Development of Sustainable Heterogeneous Catalysts for the Photocatalytic Treatment of Effluents
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Synthesis and Characterization of the Photocatalysts
2.2. Photocatalysis and Adsorption Test
3. Result and Discussion
3.1. Characterization of the Photocatalysts
3.1.1. X-Ray Diffraction (XRD)
3.1.2. Infrared Spectroscopy
3.1.3. Raman Spectroscopy
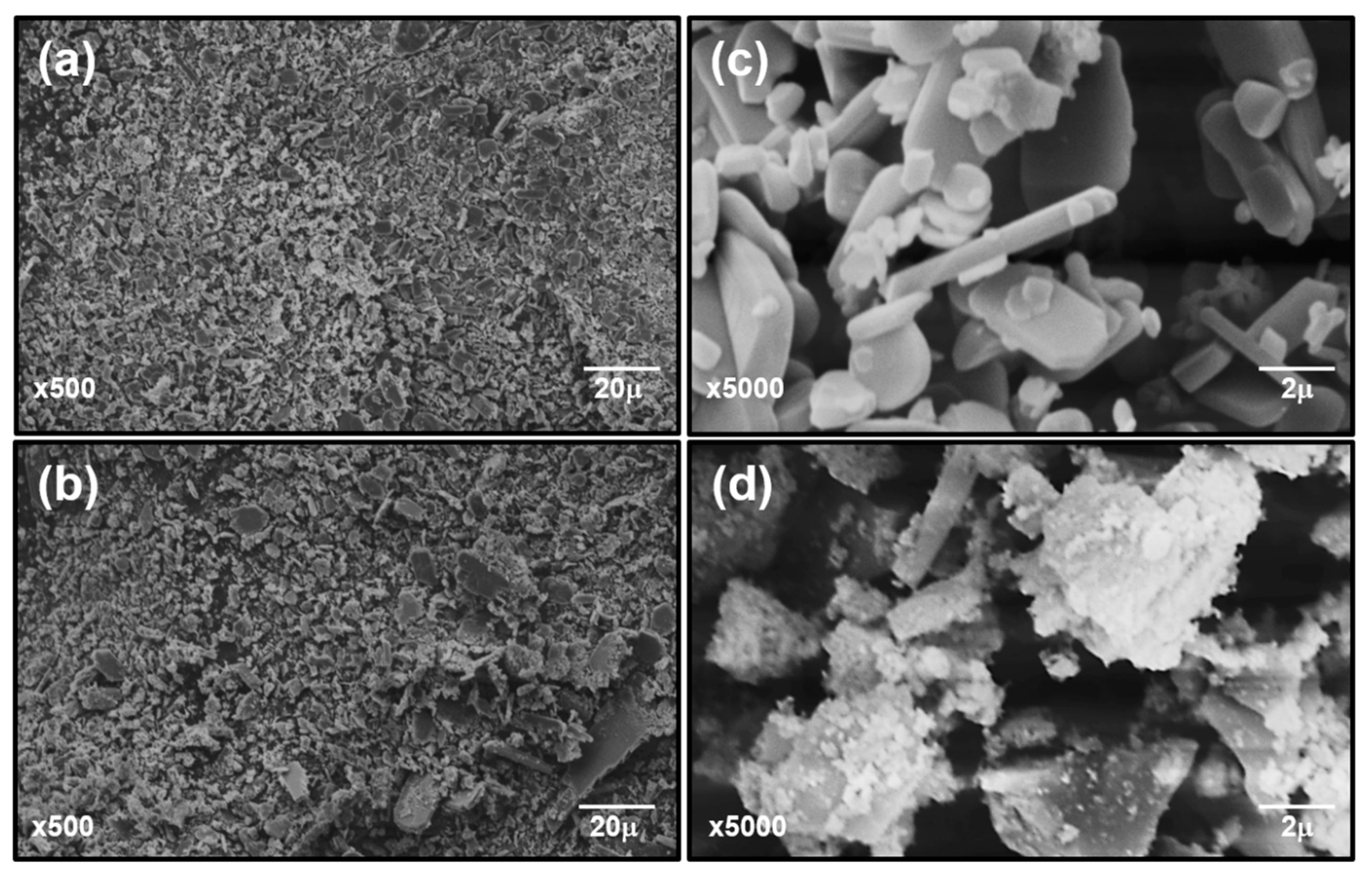
3.1.4. Microstructure Characteristics and Elemental Analysis
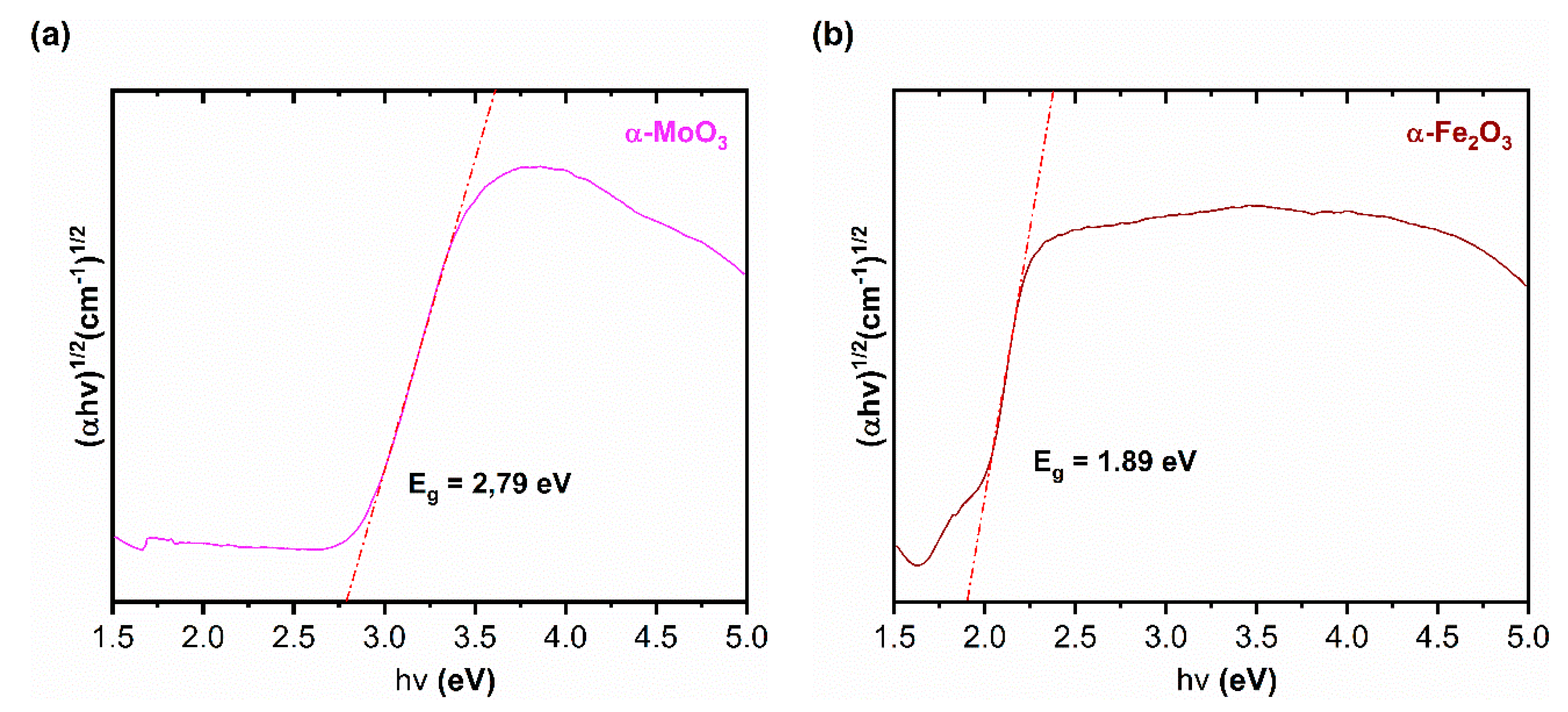
3.1.5. UV–Visible Spectroscopy
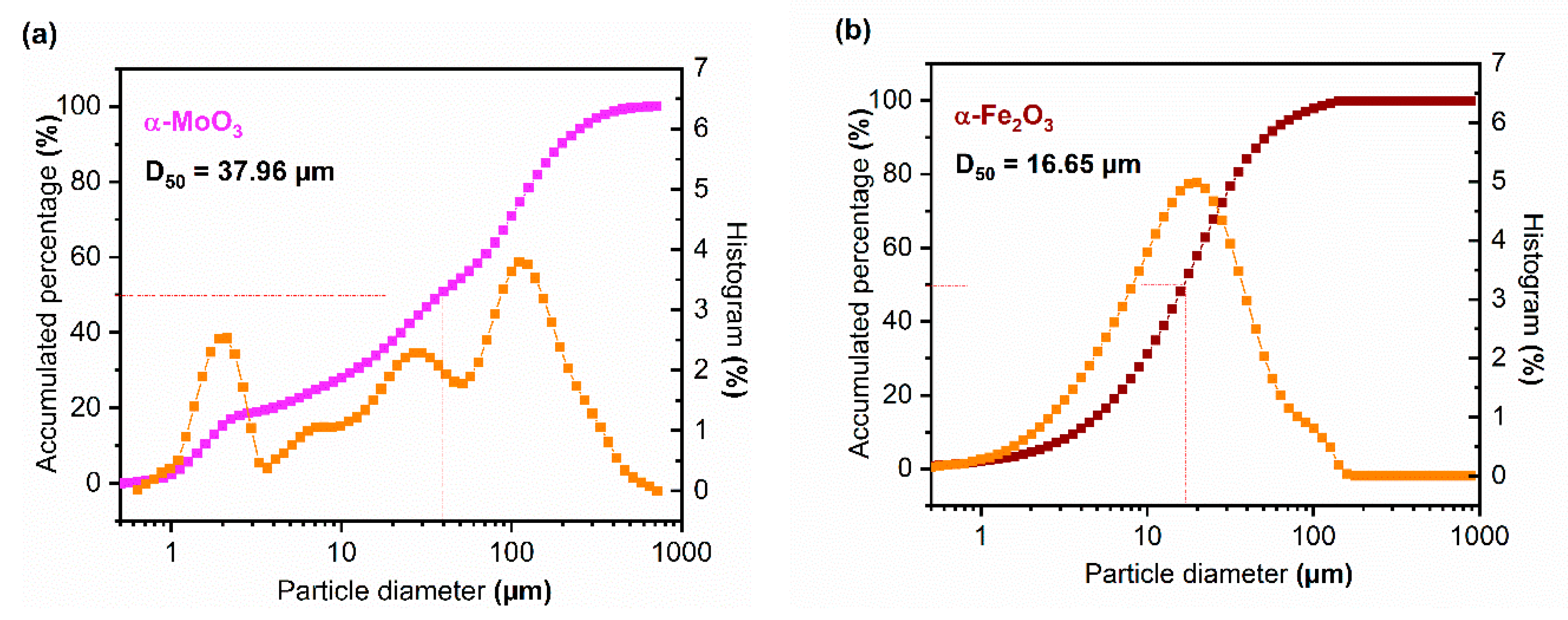
3.1.6. N2 Adsorption/Desorption Isotherms and Granulometric Analysis
3.2. Photocatalytic Experiments
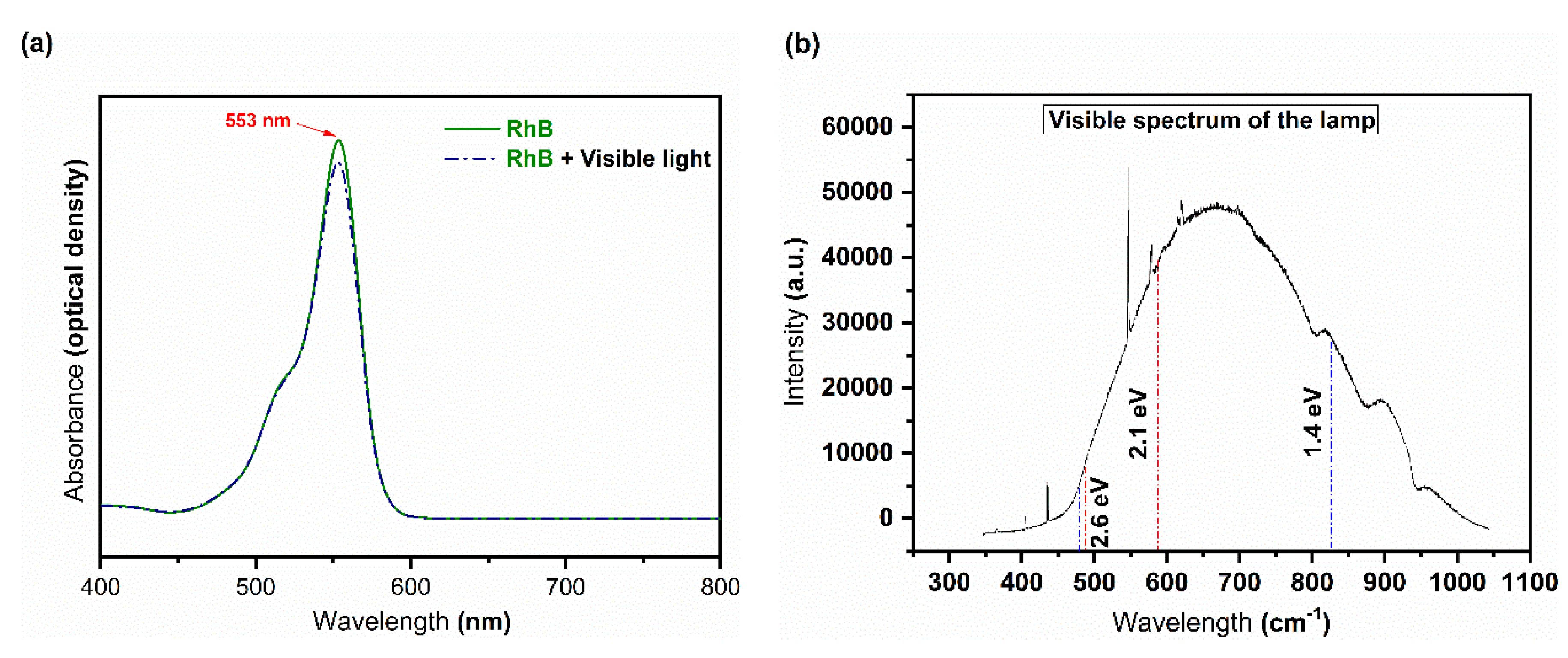
3.2.1. Photolysis
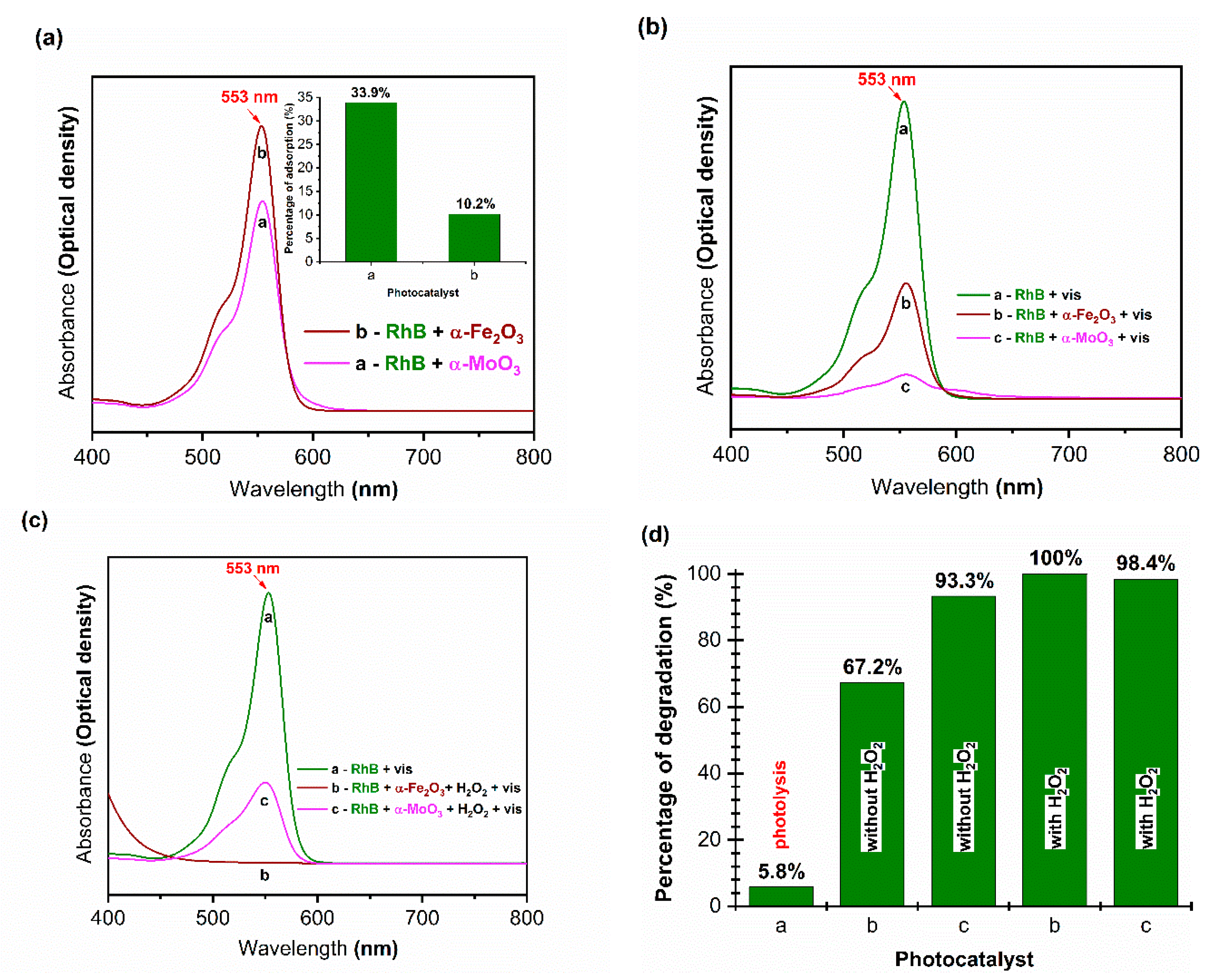
3.2.2. Photocatalytic Activity and Possible Mechanism
3.2.3. Increased Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gupta, K.; Khatri, O.P. Fast and efficient adsorptive removal of organic dyes and active pharmaceutical ingredient by microporous carbon: Effect of molecular size and charge. Chem. Eng. J. 2019, 378, 122218. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef] [PubMed]
- Hasanpour, M.; Hatami, M. Photocatalytic performance of aerogels for organic dyes removal from wastewaters: Review study. J. Mol. Liq. 2020, 309, 113094. [Google Scholar] [CrossRef]
- Santos, D.H.S.; Duarte, J.L.S.; Tavares, M.G.R.; Tavares, M.G.; Friedrich, L.C.; Meili, L.; Pimentel, W.R.O.; Tonholo, J.; Zanta, C.L.P.S. Electrochemical degradation and toxicity evaluation of reactive dyes mixture and real textile effluent over DSA® electrodes. Chem. Eng. Process. Process Intensif. 2020, 153, 107940. [Google Scholar] [CrossRef]
- Sundaran, S.P.; Reshmi, C.R.; Sagitha, P.; Manaf, O.; Sujith, A. Multifunctional graphene oxide loaded nanofibrous membrane for removal of dyes and coliform from water. J. Environ. Manag. 2019, 240, 494–503. [Google Scholar] [CrossRef]
- Puri, C.; Sumana, G. Highly effective adsorption of crystal violet dye from contaminated water using graphene oxide intercalated montmorillonite nanocomposite. Appl. Clay Sci. 2018, 166, 102–112. [Google Scholar] [CrossRef]
- Nie, G.; Xiao, L. New insight into wastewater treatment by activation of sulfite with photosensitive organic dyes under visible light irradiation. Chem. Eng. J. 2020, 389, 123446. [Google Scholar] [CrossRef]
- Wetchakun, K.; Wetchakun, N.; Sakulsermsuk, S. An overview of solar/visible light-driven heterogeneous photocatalysis for water purification: TiO2-and ZnO-based photocatalysts used in suspension photoreactors. J. Ind. Eng. Chem. 2019, 71, 19–49. [Google Scholar] [CrossRef]
- Youssef, Z.; Colombeau, L.; Yesmurzayeva, N.; Baros, F.; Vanderesse, R.; Hamieh, T.; Toufaily, J.; Frochot, C.; Roques-Carmes, T.; Acherar, S. Dye-sensitized nanoparticles for heterogeneous photocatalysis: Cases studies with TiO2, ZnO, fullerene and graphene for water purification. Dye. Pigment. 2018, 159, 49–71. [Google Scholar] [CrossRef]
- Zheng, B.; Wang, Z.; Wang, X.; Chen, Y. Enhanced photocatalytic properties of defect-rich α-MoO3 nanoflakes by cavitation and pitting effect. J. Hazard. Mater. 2019, 378, 120753. [Google Scholar] [CrossRef]
- Desseigne, M.; Dirany, N.; Chevallier, V.; Arab, M. Shape dependence of photosensitive properties of WO3 oxide for photocatalysis under solar light irradiation. Appl. Surf. Sci. 2019, 483, 313–323. [Google Scholar] [CrossRef]
- Leite, R.S.; Santos, A.M.C.; Severo, L.L.; Duarte Neto, J.F.; Castellano, L.R.C.; Neves, G.A.; Lira, H.L.; Menezes, R.R. Photocatalytic degradation of dyes and microorganism inactivation using solution blow spun silver-modified titania fibers. Ceram. Int. 2020, 46, 13482–13490. [Google Scholar] [CrossRef]
- Lops, C.; Ancona, A.; Di Cesare, K.; Dumontel, B.; Garino, N.; Canavese, G.; Hérnandez, S.; Cauda, V. Sonophotocatalytic degradation mechanisms of Rhodamine B dye via radicals generation by micro- and nano-particles of ZnO. Appl. Catal. B Environ. 2019, 243, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, C.; Sujatha, M.P.; Gomathisankar, P. Photoreduction of chromium(VI) on ZrO2 and ZnS surfaces. Mon. Chem. Chem. Mon. 2009, 140, 1269. [Google Scholar] [CrossRef]
- Ren, W.; Wan, C.; Li, Z.; Liu, X.; Zhang, R.; Yang, X.; Lee, D.-J. Functional CdS nanocomposites recovered from biomineralization treatment of sulfate wastewater and its applications in the perspective of photocatalysis and electrochemistry. Sci. Total Environ. 2020, 742, 140646. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, Y.; Zhu, G.; Li, B.; Gou, J.; Cheng, X. Synthesis of PbS/TiO2 nano-tubes photoelectrode and its enhanced visible light driven photocatalytic performance and mechanism for purification of 4-chlorobenzoic acid. Sep. Purif. Technol. 2019, 227, 115697. [Google Scholar] [CrossRef]
- Abdelkader, E.; Nadjia, L.; Naceur, B.; Noureddine, B. SnO2 foam grain-shaped nanoparticles: Synthesis, characterization and UVA light induced photocatalysis. J. Alloys Compd. 2016, 679, 408–419. [Google Scholar] [CrossRef]
- Shanenkov, I.; Sivkov, A.; Ivashutenko, A.; Medvedeva, T.; Shchetinin, I. High-energy plasma dynamic synthesis of multiphase iron oxides containing Fe3O4 and ε-Fe2O3 with possibility of controlling their phase composition. J. Alloys Compd. 2019, 774, 637–645. [Google Scholar] [CrossRef]
- AlSalka, Y.; Granone, L.I.; Ramadan, W.; Hakki, A.; Dillert, R.; Bahnemann, D.W. Iron-based photocatalytic and photoelectrocatalytic nano-structures: Facts, perspectives, and expectations. Appl. Catal. B Environ. 2019, 244, 1065–1095. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, N.; Wang, T.; Huang, H.; Chen, Y.; Li, Z.; Zou, Z. Heterogeneous degradation of organic contaminants in the photo-Fenton reaction employing pure cubic β-Fe2O3. Appl. Catal. B Environ. 2019, 245, 410–419. [Google Scholar] [CrossRef]
- Merazga, A.; Al-Zahrani, J.; Al-Baradi, A.; Omer, B.; Badawi, A.; Al-Omairy, S. Optical band-gap of reduced graphene oxide/TiO2 composite and performance of associated dye-sensitized solar cells. Mater. Sci. Eng. B 2020, 259, 114581. [Google Scholar] [CrossRef]
- Xiao, C.; Li, J.; Zhang, G. Synthesis of stable burger-like α-Fe2O3 catalysts: Formation mechanism and excellent photo-Fenton catalytic performance. J. Clean. Prod. 2018, 180, 550–559. [Google Scholar] [CrossRef]
- Pizarro, F.; Olivares, M.; Valenzuela, C.; Brito, A.; Weinborn, V.; Flores, S.; Arredondo, M. The effect of proteins from animal source foods on heme iron bioavailability in humans. Food Chem. 2016, 196, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yao, Y.; Luo, Z.; Pan, C.; Yang, J.; Fang, Y.; Deng, H.; Liu, C.; Tan, Q.; Liu, F.; et al. Nanostructured MoO3 for Efficient Energy and Environmental Catalysis. Molecules 2019, 25, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhen, Y.; Wang, J.; Fu, F.; Fu, W.; Liang, Y. The Novel Z-Scheme Ternary-Component Ag/AgI/α-MoO3 Catalyst with Excellent Visible-Light Photocatalytic Oxidative Desulfurization Performance for Model Fuel. Nanomaterials 2019, 9, 1054. [Google Scholar] [CrossRef] [Green Version]
- Boukhachem, A.; Mokhtari, M.; Benameur, N.; Ziouche, A.; Martínez, M.; Petkova, P.; Ghamnia, M.; Cobo, A.; Zergoug, M.; Amlouk, M. Structural optical magnetic properties of Co doped α-MoO3 sprayed thin films. Sens. Actuators A Phys. 2017, 253, 198–209. [Google Scholar] [CrossRef]
- Huang, L.; Fang, W.; Yang, Y.; Wu, J.; Yu, H.; Dong, X.; Wang, T.; Liu, Z.; Zhao, B. Three-dimensional MoO3 nanoflowers assembled with nanosheets for rhodamine B degradation under visible light. Mater. Res. Bull. 2018, 108, 38–45. [Google Scholar] [CrossRef]
- Xi, Q.; Liu, J.; Wu, Z.; Bi, H.; Li, Z.; Zhu, K.; Zhuang, J.; Chen, J.; Lu, S.; Huang, Y.; et al. In-situ fabrication of MoO3 nanobelts decorated with MoO2 nanoparticles and their enhanced photocatalytic performance. Appl. Surf. Sci. 2019, 480, 427–437. [Google Scholar] [CrossRef]
- Mathevula, L.E.; Noto, L.L.; Mothudi, B.M.; Chithambo, M.; Dhlamini, M.S. Structural and optical properties of sol-gel derived α-Fe2O3 nanoparticles. J. Lumin. 2017, 192, 879–887. [Google Scholar] [CrossRef]
- Trpkov, D.; Panjan, M.; Kopanja, L.; Tadić, M. Hydrothermal synthesis, morphology, magnetic properties and self-assembly of hierarchical α-Fe2O3 (hematite) mushroom-, cube- and sphere-like superstructures. Appl. Surf. Sci. 2018, 457, 427–438. [Google Scholar] [CrossRef]
- Mallesh, S.; Narsimulu, D.; Kim, K.H. High coercivity in α-Fe2O3 nanostructures synthesized by surfactant-free microwave-assisted solvothermal method. Phys. Lett. A 2020, 384, 126038. [Google Scholar] [CrossRef]
- Mirzaei, A.; Janghorban, K.; Hashemi, B.; Bonyani, M.; Leonardi, S.G.; Neri, G. Highly stable and selective ethanol sensor based on α-Fe2O3 nanoparticles prepared by Pechini sol–gel method. Ceram. Int. 2016, 42, 6136–6144. [Google Scholar] [CrossRef]
- Chantelle, L.; Oliveira, A.L.M.; Kennedy, B.J.; Maul, J.; Silva, M.R.S.; Duarte, T.M.; Albuquerque, A.R.; Sambrano, J.R.; Landers, R.; Siu-Li, M.; et al. Probing the Site-Selective Doping in SrSnO3:Eu Oxides and Its Impact on the Crystal and Electronic Structures Using Synchrotron Radiation and DFT Simulations. Inorg. Chem. 2020, 59, 7666–7680. [Google Scholar] [CrossRef]
- Chen, H.-S. Identification of Rhodamine 6g and Rhodamine B dyes present in ballpoint pen inks using high-performance liquid chromatography and UV-Vis spectrometry. Forensic Sci. J. 2007, 6, 21–37. [Google Scholar]
- Jinisha, R.; Gandhimathi, R.; Ramesh, S.T.; Nidheesh, P.V.; Velmathi, S. Removal of rhodamine B dye from aqueous solution by electro-Fenton process using iron-doped mesoporous silica as a heterogeneous catalyst. Chemosphere 2018, 200, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Saruchi; Kumar, V. Adsorption kinetics and isotherms for the removal of rhodamine B dye and Pb+2 ions from aqueous solutions by a hybrid ion-exchanger. Arab. J. Chem. 2019, 12, 316–329. [Google Scholar] [CrossRef]
- Pechini, M. Method of Preparing Lead and Alkaline Earth Titanates and Niobates and Coating Method Using the Same to Form a Capacitor. U.S. Patent 3,330,697, 11 July 1967. [Google Scholar]
- Wood, D.L.; Tauc, J. Weak Absorption Tails in Amorphous Semiconductors. Phys. Rev. B 1972, 5, 3144–3151. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Sales, H.B.; Bouquet, V.; Députier, S.; Ollivier, S.; Gouttefangeas, F.; Guilloux-Viry, M.; Dorcet, V.; Weber, I.T.; Souza, A.; Santos, I.M.G. Sr1−xBaxSnO3 system applied in the photocatalytic discoloration of an azo-dye. Solid State Sci. 2014, 28, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Rahman, Q.I.; Ahmad, M.; Misra, S.K.; Lohani, M. Effective photocatalytic degradation of rhodamine B dye by ZnO nanoparticles. Mater. Lett. 2013, 91, 170–174. [Google Scholar] [CrossRef]
- Podasca, V.E.; Buruiana, T.; Buruiana, E.C. Photocatalytic degradation of Rhodamine B dye by polymeric films containing ZnO, Ag nanoparticles and polypyrrole. J. Photochem. Photobiol. A Chem. 2019, 371, 188–195. [Google Scholar] [CrossRef]
- Layegh, M.; Ghodsi, F.E.; Hadipour, H. Improving the electrochemical response of nanostructured MoO3 electrodes by Co doping: Synthesis and characterization. J. Phys. Chem. Solids 2018, 121, 375–385. [Google Scholar] [CrossRef]
- Kim, C.-Y. Atomic structure of hematite (α-Fe2O3) nanocube surface; synchrotron X-ray diffraction study. Nano-Struct. Nano-Objects 2020, 23, 100497. [Google Scholar] [CrossRef]
- Maggi, F.; Dossi, S.; Paravan, C.; Galfetti, L.; Rota, R.; Cianfanelli, S.; Marra, G. Iron oxide as solid propellant catalyst: A detailed characterization. Acta Astronaut. 2019, 158, 416–424. [Google Scholar] [CrossRef]
- Seguin, L.; Figlarz, M.; Cavagnat, R.; Lassègues, J.-C. Infrared and Raman spectra of MoO3 molybdenum trioxides and MoO3 · xH2O molybdenum trioxide hydrates. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1995, 51, 1323–1344. [Google Scholar] [CrossRef]
- Jiebing, S.; Rui, X.; Shimin, W.; Wufeng, T.; Hua, T.; Jing, S. Preparation and Characterization of Molybdenum Oxide Thin Films by Sol-Gel Process. J. Sol-Gel Sci. Technol. 2003, 27, 315–319. [Google Scholar] [CrossRef]
- Wang, Y.; Muramatsu, A.; Sugimoto, T. FTIR analysis of well-defined α-Fe2O3 particles. Colloids Surf. A Physicochem. Eng. Asp. 1998, 134, 281–297. [Google Scholar] [CrossRef]
- Žic, M.; Ristić, M.; Musić, S. Monitoring the hydrothermal precipitation of α-Fe2O3 from concentrated Fe(NO3)3 solutions partially neutralized with NaOH. J. Mol. Struct. 2011, 993, 115–119. [Google Scholar] [CrossRef]
- Grigorie, A.C.; Muntean, C.; Stefanescu, M. Obtaining of γ-Fe2O3 nanoparticles by thermal decomposition of polyethyleneglycol–iron nitrate mixtures. Thermochim. Acta 2015, 621, 61–67. [Google Scholar] [CrossRef]
- Nazri, G.-A.; Julien, C. Far-infrared and Raman studies of orthorhombic MoO3 single crystal. Solid State Ion. 1992, 53–56, 376–382. [Google Scholar] [CrossRef]
- Faria, D.L.A.; Lopes, F.N. Heated goethite and natural hematite: Can Raman spectroscopy be used to differentiate them? Vib. Spectrosc. 2007, 45, 117–121. [Google Scholar] [CrossRef]
- Froment, F.; Tournié, A.; Colomban, P. Raman identification of natural red to yellow pigments: Ochre and iron-containing ores. J. Raman Spectrosc. 2008, 39, 560–568. [Google Scholar] [CrossRef]
- Lübbe, M.; Gigler, A.M.; Stark, R.W.; Moritz, W. Identification of iron oxide phases in thin films grown on Al2O3(0001) by Raman spectroscopy and X-ray diffraction. Surf. Sci. 2010, 604, 679–685. [Google Scholar] [CrossRef]
- Chen, D.; Liu, M.; Yin, L.; Li, T.; Yang, Z.; Li, X.; Fan, B.; Wang, H.; Zhang, R.; Li, Z.; et al. Single-crystalline MoO3 nanoplates: Topochemical synthesis and enhanced ethanol-sensing performance. J. Mater. Chem. 2011, 21, 9332–9342. [Google Scholar] [CrossRef]
- Manukyan, K.; Davtyan, D.; Bossert, J.; Kharatyan, S. Direct reduction of ammonium molybdate to elemental molybdenum by combustion reaction. Chem. Eng. J. 2011, 168, 925–930. [Google Scholar] [CrossRef]
- Li, L.; Liang, P.; Liu, C.; Zhang, H.; Mitsuzaki, N.; Chen, Z. New method for improving the bulk charge separation of hematite with enhanced water splitting. Int. J. Hydrogen Energy 2019, 44, 4208–4217. [Google Scholar] [CrossRef]
- Scanlon, D.O.; Watson, G.W.; Payne, D.J.; Atkinson, G.R.; Egdell, R.G.; Law, D.S.L. Theoretical and Experimental Study of the Electronic Structures of MoO3 and MoO2. J. Phys. Chem. C 2010, 114, 4636–4645. [Google Scholar] [CrossRef]
- Amlouk, A.; Boubaker, K.; Amlouk, M.; Bouhafs, M. Study of ytterbium doping effects on structural, mechanical and opto-thermal properties of sprayed ZnO thin films using the Boubaker Polynomials Expansion Scheme (BPES). J. Alloys Compd. 2009, 485, 887–891. [Google Scholar] [CrossRef]
- Filatova, E.O.; Konashuk, A.S. Interpretation of the Changing the Band Gap of Al2O3 Depending on Its Crystalline Form: Connection with Different Local Symmetries. J. Phys. Chem. C 2015, 119, 20755–20761. [Google Scholar] [CrossRef]
- Marcos-Hernández, M.; Villagrán, D. 11-Mesoporous Composite Nanomaterials for Dye Removal and Other Applications. In Composite Nanoadsorbents; Kyzas, G.Z., Mitropoulos, A.C., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 265–293. ISBN 978-0-12-814132-8. [Google Scholar]
- Muruganandham, M.; Swaminathan, M. Decolourisation of Reactive Orange 4 by Fenton and photo-Fenton oxidation technology. Dye. Pigment. 2004, 63, 315–321. [Google Scholar] [CrossRef]
- Xing, Z.; Zhang, J.; Cui, J.; Yin, J.; Zhao, T.; Kuang, J.; Xiu, Z.; Wan, N.; Zhou, W. Recent advances in floating TiO2-based photocatalysts for environmental application. Appl. Catal. B Environ. 2018, 225, 452–467. [Google Scholar] [CrossRef]
- Jing, X.; Peng, X.; Sun, X.; Zhou, W.; Wang, W.; Wang, S. Design and synthesis of Mo2C/MoO3 with enhanced visible-light photocatalytic performance for reduction of Cr (VI) and degradation of organic pollutants. Mater. Sci. Semicond. Process. 2019, 100, 262–269. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Deng, R.; Xv, S.; Lv, X.; Zhou, J.; Li, S.; Cao, F.; Qin, G. Preparation and photocatalytic property of porous α-Fe2O3 nanoflowers. Mater. Res. Bull. 2018, 107, 94–99. [Google Scholar] [CrossRef]
- Luo, H.; Zhao, Y.; He, D.; Ji, Q.; Cheng, Y.; Zhang, D.; Pan, X. Hydroxylamine-facilitated degradation of rhodamine B (RhB) and p-nitrophenol (PNP) as catalyzed by Fe@Fe2O3 core-shell nanowires. J. Mol. Liq. 2019, 282, 13–22. [Google Scholar] [CrossRef]
- Honorio, L.M.C.; de Oliveira, A.L.M.; da Silva Filho, E.C.; Osajima, J.A.; Hakki, A.; Macphee, D.E.; Santos, I.M.G. dos Supporting the photocatalysts on ZrO2: An effective way to enhance the photocatalytic activity of SrSnO3. Appl. Surf. Sci. 2020, 528, 146991. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Dhanavel, L.; Deogratias, T.; Sivaranjani, S.; Narayanan, V.; Stephen, A. Facile synthesis of 1D/1D ZnO@h-MoO3 for enhanced visible light driven photo degradation of industrial textile effluent. Mater. Lett. 2020, 262, 127049. [Google Scholar] [CrossRef]
- Adhikari, S.; Kim, D.-H. Heterojunction C3N4/MoO3 microcomposite for highly efficient photocatalytic oxidation of Rhodamine B. Appl. Surf. Sci. 2020, 511, 145595. [Google Scholar] [CrossRef]
- Mohamed, M.M.; Salama, T.M.; Morsy, M.; Shahba, R.M.A.; Mohamed, S.H. Facile strategy of synthesizing α-MoO3−x nanorods boosted as traced by 1% graphene oxide: Efficient visible light photocatalysis and gas sensing applications. Sens. Actuators B Chem. 2019, 299, 126960. [Google Scholar] [CrossRef]


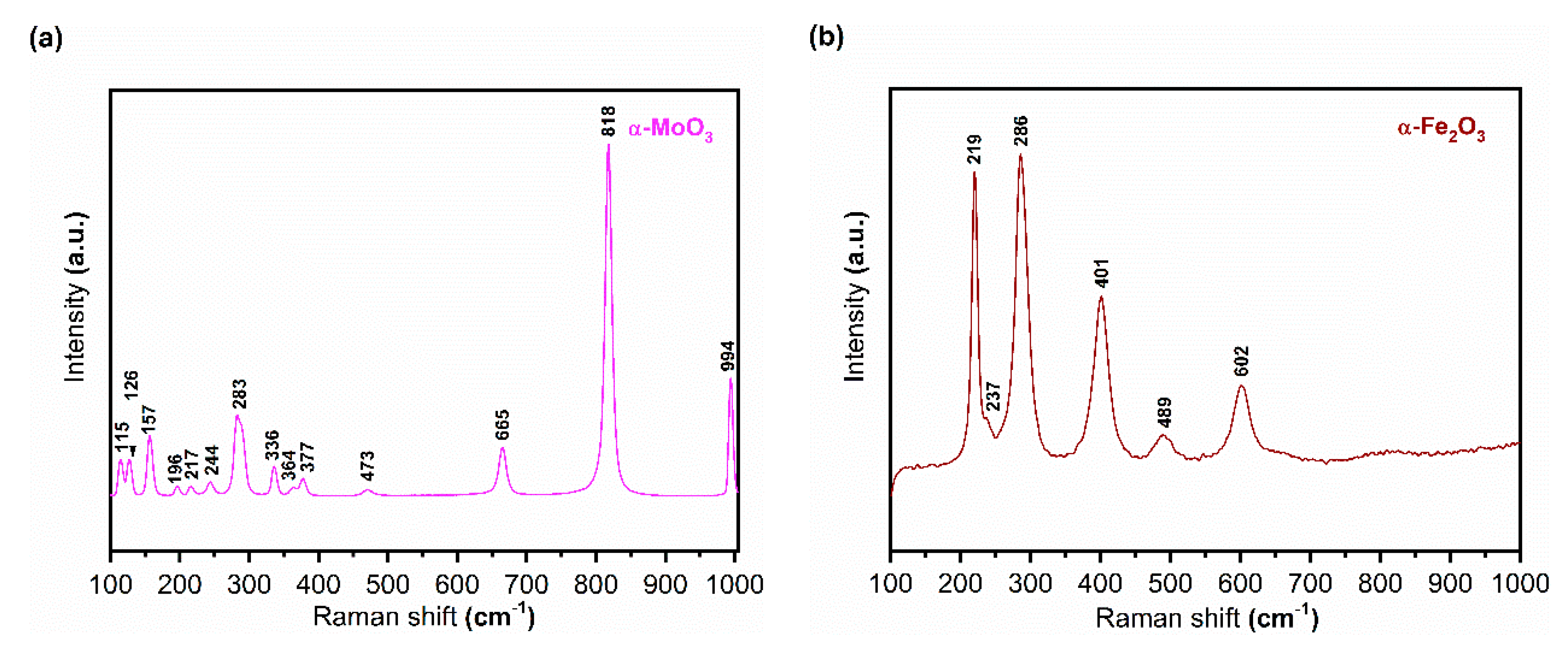

| Band Position (cm−1) | Assignments | ||
|---|---|---|---|
| This Work | Seguin et al. [46] | Jiebing et al. [47] | |
| 997 | 990 | 995 | ν(Mo=O) |
| 852 | 876 | 868 | δ(Mo–O–Mo) |
| 817 | 818 | 818 | ν(Mo=O) |
| 592 | 603 | 557 | ν(Mo–O) |
| 487 | 509 | 513 | ν(Mo–O) |
| Band Position (cm−1) | Assignments | |||
|---|---|---|---|---|
| This Work | Wang et al. [48] | Žic et. al. [49] | Grigorie et al. [50] | |
| 532 | 578 | 571 | 542 | Fe–O stretching mode |
| 449 | 485 | 461 | 488 | Fe–O stretching mode |
| 380 | 397 | α-Fe2O3 |
| Sample | Chemical Composition (%) | |||
|---|---|---|---|---|
| MoO3 | Fe2O3 | SO3 | P2O5 | |
| α-MoO3 | 83 | 0.1 | 16 | 0.8 |
| α-Fe2O3 | 100 | - | - | - |
| Sample | SBET (m2 g−1) | Pore Diameter (nm) | Pore Volume (cm3 g−1) |
|---|---|---|---|
| α-MoO3 | 3.24 | 5.80 | 4.70 × 10−3 |
| α-Fe2O3 | 8.23 | 7.39 | 1.52 × 10−2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sales, H.B.; Menezes, R.R.; Neves, G.A.; Souza, J.J.N.d.; Ferreira, J.M.; Chantelle, L.; Menezes de Oliveira, A.L.; Lira, H.d.L. Development of Sustainable Heterogeneous Catalysts for the Photocatalytic Treatment of Effluents. Sustainability 2020, 12, 7393. https://doi.org/10.3390/su12187393
Sales HB, Menezes RR, Neves GA, Souza JJNd, Ferreira JM, Chantelle L, Menezes de Oliveira AL, Lira HdL. Development of Sustainable Heterogeneous Catalysts for the Photocatalytic Treatment of Effluents. Sustainability. 2020; 12(18):7393. https://doi.org/10.3390/su12187393
Chicago/Turabian StyleSales, Herbet B., Romualdo R. Menezes, Gelmires A. Neves, João J. N. de Souza, Jailson M. Ferreira, Laís Chantelle, André L. Menezes de Oliveira, and Hélio de L. Lira. 2020. "Development of Sustainable Heterogeneous Catalysts for the Photocatalytic Treatment of Effluents" Sustainability 12, no. 18: 7393. https://doi.org/10.3390/su12187393








