Climate Change Affects Forest Productivity in a Typical Climate Transition Region of China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field-Based Forest NPP
2.3. Modeling Forest NPP
2.4. Anomaly and Trend Analyses
3. Results
3.1. Climate Anomaly and Trend
3.2. Model Validation
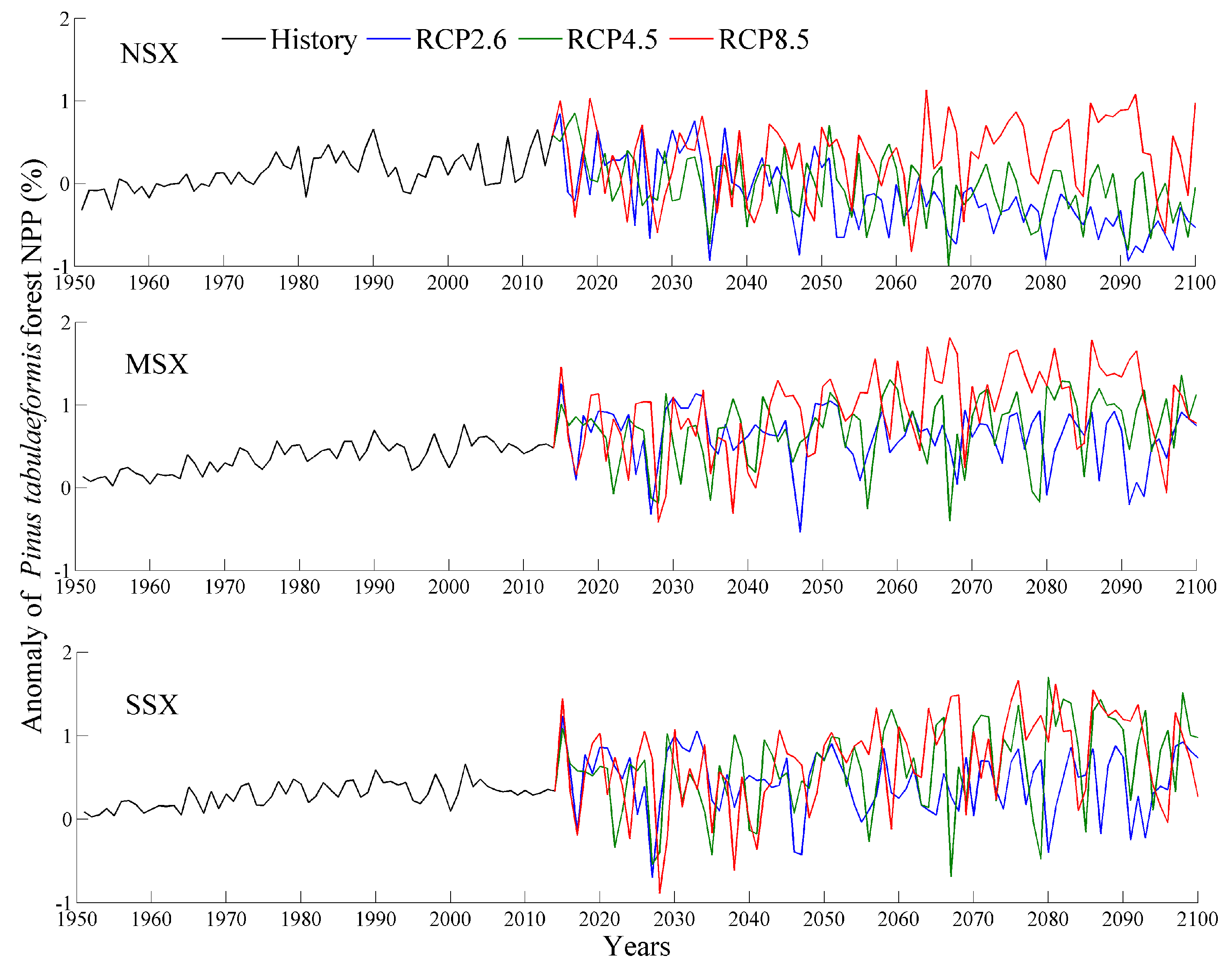
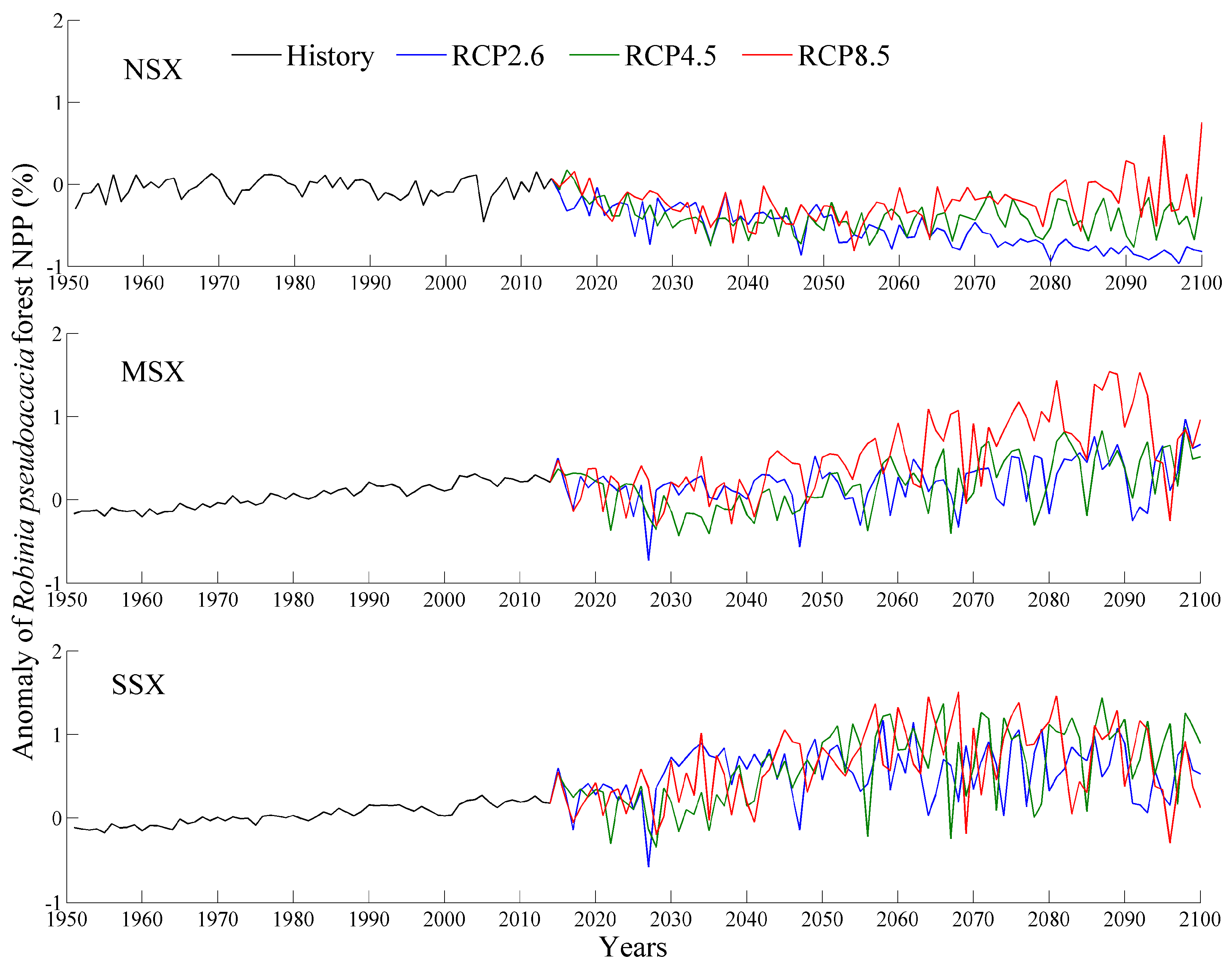
3.3. Dynamic Changes in Forest NPP
3.4. Trends in Forest NPP
4. Discussion
4.1. Future Climate Change
4.2. Response of Forest NPP to Climate Change
4.3. Uncertainty Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; p. 1535. [Google Scholar]
- Xu, Z.L.; Zhao, C.Y.; Feng, Z.D.; Zhang, F.; Sher, H.; Wang, C.; Peng, H.H.; Wang, Y.; Zhao, Y.; Wang, Y.; et al. Estimating realized and potential carbon storage benefits from reforestation and afforestation under climate change: A case study of the Qinghai spruce forests in the Qilian Mountains, northwestern China. Mitig. Adapt. Strat. 2013, 18, 1257–1268. [Google Scholar] [CrossRef]
- Grimm, N.B.; Chapin, F.S.; Bierwagen, B.; Gonzalez, P.; Groffman, P.M.; Luo, Y.Q.; Melton, F.; Nadelhoffer, K.J.; Pairis, A.; Raymond, P.A.; et al. The impacts of climate change on ecosystem structure and function. Front. Ecol. Environ. 2013, 11, 474–482. [Google Scholar] [CrossRef] [Green Version]
- Friend, A.D.; Lucht, W.; Rademacher, T.T.; Keribin, R.; Betts, R.; Cadule, P.; Ciais, P.; Clark, D.B.; Dankers, R.; Falloon, P.D.; et al. Carbon residence time dominates uncertainty in terrestrial vegetation responses to future climate and atmospheric CO2. Proc. Natl. Acad. Sci. USA 2014, 111, 3280. [Google Scholar] [CrossRef]
- Heimann, M.; Reichstein, M. Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature 2008, 451, 289–292. [Google Scholar] [CrossRef] [Green Version]
- Miles, L.; Kapos, V. Reducing greenhouse gas emissions from deforestation and forest degradation: Global land-use implications. Science 2008, 320, 1454–1455. [Google Scholar] [CrossRef]
- Li, P.; Peng, C.H.; Wang, M.; Li, W.Z.; Zhao, P.X.; Wang, K.F.; Yang, Y.Z.; Zhu, Q.A. Quantification of the response of global terrestrial net primary production to multifactor global change. Ecol. Indic. 2017, 76, 245–255. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, C.Q.; Zhang, X.Z.; Chen, B.X.; Wu, J.X.; Tu, Y.L.; Miao, Y.J.; Luo, L.M. A modified framework for the regional assessment of climate and human impacts on net primary productivity. Ecol. Indic. 2016, 60, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Liu, G.H.; Li, Z.S.; Wang, P.T.; Wang, Z.Z. Assessing the driving forces in vegetation dynamics using net primary productivity as the indicator: A case study in Jinghe river basin in the Loess Plateau. Forests 2018, 9, 374. [Google Scholar] [CrossRef]
- Sallaba, F.; Lehsten, D.; Seaquist, J.; Sykes, M.T. A rapid NPP meta-model for current and future climate and CO2 scenarios in Europe. Ecol. Model. 2015, 302, 29–41. [Google Scholar] [CrossRef]
- Deleuze, C.; Houllier, F. Prediction of stem profile of Picea abies using a process-based tree growth model. Tree Physiol. 1995, 15, 113–120. [Google Scholar] [CrossRef]
- Melillo, J.M.; Mcguire, A.D.; Kicklighter, D.W.; Moore, B.; Vorosmarty, C.J.; Schloss, A.L. Global climate change and terrestrial net primary production. Nature 1993, 363, 234–240. [Google Scholar] [CrossRef]
- Thornton, P.E.; Law, B.E.; Gholz, H.L.; Clark, K.L.; Falge, E.; Ellsworth, D.S.; Goldstein, A.H.; Monson, R.K.; Hollinger, D.; Falk, M.; et al. Modeling and measuring the effects of disturbance history and climate on carbon and water budgets in evergreen needleleaf forests. Agric. For. Meteorol. 2002, 113, 185–222. [Google Scholar] [CrossRef]
- Smith, B.; Wårlind, D.; Arneth, A.; Hickler, T.; Leadley, P.; Siltberg, J.; Zaehle, S. Implications of incorporating N cycling and N limitations on primary production in an individual-based dynamic vegetation model. Biogeosciences 2014, 11, 2027–2054. [Google Scholar] [CrossRef] [Green Version]
- Sato, H.; Itoh, A.; Kohyama, T. SEIB–DGVM: A new dynamic global vegetation model using aspatially explicit individual–based approach. Ecol. Model. 2007, 200, 279–307. [Google Scholar] [CrossRef]
- Su, H.X.; Sang, W.G.; Wang, Y.X.; Ma, K.P. Simulating Picea schrenkiana forest productivity under climatic changes and atmospheric CO2 increase in Tianshan Mountains, Xinjiang Autonomous Region, China. For. Ecol. Manag. 2007, 246, 273–284. [Google Scholar] [CrossRef]
- Peng, S.Z.; Chen, Y.M.; Zhao, C.Y.; Xu, Z.L. Simulating the productivity of a subalpine forest at high elevations under representative concentration pathway scenarios in the Qilian Mountains of northwest China. Scand. J. Forest. Res. 2017, 32, 166–173. [Google Scholar] [CrossRef]
- Poulter, B.; Hattermann, F.; Hawkins, E.; Zaehle, S.; Sitch, S.; Restrepo-Coupe, N.; Heyder, U.; Cramer, W. Robust dynamics of Amazon dieback to climate change with perturbed ecosystem model parameters. Glob. Change Biol. 2010, 16, 2476–2495. [Google Scholar] [CrossRef]
- Restrepo-Coupe, N.; Levine, N.M.; Christoffersen, B.O.; Albert, L.P.; Wu, J.; Costa, M.H.; Galbraith, D.; Imbuzeiro, H.; Martins, G.; da Araujo, A.C.; et al. Do dynamic global vegetation models capture the seasonality of carbon fluxes in the Amazon basin? A data-model intercomparison. Glob. Change Biol. 2017, 23, 191–208. [Google Scholar] [CrossRef]
- Feng, X.H.; Uriarte, M.; González, G.; Reed, S.; Thompson, J.; Zimmerman, J.K.; Murphy, L. Improving predictions of tropical forest response to climate change through integration of field studies and ecosystem modeling. Glob. Change Biol. 2017, 24, 1–20. [Google Scholar] [CrossRef]
- Peng, S.Z.; Chen, Y.M.; Cao, Y. Simulating water-use efficiency of Picea crassifolia forest under representative concentration pathway scenarios in the Qilian Mountains of northwest China. Forests 2016, 7, 140. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, K.; Zhang, H. Carbon storage by forest vegetation and its spatial distribution in Shaanxi. Resour. Sci. 2012, 34, 1781–1789. (In Chinese) [Google Scholar]
- Sitch, S.; Smith, B.; Prentice, I.C.; Arneth, A.; Bondeau, A.; Cramer, W.; Kaplan, J.O.; Levis, S.; Lucht, W.; Sykes, M.T.; et al. Evaluation of ecosystem dynamics, plant geography and terrestrial carbon cycling in the LPJ dynamic global vegetation model. Glob. Change Biol. 2003, 9, 161–185. [Google Scholar] [CrossRef]
- Bugmann, H. A review of forest gap models. Clim. Change 2001, 51, 259–305. [Google Scholar] [CrossRef]
- Hickler, T.; Vohland, K.; Feehan, J.; Miller, P.A.; Smith, B.; Costa, L.; Giesecke, T.; Fronzek, S.; Carter, T.R.; Cramer, W.; et al. Projecting the future distribution of European potential natural vegetation zones with a generalized, tree species-based dynamic vegetation mode. Glob. Ecol. Biogeogr. 2012, 21, 50–63. [Google Scholar] [CrossRef]
- Harris, I.; Jones, P.D.; Osborn, T.J.; Lister, D.H. Updated high-resolution grids of monthly climatic observations–the CRU TS3.10 Dataset. Int. J. Climatol. 2014, 34, 623–642. [Google Scholar] [CrossRef]
- Brekke, L.; Wood, A.; Pruitt, T. Data from: Downscaled CMIP3 and CMIP5 Climate and Hydrology Projections: Release of Downscaled CMIP5 Climate Projections, Comparison with Preceding Information, and Summary of User Needs. 2014. Available online: https://gdo-dcp.ucllnl.org/downscaled_cmip_projections/ (accessed on 17 May 2019).
- Peng, S.Z.; Gang, C.C.; Cao, Y.; Chen, Y.M. Assessment of climate change trends over the Loess Plateau in China from 1901 to 2100. Int. J. Climatol. 2018, 38, 2250–2264. [Google Scholar] [CrossRef]
- Wu, T.; Song, L.; Li, W.; Wang, Z.; Zhang, H.; Xin, X.; Zhang, Y.; Zhang, L.; Li, J.; Wu, F.; et al. An overview of BCC climate system model development and application for climate change studies. Acta Meteorol. Sin. 2014, 28, 34–56. [Google Scholar] [CrossRef]
- Peng, S.Z.; Ding, Y.X.; Wen, Z.M.; Chen, Y.M.; Cao, Y.; Ren, J.Y. Spatiotemporal change and trend analysis of potential evapotranspiration over the Loess Plateau of China during 2011–2100. Agric. For. Meteorol. 2017, 233, 183–194. [Google Scholar] [CrossRef]
- Sicard, P.; Mangin, A.; Hebel, P.; Malléab, P. Detection and estimation trends linked to air quality and mortality on French Riviera over the 1990-2005 period. Sci. Total Environ. 2010, 408, 1943–1950. [Google Scholar] [CrossRef]
- Gocic, M.; Trajkovic, S. Analysis of changes in meteorological variables using Mann-Kendall and Sen’s slope estimator statistical tests in Serbia. Glob. Planet. Change 2013, 100, 172–182. [Google Scholar] [CrossRef]
- Atta-ur-Rahman; Dawood, M. Spatio-statistical analysis of temperature fluctuation using Mann–Kendall and Sen’s slope approach. Clim. Dyn. 2017, 48, 783–797. [Google Scholar] [CrossRef]
- Xu, C.H.; Xu, Y. The projection of temperature and precipitation over China under RCP scenarios using a CMIP5 multi-model ensemble. Atmos. Ocean. Sci. Lett. 2012, 5, 527–533. [Google Scholar] [CrossRef]
- Wang, L.; Chen, W. A CMIP5 multimodel projection of future temperature, precipitation, and climatological drought in China. Int. J. Climatol. 2014, 34, 2059–2078. [Google Scholar] [CrossRef]
- Cook, B.I.; Smerdon, J.E.; Seager, R.; Coats, S. Global warming and 21st century drying. Clim. Dyn. 2014, 43, 2607–2627. [Google Scholar] [CrossRef] [Green Version]
- Potter, C.; Klooster, S.; Carvalho, C.R.D.; Genovese, V.B.; Torregrosa, T.; Dungan, J.; Bobo, M.; Coughlan, J. Modeling seasonal and interannual variability in ecosystem carbon cycling for the Brazilian Amazon region. J. Geophys. Res. 2001, 106, 10423–10446. [Google Scholar] [CrossRef] [Green Version]
- Girardin, M.P.; Hogg, E.H.; Bernier, P.Y.; Kurz, W.A.; Guo, X.J.; Cyr, G. Negative impacts of high temperatures on growth of black spruce forests intensify with the anticipated climate warming. Glob. Change Biol. 2016, 22, 627–643. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005, 165, 351–372. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.G.; Li, N.; Su, H.X.; Sang, W.G. Simulation and analysis on future carbon balance of three deciduous forests in Beijing mountain area, warm temperate zone of China. Chin. J. Plant Ecol. 2009, 33, 516–534. (In Chinese) [Google Scholar]
- Digrade, A.; Mote, L.; Bachy, A.; Mozaffar, A.; Schoon, N.; Bussoti, F.; Amelynck, C.; Dalcq, A.C.; Fauconnier, M.L.; Aubinet, M.; et al. Decrease in the photosynthetic performance of temperate grassland species does not lead to a decline in the gross primary production of the ecosystem. Front. Plant Sci. 2018, 9, 67. [Google Scholar] [CrossRef]
- Pšidová, E.; Živčák, M.; Stojnić, C.; Orlović, S.; Gömöry, D.; Kučerová, J.; Ditmarová, L.; Střelcová, D.; Brestičb, M.; Kalajie, H.M. Altitude of origin influences the responses of PSII photochemistry to heat waves in European beech (Fagus sylvatica L.). Environ. Exp. Bot. 2018, 152, 97–106. [Google Scholar] [CrossRef]
- Yang, Y.J. Study on Carbon Pool of Typical Forest Ecosystem in Loess Hilly Area of Northern Shaanxi Province. Master’s Thesis, Chinese Academy of Sciences, Beijing, China, 2014. [Google Scholar]
- Li, W.H. Study on Transpirational Water Consumption and Photosynthetic Characteristics of Main Afforestation Species in the Loess Plateau of Northern Shaanxi. Doctor’s Thesis, Beijing Forestry University, Beijing, China, 2007. [Google Scholar]
- Wang, Y.L. Photosynthetic Water Consumption Characteristics and Influencing Factors of Main Tree Species in Two Typical Forests in the Semi-Arid Region of the Loess Plateau. Doctor’s Thesis, Chinese Academy of Sciences, Beijing, China, 2010. [Google Scholar]
- Yang, J.W.; Liang, Z.S. Characteristics of growth and water use of Robinia pseudoacacia under different soil water conditions. Sci. Silvae Sin. 2004, 40, 93–98. [Google Scholar] [CrossRef]
- Dai, E.F.; Wu, Z.; Ge, Q.S.; Xi, W.M.; Wang, X.F. Predicting the responses of forest distribution and aboveground biomass to climate change under RCPs scenarios in southern China. Glob. Change Biol. 2016, 22, 3642–3661. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.R.; Zavaleta, E.S.; Chiariello, N.R.; Cleland, E.E.; Mooney, H.A.; Field, C.B. Grassland responses to global environmental changes suppressed by elevated CO2. Science 2002, 298, 1987–1990. [Google Scholar] [CrossRef]
- Bonan, G.B. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef]
- Scheiter, S.; Langan, L.; Higgins, S.I. Next-generation dynamic global vegetation models: Learning from community ecology. New Phytol. 2013, 198, 957–969. [Google Scholar] [CrossRef] [PubMed]
- Hickler, T.; Smith, B.; Sykes, M.T.; Davis, M.B.; Sugita, S.; Walker, K. Using a generalized vegetation model to simulate vegetation dynamics in Northeastern USA. Ecology 2004, 85, 519–530. [Google Scholar] [CrossRef]



| Sites | Species | Number of Plots | Tree Density (Trees ha−1) | DBH (cm) | Height (m) | P (mm) | T (°C) |
|---|---|---|---|---|---|---|---|
| NSX | QW | 12 | 746 (150–900) | 10.8 ± 0.3 | 7.6 ± 0.2 | 549.9 ± 15.4 | 9.0 ± 0.1 |
| PT | 5 | 2239 (1280–3570) | 10.4 ± 0.1 | 7.8 ± 0.1 | |||
| RP | 5 | 855 (166–1917) | 11.5 ± 0.4 | 9.5 ± 0.2 | |||
| MSX | QW | 8 | 776 (233–1550) | 11.5 ± 0.4 | 9.8 ± 0.2 | 732.0 ± 19.9 | 9.6 ± 0.1 |
| PT | 6 | 1940 (380–4370) | 11.0 ± 0.1 | 9.6 ± 0.1 | |||
| RP | 7 | 1163 (550–1860) | 11.2 ± 0.2 | 9.6 ± 0.1 | |||
| SSX | QW | 10 | 975 (300–2333) | 12.1 ± 0.3 | 10.4 ± 0.2 | 853.2 ± 21.6 | 12.4 ± 0.1 |
| PT | 5 | 1628 (783–2830) | 11.5 ± 0.2 | 10.0 ± 0.1 | |||
| RP | 4 | 1050 (617–1300) | 8.6 ± 0.2 | 10.1 ± 0.1 |
| Sites | 1951–2014 | 2015–2100 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| RCP2.6 | RCP4.5 | RCP8.5 | |||||||
| T | P | T | P | T | P | T | P | ||
| NSX | Av | 9.2 ± 0.1 | 547.4 ± 11.7 | 10.7 ± 0.1 | 562.1 ± 11.6 | 11.2 ± 0.1 | 569.6 ± 13.2 | 12.2 ± 0.2 | 612.6 ± 14 |
| An | 0.4 | −2.5 | 1.9 | 0.2 | 2.4 | 1.5 | 3.4 | 9.2 | |
| trend | 0.3 ** | −8.1 | 0.1 ** | −1.2 | 0.2 ** | 5.5 | 0.5 ** | 6.6 | |
| MSX | Av | 9.8 ± 0.1 | 738.4 ± 15.4 | 11.2 ± 0.1 | 721.3 ± 13.5 | 11.7±0.1 | 731.5 ± 15.2 | 12.9 ± 0.2 | 744.8 ± 16.5 |
| An | 0.4 | −0.9 | 1.8 | −3.2 | 2.2 | −1.8 | 3.4 | 0.0 | |
| trend | 0.2 ** | −4.2 | 0.1 ** | −0.2 | 0.2 ** | 15.2 * | 0.5 ** | 7.7 | |
| SSX | Av | 12.5 ± 0.1 | 852.8 ± 16.2 | 13.9 ± 0.1 | 823.3 ± 15.4 | 14.4 ± 0.1 | 835.6 ± 16.3 | 15.7 ± 0.2 | 830.0 ± 17.9 |
| An | 0.3 | −1.8 | 1.7 | −5.2 | 2.1 | −3.8 | 3.5 | −4.4 | |
| trend | 0.2 ** | 0.5 | 0.1 ** | −1.0 | 0.2 ** | 18.8 ** | 0.6 ** | 6.9 | |
| Site | Species | 1951–2014 | 2015–2100 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| RCP2.6 | RCP4.5 | RCP8.5 | |||||||
| Av | An | Av | An | Av | An | Av | An | ||
| NSX | QW | 357.2 | 0.8% | 249.9 | −29.5% | 273.1 | −23.0% | 337.0 | −4.9% |
| PT | 402.1 | −2.2% | 289.8 | −29.5% | 334.8 | −18.5% | 464.0 | 12.9% | |
| RP | 281.7 | −4.1% | 128.8 | −56.2% | 227.8 | −22.5% | 319.0 | 8.6% | |
| MSX | QW | 445.9 | 0.6% | 536.4 | 19.5% | 586.7 | 30.7% | 668.5 | 49.0% |
| PT | 465.9 | 0.7% | 551.7 | 19.3% | 583.0 | 26.0% | 659.1 | 42.5% | |
| RP | 369.5 | 5.2% | 425.8 | 21.3% | 450.1 | 28.2% | 536.5 | 52.8% | |
| SSX | QW | 384.5 | −3.1% | 448.7 | 13.0% | 491.8 | 23.9% | 460.4 | 16.0% |
| PT | 452.6 | −0.4% | 507.5 | 11.6% | 573.4 | 26.1% | 598.1 | 31.6% | |
| RP | 316.5 | 4.0% | 472.2 | 55.2% | 454.4 | 49.3% | 495.6 | 62.9% | |
| Site | Species | 1951–2014 | 2015–2100 | ||
|---|---|---|---|---|---|
| RCP2.6 | RCP4.5 | RCP8.5 | |||
| NSX | QW | 23.8 * | −38.4 * | −20.7 * | −8.8 * |
| PT | 25.9 * | −40.6 * | −20.5 * | 15.3 * | |
| RP | 0.7 | −24.1 * | −4.3 | 28.0 * | |
| MSX | QW | 17.7 * | −7.8 * | 20.9 * | 32.6 * |
| PT | 23.8 * | −6.1 | 14.7 * | 26.5 * | |
| RP | 26.2 * | 15.1 * | 26.3 * | 46.0 * | |
| SSX | QW | 6.4 * | −10.2 * | 17.2 * | 6.7 |
| PT | 15.6 * | −3.7 | 28.4 * | 29.8 * | |
| RP | 17.9 * | 7.4 | 33.5 * | 23.9 * | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Liang, S.; Peng, S. Climate Change Affects Forest Productivity in a Typical Climate Transition Region of China. Sustainability 2019, 11, 2856. https://doi.org/10.3390/su11102856
Ding Y, Liang S, Peng S. Climate Change Affects Forest Productivity in a Typical Climate Transition Region of China. Sustainability. 2019; 11(10):2856. https://doi.org/10.3390/su11102856
Chicago/Turabian StyleDing, Yongxia, Siqi Liang, and Shouzhang Peng. 2019. "Climate Change Affects Forest Productivity in a Typical Climate Transition Region of China" Sustainability 11, no. 10: 2856. https://doi.org/10.3390/su11102856




