A Forecasting and Prediction Methodology for Improving the Blue Economy Resilience to Climate Change in the Romanian Lower Danube Euroregion
Abstract
:1. Introduction
1.1. EU Blue Economy and Blue Growth
1.2. Impact of Climate Change on Aquatic Ecosystems
1.3. Romania BE in Relation to Climate Change
1.4. Deep Learning Approach in Aquatic Ecosystems
1.5. Aim and Uniqueness of This Study
2. Materials and Methods
2.1. Study Area
2.2. Dataset Description
2.3. The Analytical Framework Forecasting and Prediction Methodology
2.3.1. Multiple Linear Regression (MLR) Method
2.3.2. Time Series Analysis
3. Results and Discussion
3.1. Water Level Forecast Analysis
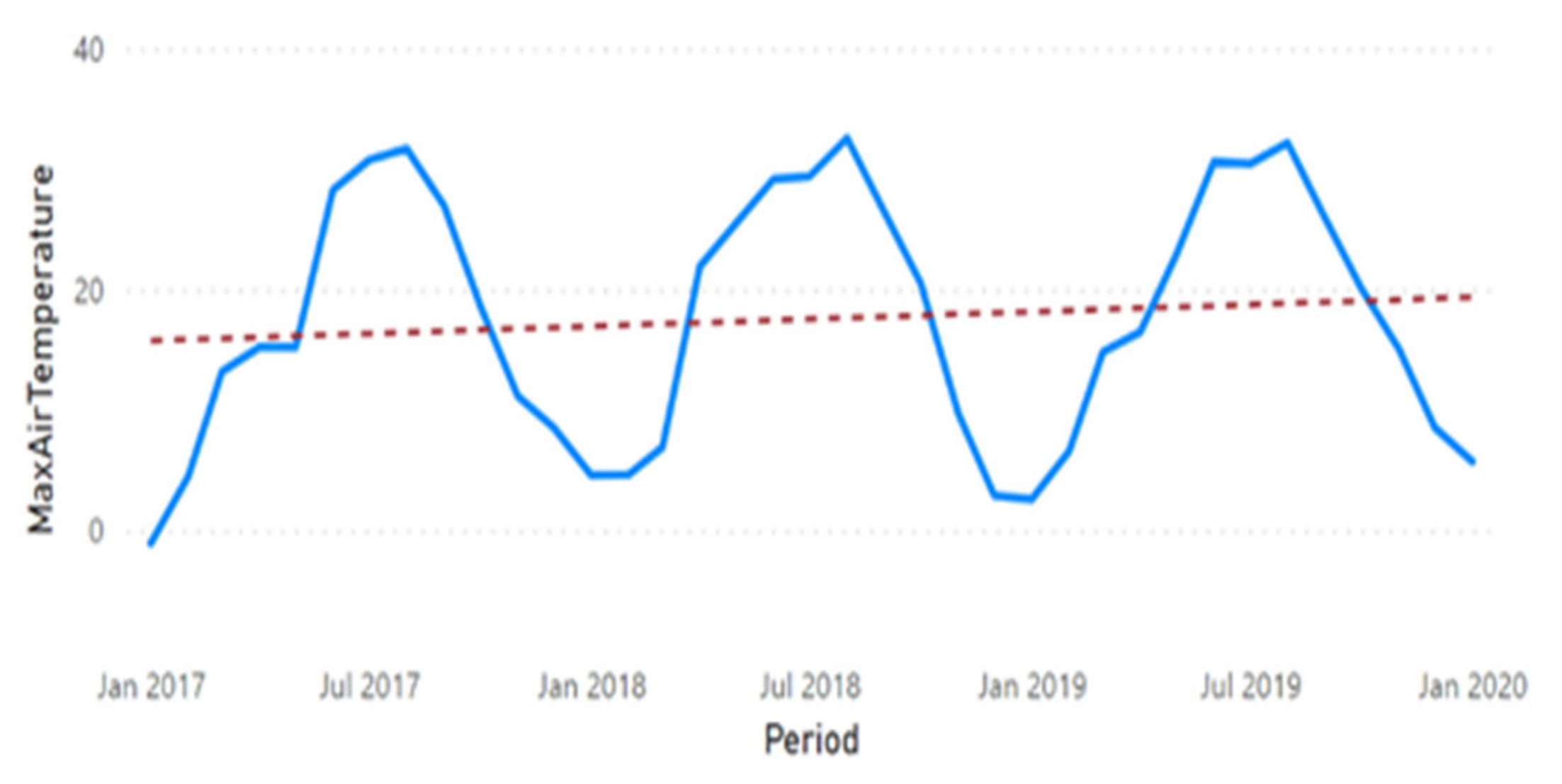
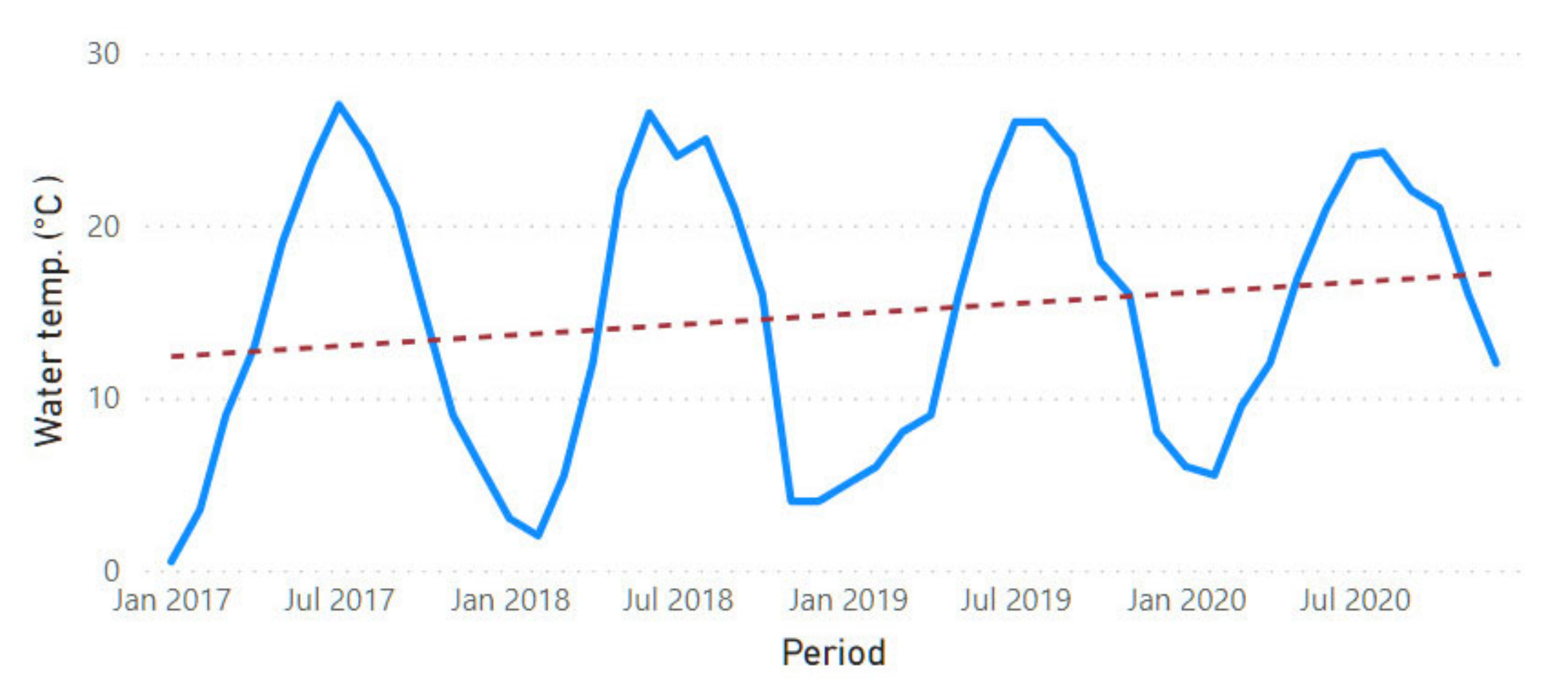
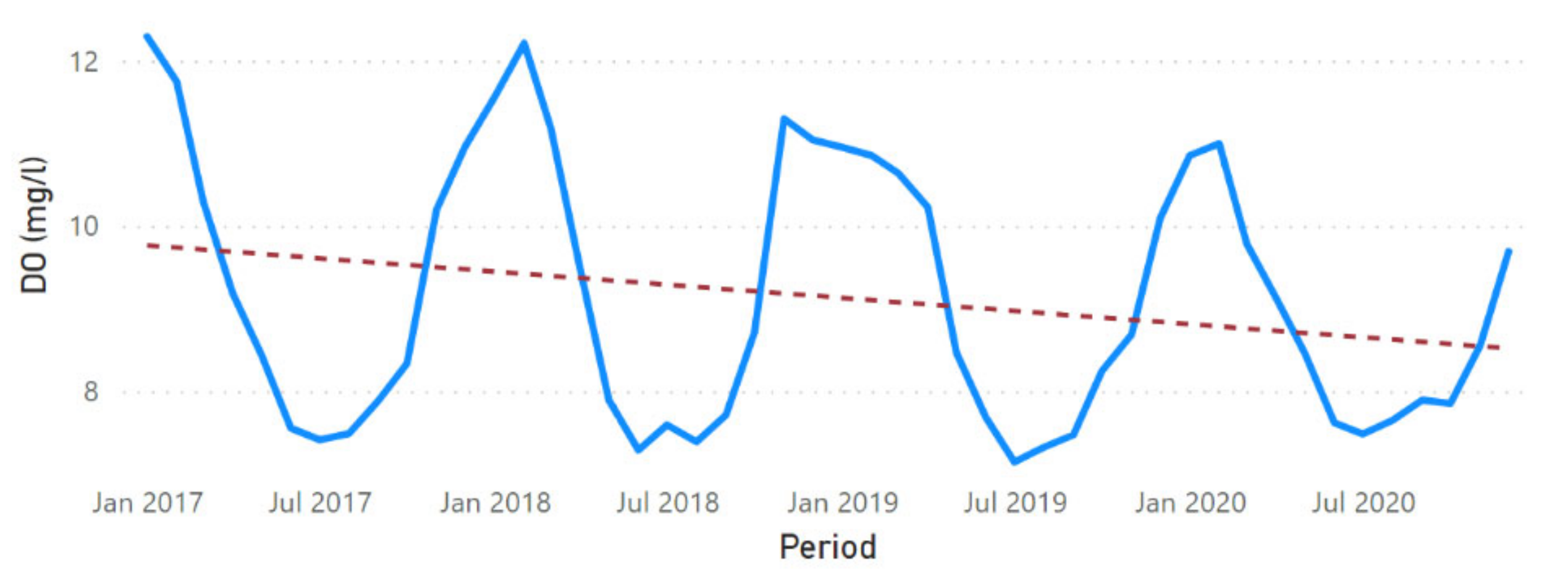
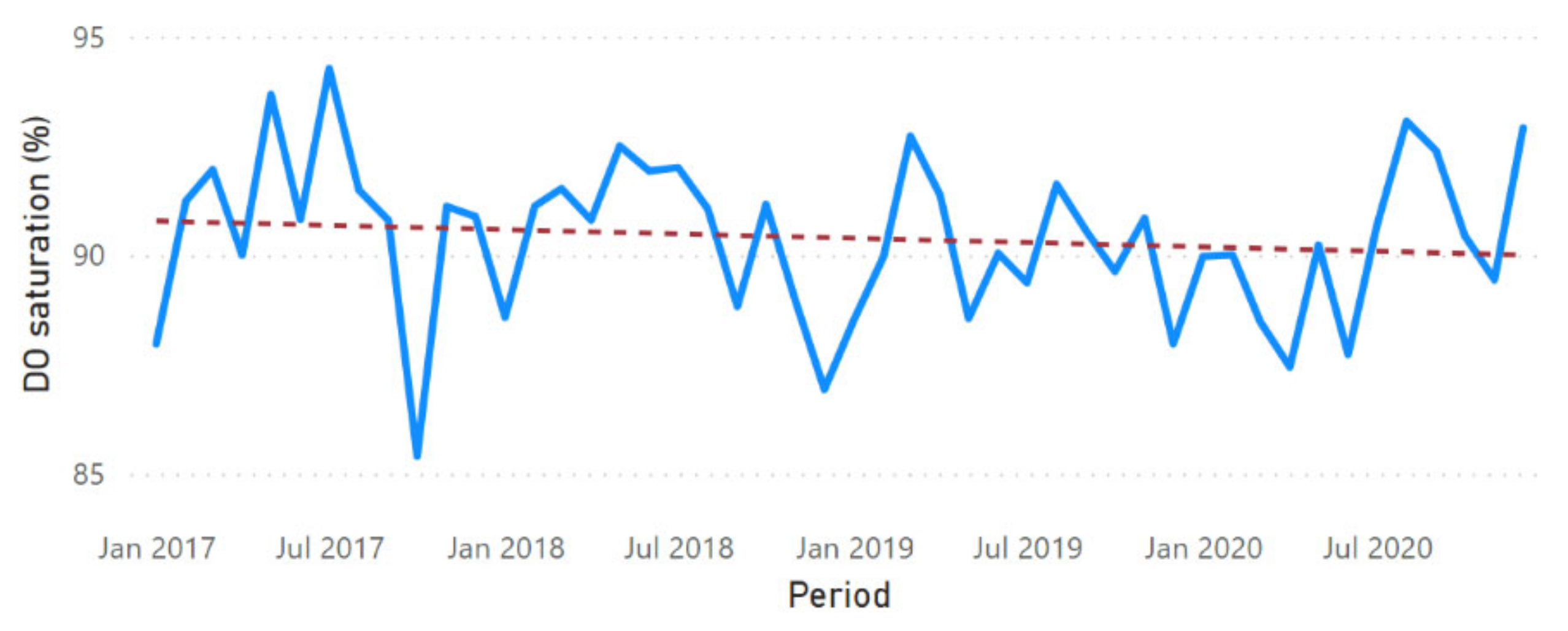
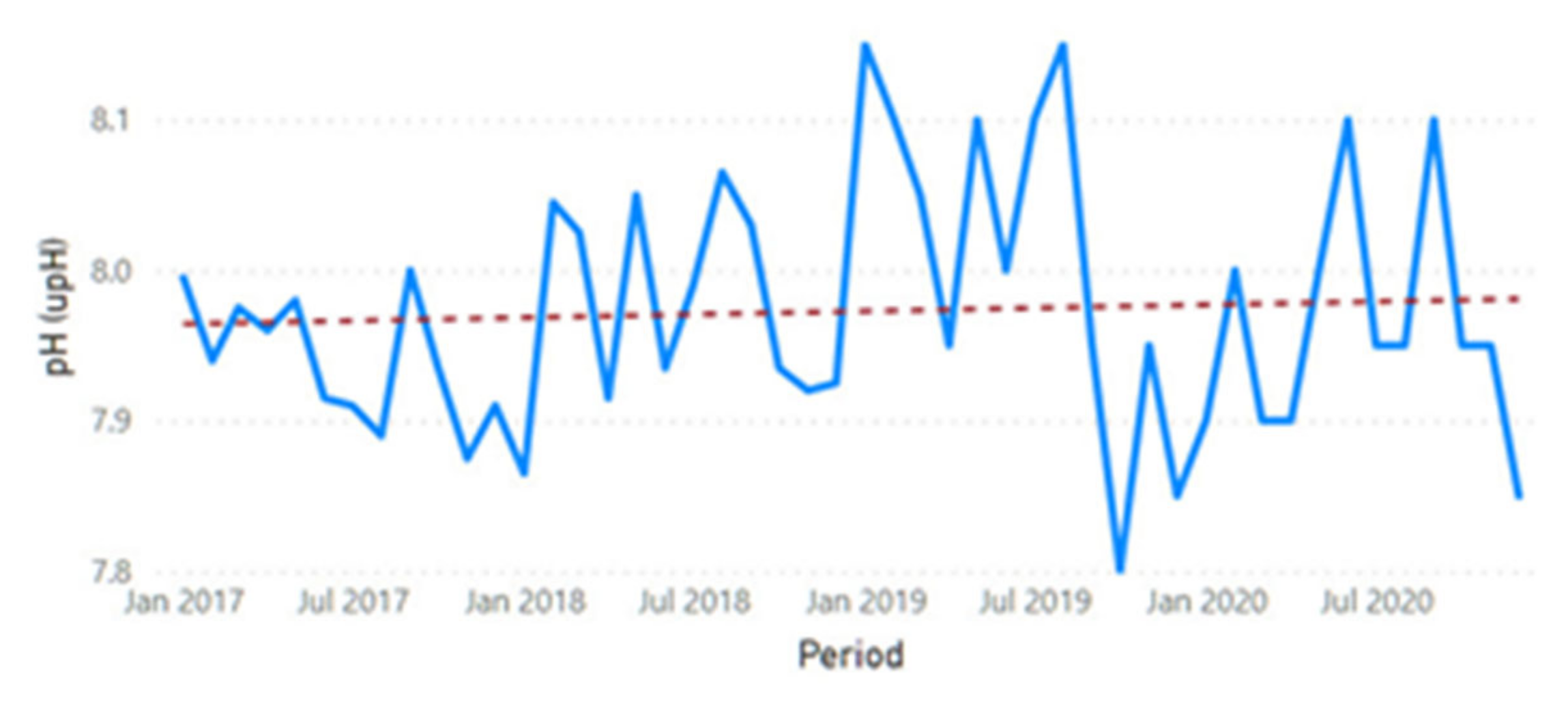
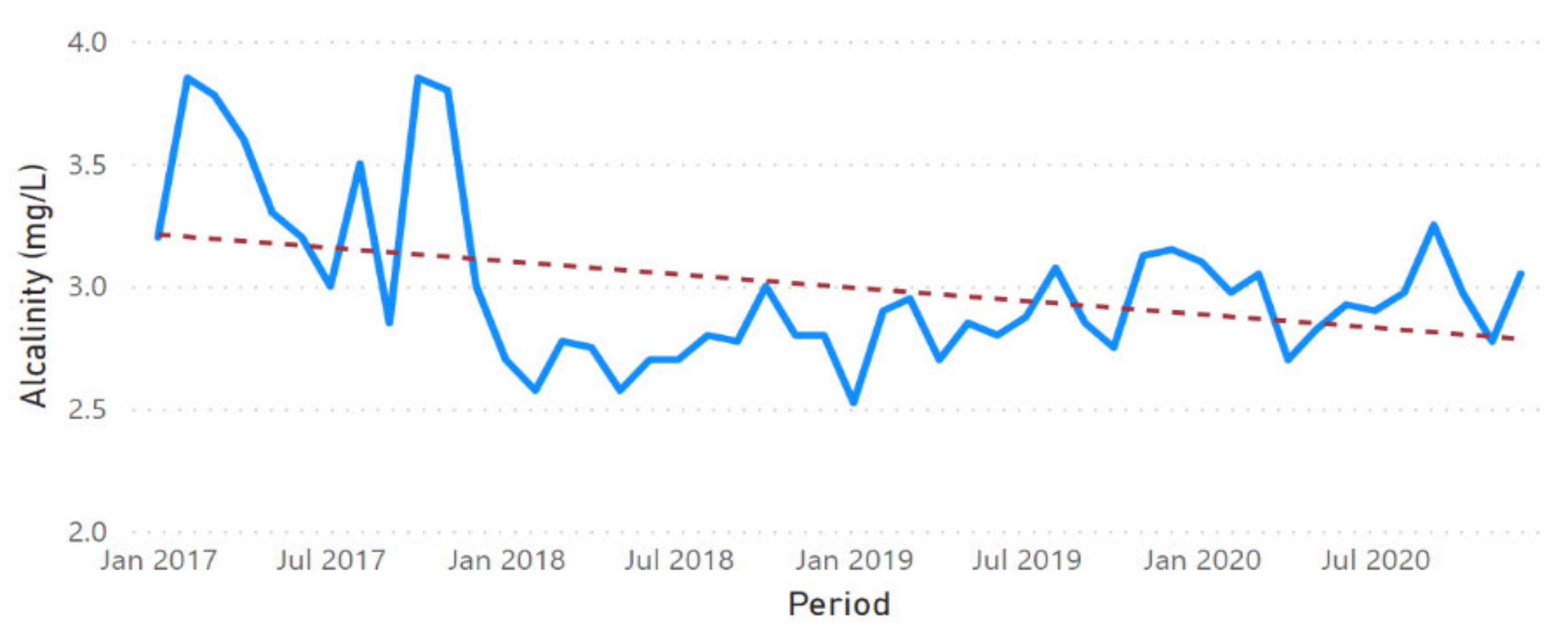
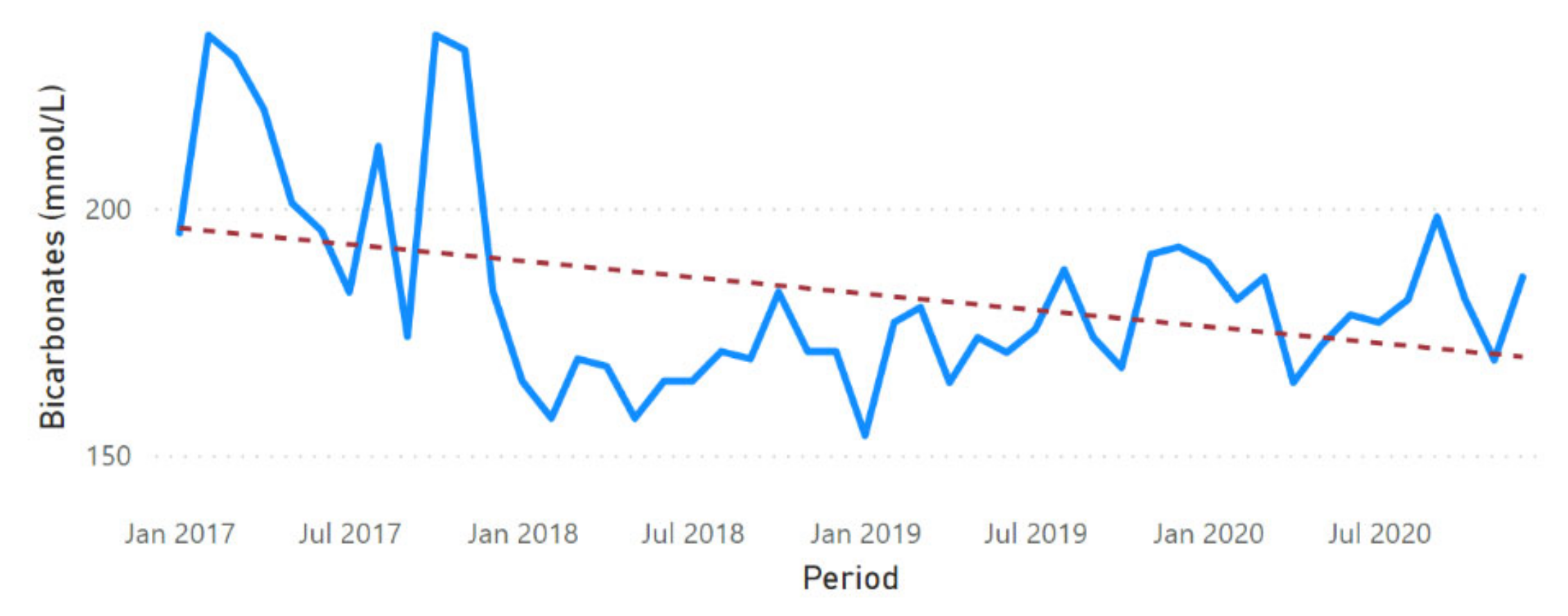
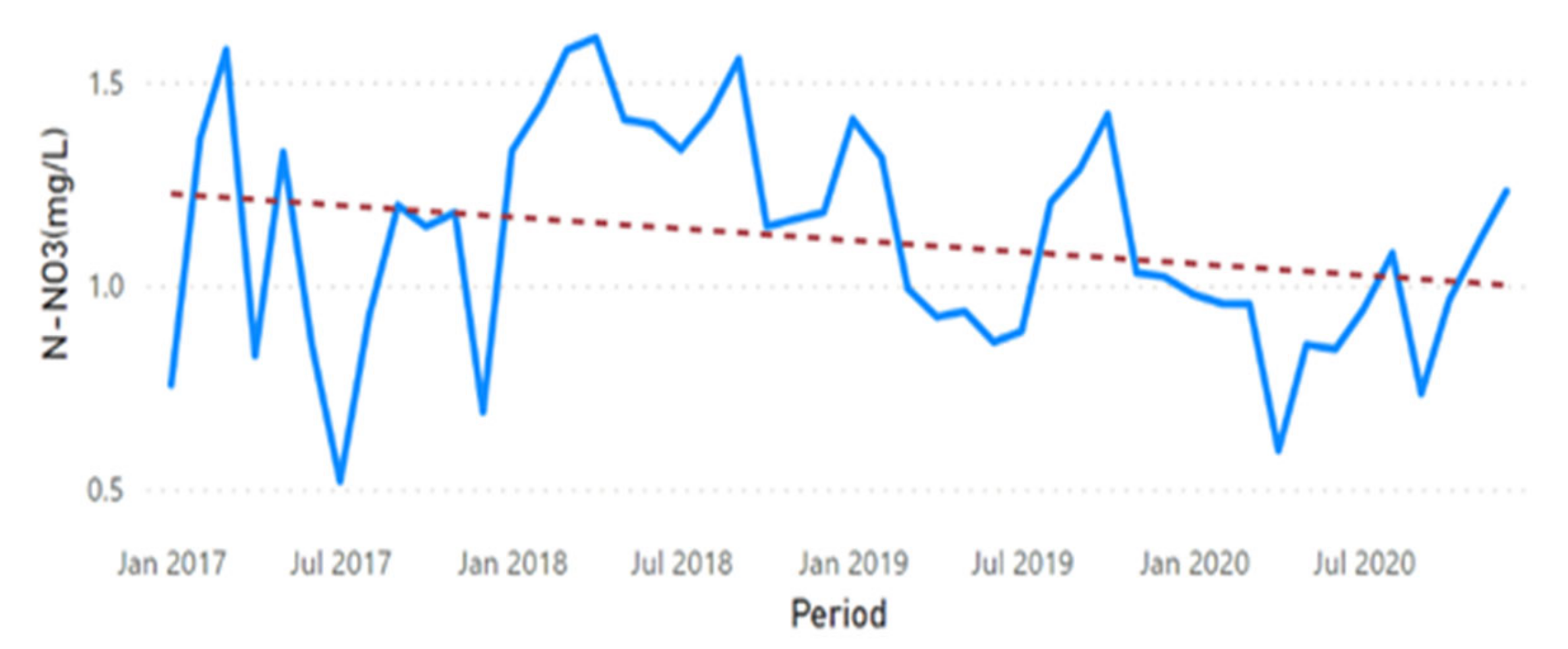
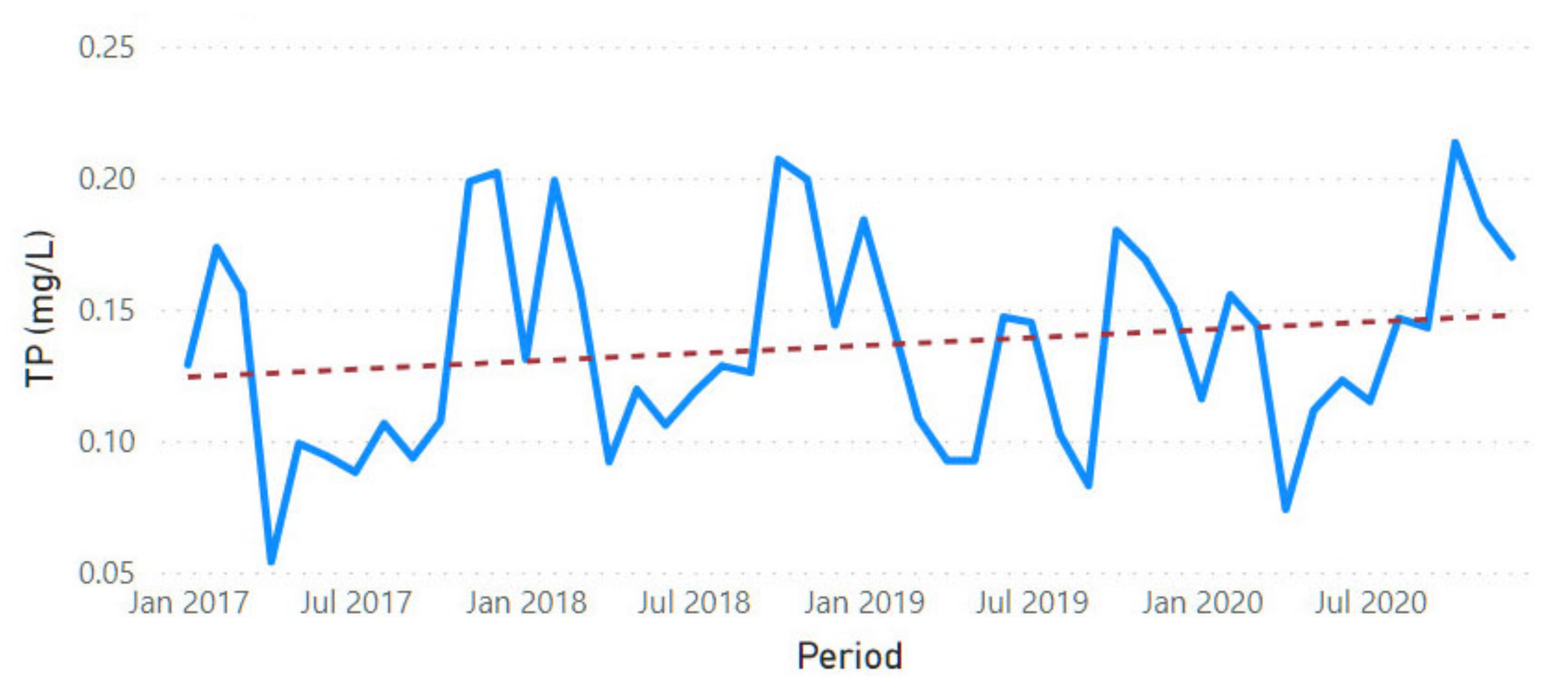
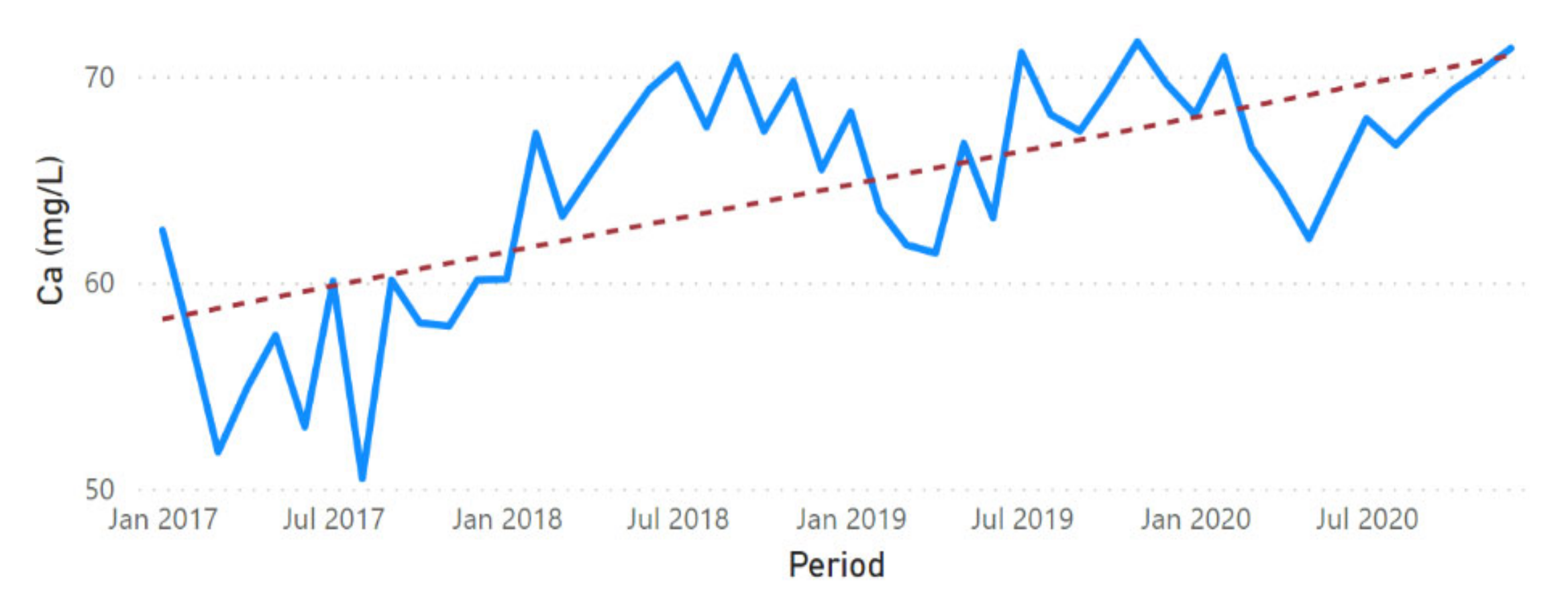
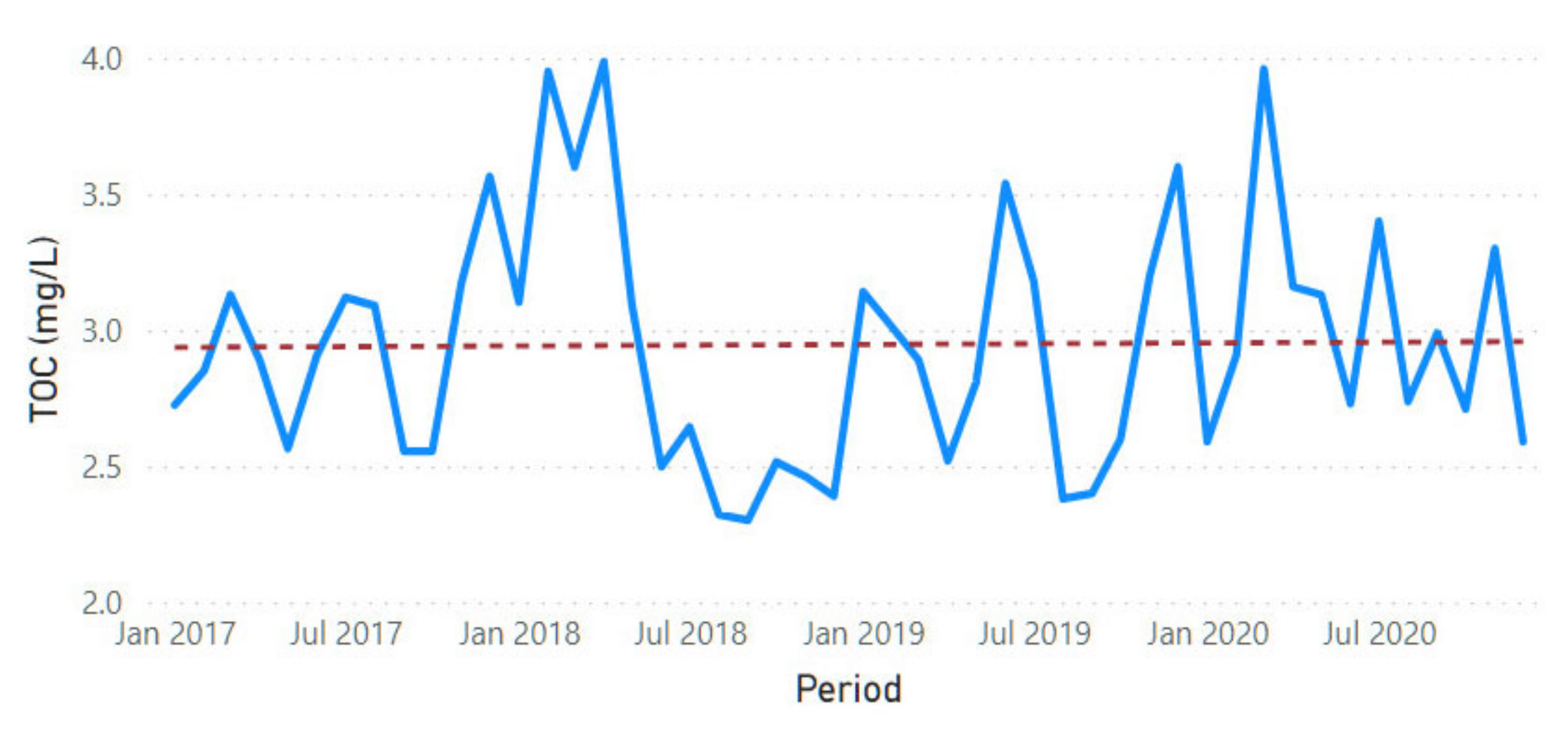
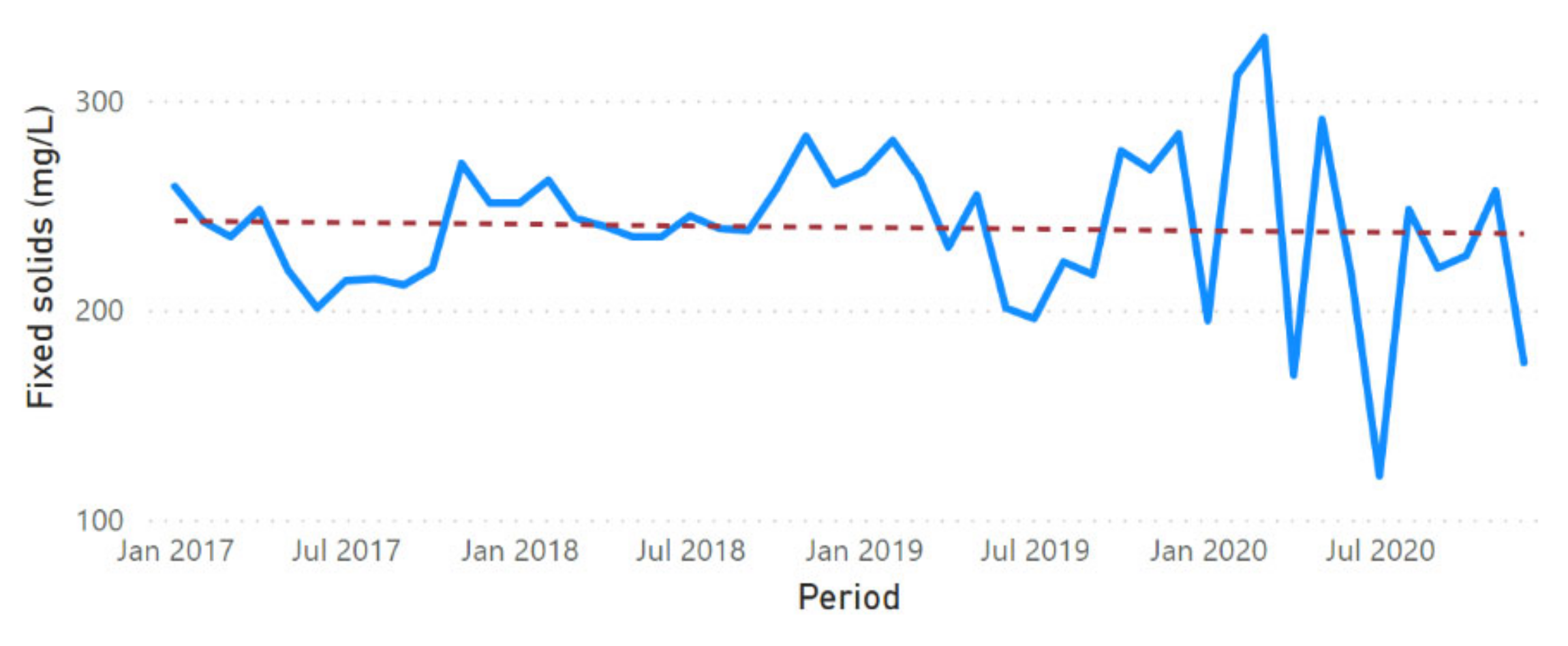
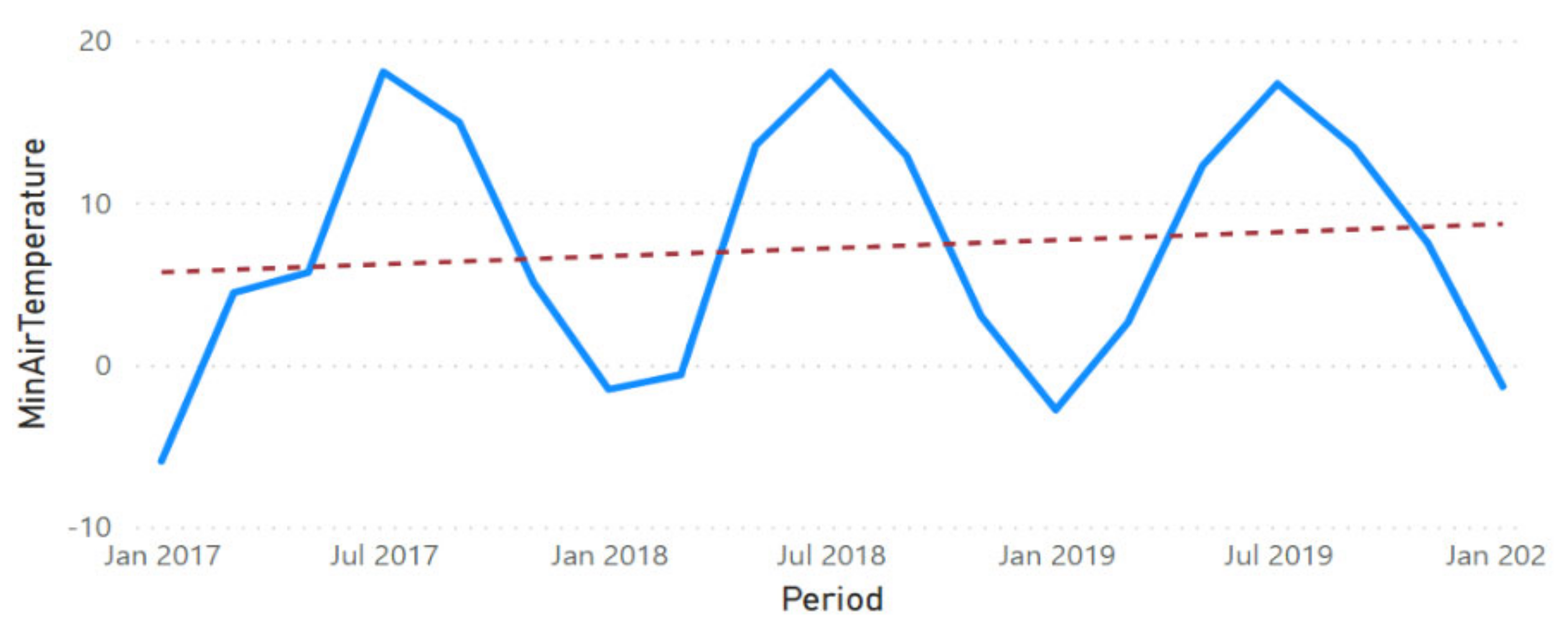
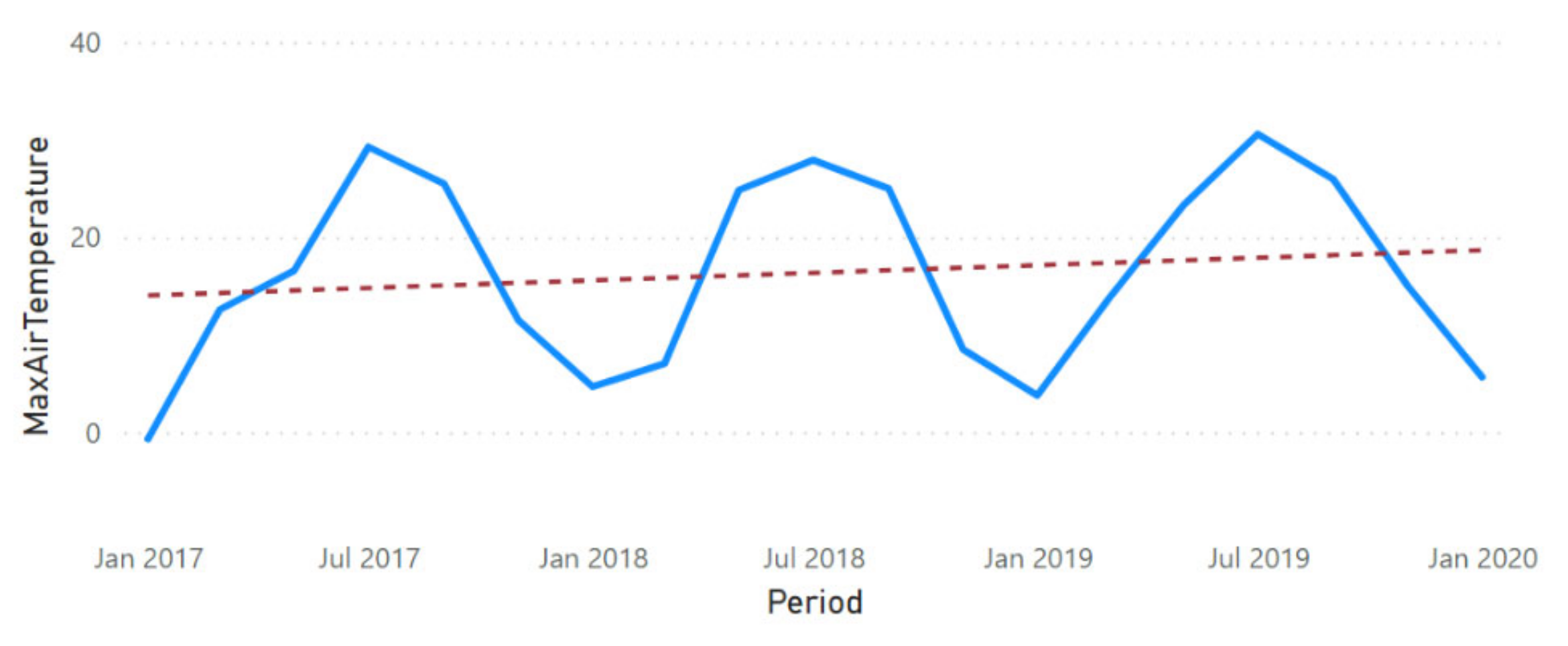
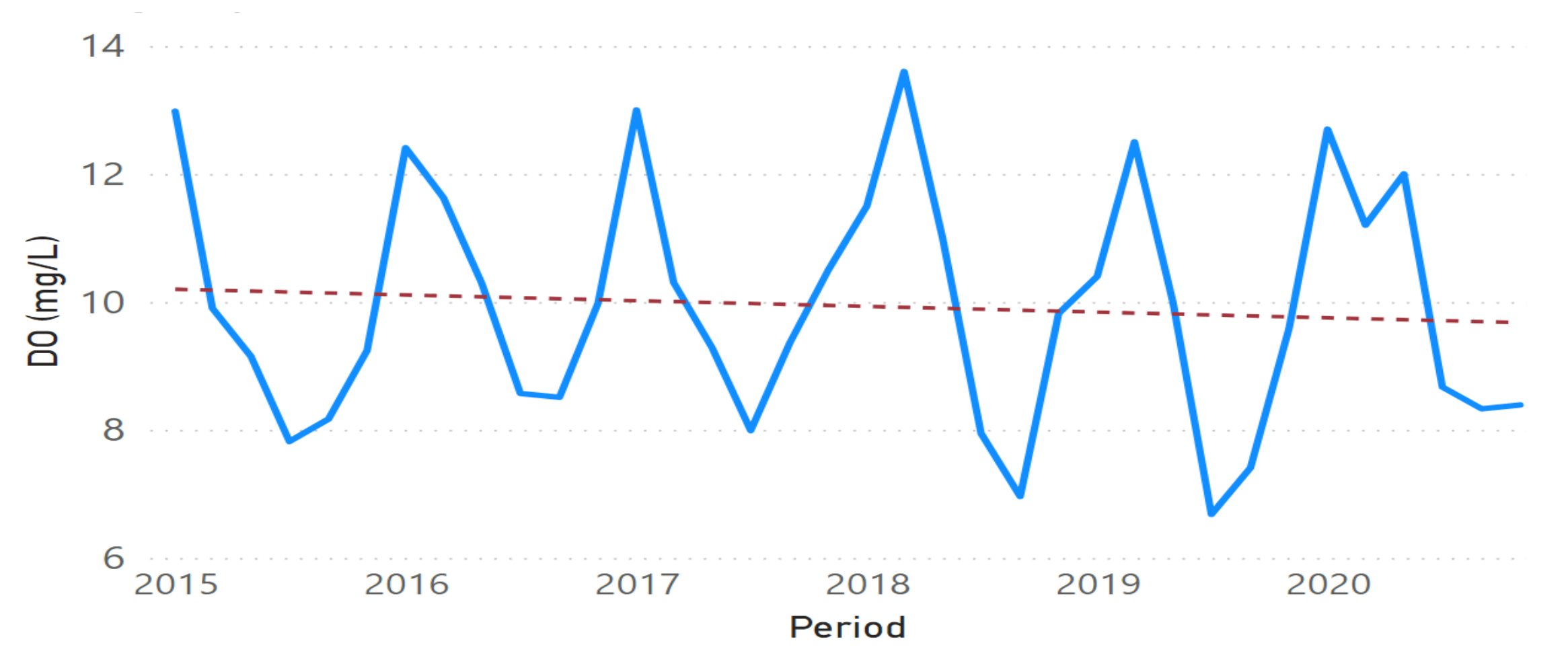
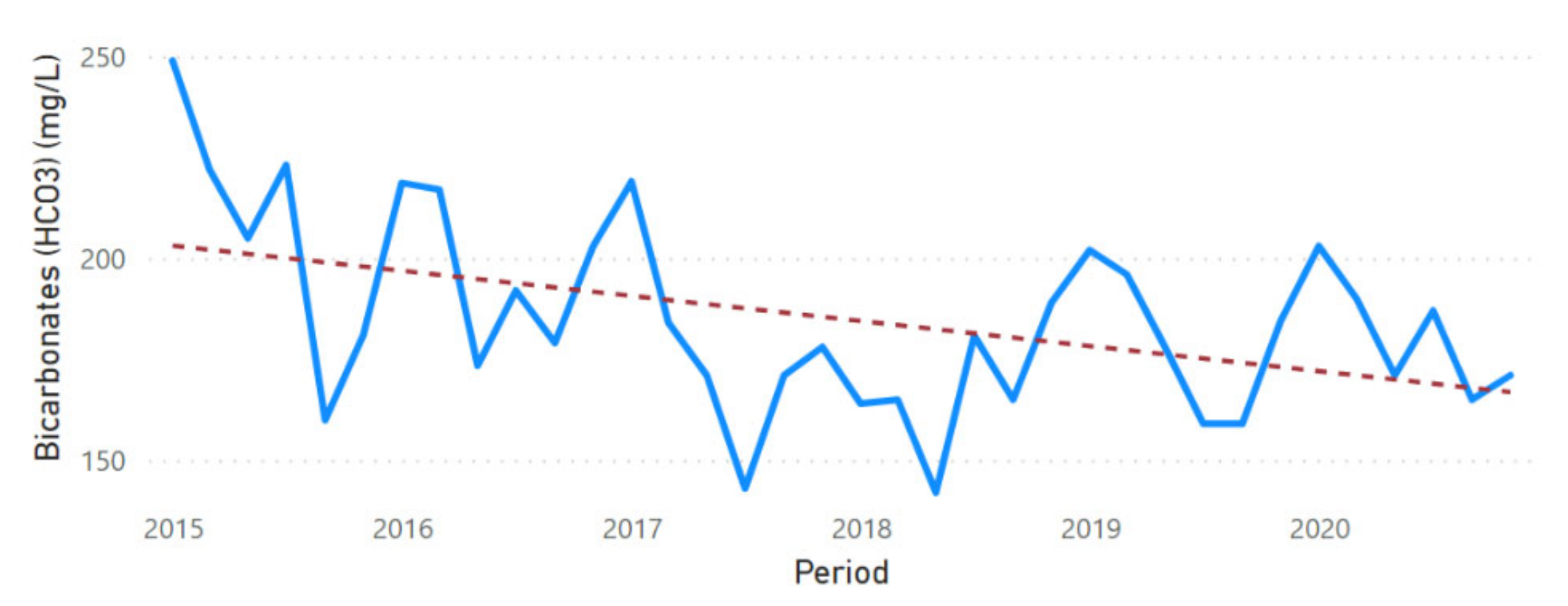
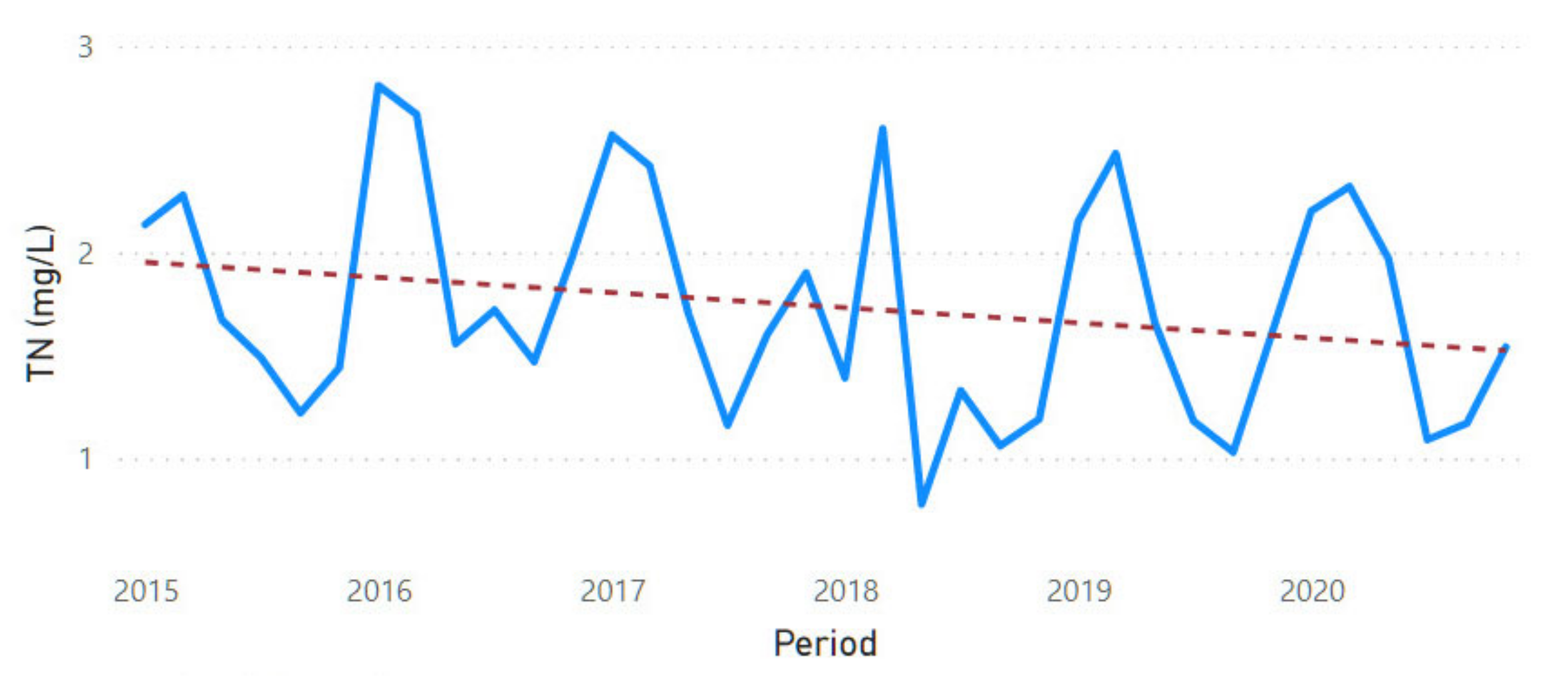
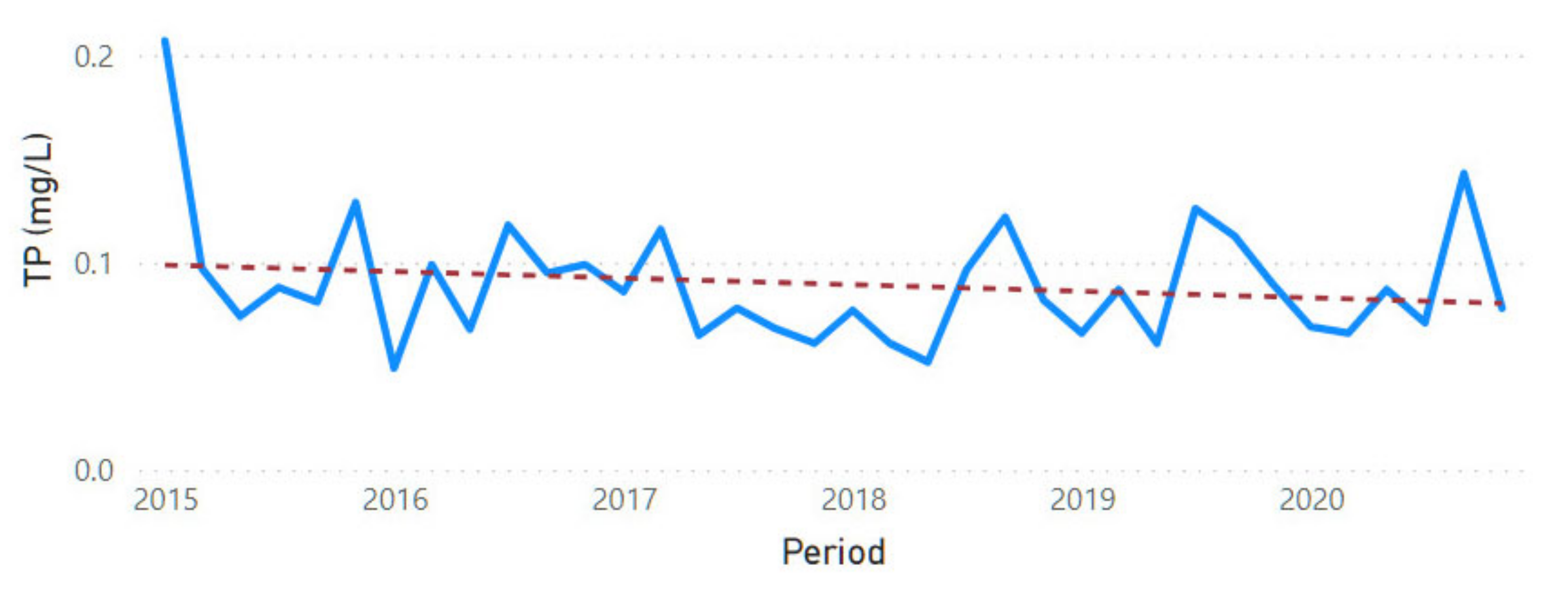
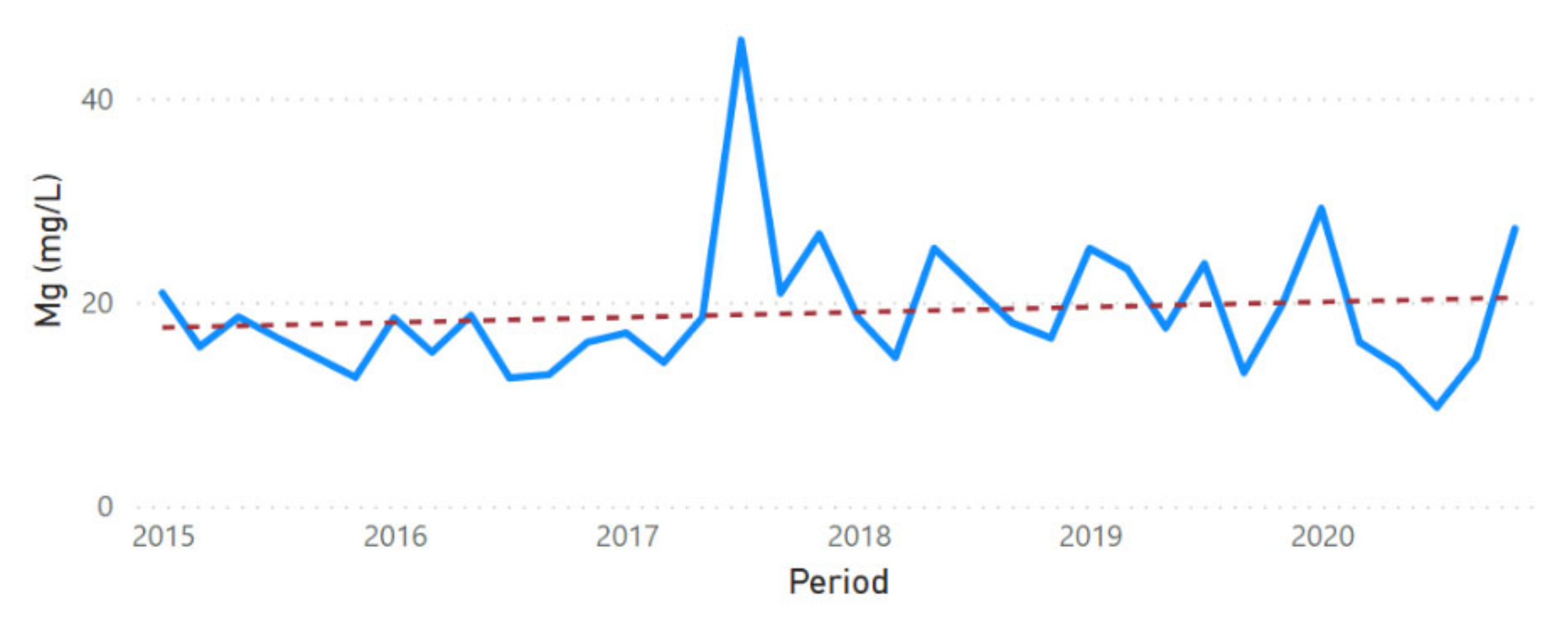
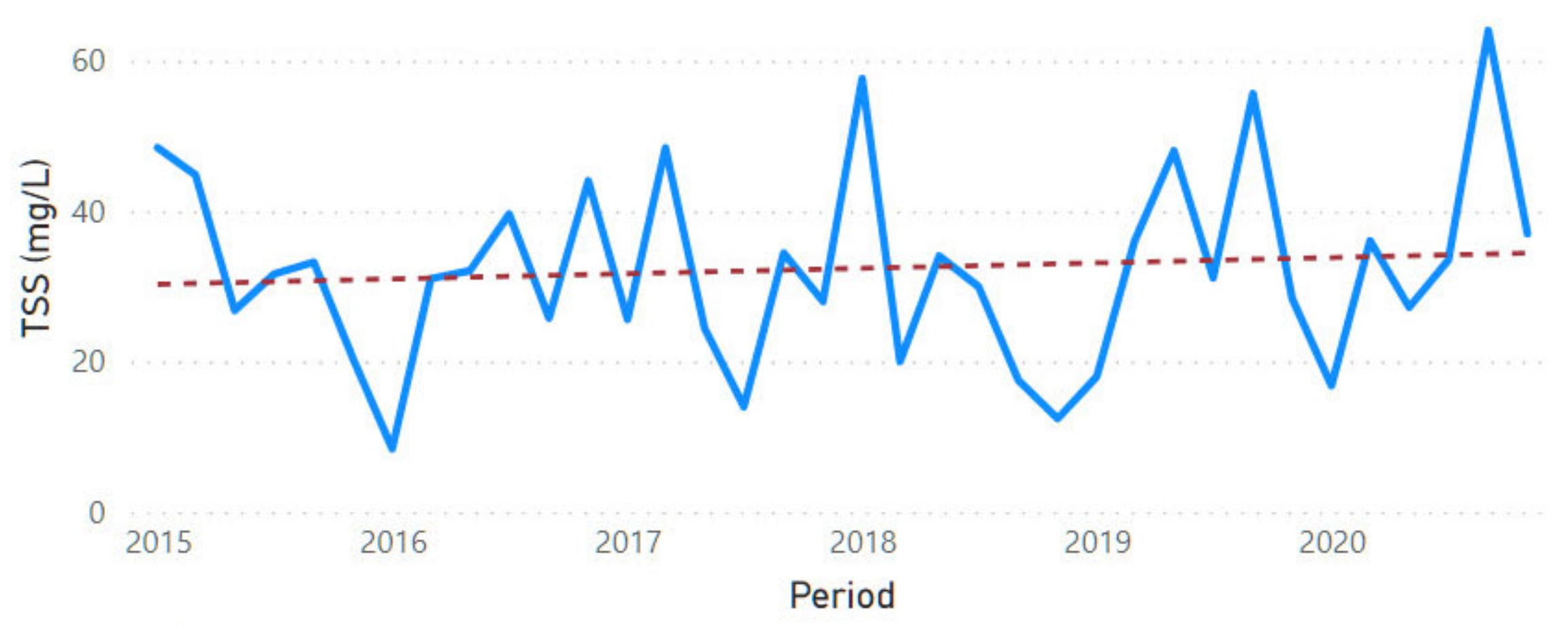
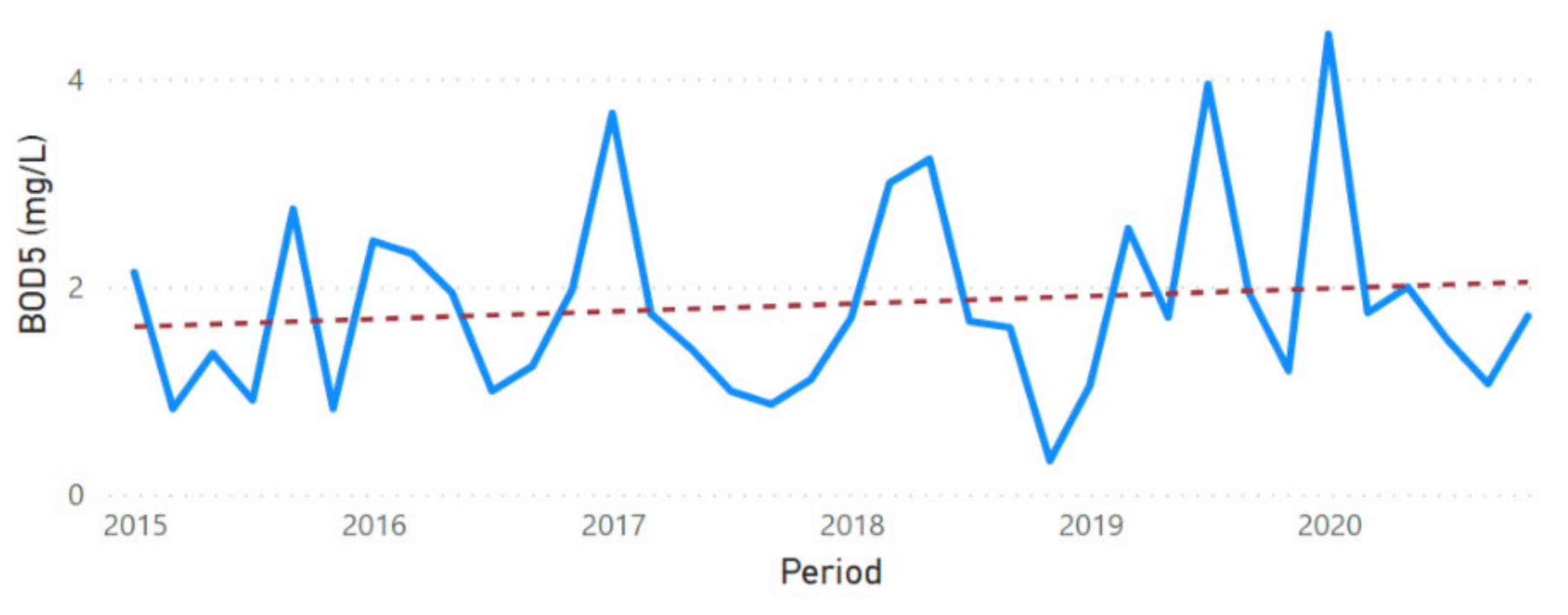
3.2. Physicochemical Parameter Trend Lines
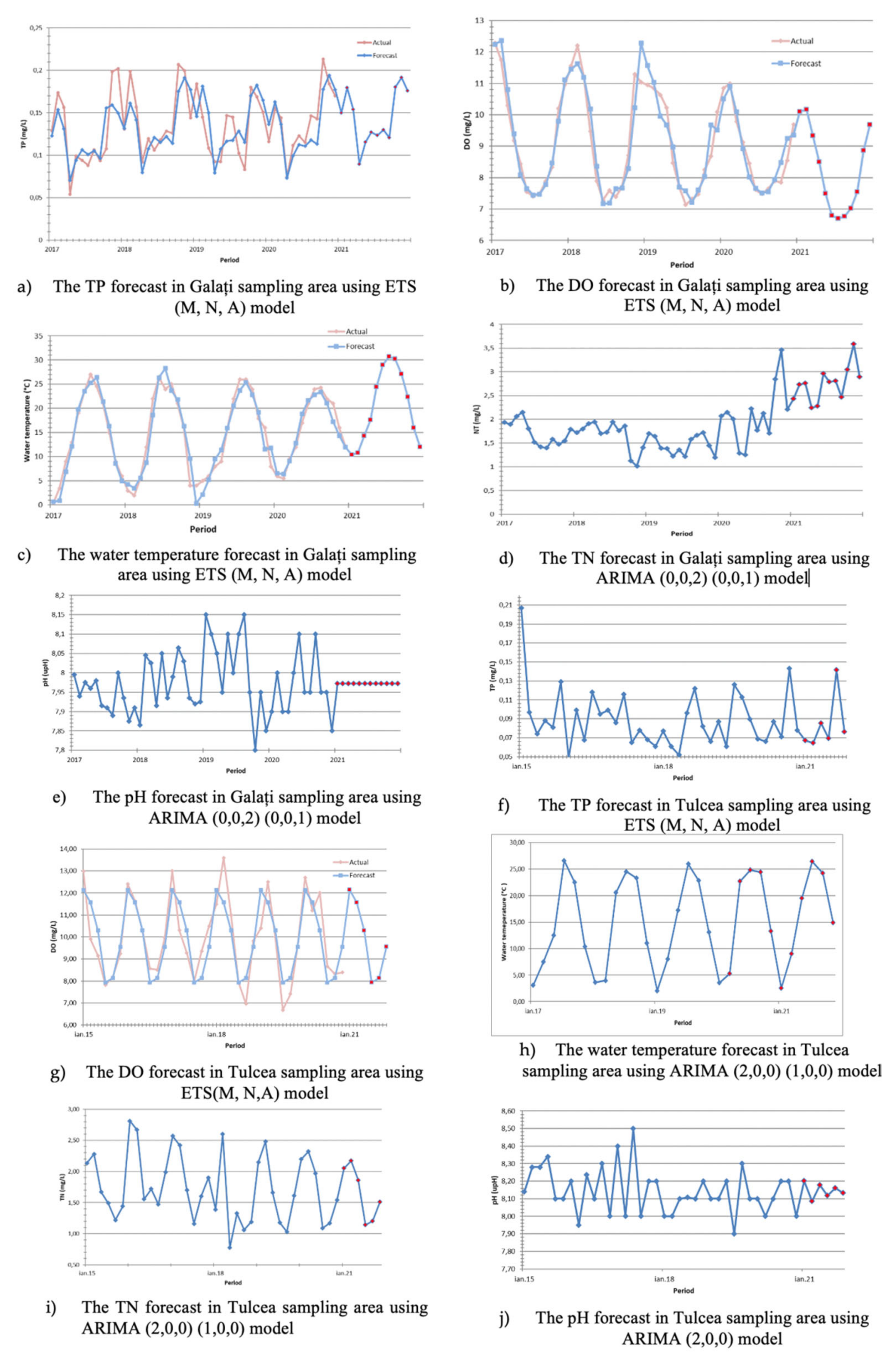
3.3. Physicochemical Parameter Forecast Analysis
3.4. Physicochemical Parameter Multiple Linear Regression (MLR) Models
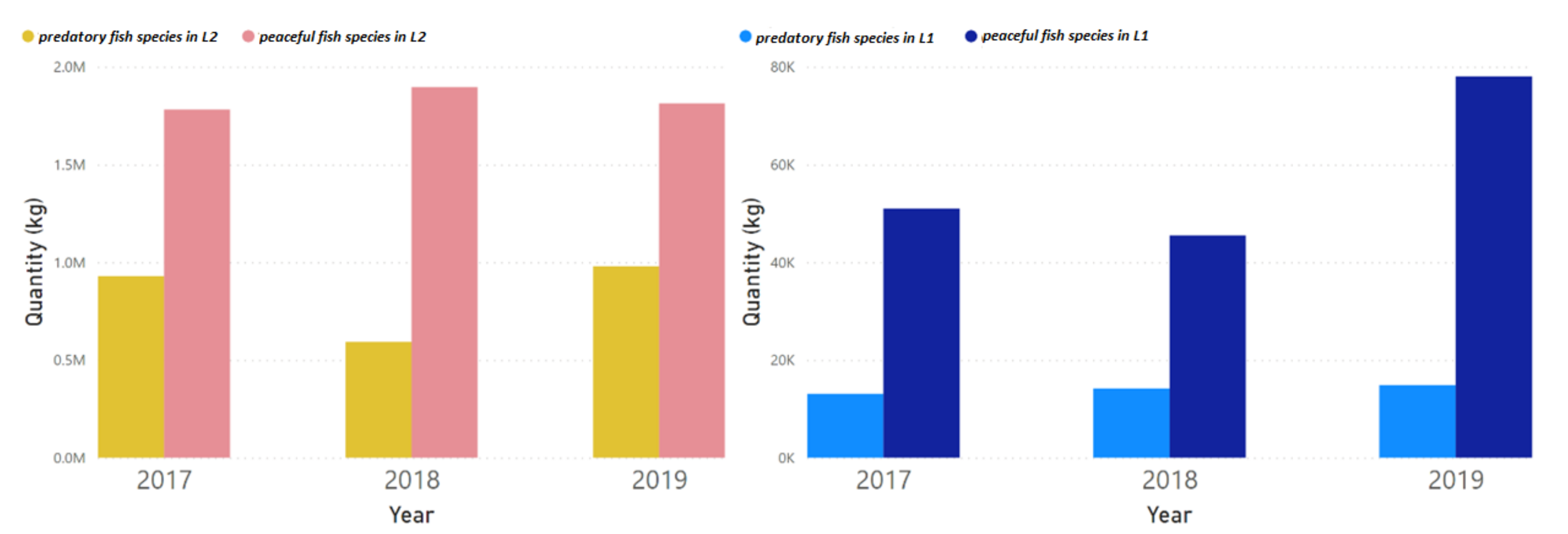
3.5. Multiple Linear Regression (MLR) Models Based on the Fish Catch Dataset for the L1 and L2 Study Area
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A















References
- Badîrcea, R.M.; Manta, A.G.; Florea, N.M.; Puiu, S.; Manta, L.F.; Doran, M.D. Connecting Blue Economy and Economic Growth to Climate Change: Evidence from European Union Countries. Energies 2021, 14, 4600. [Google Scholar] [CrossRef]
- UNEP Annual Report 2015. Available online: https://www.unep.org/annualreport/2015/en/index.html (accessed on 2 October 2021).
- Nagy, H.; Nene, S. Blue Gold: Advancing Blue Economy Governance in Africa. Sustainability 2021, 13, 7153. [Google Scholar] [CrossRef]
- Tianming, G.; Bobylev, N.; Gadal, S.; Lagutina, M.; Sergunin, A.; Erokhin, V. Planning for Sustainability: An Emerging Blue Economy in Russia’s Coastal Arctic? Sustainability 2021, 13, 4957. [Google Scholar] [CrossRef]
- Graziano, M.; Alexander, K.A.; Liesch, M.; Lema, E.; Torres, J.A. Understanding an Emerging Economic Discourse through Regional Analysis: Blue Economy Clusters in the U.S. Great Lakes Basin. Appl. Geogr. 2019, 105, 111–123. [Google Scholar] [CrossRef]
- Schutter, M.S.; Hicks, C.C. Networking the Blue Economy in Seychelles: Pioneers, Resistance, and the Power of Influence. J. Political Ecol. 2019, 26, 425–447. [Google Scholar] [CrossRef] [Green Version]
- The EU Blue Economy Report 2019—Publications Office of the EU. Available online: https://op.europa.eu/en/publication-detail/-/publication/676bbd4a-7dd9-11e9-9f05-01aa75ed71a1/language-en/ (accessed on 30 May 2021).
- Rountrey, A.N.; Coulson, P.G.; Meeuwig, J.J.; Meekan, M. Water Temperature and Fish Growth: Otoliths Predict Growth Patterns of a Marine Fish in a Changing Climate. Glob. Chang. Biol. 2014, 20, 2450–2458. [Google Scholar] [CrossRef]
- The EU Blue Economy Report 2021—Publications Office of the EU. Available online: https://op.europa.eu/en/publication-detail/-/publication/0b0c5bfd-c737-11eb-a925-01aa75ed71a1 (accessed on 2 October 2021).
- Boonstra, W.J.; Valman, M.; Björkvik, E. A Sea of Many Colours—How Relevant Is Blue Growth for Capture Fisheries in the Global North, and Vice Versa? Mar. Policy 2018, 87, 340–349. [Google Scholar] [CrossRef]
- Martínez-Vázquez, R.M.; Milán-García, J.; de Pablo Valenciano, J. Challenges of the Blue Economy: Evidence and Research Trends. Environ. Sci. Eur. 2021, 33, 61. [Google Scholar] [CrossRef]
- Ehlers, P. Blue Growth and Ocean Governance—How to Balance the Use and the Protection of the Seas. WMU J. Marit. Aff. 2016, 15, 187–203. [Google Scholar] [CrossRef] [Green Version]
- Bennett, A.; Basurto, X.; Virdin, J.; Lin, X.; Betances, S.J.; Smith, M.D.; Allison, E.H.; Best, B.A.; Brownell, K.D.; Campbell, L.M.; et al. Recognize Fish as Food in Policy Discourse and Development Funding. Ambio 2021, 50, 981–989. [Google Scholar] [CrossRef]
- Omar, M.E.D.M.; Moussa, A.M.A.; Hinkelmann, R. Impacts of Climate Change on Water Quantity, Water Salinity, Food Security, and Socioeconomy in Egypt. Water Sci. Eng. 2021, 14, 17–27. [Google Scholar] [CrossRef]
- Letelier, R.M.; Björkman, K.M.; Church, M.J.; Hamilton, D.S.; Mahowald, N.M.; Scanza, R.A.; Schneider, N.; White, A.E.; Karl, D.M. Climate-Driven Oscillation of Phosphorus and Iron Limitation in the North Pacific Subtropical Gyre. Proc. Natl. Acad. Sci. USA 2019, 116, 12720–12728. [Google Scholar] [CrossRef] [Green Version]
- Mates, D.; Grosu, V.; Hlaciuc, E.; Bostan, I.; Bunget, O.; Domil, A.; Moraru, M.; Artene, A. Biological Assets and the Agricultural Products in the Context of the Implementation of the IAS 41: A Case Study of the Romanian Agro-Food System. Arch. Biol. Sci. 2015, 67, 705–714. [Google Scholar] [CrossRef]
- Stenevik, E.K.; Sundby, S. Impacts of Climate Change on Commercial Fish Stocks in Norwegian Waters. Mar. Policy 2007, 31, 19–31. [Google Scholar] [CrossRef]
- Doney, S.C.; Ruckelshaus, M.; Emmett Duffy, J.; Barry, J.P.; Chan, F.; English, C.A.; Galindo, H.M.; Grebmeier, J.M.; Hollowed, A.B.; Knowlton, N.; et al. Climate Change Impacts on Marine Ecosystems. Annu. Rev. Mar. Sci. 2012, 4, 11–37. [Google Scholar] [CrossRef] [Green Version]
- Okey, T.A.; Alidina, H.M.; Agbayani, S. Mapping Ecological Vulnerability to Recent Climate Change in Canada’s Pacific Marine Ecosystems. Ocean Coast. Manag. 2015, 106, 35–48. [Google Scholar] [CrossRef]
- Madeira, D.; Araújo, J.E.; Vitorino, R.; Capelo, J.L.; Vinagre, C.; Diniz, M.S. Ocean Warming Alters Cellular Metabolism and Induces Mortality in Fish Early Life Stages: A Proteomic Approach. Environ. Res. 2016, 148, 164–176. [Google Scholar] [CrossRef]
- Hiddink, J.G.; ter Hofstede, R. Climate Induced Increases in Species Richness of Marine Fishes. Glob. Chang. Biol. 2008, 14, 453–460. [Google Scholar] [CrossRef]
- Cheung, W.W.L.; Lam, V.W.Y.; Sarmiento, J.L.; Kearney, K.; Watson, R.; Pauly, D. Projecting Global Marine Biodiversity Impacts under Climate Change Scenarios. Fish Fish. 2009, 10, 235–251. [Google Scholar] [CrossRef]
- Munday, P.L.; Jones, G.P.; Pratchett, M.S.; Williams, A.J. Climate Change and the Future for Coral Reef Fishes. Fish Fish. 2008, 9, 261–285. [Google Scholar] [CrossRef]
- Allison, E.H.; Perry, A.L.; Badjeck, M.C.; Neil Adger, W.; Brown, K.; Conway, D.; Halls, A.S.; Pilling, G.M.; Reynolds, J.D.; Andrew, N.L.; et al. Vulnerability of National Economies to the Impacts of Climate Change on Fisheries. Fish Fish. 2009, 10, 173–196. [Google Scholar] [CrossRef] [Green Version]
- Cheung, W.W.L.; Close, C.; Lam, V.; Watson, R.; Pauly, D. Application of Macroecological Theory to Predict Effects of Climate Change on Global Fisheries Potential. Mar. Ecol. Prog. Ser. 2008, 365, 187–197. [Google Scholar] [CrossRef] [Green Version]
- Storch, D.; Menzel, L.; Frickenhaus, S.; Pörtner, H.O. Climate Sensitivity across Marine Domains of Life: Limits to Evolutionary Adaptation Shape Species Interactions. Glob. Chang. Biol. 2014, 20, 3059–3067. [Google Scholar] [CrossRef] [Green Version]
- Bradley, M.; van Putten, I.; Sheaves, M. The Pace and Progress of Adaptation: Marine Climate Change Preparedness in Australia’s Coastal Communities. Mar. Policy 2015, 53, 13–20. [Google Scholar] [CrossRef]
- Diop, B.; Blanchard, F.; Sanz, N. Mangrove Increases Resiliency of the French Guiana Shrimp Fishery Facing Global Warming. Ecol. Model. 2018, 387, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Smederevac-Lalić, M.M.; Kalauzi, A.J.; Regner, S.B.; Lenhardt, M.B.; Naunovic, Z.Z.; Hegediš, A.E. Prediction of Fish Catch in the Danube River Based on Long-Term Variability in Environmental Parameters and Catch Statistics. Sci. Total Environ. 2017, 609, 664–671. [Google Scholar] [CrossRef]
- Kahl, U.; Hülsmann, S.; Radke, R.J.; Benndorf, J. The Impact of Water Level Fluctuations on the Year Class Strength of Roach: Implications for Fish Stock Management. Limnologica 2008, 38, 258–268. [Google Scholar] [CrossRef] [Green Version]
- Górski, K.; van den Bosch, L.V.; van de Wolfshaar, K.E.; Middelkoop, H.; Nagelkerke, L.A.J.; Filippov, O.V.; Zolotarev, D.V.; Yakovlev, S.V.; Minin, A.E.; Winter, H.V.; et al. Post-Damming Flow Regime Development in a Large Lowland River (Volga, Russian Federation): Implications For Floodplain Inundation And Fisheries. River Res. Appl. 2012, 28, 1121–1134. [Google Scholar] [CrossRef]
- Liang, J.; Yu, X.; Zeng, G.; Wu, H.; Lai, X.; Li, X.; Huang, L.; Yuan, Y.; Guo, S.; Dai, J. A Hydrologic Index Based Method for Determining Ecologically Acceptable Water-Level Range of Dongting Lake. J. Limnol. 2014, 73, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Dasgupta, S.; Laplante, B.; Meisner, C.; Wheeler, D.; Yan, J. The Impact of Sea Level Rise on Developing Countries: A Comparative Analysis. Clim. Chang. 2009, 93, 379–388. [Google Scholar] [CrossRef]
- De Lima, F.T.; Reynalte-Tataje, D.A.; Zaniboni-Filho, E. Effects of Reservoirs Water Level Variations on Fish Recruitment. Neotrop. Ichthyol. 2017, 15, e160084. [Google Scholar] [CrossRef] [Green Version]
- Ecologia e Manejo Recursos Pesqueiros em Reservatórios do Brasil—PDF Free Download. Available online: https://docplayer.com.br/8700634-Ecologia-e-manejo-recursos-pesqueiros-em-reservatorios-do-brasil.html (accessed on 2 October 2021).
- Feuchtmayr, H.; Moran, R.; Hatton, K.; Connor, L.; Heyes, T.; Moss, B.; Harvey, I.; Atkinson, D. Global Warming and Eutrophication: Effects on Water Chemistry and Autotrophic Communities in Experimental Hypertrophic Shallow Lake Mesocosms. J. Appl. Ecol. 2009, 46, 713–723. [Google Scholar] [CrossRef]
- Suddick, E.C.; Whitney, P.; Townsend, A.R.; Davidson, E.A. The Role of Nitrogen in Climate Change and the Impacts of Nitrogen-Climate Interactions in the United States: Foreword to Thematic Issue. Biogeochemistry 2013, 114, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, C.; Greuell, W.; Andersson, J.; Gerten, D.; Pisacane, G.; Roudier, P.; Ludwig, F. Impacts of Climate Change on European Hydrology at 1.5, 2 and 3 Degrees Mean Global Warming above Preindustrial Level. Clim. Chang. 2017, 143, 13–26. [Google Scholar] [CrossRef] [Green Version]
- Kiesel, J.; Gericke, A.; Rathjens, H.; Wetzig, A.; Kakouei, K.; Jähnig, S.C.; Fohrer, N. Climate Change Impacts on Ecologically Relevant Hydrological Indicators in Three Catchments in Three European Ecoregions. Ecol. Eng. 2019, 127, 404–416. [Google Scholar] [CrossRef]
- Stefan, D.S.; Stefan, M. Water Stress Induced by Enrichment of Nutrient and Climate Change Factors. In Water Stress in Plants; Intechopen: London, UK, 2016. [Google Scholar]
- Oschlies, A.; Brandt, P.; Stramma, L.; Schmidtko, S. Drivers and Mechanisms of Ocean Deoxygenation. Nat. Geosci. 2018, 11, 467–473. [Google Scholar] [CrossRef]
- Schmidtko, S.; Stramma, L.; Visbeck, M. Decline in Global Oceanic Oxygen Content during the Past Five Decades. Nature 2017, 542, 335–339. [Google Scholar] [CrossRef]
- Helm, K.P.; Bindoff, N.L.; Church, J.A. Observed Decreases in Oxygen Content of the Global Ocean. Geophys. Res. Lett. 2011, 38. [Google Scholar] [CrossRef]
- Hennige, S.; Roberts, J.; Williamson, P. Secretariat of Convention on Biological Diversity: An. Updated Synthesis of the Impacts of Ocean. Acidification on Marine Biodiversity; CBD Technical Series; Secretariat of the Convention on Biological Diversity: Montreal, QC, Canada, 2014. [Google Scholar]
- Griffin, R.C. Water Resource Economics: The Analysis of Scarcity, Policies, and Projects; The MIT Press: Cambridge, MA, USA, 2006; 402p. [Google Scholar]
- Zhang, N.; Wu, T.; Wang, B.; Dong, L.; Ren, J. Sustainable Water Resource and Endogenous Economic Growth. Technol. Forecast. Soc. Chang. 2016, 112, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Kung, C.C.; Wu, T. Influence of Water Allocation on Bioenergy Production under Climate Change: A Stochastic Mathematical Programming Approach. Energy 2021, 231, 120955. [Google Scholar] [CrossRef]
- Nuţă, A.C. The Incidence of Public Spending on Economic Growth. Euro Econ. 2010, 20, 65–68. [Google Scholar]
- Xenopoulos, M.A.; Lodge, D.M.; Alcamo, J.; Märker, M.; Schulze, K.; van Vuuren, D.P. Scenarios of Freshwater Fish Extinctions from Climate Change and Water Withdrawal. Glob. Chang. Biol. 2005, 11, 1557–1564. [Google Scholar] [CrossRef]
- Huang, M.; Ding, L.; Wang, J.; Ding, C.; Tao, J. The Impacts of Climate Change on Fish Growth: A Summary of Conducted Studies and Current Knowledge. Ecol. Indic. 2021, 121, 106976. [Google Scholar] [CrossRef]
- Free, C.M.; Thorson, J.T.; Pinsky, M.L.; Oken, K.L.; Wiedenmann, J.; Jensen, O.P. Impacts of Historical Warming on Marine Fisheries Production. Science 2019, 363, 979–983. [Google Scholar] [CrossRef]
- Tao, J.; He, D.; Kennard, M.J.; Ding, C.; Bunn, S.E.; Liu, C.; Jia, Y.; Che, R.; Chen, Y. Strong Evidence for Changing Fish Reproductive Phenology under Climate Warming on the Tibetan Plateau. Glob. Chang. Biol. 2018, 24, 2093–2104. [Google Scholar] [CrossRef] [Green Version]
- Vuorinen, I.; Hänninen, J.; Rajasilta, M.; Laine, P.; Eklund, J.; Montesino-Pouzols, F.; Corona, F.; Junker, K.; Meier, H.E.M.; Dippner, J.W. Scenario Simulations of Future Salinity and Ecological Consequences in the Baltic Sea and Adjacent North Sea Areas-Implications for Environmental Monitoring. Ecol. Indic. 2015, 50, 196–205. [Google Scholar] [CrossRef]
- Tedesco, P.A.; Oberdorff, T.; Cornu, J.F.; Beauchard, O.; Brosse, S.; Dürr, H.H.; Grenouillet, G.; Leprieur, F.; Tisseuil, C.; Zaiss, R.; et al. A Scenario for Impacts of Water Availability Loss Due to Climate Change on Riverine Fish Extinction Rates. J. Appl. Ecol. 2013, 50, 1105–1115. [Google Scholar] [CrossRef]
- Heather, F.J.; Childs, D.Z.; Darnaude, A.M.; Blanchard, J.L. Using an Integral Projection Model to Assess the Effect of Temperature on the Growth of Gilthead Seabream Sparus Aurata. PLoS ONE 2018, 13, e0196092. [Google Scholar] [CrossRef]
- Carozza, D.A.; Bianchi, D.; Galbraith, E.D. Metabolic Impacts of Climate Change on Marine Ecosystems: Implications for Fish Communities and Fisheries. Glob. Ecol. Biogeogr. 2019, 28, 158–169. [Google Scholar] [CrossRef] [Green Version]
- Ding, C.Z.; Jiang, X.M.; Chen, L.Q.; Juan, T.; Chen, Z.M. Growth Variation of Schizothorax Dulongensis Huang, 1985 along Altitudinal Gradients: Implications for the Tibetan Plateau Fishes under Climate Change. J. Appl. Ichthyol. 2016, 32, 729–733. [Google Scholar] [CrossRef]
- Tao, J.; Kennard, M.J.; Jia, Y.; Chen, Y. Climate-Driven Synchrony in Growth-Increment Chronologies of Fish from the World’s Largest High-Elevation River. Sci. Total Environ. 2018, 645, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Smalås, A.; Strøm, J.F.; Amundsen, P.A.; Dieckmann, U.; Primicerio, R. Climate Warming Is Predicted to Enhance the Negative Effects of Harvesting on High-Latitude Lake Fish. J. Appl. Ecol. 2020, 57, 270–282. [Google Scholar] [CrossRef]
- Birk, S.; van Kouwen, L.; Willby, N. Harmonising the Bioassessment of Large Rivers in the Absence of Near-Natural Reference Conditions—A Case Study of the Danube River. Freshw. Biol. 2012, 57, 1716–1732. [Google Scholar] [CrossRef]
- Chapman, D.V.; Bradley, C.; Gettel, G.M.; Hatvani, I.G.; Hein, T.; Kovács, J.; Liska, I.; Oliver, D.M.; Tanos, P.; Trásy, B.; et al. Developments in Water Quality Monitoring and Management in Large River Catchments Using the Danube River as an Example. Environ. Sci. Policy 2016, 64, 141–154. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Song, X.; Wan, R.; Gao, L. A Modified Water Quality Index for Intensive Shrimp Ponds of Litopenaeus Vannamei. Ecol. Indic. 2013, 24, 287–293. [Google Scholar] [CrossRef]
- Wang, Q.; Li, S.; Jia, P.; Qi, C.; Ding, F. A Review of Surface Water Quality Models. Sci. World J. 2013, 2013, 231768. [Google Scholar] [CrossRef] [Green Version]
- Mănoiu, V.-M.; Crăciun, A.-I. Danube River Water Quality Trends: A Qualitative Review Based on the Open Access Web of Science Database. Ecohydrol. Hydrobiol. 2021. [Google Scholar] [CrossRef]
- Bărbulescu, A.; Barbeş, L. Assessing the Water Quality of the Danube River (at Chiciu, Romania) by Statistical Methods. Environ. Earth Sci. 2020, 79, 122. [Google Scholar] [CrossRef]
- Iticescu, C.; Georgescu, L.P.; Murariu, G.; Topa, C.; Timofti, M.; Pintilie, V.; Arseni, M. Lower Danube Water Quality Quantified through WQI and Multivariate Analysis. Water 2019, 11, 1305. [Google Scholar] [CrossRef] [Green Version]
- Calmuc, M.; Calmuc, V.; Arseni, M.; Topa, C.; Timofti, M.; Georgescu, L.P.; Iticescu, C. A Comparative Approach to a Series of Physico-Chemical Quality Indices Used in Assessing Water Quality in the Lower Danube. Water 2020, 12, 3239. [Google Scholar] [CrossRef]
- Frîncu, R.M. Long-Term Trends in Water Quality Indices in the Lower Danube and Tributaries in Romania (1996–2017). Int. J. Environ. Res. Public Health 2021, 18, 1665. [Google Scholar] [CrossRef]
- Krtolica, I.; Cvijanović, D.; Obradović, Đ.; Novković, M.; Milošević, D.; Savić, D.; Vojinović-Miloradov, M.; Radulović, S. Water Quality and Macrophytes in the Danube River: Artificial Neural Network Modelling. Ecol. Indic. 2021, 121, 107076. [Google Scholar] [CrossRef]
- Antanasijević, D.; Pocajt, V.; Perić-Grujić, A.; Ristić, M. Modelling of Dissolved Oxygen in the Danube River Using Artificial Neural Networks and Monte Carlo Simulation Uncertainty Analysis. J. Hydrol. 2014, 519, 1895–1907. [Google Scholar] [CrossRef]
- Antanasijević, D.; Pocajt, V.; Povrenović, D.; Perić-Grujić, A.; Ristić, M. Modelling of Dissolved Oxygen Content Using Artificial Neural Networks: Danube River, North Serbia, Case Study. Environ. Sci. Pollut. Res. 2013, 20, 9006–9013. [Google Scholar] [CrossRef] [PubMed]
- Milošević, D.; Mančev, D.; Čerba, D.; Stojković Piperac, M.; Popović, N.; Atanacković, A.; Đuknić, J.; Simić, V.; Paunović, M. The Potential of Chironomid Larvae-Based Metrics in the Bioassessment of Non-Wadeable Rivers. Sci. Total Environ. 2018, 616–617, 472–479. [Google Scholar] [CrossRef]
- Ragavan, A.J.; Fernandez, G.C. Modeling Water Quality Trend in Long Term Time Series. In Statistics and Data Analysis; SAS Institute: San Francisco, CA, USA; Available online: https://www.researchgate.net/publication/238597459_Modeling_Water_Quality_Trend_in_Long_Term_Time_Series (accessed on 12 August 2021).
- Katimon, A.; Shahid, S.; Mohsenipour, M. Modeling Water Quality and Hydrological Variables Using ARIMA: A Case Study of Johor River, Malaysia. Sustain. Water Resour. Manag. 2018, 4, 991–998. [Google Scholar] [CrossRef]
- Jain, G.; Mallick, B. A Study of Time Series Models ARIMA and ETS. SSRN Electron. J. 2017. [Google Scholar] [CrossRef]
- Saleh, A.Y.; Tei, R. Flood Prediction Using Seasonal Autoregressive Integrated Moving Average (SARIMA) Model. Int. J. Innov. Technol. Explor. Eng. 2019, 8, 1037–1042. [Google Scholar]
- Arawo, C.C. Multiple Linear Regression (MLR) Model: A Tool for Water Quality Interpretation. Momona Ethiop. J. Sci. 2020, 12, 123–134. [Google Scholar] [CrossRef]
- Du, S.; Hu, L.; Song, M. Production Optimization Considering Environmental Performance and Preference in the Cap-and-Trade System. J. Clean. Prod. 2016, 112, 1600–1607. [Google Scholar] [CrossRef]
- Choi, Y.; Oh, D.-H.; Zhang, N. Environmentally Sensitive Productivity Growth and Its Decompositions in China: A Metafrontier Malmquist–Luenberger Productivity Index Approach. Empir. Econ. 2015, 49, 1017–1043. [Google Scholar] [CrossRef]
- Ewaid, S.H.; Abed, S.A.; Kadhum, S.A. Predicting the Tigris River Water Quality within Baghdad, Iraq by Using Water Quality Index and Regression Analysis. Environ. Technol. Innov. 2018, 11, 390–398. [Google Scholar] [CrossRef]
- Easterling, W.E.; Crosson, P.R.; Rosenberg, N.J.; McKenney, M.S.; Katz, L.A.; Lemon, K.M. Paper 2. Agricultural Impacts of and Responses to Climate Change in the Missouri-Iowa-Nebraska-Kansas (MINK) Region. Clim. Chang. 1993, 24, 23–61. [Google Scholar] [CrossRef]
- McCarl, B.A.; Dillon, C.R.; Keplinger, K.O.; Williams, R.L. Limiting Pumping from the Edwards Aquifer: An Economic Investigation of Proposals, Water Markets, and Spring Flow Guarantees. Water Resour. Res. 1999, 35, 1257–1268. [Google Scholar] [CrossRef]
- Ministry of Environment and Climate Change. Romania’s Sixth National Communication on Climate Change and First Biennial Report; Ministry of Environment and Climate Change: Bucharest, Romania, 2013. [Google Scholar]
- Davies, O.L.; Brown, R.G. Statistical Forecasting for Inventory Control. J. R. Stat. Soc. Ser. A (Gen.) 1960, 123, 348–349. [Google Scholar] [CrossRef]
- Winters, P.R. Forecasting Sales by Exponentially Weighted Moving Averages. Manag. Sci. 1960, 6, 324–342. [Google Scholar] [CrossRef]
- Holt, C.C. Forecasting Seasonals and Trends by Exponentially Weighted Moving Averages. Int. J. Forecast. 2004, 20, 5–10. [Google Scholar] [CrossRef]
- Metcalfe, A.V.; Cowpertwait, P.S.P. Introductory Time Series with R; Springer: New York, NY, USA, 2009. [Google Scholar]
- Rosenzweig, C.; Casassa, G.; Karoly, D.J.; Imeson, A.; Liu, C.; Menzel, A.; Rawlins, S.; Root, T.L.; Seguin, B.; Tryjanowski, P. Assessment of Observed Changes and Responses in Natural and Managed Systems. In Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Matear, R.J.; Hirst, A.C. Long-Term Changes in Dissolved Oxygen Concentrations in the Ocean Caused by Protracted Global Warming. Glob. Biogeochem. Cycles 2003, 17, 35–67. [Google Scholar] [CrossRef]
- Li, H.; Liu, L.; Li, M.; Zhang, X. Effects of PH, Temperature, Dissolved Oxygen, and Flow Rate on Phosphorus Release Processes at the Sediment and Water Interface in Storm Sewer. J. Anal. Methods Chem. 2013, 2013, 104316. [Google Scholar] [CrossRef]
- Luz Rodríguez-Blanco, M.; Mercedes Taboada-Castro, M.; Arias, R.; Teresa Taboada-Castro, M. Assessing the Expected Impact of Climate Change on Nitrate Load in a Small Atlantic Agro-Forested Catchment. In Climate Change and Global Warming; IntechOpen: London, UK, 2019. [Google Scholar]
- Influence of Dissolved Oxygen on Nitrates Concentration in Zagreb Aquifer—CROSBI. Available online: https://www.bib.irb.hr/818575 (accessed on 2 October 2021).
- Calvi, C.; Dapeña, C.; Martinez, D.E.; Quiroz Londoño, O.M. Relationship between Electrical Conductivity, 18O of Water and NO3 Content in Different Streamflow Stages. Environ. Earth Sci. 2018, 77, 248. [Google Scholar] [CrossRef]
- Miyamoto, T.; Kameyama, K.; Iwata, Y. Monitoring Electrical Conductivity and Nitrate Concentrations in an Andisol Field Using Time Domain Reflectometry. Jpn. Agric. Res. Q. 2015, 49, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, O.; Bernet, N.; Delgenès, J.-P. Effect of Dissolved Oxygen Concentration on Nitrite Accumulation in Nitrifying Sequencing Batch Reactor. Water Environ. Res. 2007, 79, 845–850. [Google Scholar] [CrossRef]
- Weon, S.Y.; Lee, S.I.; Koopman, B. Effect of Temperature and Dissolved Oxygen on Biological Nitrification at High Ammonia Concentrations. Environ. Technol. 2004, 25, 1211–1219. [Google Scholar] [CrossRef]
- Paudel, B.; Montagna, P.A.; Adams, L. The Relationship between Suspended Solids and Nutrients with Variable Hydrologic Flow Regimes. Reg. Stud. Mar. Sci. 2019, 29, 100657. [Google Scholar] [CrossRef]
- Wang, S.; Jin, X.; Bu, Q.; Jiao, L.; Wu, F. Effects of Dissolved Oxygen Supply Level on Phosphorus Release from Lake Sediments. Colloids Surf. A Physicochem. Eng. Asp. 2008, 316, 245–252. [Google Scholar] [CrossRef]
- Sun, X.; Ji, Q.; Jayakumar, A.; Ward, B.B. Dependence of Nitrite Oxidation on Nitrite and Oxygen in Low-Oxygen Seawater. Geophys. Res. Lett. 2017, 44, 7883–7891. [Google Scholar] [CrossRef]
- Cai, Q.; Zhang, W.; Yang, Z. Stability of Nitrite in Wastewater and Its Determination by Ion Chromatography. Anal. Sci. 2002, 17, i917–i920. [Google Scholar]
- Zuo, X.; He, H.; Yang, Y.; Yan, C.; Zhou, Y. Study on Control of NH4+-N in Surface Water by Photocatalytic. IOP Conf. Ser. Earth Environ. Sci. 2018, 108, 022031. [Google Scholar] [CrossRef]
- Simionov, I.-A.; Petrea, S.-M.; Mogodan, A.; Nica, A. Effect of Changes in the Romanian Lower Sector Danube River Hydrological and Hydrothermal Regime on Fish Diversity. Sci. Papers. Ser. E. Land Reclam. Earth Obs. Surv. Environ. Eng. 2020, IX, 106–111. [Google Scholar]
- Wysujack, K.; Mehner, T. Can Feeding of European Catfish Prevent Cyprinids from Reaching a Size Refuge? Ecol. Freshw. Fish. 2005, 14, 87–95. [Google Scholar] [CrossRef]
- Özdilek, Ş.Y.; Jones, R.I. The Diet Composition and Trophic Position of Introduced Prussian Carp Carassius Gibelio (Bloch, 1782) and Native Fish Species in a Turkish River. Turk. J. Fish. Aquat. Sci. 2014, 14, 769–776. [Google Scholar] [CrossRef]
- Yazıcıoğlu, O.; Yılmaz, S.; Yazıcı, R.; Yılmaz, M.; Polat, N. Food Items and Feeding Habits of White Bream, Blicca Bjoerkna (Linnaeus, 1758) Inhabiting Lake Ladik (Samsun, Turkey). Turk. J. Fish. Aquat. Sci. 2017, 17, 371–378. [Google Scholar] [CrossRef]
- Ferreira, M.; Gago, J.; Ribeiro, F. Diet of European Catfish in a Newly Invaded Region. Fishes 2019, 4, 58. [Google Scholar] [CrossRef] [Green Version]
- Kuzishchin, K.V.; Gruzdeva, M.A.; Pavlov, D.S. Traits of Biology of European Wels Catfish Silurus Glanis from the Volga–Ahtuba Water System, the Lower Volga. J. Ichthyol. 2018, 58, 833–844. [Google Scholar] [CrossRef]
- Yazicioglu, O.; Polat, N.; Yilmaz, S. Feeding Biology of Pike, Esox lucius L., 1758 Inhabiting Lake Ladik, Turkey. Turk. J. Fish. Aquat. Sci. 2018, 18, 1215–1226. [Google Scholar] [CrossRef]
- Ivanovs, K. Pike Esox Lucius Distribution and Feeding Comparisons in Natural and Historically Channelized River Sections. Environ. Clim. Technol. 2016, 18, 33–41. [Google Scholar] [CrossRef] [Green Version]

| Parameter | Measurement Unit | Standard Method of Determination | Sampling Area |
|---|---|---|---|
| Water level (wt) | (cm) | SOP 2043 | Brăila, Galați, Tulcea, Sulina |
| Water temperature (wt) | (°C) | Sensor’s methods | Galați, Tulcea |
| Air temperature (aw) | (°C) | Sensor’s method | Galați, Tulcea |
| Total suspended solids (TSS) | (mg/L) | SE EN 875:2005 | Galați, Tulcea |
| Turbidity (tb) | (NTU) | SR EN ISO 7027:2001 | Galați |
| Dissolved oxygen (DO) | (mg/L) | SR EN ISO 5814:2013 | Galați, Tulcea |
| Dissolved oxygen saturation (DO sat.) | (%) | - | Galați, Tulcea |
| Biochemical oxygen demand (BOD5) | (mg/L) | SR EN 1899:2:2002 | Galați, Tulcea |
| Chemical oxygen demand (COD) | (mg/L) | SR EN 1484:2001 | Galați |
| Total organic carbon (TOC) | (mg/L) | SR EN 1484:2001 | Galați |
| Electrical conductivity (EC) | (µS/cm) | SE EN 27888:1997 | Galați, Tulcea |
| Fixed solids (FS) | (mg/L) | STAS 9187:1984 | Galați, Tulcea |
| Calcium (Ca) | (mg/L) | SE ISO 6058:2008 | Galați, Tulcea |
| Magnesium (Mg) | (mg/L) | SE ISO 6059:2008 | Galați, Tulcea |
| Total hardness (TH) | (°G) | SR ISO 6059:2008 | Galați |
| Chloride (Cl) | (mg/L) | SE ISO 9297:2001 | Galați, Tulcea |
| Sulfate (SO4) | (mg/L) | EPA 9038:1986 | Galați, Tulcea |
| pH | (upH) | SR ISO 10523:2012 | Galați, Tulcea |
| Alkalinity (Alk) | (mmol/L) | SR ISO 9963-1/A99:2002 | Galați, Tulcea |
| Bicarbonates (Bi) | (mg/L) | SR ISO 9963-1/A99:2002 | Galați, Tulcea |
| Ammonium-nitrogen (N-NH4) | (mg/L) | SR ISO 7150-1:2001 | Galați, Tulcea |
| Nitrite-nitrogen (N-NO2) | (mg/L) | SR ISO 26777/C91:2006 | Galați, Tulcea |
| Nitrate-nitrogen (N-NO3) | (mg/L) | SR ISO 7890-3:2000 | Galați, Tulcea |
| Total nitrogen (TN) | (mg/L) | SR EN 12260:2004 | Galați, Tulcea |
| Orthophosphate as phosphorus (P-PO4) | (mg/L) | SR EN ISO6878:2005 | Galați, Tulcea |
| Total phosphorus (TP) | (mg/L) | SR EN ISO 6878:2005 | Galați, Tulcea |
| Fish Species | Abbreviation | Study Area |
|---|---|---|
| Abramis brama danubii (freshwater bream) | Fbr | L1, L2 |
| Alburnus alburnus (common bleak) | Cbl | L1 |
| Alosa caspia (Caspian shad) | Csh | L2 |
| Alosa immaculata (Pontic shad) | Psh | L1, L2 |
| Aspius aspius (asp) | Asp | L1, L2 |
| Barbus barbus (common barbel) | Cbb | L1, L2 |
| Blicca bjoerkna (white bream) | Wbr | L1, L2 |
| Carassius gibelio (Prusian carp) | Pcp | L1, L2 |
| Other cyprinids | Ocp | L1, L2 |
| Cyprinus carpio carpio (common carp) | Ccp | L1, L2 |
| Esox lucius (pike) | Pk | L1, L2 |
| Liza aurata (golden grey mullet) | Ggm | L2 |
| Mullus barbatus ponticus (red mullet) | Rmt | L2 |
| Neogobius kessleri (bighead goby) | Bgb | L2 |
| Pelecus cultratus (sabre carp) | Scp | L2 |
| Perca fluviatilis fluviatilis (perch) | Prc | L2 |
| Psetta maxima maeotica (turbot) | Tbt | L2 |
| Raja clavata (thornback ray) | Tr | L2 |
| Rutilus rutilus carpathorosicus (roach) | Rch | L1, L2 |
| Sander lucioperca (pikeperch) | PkPrc | L1, L2 |
| Scardinius erytrophthalmus (common rudd) | Crd | L1, L2 |
| Silurus glanis(catfish) | Ctf | L1, L2 |
| Sprattus sprattus (European sprat) | Esp | L2 |
| Tinca tinca (tench) | Tnch | L2 |
| Trachurus ponticus (horse mackerel) | Hmk | L2 |
| Vimba vimba carinata (vimba) | Vmb | L1, L2 |
| Sampling Point | MLR Prediction Model | Model No. |
|---|---|---|
| Galati | P-PO4 = −2.918 − 0.276 SO4 + 1.162 Bicarbonates + 0.457 N-NO3 + 0.8701 TP + 0.095 water level | M1 |
| N-NO3 = -3.818 + 0.514 EC + 1.051 Mg + 0.401 SO4 − 0.166 N-NO2 + 1.047 NT | M2 | |
| N-NO2 = −5.285 − 0.378 DO + 1.618 Mg + 0.497 SO4 − 0.775 N-NO3 + 1.277 NT + 0.313 water level | M3 | |
| N-NH4 = -6.077 + 0.557 TSS + 0.667 Mg + 0.772 Chloride + 1.004 Bicarbonates | M4 | |
| SO4 = 3.523 + 0.701 COD − 0.987 Ca − 0.614 Mg + 0.588 N-NO3 − 0.386 NT | M5 | |
| Tulcea | P-PO4 = 3.010 + 3.344 DO − 0.671 BOD5 + 0.275 N-NH4 + 0.605 N-NO2 − 0.776 N-NO3 + 0.472 TP − 0.402 TSS − 0.426 FS + 0.819 Mg − 1.975 TH − 1.254 Cl − 0.860 SO4 | M6 |
| N-NO3 = −0.178 + 0.940 TN | M7 | |
| N-NO2 = 1.340 − 6.920 pH + 0.992 EC − 2.787 DO − 0.240 COD + 0.5887 BOD5 − 0.237 N-NH4 + 0.650 N-NO3 + 0.227 P-PO4 + 0.2435 TSS + 0.3081 FS − 0.694 Mg + 1.391 TH + 0.648 Chloride + 0.669 SO4 | M8 | |
| N-NH4 = 2.080 − 17.370 pH + 4.430 EC − 7.310 DO − 0.780 COD + 1.492 BOD5 − 1.785 N-NO2 + 1.520 N-NO3 + 0.541 P-PO4 + 0.548 TSS + 0.906 FS − 1.658 Mg + 3.168 TH + 1.608 SO4 | M9 | |
| SO4 = −1.430 + 4.690 pH + 2.667 DO − 0.511 BOD5 + 0.213 N-NH4 + 0.643 N-NO2 − 0.644 N-NO3 − 0.318 P-PO4 − 0.275 TSS − 0.324 FS + 0.723 Mg − 1.389 TH − 0.891 Cl | M10 |
| Study Area | MLR Prediction Model | Model No. |
|---|---|---|
| L1 | Ccp = −0.413 + 0.350 Pcp + 0.2316 Wbr + 0.607 Ctf | L1M1 |
| Pcp = −0.182 + 1.0377 Fbr | L1M2 | |
| Fbr = 0.289 + 0.6836 Pcp + 0.2827 PkPrc | L1M3 | |
| Vmb = 0.256 + 0.780 Psh | L1M4 | |
| Cbb = 0.688 + 0.783 PkPrc | L1M5 | |
| Rch = −0.198 + 0.4816 Pcp + 0.452 Wbr | L1M6 | |
| Wbr = 0.202 + 0.375 Vmb + 0.926 Rch − 0.447 Asp | L1M7 | |
| Asp = −0.010 + 0.9502 PkPrc | L1M8 | |
| Ctf = 0.385 + 0.6213 Ccp + 0.748 PkPrc − 0.3336 Pk − 0.2185 Psh | L1M9 | |
| PhPrc = −0.003 + 0.370 Asp + 0.475 Ctf + 0.2058 Pk | L1M10 | |
| Pk = 1.007 + 0.754 Rch + 1.037 PkPrc − 1.159 Ocp | L1M11 | |
| Ocp = 0.346 + 0.335 Rch + 0.365 Ctf − 0.3086 Pk + 0.449 Psh | L1M12 | |
| Psh= 0.243 + 0.3145 Vmb + 0.7288 Ocp | L1M13 | |
| L2 | Fbr = −3745 − 1.258 Pk + 1.906 Rch + 2.188 PkPrc | L2M1 |
| Pcp = 10049 + 7.092 Rch − 1.970 PkPrc | L2M2 | |
| Ocp = −2419 + 0.1091 Fbr + 0.700 Ccp | L2M3 | |
| Ccp = 231 + 0.364 Ocp + 0.798 Ctf − 0.1683 PkPrc | L2M4 | |
| Pk = −2032 − 0.1722 Fbr + 0.0290 Pcp + 0.725 Rch + 1.503 Tnch | L2M5 | |
| Rch = 1391 + 0.1286 Fbr + 0.0752 Pcp + 0.2934 Pk | L2M6 | |
| Ctf = 3003 + 0.6943 Ccp + 0.2869 Pk − 1.215 Tnch | L2M7 | |
| Tnch = 2746 + 0.0794 Ocp + 0.1264 Pk − 0.1522 Ctf | L2M8 | |
| Prc = 1259 + 0.1830 Rch | L2M9 | |
| PkPrc = −643 + 0.2382 Fbr | L2M10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrea, S.M.; Zamfir, C.; Simionov, I.A.; Mogodan, A.; Nuţă, F.M.; Rahoveanu, A.T.; Nancu, D.; Cristea, D.S.; Buhociu, F.M. A Forecasting and Prediction Methodology for Improving the Blue Economy Resilience to Climate Change in the Romanian Lower Danube Euroregion. Sustainability 2021, 13, 11563. https://doi.org/10.3390/su132111563
Petrea SM, Zamfir C, Simionov IA, Mogodan A, Nuţă FM, Rahoveanu AT, Nancu D, Cristea DS, Buhociu FM. A Forecasting and Prediction Methodology for Improving the Blue Economy Resilience to Climate Change in the Romanian Lower Danube Euroregion. Sustainability. 2021; 13(21):11563. https://doi.org/10.3390/su132111563
Chicago/Turabian StylePetrea, Stefan Mihai, Cristina Zamfir, Ira Adeline Simionov, Alina Mogodan, Florian Marcel Nuţă, Adrian Turek Rahoveanu, Dumitru Nancu, Dragos Sebastian Cristea, and Florin Marian Buhociu. 2021. "A Forecasting and Prediction Methodology for Improving the Blue Economy Resilience to Climate Change in the Romanian Lower Danube Euroregion" Sustainability 13, no. 21: 11563. https://doi.org/10.3390/su132111563










