. Sustainability 2018. under revision. [Google Scholar]
- López, L.; Betrán, J.; Ramos, Á.; López, H.; López, P.; Bermejo, J.L.; Urbano, P.; Piñeiro, J.; Castro, J.; Blázquez, R.; et al. Parte II: Abonado de los principales cultivos en España. In Guía Práctica de la Fertilización Racional de Los Cultivos en España; Ministerio de Medio Ambiente y Medio Rural y Marino: Gobierno de España, Spain, 2010; pp. 123–259. ISBN 978-8-44-910997-3. [Google Scholar]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Sommer, S.G.; Kjellerup, V.; Kristjansen, O. Determination of total ammonium nitrogen in pig and cattle slurry: Sample preparation and analysis. Acta Agric. Scand. B Plant Soil Sci. 1992, 42, 146–151. [Google Scholar] [CrossRef]
- Kuo, S. Phosphorus. In Methods of Soil Analysis Part 3—Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Eds.; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1996; pp. 869–919. ISBN 978-0-89-118825-4. [Google Scholar]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Zucconi, F.; Pera, A.; Forte, M.; De Bertoldi, M. Evaluating toxicity of immature compost. Biocycle 1981, 22, 54–57. [Google Scholar]
- Tammeorg, P.; Brandstaka, T.; Simojoki, A.; Helenius, J. Nitrogen mineralisation dynamics of meat bone meal and cattle manure as affected by the application of softwood chip biochar in soil. Earth Environ. Sci. Trans. R. Soc. Edinburgh 2012, 103, 19–30. [Google Scholar] [CrossRef]
- Moll, R.H.; Kamprath, E.J.; Jackson, W.A. Analysis and interpretation of factors which contribute to efficiency of nitrogen utilization. Agron. J. 1982, 74, 562–564. [Google Scholar] [CrossRef]
- Singh, B.P.; Hatton, B.J.; Singh, B.; Cowie, A.L.; Kathuria, A. Influence of biochars on nitrous oxide emission and nitrogen leaching from two contrasting soils. J. Environ. Qual. 2010, 39, 1224–1235. [Google Scholar] [CrossRef] [PubMed]
- Steiner, C.; Glaser, B.; Geraldes Teixeira, W.; Lehmann, J.; Blum, W.E.; Zech, W. Nitrogen retention and plant uptake on a highly weathered central Amazonian Ferralsol amended with compost and charcoal. J. Plant Nutr. Soil Sci. 2008, 171, 893–899. [Google Scholar] [CrossRef]
- Bruun, E.W.; Ambus, P.; Egsgaard, H.; Hauggaard-Nielsen, H. Effects of slow and fast pyrolysis biochar on soil C and N turnover dynamics. Soil Biol. Biochem. 2012, 46, 73–79. [Google Scholar] [CrossRef]
- MacDonald, G.K.; Bennett, E.M.; Potter, P.A.; Ramankutty, N. Agronomic phosphorus imbalances across the world’s croplands. Proc. Natl. Acad. Sci. USA 2011, 108, 3086–3091. [Google Scholar] [CrossRef] [PubMed]
- Sharpley, A.; Moyer, B. Phosphorus forms in manure and compost and their release during simulated rainfall. J. Environ. Qual. 2000, 29, 1462–1469. [Google Scholar] [CrossRef]
- Wang, T.; Camps-Arbestain, M.; Hedley, M.; Bishop, P. Predicting phosphorus bioavailability from high-ash biochars. Plant Soil 2012, 357, 173–187. [Google Scholar] [CrossRef]
- Knicker, H. How does fire affect the nature and stability of soil organic nitrogen and carbon? A review. Biogeochemistry 2007, 85, 91–118. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, S.; Forster, H.S. Boron sorption on calcareous soils and reference calcites. Soil Sci. 1991, 152, 304–310. [Google Scholar] [CrossRef]
- European Biochar Foundation (EBC). European Biochar Certificate—Guidelines for a Sustainable Production of Biochar, Version 6.1 ed.; European Biochar Foundation (EBC): Arbaz, Switzerland, 2018. [Google Scholar]
- Munzuroglu, O.; Geckil, H. Effects of metals on seed germination, root elongation, and coleoptile and hypocotyl growth in Triticum aestivum and Cucumis sativus. Arch. Environ. Contam. Toxicol. 2002, 43, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Alburquerque, J.A.; Calero, J.M.; Barrón, V.; Torrent, J.; del Campillo, M.C.; Gallardo, A.; Villar, R. Effects of biochars produced from different feedstocks on soil properties and sunflower growth. J. Plant Nutr. Soil Sci. 2014, 177, 16–25. [Google Scholar] [CrossRef]
- Atwell, B.J.; Kriedemann, P.E.; Turnbull, C.G.N. Plants in Action: Adaptation in Nature, Performance in Cultivation, 1st ed.; Macmillan Education Australia Pty Ltd.: Melbourne, Australia, 1999; p. 664. ISBN D 7329 4439 2. [Google Scholar]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Wilson, J.B. A review of evidence on the control of shoot: Root ratio, in relation to models. Ann. Bot. Lond. 1988, 61, 433–449. [Google Scholar] [CrossRef]
- Wu, F.; Bao, W.; Li, F.; Wu, N. Effects of drought stress and N supply on the growth, biomass partitioning and water-use efficiency of Sophora davidii seedlings. Environ. Exp. Bot. 2008, 63, 248–255. [Google Scholar] [CrossRef] [Green Version]
- Sharp, R.E.; Davies, W.J. Solute regulation and growth by roots and shoots of water-stressed maize plants. Planta 1979, 147, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Prendergast-Miller, M.T.; Duvall, M.; Sohi, S.P. Biochar-root interactions are mediated by biochar nutrient content and impacts on soil nutrient availability. Eur. J. Soil Sci. 2014, 65, 173–185. [Google Scholar] [CrossRef]
- Lehmann, J.; Kuzyakov, Y.; Pan, G.; Ok, Y.S. Biochars and the plant-soil interface. Plant Soil 2015, 395, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Camps-Arbestain, M.; Amonette, J.E.; Singh, B.; Wang, T.; Schmidt, H.P. A biochar classification system and associated test methods. In Biochar for Environmental Management: Science, Technology and Implementation, 2nd ed.; Lehmann, J., Joseph, S., Eds.; Rouledge: New York, NY, USA, 2015; pp. 165–194. ISBN 978-0-41-570415-1. [Google Scholar]
- European Commission. Commision Regulation (EU) No 420/2011 of 29 April 2011 amending regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2011, L111/113. Available online: https://www.fsai.ie/uploadedFiles/Reg420_2011.pdf (accessed on 19 May 2018).
- European Commission. Commision Regulation (EU) No 488/2014 of 12 May 2014 amending Regulation (EC) No 1881/2006 as regards maximum levels of cadmium in foodstuffs. Off. J. Eur. Union 2014, L138/175. Available online: https://www.fsai.ie/uploadedFiles/Reg488_2014.pdf (accessed on 19 May 2018).
| pH a | EC a | OM | C org | C/N | C ext | N | P | K | |
|---|---|---|---|---|---|---|---|---|---|
| (mS cm−1) | (g 100 g−1) | (mg g−1) | (g 100 g−1) | ||||||
| Soil | 8.1 | 0.51 | 2.9 | 1.7 | 5.7 | 10.9 | 0.3 | - | - |
| Manure | 9.1 | 3.9 | 45.6 | 18.1 | 9.1 | 40.4 | 2.0 | 1.6 | 2.4 |
| Fertiliser | - | - | - | - | - | - | 18.0 | 26.0 | 0.0 |
| Biochar | Fe | Cu | Mn | Zn | B |
|---|---|---|---|---|---|
| (mg kg−1) | |||||
| OC650 | 5.1 | 0.7 | 59.3 | 11.9 | 0.24 |
| (0.0)1 | (0.0) | (1.4) | (0.1) | (0.01) | |
| OC450 | 0.9 | 0.2 | 24.4 | 1.5 | 0.45 |
| (0.0) | (0.1) | (0.0) | (0.0) | (0.00) | |
| O400 | 0.1 | 0.4 | 1.6 | 5.6 | 0.39 |
| (0.1) | (0.1) | (1.0) | (0.6) | (0.33) | |
| GH400 | 0.1 | 0.5 | 15.2 | 2.0 | 0.82 |
| (0.0) | (0.3) | (1.4) | (0.1) | (0.04) | |
| GW400 | 2.9 | 0.7 | 437.0 | 5.4 | 2.12 |
| (0.5) | (0.0) | (1.6) | (0.1) | (0.00) | |
| CM400 | 106.9 | 1.4 | 4.4 | 3.9 | 0.82 |
| (1.6) | (0.0) | (0.1) | (0.0) | (0.01) | |
| PM400 | 4.2 | 3.3 | 45.8 | 21.5 | 5.58 |
| (0.4) | (0.3) | (0.5) | (0.4) | (0.33) | |
| PC400 | 18.9 | 2.7 | 16.1 | 9.1 | 2.07 |
| (0.6) | (0.0) | (0.2) | (0.4) | (0.01) | |
| Biochar | Cd | Cr | Ni | Pb |
|---|---|---|---|---|
| (mg kg−1) | ||||
| OC650 | <0.01 | <0.01 | 0.44 | 0.1 |
| (0.03)1 | (0.0) | |||
| OC450 | <0.01 | <0.01 | 0.07 | 0.1 |
| (0.00) | (0.0) | |||
| O400 | <0.01 | <0.01 | 0.32 | 2.8 |
| (0.25) | (2.0) | |||
| GH400 | 0.02 | <0.01 | 0.17 | 0.6 |
| (0.00) | (0.01) | (0.1) | ||
| GW400 | 0.05 | 0.08 | 0.19 | 1.9 |
| (0.01) | (0.00) | (0.01) | (0.0) | |
| CM400 | 0.11 | 0.04 | 0.29 | 21.7 |
| (0.02) | (0.00) | (0.01) | (0.0) | |
| PM400 | <0.01 | <0.01 | 0.04 | 0.2 |
| (0.00) | (0.0) | |||
| PC400 | 0.01 | <0.01 | 0.32 | 5.0 |
| (0.00) | (0.01) | (0.3) | ||
| Biochar | GI 1 | G 2 | Root | Shoot | Total | Root/Shoot | Leaf SPAD | Chlorophyll Fluorescence | N | N Uptake | NUE 4 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| % | Biomass (g) | (Fv/Fm) 3 | mg g−1 | mg N kg−1 Soil | % | ||||||
| Soil | - | - | 0.82 | 3.85 ab | 4.67 ab | 0.21 b | 27.8 | 0.825 | 7.98 ab | 28.7 | 67.5 ab |
| (0.15) 5 | (0.60) | (0.73) | (0.02) | (2.4) | (0.007) | (0.65) | (4.9) | (11.5) | |||
| OC450 | 101 a | 117 ab | 0.89 | 4.24 a | 5.14 a | 0.21 b | 27.2 | 0.826 | 7.59 b | 30.2 | 71.4 ab |
| (1) | (10) | (0.19) | (0.21) | (0.30) | (0.03) | (1.9) | (0.008) | (0.62) | (3.5) | (3.5) | |
| O400 | 88 ab | 117 ab | 0.75 | 3.79 ab | 4.54 ab | 0.20 b | 24.9 | 0.827 | 8.02 ab | 28.1 | 66.4 ab |
| (6) | (16) | (0.10) | (0.33) | (0.35) | (0.03) | (1.2) | (0.003) | (0.62) | (2.1) | (4.9) | |
| GH400 | 69 ab | 108 ab | 0.90 | 3.18 b | 4.08 b | 0.29 a | 26.8 | 0.829 | 9.07 a | 26.7 | 63.1 b |
| (5) | (23) | (0.11) | (0.28) | (0.31) | (0.04) | (1.1) | (0.009) | (0.73) | (2.2) | (5.3) | |
| GW400 | 85 ab | 83 b | 0.80 | 4.08 a | 4.88 a | 0.20 b | 26.7 | 0.825 | 8.11 ab | 30.9 | 73.0 ab |
| (9) | (19) | (0.09) | (0.24) | (0.21) | (0.03) | (2.6) | (0.008) | (0.69) | (2.1) | (5.0) | |
| CM400 | 61 b | 133 a | 0.86 | 3.71 ab | 4.58 ab | 0.20 b | 27.2 | 0.826 | 8.42 ab | 29.2 | 69.1 ab |
| (7) | (10) | (0.11) | (0.29) | (0.36) | (0.2) | (1.2) | (0.006) | (0.63) | (1.7) | (4.0) | |
| PC400 | 85 ab | 100 ab | 0.83 | 4.20 a | 5.03 a | 0.23b | 28.3 | 0.824 | 8.08 ab | 31.7 | 75.0 a |
| (5) | (19) | (0.07) | (0.33) | (0.34) | (0.02) | (2.5) | (0.014) | (0.6) | (3.5) | (8.3) | |
| Biochar | Cd | Cr | Ni | Pb |
|---|---|---|---|---|
| (mg kg−1) | ||||
| Soil | 0.02 | 0.03 | 0.03 bc | 0.07 |
| (0.00)1 | (0.01) | (0.01) | (0.01) | |
| OC450 | 0.02 | 0.01 | 0.01 c | 0.09 |
| (0.01) | (0.01) | (0.01) | (0.01) | |
| O400 | 0.02 | 0.04 | 0.07 a | 0.11 |
| (0.01) | (0.04) | (0.02) | (0.01) | |
| GH400 | 0.02 | 0.01 | 0.01 c | 0.16 |
| (0.01) | (0.02) | (0.02) | (0.01) | |
| GW400 | 0.02 | 0.01 | 0.01 c | 0.12 |
| (0.01) | (0.02) | (0.02) | (0.01) | |
| CM400 | 0.02 | 0.06 | 0.06 ab | 0.07 |
| (0.01) | (0.02) | (0.02) | (0.01) | |
| PC400 | 0.02 | 0.02 | 0.02 c | 0.13 |
| (0.01) | (0.02) | (0.01) | (0.01) | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Cano, I.; Cayuela, M.L.; Sánchez-García, M.; Sánchez-Monedero, M.A. Suitability of Different Agricultural and Urban Organic Wastes as Feedstocks for the Production of Biochar—Part 2: Agronomical Evaluation as Soil Amendment. Sustainability 2018, 10, 2077. https://doi.org/10.3390/su10062077
López-Cano I, Cayuela ML, Sánchez-García M, Sánchez-Monedero MA. Suitability of Different Agricultural and Urban Organic Wastes as Feedstocks for the Production of Biochar—Part 2: Agronomical Evaluation as Soil Amendment. Sustainability. 2018; 10(6):2077. https://doi.org/10.3390/su10062077
Chicago/Turabian StyleLópez-Cano, Inés, María Luz Cayuela, María Sánchez-García, and Miguel A. Sánchez-Monedero. 2018. "Suitability of Different Agricultural and Urban Organic Wastes as Feedstocks for the Production of Biochar—Part 2: Agronomical Evaluation as Soil Amendment" Sustainability 10, no. 6: 2077. https://doi.org/10.3390/su10062077
APA StyleLópez-Cano, I., Cayuela, M. L., Sánchez-García, M., & Sánchez-Monedero, M. A. (2018). Suitability of Different Agricultural and Urban Organic Wastes as Feedstocks for the Production of Biochar—Part 2: Agronomical Evaluation as Soil Amendment. Sustainability, 10(6), 2077. https://doi.org/10.3390/su10062077






