Grass-Legume Mixtures for Improved Soil Health in Cultivated Agroecosystem
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Experiments
2.2. Soil Sampling
2.3. Soil Sample Analysis for Basic Physical and Chemical Properties
2.4. Soil Phospholipid Fatty Acid Analysis
2.5. Soil Mineralizable Carbon and Nitrogen Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Results
3.1.1. Plant Aboveground Biomass
3.1.2. Soil Mineralizable Carbon and Nitrogen
3.1.3. Soil Microbial Biomass
3.1.4. Fungi to Bacteria Ratio
3.1.5. Soil Microbial Community Structure
3.2. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liebig, M.; Carpenter-Boggs, L.; Johnson, J.M.F.; Wright, S.; Barbour, N. Cropping system effects on soil biological characteristics in the Great Plains. Renew. Agric. Food Syst. 2006, 21, 36–48. [Google Scholar] [CrossRef]
- Jackson, R.B.; Fierer, N.; Schimel, J.P. New directions in microbial ecology. Ecology 2007, 88, 1343–1344. [Google Scholar] [CrossRef]
- Karolien, D.; Dries, R.; Mihiri, C.W.M.W.; Peter, L.; Pascal, B. Microbial community composition and rhizodeposit-carbon assimilation in differently managed temperate grassland soils. Soil Biol. Biochem. 2009, 41, 144–153. [Google Scholar]
- Singh, B.K.; Millard, P.; Whiteley, A.S.; Murrell, J.C. Unraveling rhizosphere-microbial interactions: Opportunities and limitations. Trends Microbiol. 2004, 12, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.L.; Hodge, A.; Kuzyakov, Y. Plant and mycorrhizal regulation of rhizodeposition. New Phytol. 2004, 163, 459–480. [Google Scholar] [CrossRef] [Green Version]
- Wei, D.; Yang, Q.; Zhang, J.Z.; Wang, S.; Chen, X.L.; Zhang, X.L.; Li, W.Q. Bacterial community structure and diversity in a black soil as affected by long-term fertilization. Pedosphere 2008, 18, 582–592. [Google Scholar] [CrossRef]
- Ngosong, C.; Jarosch, M.; Raupp, J.; Neumann, E.; Ruess, L. The impact of farming practice on soil microorganisms and arbuscular mycorrhizal fungi: Crop type versus long-term mineral and organic fertilization. Appl. Soil Ecol. 2010, 46, 134–142. [Google Scholar] [CrossRef]
- Insam, H. Developments in soil microbiology since the mid 1960’s. Geoderma 2001, 100, 389–402. [Google Scholar] [CrossRef]
- McKinley, V.L.; Peacock, A.D.; White, D.C. Microbial community PLFA and PHB responses to ecosystem restoration in tallgrass prairie soils. Soil Biol. Biochem. 2005, 37, 1946–1958. [Google Scholar] [CrossRef]
- Acosta-Martinez, V.; Mikha, M.M.; Vigil, M.F. Microbial communities and enzyme activities in soils under alternative crop rotations compared to wheat-fallow for the Central Great Plains. Appl. Soil Ecol. 2007, 37, 41–52. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setala, H.; van der Putten, W.H.; Wall, D.H. Ecological linkages between aboveground and belowground biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Grayston, S.J.; Wang, S.; Campbell, C.D.; Edwards, A.C. Selective influence of plant species on microbial diversity in the rhizosphere. Soil Biol. Biochem. 1998, 30, 369–378. [Google Scholar] [CrossRef]
- Soderberg, K.H.; Olsson, P.A.; Baath, E. Structure and activity of the bacterial community in the rhizosphere of different plant species and the effect of arbuscular mycorrhizal colonization. FEMS Microbiol. Ecol. 2002, 40, 223–231. [Google Scholar] [CrossRef]
- Nair, A.; Ngouajio, M. Soil microbial biomass, functional microbial diversity, and nematode community structure as affected by cover crops and compost in an organic vegetable production system. Appl. Soil Ecol. 2012, 58, 45–55. [Google Scholar] [CrossRef]
- Gangatharan, R.; Neri, D. Can biodiversity improve soil fertility resilience in agroecosystems? New Medit 2012, 4, 11–18. [Google Scholar]
- Kirkegaard, J.; Christen, O.; Krupinsky, J.; Layzell, D. Break crop benefits in temperate wheat production. Field Crops Res. 2008, 107, 185–195. [Google Scholar] [CrossRef]
- Bauhus, J.; Khanna, P.K.; Menden, N. Aboveground and belowground interactions in mixed plantations of Eucalyptus globulus and Acacia mearnsii. Can. J. For. Res. 2000, 30, 1886–1894. [Google Scholar] [CrossRef]
- Vancura, V.; Hanzlikova, A. Root exudates of plants IV. Differences in chemical composition of seed and seeding exudates. Plant Soil 1972, 36, 271–282. [Google Scholar]
- Martin, J.K. 14C-labelled material leached from the rhizosphere of plants supplied with 14CO2. Aust. J. Biol. Sci. 1971, 24, 1131–1142. [Google Scholar] [CrossRef]
- Wyoming Agricultural Statistics (WAS); United States Department of Agriculture, National Agricultural Statistics Service, Wyoming Field Office: Washington, DC, USA, 2015; p. 88.
- Skinner, Q.D. A Field Guide to Wyoming Grasses; Education Resources Publishing: Cumming, GA, USA, 2010; p. 595. [Google Scholar]
- Marschner, P.; Kandeler, E.; Marschner, B. Structure and function of the soil microbial community in a long term fertilizer experiment. Soil Biol. Biochem. 2003, 35, 453–461. [Google Scholar] [CrossRef]
- United States Department of Agriculture-Natural Resources Conservation Service. On-line Database. 2015. Available online: http://websoilsurvey.nrcs.usda.gov (accessed on 19 February 2015).
- Gee, G.W.; Bauder, J.W. Particle-size analysis. In Methods of Soil Analysis. Physical and Mineralogical Methods. Part 1, Agronomy Monographs, 2th ed.; Klute, A., Ed.; ASA and SSSA: Madison, WI, USA, 1986; pp. 383–411. [Google Scholar]
- Thomas, G.W. Soil pH and soil acidity. In Methods of Soil Analysis. Part 3: Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 475–490. [Google Scholar]
- Schhulte, E.E.; Hopkins, B.G. Estimation of soil organic matter by weight loss-on-ignition. In Soil Organic Matter: Analysis and Interpretation; Magdoff, F.R., Tabatabai, M.A., Hanlon, E.A., Eds.; Soil Science Society of America Special Publication 46: Madison, WI, USA, 1996; pp. 21–31. [Google Scholar]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis. Chemical and Biological Methods. Part 2, 2nd ed.; Page, A.L., Ed.; ASA and SSSA: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Knudsen, D.; Peterson, G.A.; Pratt, P.F. Lithium, sodium, and potassium. In Methods of Soil Analysis. Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; No. 9; ASA Monograph: Madison, WI, USA, 1982; pp. 225–246. [Google Scholar]
- Buyer, J.S.; Teasdale, J.R.; Roberts, D.P.; Zasada, I.A.; Maul, J.E. Factors affecting soil microbial community structure in tomato cropping systems. Soil Biol. Biochem. 2010, 42, 831–841. [Google Scholar] [CrossRef]
- Frostegard, A.; Tundlid, A.; Baath, E. Phospholipid fatty acids composition, biomass, and activity of microbial communities from two soil types experimentally exposed to different heavy metals. Appl. Environ. Microbiol. 1993, 59, 3605–3617. [Google Scholar] [PubMed]
- Blackwood, C.B.; Buyer, J.S. Soil microbial communities grown with Bt and non-Bt corn in three soils. J. Environ. Qual. 2004, 33, 832–836. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Wu, F. Soil microbial community structure in cucumber rhizosphere of different resistance cultivars to fusarium wilt. FEMS Microbiol. Ecol. 2010, 72, 456–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zibilske, L.M. Carbon mineralization. In Methods of Soil Analysis. Part 2: Microbiological and Biochemical Properties; Weaver, R.W., Angle, S., Bottomley, P., Bezdicek, D., Smith, S., Tabatabai, A., Wollum, A., Mickelson, S.H., Bigham, J.M., Eds.; Soil Science Society of America: Madison, WI, USA, 1994; pp. 15–40. [Google Scholar]
- Doane, T.A.; Horwath, W.R. Spectrophotometric determination of nitrate with a single reagent. Anal. Lett. 2003, 36, 2713–2722. [Google Scholar] [CrossRef]
- Weatherburn, M.W. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H. Principles and Procedures of Statistics: A Biometrical Approach; McGraw-Hill Book Company: New York, NY, USA, 1980. [Google Scholar]
- Cardinale, B.J.; Wright, J.P.; Cadotte, M.W.; Carroll, I.T.; Hector, A.; Srivastava, D.S.; Loreau, M.; Weis, J.J. Impacts of plant diversity on biomass production increase through time because of species complementarity. Proc. Natl. Acad. Sci. USA 2007, 104, 18123–18128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebhart, D.L.; Call, C.A.; Weaver, R.W. Dinitrogen fixation and transfer in legume crested wheatgrass mixtures. J. Range Manag. 1993, 46, 431–435. [Google Scholar] [CrossRef]
- Mooso, G.D.; Wedin, W.F. Yield dynamics of canopy components in alfalfa-grass mixtures. Agron. J. 1990, 82, 696–701. [Google Scholar] [CrossRef]
- Zelles, L. Fatty acid patterns of phospholipids and lipopolysaccharides in the characterization of microbial communities in soil: A review. Biol. Fertil. Soils 1999, 29, 111–129. [Google Scholar] [CrossRef]
- Toyota, K.; Kuninaga, S. Comparison of soil microbial community between soils amended with or without farmyard manure. Appl. Soil Ecol. 2006, 33, 39–48. [Google Scholar] [CrossRef]
- Esperschutz, J.; Gattinger, A.; Mader, P.; Schloter, M.; Fliebach, A. Response of soil microbial biomass and community structures to conventional and organic farming systems under identical crop rotations. Microb. Ecol. 2007, 61, 26–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grayston, S.J.; Griffith, G.S.; Mawdsley, J.L.; Campbell, C.D.; Bardgett, R.D. Accounting for variability in soil microbial communities of temperate upland grassland ecosystems. Soil Biol. Biochem. 2001, 33, 533–551. [Google Scholar] [CrossRef]
- Chen, M.; Chen, B.; Marschner, P. Plant growth and soil microbial community structure of legumes and grasses grown in monoculture or mixture. J. Environ. Sci. 2008, 20, 1231–1237. [Google Scholar] [CrossRef] [Green Version]
- Rillig, M.C.; Wright, S.F.; Eviner, V.T. The role of arbuscular mycorrhizal fungi and glomalin in soil aggregation: Comparing effects of five plant species. Plant Soil 2002, 238, 325–333. [Google Scholar] [CrossRef]
- De Vries, F.T.; Hoffland, E.; van Eekeren, N.; Brussaard, L.; Bloem, J. Fungal/bacterial ratios in grasslands with contrasting nitrogen management. Soil Biol. Biochem. 2006, 38, 2092–2103. [Google Scholar] [CrossRef] [Green Version]
- Hogberg, M.N.; Chen, Y.; Hogberg, P. Gross nitrogen mineralization and fungi-to-bacteria ratios are negatively correlated in boreal forests. Biol. Fertil. Soils 2007, 44, 363–366. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Smith, R.S.; Shiel, R.S.; Peacock, S.; Simkin, J.M.; Quirk, H.; Hobbs, P.J. Parasitic plants indirectly regulate below-ground properties in grassland ecosystems. Nature 2006, 439, 969–972. [Google Scholar] [CrossRef] [PubMed]
- Donnison, L.M.; Griffith, G.S.; Hedger, J.; Hobbs, P.J.; Bardgett, R.D. Management influences on soil microbial communities and their function in botanically diverse haymeadows of northern England and Wales. Soil Biol. Biochem. 2000, 32, 253–263. [Google Scholar] [CrossRef]
- Strickland, M.S.; Rousk, J. Considering fungal: Bacterial dominance in soils—Methods, controls, and ecosystem implications. Soil Biol. Biochem. 2010, 42, 1385–1395. [Google Scholar] [CrossRef]
- Bittman, S.; Forge, T.A.; Kowalenko, C.G. Responses of the bacterial and fungal biomass in a grassland soil to multi-year applications of dairy manure slurry and fertilizer. Soil Biol. Biochem. 2005, 37, 613–623. [Google Scholar] [CrossRef]
- Benizri, E.; Amiaud, B. Relationship between plants and soil microbial communities in fertilized grasslands. Soil Biol. Biochem. 2005, 37, 2055–2064. [Google Scholar] [CrossRef]
- De Vries, F.T.; Bloem, J.; van Eekeren, N.; Brussaard, L.; Hoffland, E. Fungal biomass in pastures increases with age and reduced N input. Soil Biol. Biochem. 2007, 39, 1620–1630. [Google Scholar] [CrossRef]
- De Vries, F.T.; Groenigen, J.W.; Hofland, E.; Bloem, J. Nitrogen losses from two grassland soils with different fungal biomass. Soil Biol. Biochem. 2011, 43, 997–1005. [Google Scholar] [CrossRef]
- Ghimire, R.; Norton, J.B.; Stahl, P.D.; Norton, U. Soil microbial substrate properties and microbial community responses under irrigated organic and reduced-tillage crop and forage production systems. PLoS ONE 2014, 9, e103901. [Google Scholar] [CrossRef] [PubMed]
- Bardgett, R.D.; Hobbs, P.J.; Frostegard, A. Changes in soil fungal: Bacterial biomass ratios following reductions in the intensity of management of an upland grassland. Biol. Fertil. Soils 1996, 22, 261–264. [Google Scholar] [CrossRef]
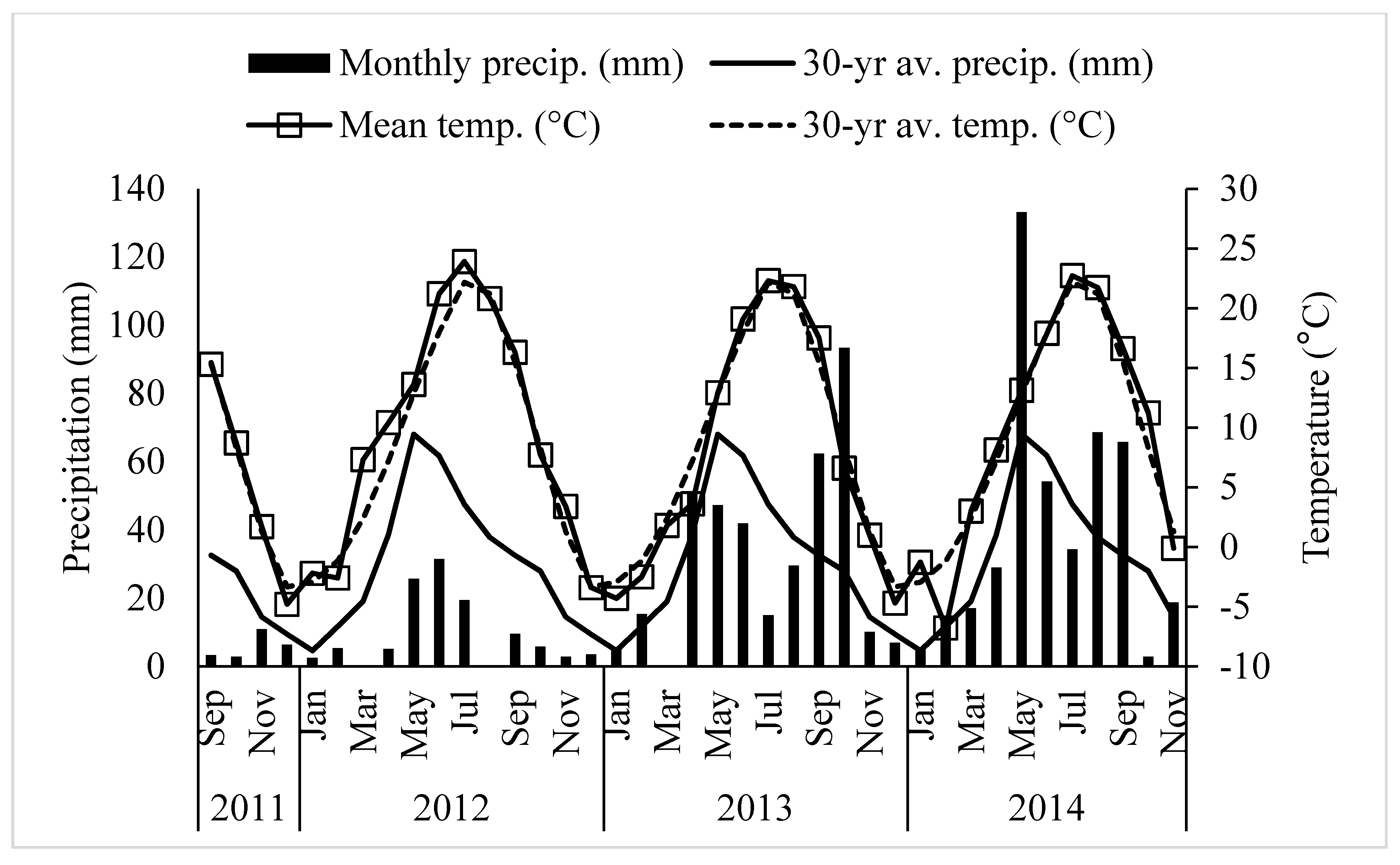


| Properties | Units | Analytical Results (n = 7) |
|---|---|---|
| Physical | ||
| Sand † (0.2–2.0 mm) | (%, w/w) | 44.3 (±0.7) |
| Silt † (0.002–0.2 mm) | (%, w/w) | 34.6 (±0.7) |
| Clay † (<0.002 mm) | (%, w/w) | 21.1 (±0.5) |
| Texture [24] | Loam | |
| Gravel | (%, w/w) | 0.8 (±0.2) |
| Chemical | ||
| pH | 8.0 (±0.02) | |
| Electrical conductivity [25] | (ds m−1) | 1.7 (±0.08) |
| Organic matter [26] | (g kg−1) | 17 (±0.4) |
| Total N # | (g kg−1) | 0.89 (±0.02) |
| Total SOC | (g kg−1) | 8.56 (±0.04) |
| Inorganic-N | (mg kg−1) | 2.36 (±0.07) |
| Available P [27] | (mg kg−1) | 77.1 (±1.0) |
| Exchangeable cations [28] | ||
| K | (mg kg−1) | 328.7 (±20.6) |
| Ca | (%, w/w) | 0.6 (±0.01) |
| Mg | (mg kg−1) | 402.9 (±12.5) |
| Na | (mg kg−1) | 203.7 (±10.6) |
| Treatments (ALF-MB-OG †) | Proportion of Seed in Mixtures § | N Fertilizer Applied | ||
|---|---|---|---|---|
| ALF | MB | OG | ||
| % | kg N ha−1 | |||
| 100-0-0 | 100 | 0 | 0 | 0 |
| 50-50-0 | 50 | 50 | 0 | 0 |
| 50-0-50 | 50 | 0 | 50 | 0 |
| 50-25-25 | 50 | 25 | 25 | 0 |
| 0-100-0 | 0 | 100 | 0 | 0 |
| 0-0-100 | 0 | 0 | 100 | 0 |
| 0-100-0 + N ‡ | 0 | 100 | 0 | 150 |
| 0-0-100 + N | 0 | 0 | 100 | 150 |
| 0-0-0 ¶ | 0 | 0 | 0 | 0 |
| Treatments (ALF-MB-OG †) | Forage Dry Matter Yield | |||
|---|---|---|---|---|
| 2012 | 2013 | 2014 | 3 year Total | |
| kg ha−1 | ||||
| 100-0-0 | 9738 b § | 8488 cd | 4880 d | 23,106 c |
| 50-50-0 | 12,463 a | 11,560 a | 8773 a | 32,796 a |
| 50-0-50 | 10,481 b | 10,495 b | 8106 ab | 29,082 b |
| 50-25-25 | 10,280 b | 9444 bc | 7387 b | 27,111 b |
| 0-100-0 | 9311 b | 5067 e | 3760 e | 18,138 d |
| 0-0-100 | 6063 c | 5549 e | 4160 de | 15,772 e |
| 0-100-0 + N ‡ | 10,621 b | 7400 d | 5960 c | 23,961 c |
| 0-0-100 + N | 6983 c | 7375 d | 5734 cd | 20,092 d |
| Treatments (ALF-MB-OG †) | Mineralizable C | Mineralizable N | ||||||
|---|---|---|---|---|---|---|---|---|
| 2011 | 2013 | 2014 | ∆ in 3 year | 2011 | 2013 | 2014 | ∆ in 3 year | |
| mg kg−1 d−1 | % | mg kg−1 d−1 | % | |||||
| 100-0-0 | 3.76 | 20.06 a § | 12.27 a | 226 * | 3.62 | 9.41 abc | 50.63 ab | 1299 * |
| 50-50-0 | 3.66 | 14.53 bc | 7.90 bc | 116 * | 3.75 | 9.50 abc | 47.64 abc | 1170 * |
| 50-0-50 | 3.72 | 14.70 bc | 7.71 bc | 107 * | 3.71 | 5.72 cde | 54.90 a | 1380 * |
| 50-25-25 | 3.85 | 13.27 cd | 7.81 bc | 103 * | 3.66 | 6.46 cdef | 45.44 bc | 1142 * |
| 0-100-0 | 3.79 | 5.54 i | 3.88 de | 2 | 4.05 | 3.76 def | 4.12 e | 2 |
| 0-0-100 | 3.62 | 6.00 hi | 3.69 de | 2 | 3.76 | 3.02 ef | 5.62 de | 49 |
| 0-100-0+ N ‡ | 3.76 | 9.62 ef | 5.07 cd | 35 | 3.64 | 2.93 ef | 9.78 de | 169 * |
| 0-0-100 + N | 3.80 | 8.54 fg | 4.94 cd | 30 | 3.59 | 2.64 f | 14.37 d | 300 * |
| 0-0-0 (control) | 3.64 | 4.08 i | 3.67 de | 1 | 3.71 | 2.60 f | 3.74 e | 1 |
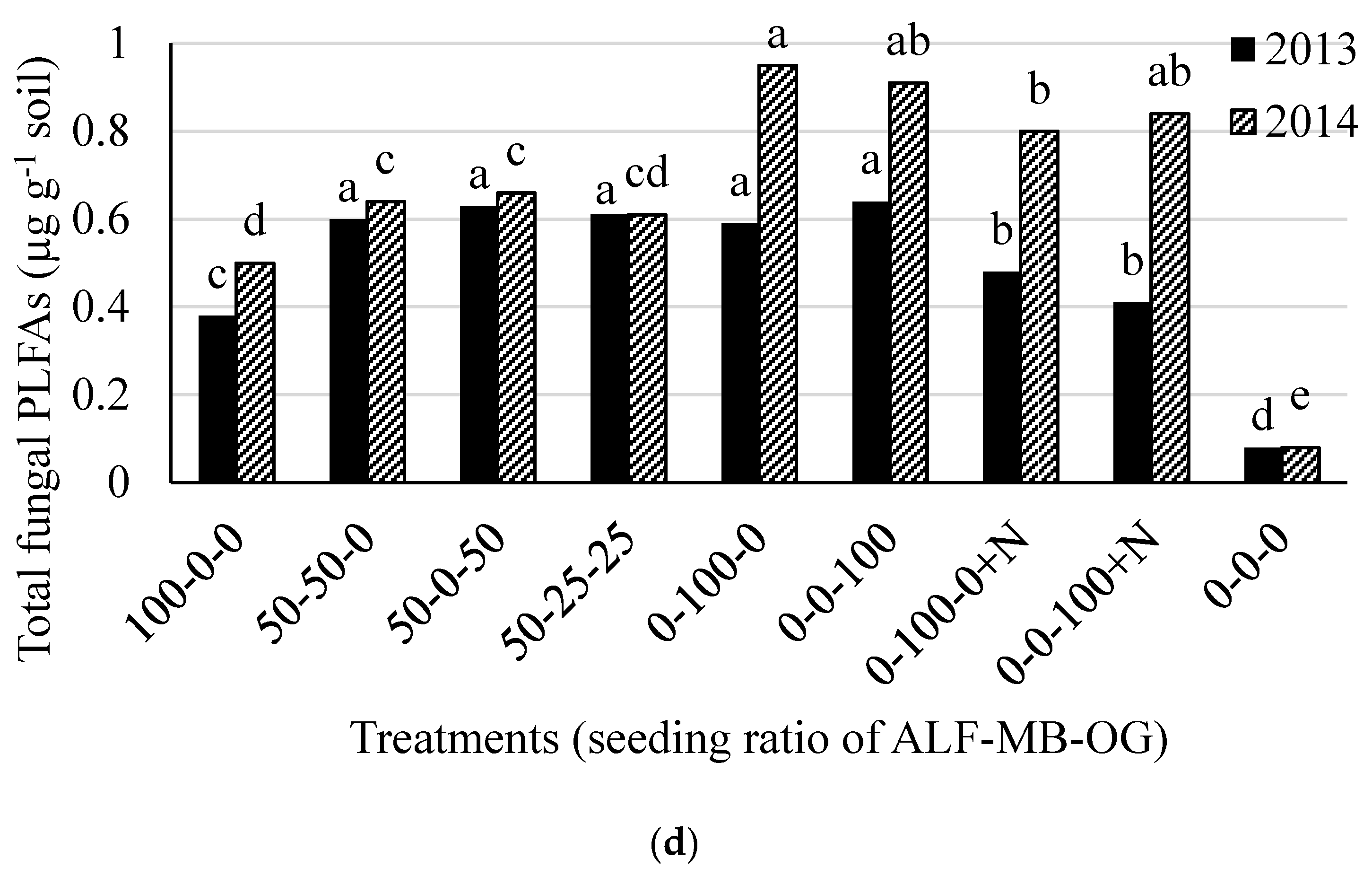
| Treatments (ALF-MB-OG †) | Gram Positive Bacteria | Gram Negative Bacteria | Actinomycetes | AMF ¶ | Other Fungi | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2013 | 2014 | 2013 | 2014 | 2013 | 2014 | 2013 | 2014 | 2013 | 2014 | |
| (µg g−1 soil) | ||||||||||
| 100-0-0 | 0.88 b § | 0.89 b | 1.91 b | 2.59 a | 0.66 b | 0.82 c | 0.19 c | 0.22 cd | 0.19 d | 0.28 c |
| 50-50-0 | 1.04 a | 1.13 a | 2.22 a | 2.19 b | 0.85 a | 0.92 b | 0.28 b | 0.16 d | 0.32 c | 0.48 b |
| 50-0-50 | 1.08 a | 1.17 a | 1.73 b | 2.19 b | 0.92 a | 0.96 ab | 0.28 b | 0.21 cd | 0.35 bc | 0.45 b |
| 50-25-25 | 1.05 a | 1.25 a | 2.01 ab | 2.18 b | 0.99 a | 1.02 a | 0.32 a | 0.16 d | 0.29 c | 0.46 b |
| 0-100-0 | 0.19 de | 0.21 d | 0.24 c | 0.27 d | 0.16 c | 0.22 f | 0.15 d | 0.28 bc | 0.44 ab | 0.67 a |
| 0-0-100 | 0.18 e | 0.20 d | 0.23 c | 0.26 d | 0.16 c | 0.24 f | 0.14 d | 0.37 a | 0.49 a | 0.54 b |
| 0-100-0 + N ‡ | 0.24 c | 0.69 c | 0.45 c | 1.13 c | 0.20 c | 0.70 d | 0.16 cd | 0.31 ab | 0.32 c | 0.48 b |
| 0-0-100 + N | 0.23 cd | 0.64 c | 0.41 c | 1.10 c | 0.21 c | 0.60 e | 0.14 d | 0.31 ab | 0.27 cd | 0.53 b |
| 0-0-0 (control) | 0.08 f | 0.14 d | 0.23 c | 0.33 d | 0.10 c | 0.14 g | 0.04 e | 0.04 e | 0.04 e | 0.04 d |
| Treatments (ALF-MB-OG †) | Gram Positive Bacteria | Gram Negative Bacteria | Actinomycetes | AMF ¶ | Other Fungi | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2013 | 2014 | 2013 | 2014 | 2013 | 2014 | 2013 | 2014 | 2013 | 2014 | |
| (%) | ||||||||||
| 100-0-0 | 23.2 a § | 18.4 c | 49.5 a | 53.6 a | 17.5 bcd | 17.2 b | 5.0 d | 4.8 d | 5.0 c | 6.0 de |
| 50-50-0 | 22.2 a | 23.1 ab | 47.1 a | 44.9 b | 18.1 abc | 18.9 ab | 6.0 cd | 3.3 d | 6.6 c | 9.8 d |
| 50-0-50 | 24.9 a | 23.4 ab | 39.2 c | 44.2 b | 21.3 a | 19.3 ab | 6.5 c | 4.2 d | 8.1 c | 9.0 de |
| 50-25-25 | 23.5 a | 24.6 a | 41.9 bc | 43.2 b | 21.0 ab | 20.2 a | 7.2 bc | 3.1 d | 6.3 c | 8.9 de |
| 0-100-0 | 15.9 bc | 12.8 d | 19.9 e | 16.0 d | 14.0 de | 12.9 c | 12.8 a | 17.8 b | 37.4 a | 40.5 a |
| 0-0-100 | 14.8 c | 12.8 d | 19.2 e | 15.7 d | 13.4 e | 14.5 c | 11.9 a | 23.5 a | 40.6 a | 33.7 b |
| 0-100-0 + N ‡ | 17.8 b | 20.9 abc | 33.0 d | 34.0 c | 14.5 cde | 21.2 a | 12.1 a | 9.4 c | 22.6 b | 14.4 c |
| 0-0-100 + N | 17.9 b | 20.0 bc | 33.3 d | 34.5 c | 16.7 cde | 19.1 ab | 11.5 a | 10.1 c | 20.6 b | 16.4 c |
| 0-0-0 (control) | 18.6 b | 20.7 bc | 46.9 ab | 46.7 b | 18.1 abc | 21.3 a | 8.2 b | 5.7 d | 8.3 c | 5.6 e |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhakal, D.; Islam, M.A. Grass-Legume Mixtures for Improved Soil Health in Cultivated Agroecosystem. Sustainability 2018, 10, 2718. https://doi.org/10.3390/su10082718
Dhakal D, Islam MA. Grass-Legume Mixtures for Improved Soil Health in Cultivated Agroecosystem. Sustainability. 2018; 10(8):2718. https://doi.org/10.3390/su10082718
Chicago/Turabian StyleDhakal, Dhruba, and M. Anowarul Islam. 2018. "Grass-Legume Mixtures for Improved Soil Health in Cultivated Agroecosystem" Sustainability 10, no. 8: 2718. https://doi.org/10.3390/su10082718





