Climate Change Impact on the Distribution of Forest Species in the Brazilian Amazon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Environmental Variables
2.3. Creation and Predictor Modeling Analysis
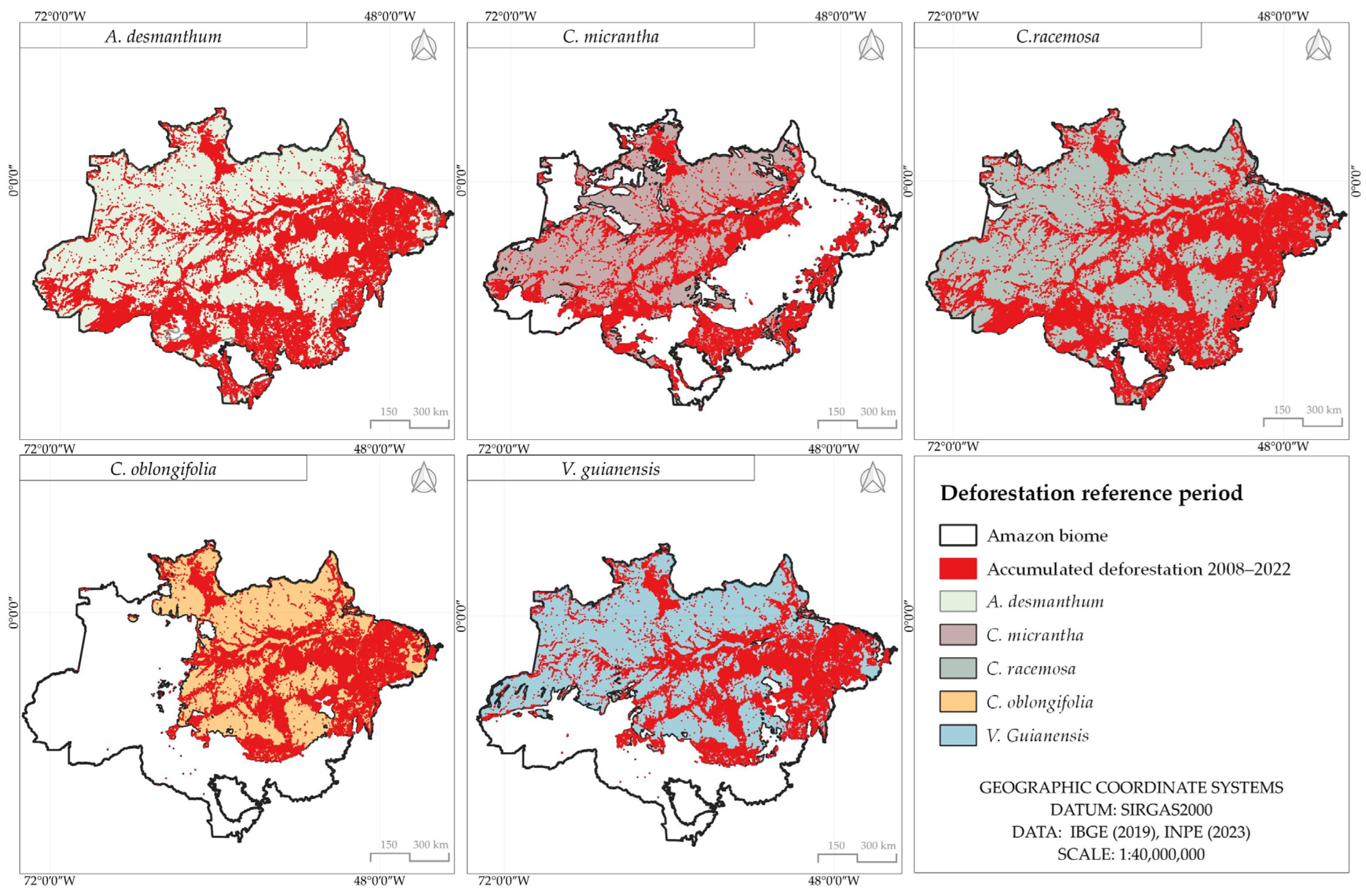
2.4. Loss Analysis of the Environmental Suitability Areas Due to Deforestation
3. Results
3.1. Distribution Analysis of Species Occurrence
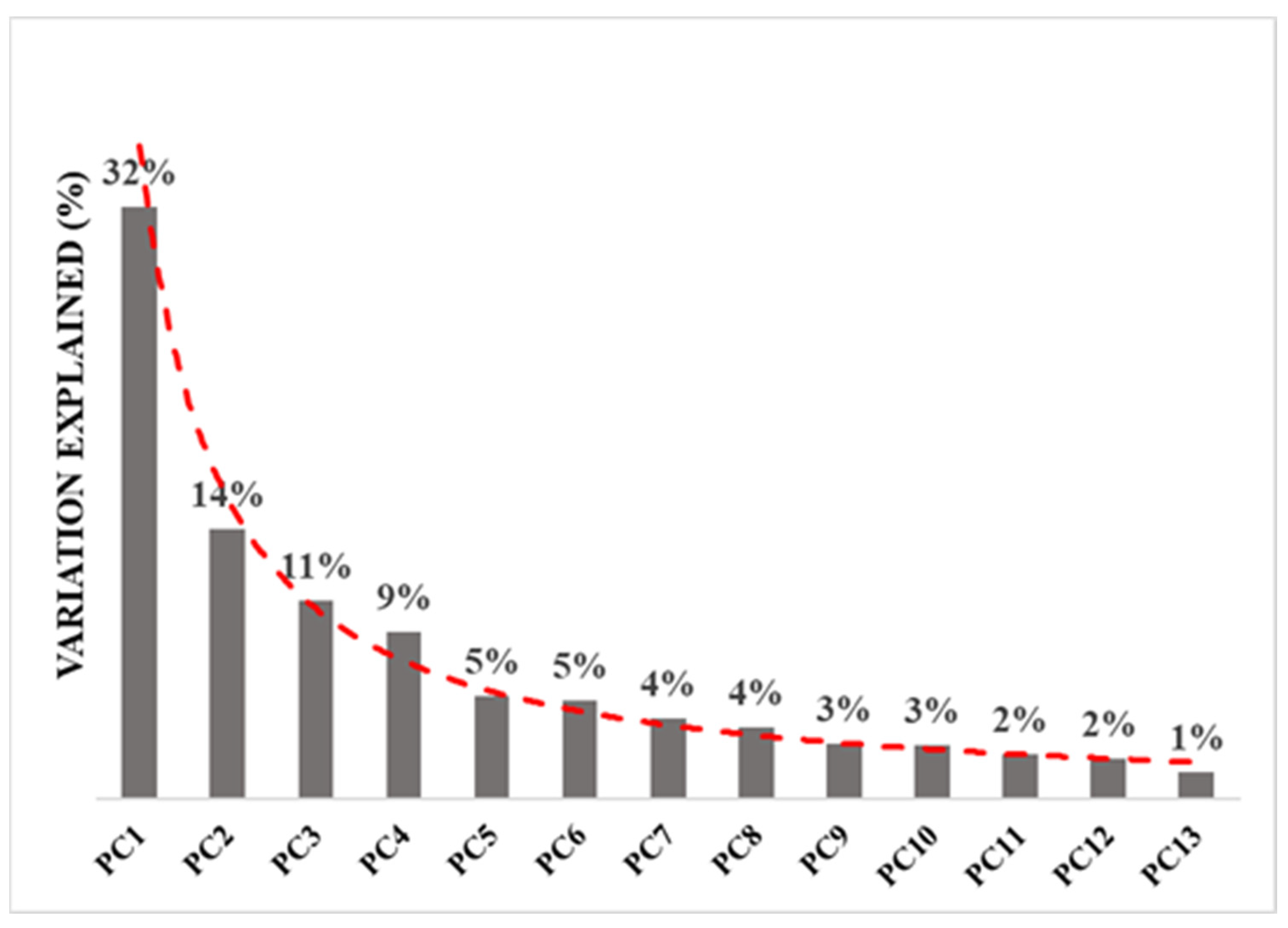
3.2. Environmental Variable Analysis
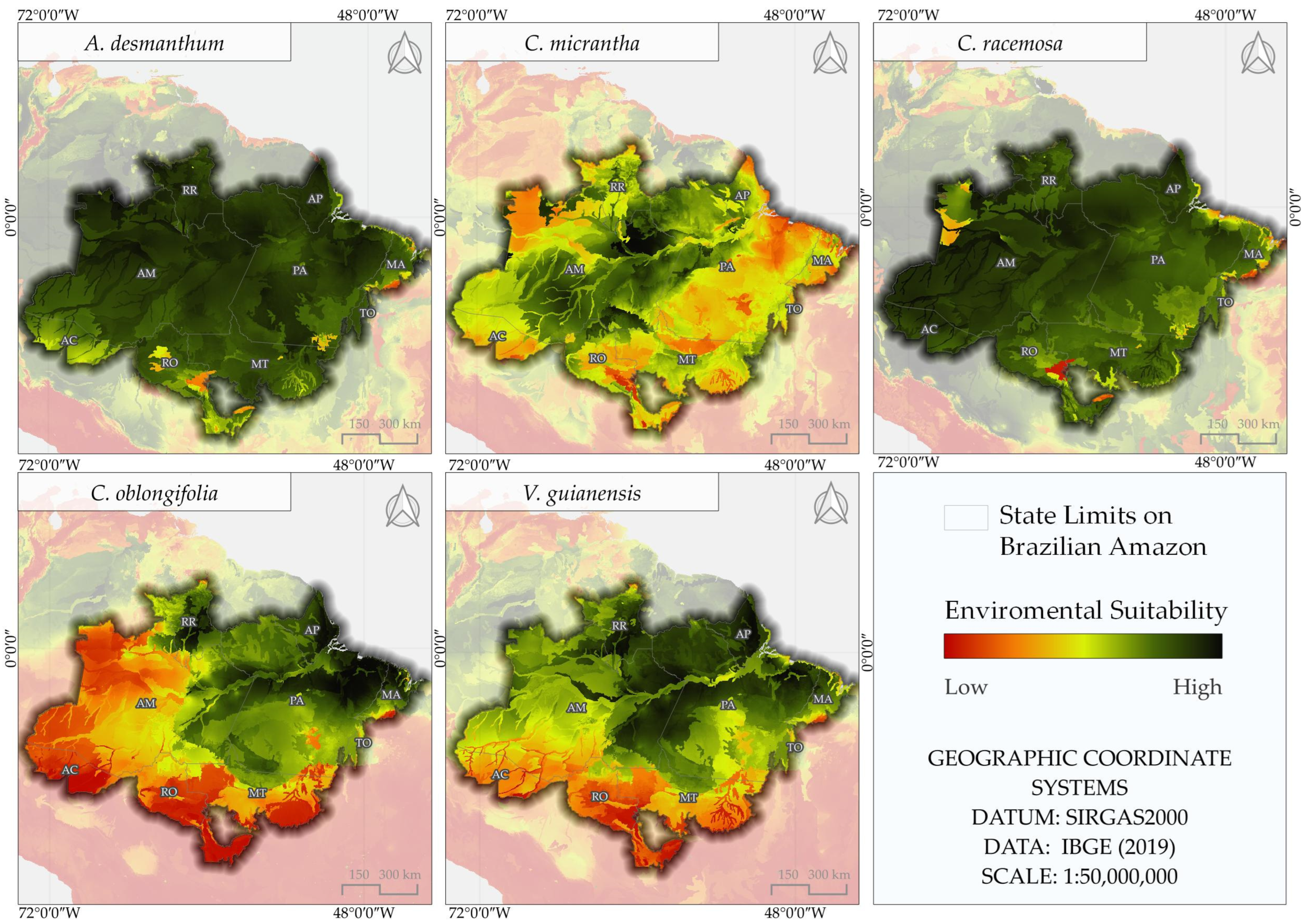
3.3. Areas of Environmental Suitability for the Reference Period
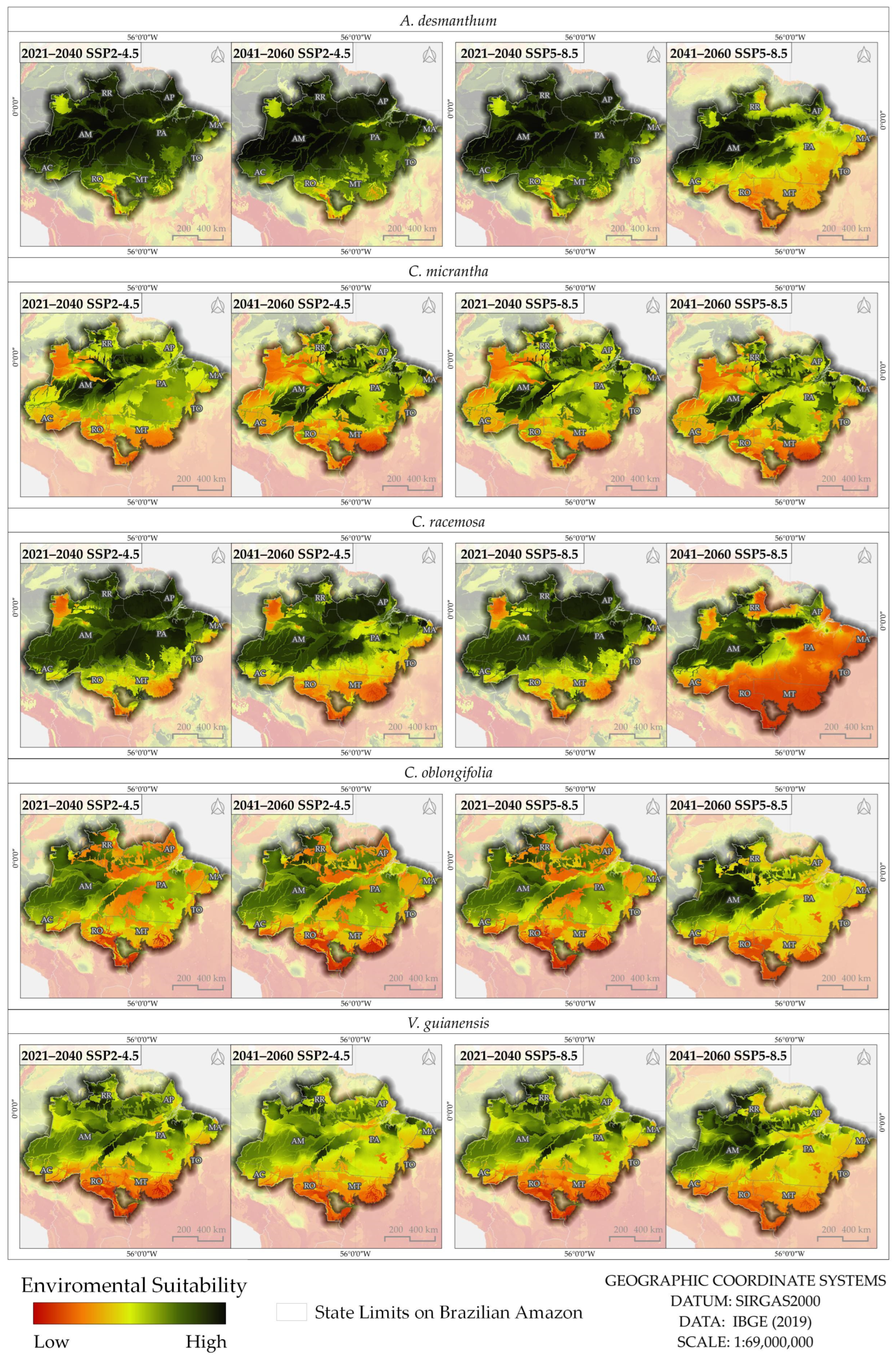
3.4. Area of Environmental Suitability for the Future
3.5. Accumulated Deforestation in Potential Areas of Species Occurrence
3.6. Model Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Artaxo, P.; Hansson, H.C.; Machado, L.A.T.; Rizzo, L.V. Tropical forests are crucial in regulating on Earth. PLoS Clim. 2022, 1, e0000054. [Google Scholar] [CrossRef]
- Val, A.L.; Wood, C.M. Global changes and physiological challenges for fish of the Amazon today and in the near future. J. Exp. Biol. 2022, 225, jeb216440. [Google Scholar]
- Qin, Y.; Xiao, X.; Liu, F.; de Sa e Silva, F.; Shimabukuro, Y.; Arai, E.; Fearnside, P.M. Forest Conservation in Indigenous Territories and Protected Areas in the Brazilian Amazon. Nat. Sustain. 2023, 6, 295–305. [Google Scholar] [CrossRef]
- Green, J.M.H.; Croft, S.A.; Durán, A.P.; West, C.D. Linking global drivers of agricultural trade to on-the-ground impacts on biodiversity. Proc. Natl. Acad. Sci. USA 2019, 116, 23202–23208. [Google Scholar] [CrossRef]
- Linares, L.M.D. The awkward question: What baseline should be used to measure biodiversity loss? The role of history, biology and politics in setting up an objective and fair baseline for the international biodiversity regime. Environ. Sci. Policy 2022, 135, 137–146. [Google Scholar] [CrossRef]
- Sintayehu, D. Impact of climate change on biodiversity and associated key ecosystem services in Africa: A systematic review. Ecosyst. Health Sustain. 2018, 4, 225–239. [Google Scholar] [CrossRef]
- Habibullah, M.S.; Din, B.H.; Tan, S.H.; Zahid, H. Impact of climate change on biodiversity loss: Global evidence. Environ. Sci. Pollut. Res. Int. 2022, 29, 1073–1086. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.B.; Oliveira, G.; Frederico, R.G.; Loyola, R.; Zacarias, D.; Ribeiro, B.R.; Mendes-Oliveira, A.C. How future climate change and deforestation can drastically affect the species of monkeys endemic to the eastern Amazon, and priorities for conservation. Biodivers. Conserv. 2022, 31, 971–988. [Google Scholar] [CrossRef]
- Inpe—Instituto Nacional de Pesquisas Espaciais. Coordenação Geral de Observação da Terra. Programa de Monitoramento da Amazônia e Demais Biomas. Desmatamento—Amazonia. Available online: http://terrabrasilis.dpi.inpe.br/app/dashboard/deforestation/biomes/legal_amazon/rates (accessed on 30 July 2023).
- Jardim, F.C.S. Natural regeneration in tropical forests. Amaz. J. Agric. Environ. Sci. 2015, 58, 105–113. [Google Scholar]
- Diosinio, L.F.; Schwartz, G.; Lopes, J.C.; Oliveira, F.A. Growth, mortality, and recruitment of tree species in a Amazonian rainforest over 13 years of reduced impact logging. For. Ecol. Manag. 2018, 430, 150–156. [Google Scholar]
- Dionisio, L.F.S. Mid-term effects of selective logging on the growth, mortality, and recruitment of Manilkara huberi (Ducke) A. Chev. in an Amazonian rainforest. Sci. For. 2020, 48, e3154. [Google Scholar]
- Jump, A.S.; Ruiz-Benito, P.; Greenwood, S.; Allen, C.D.; Kitzbeger, T.; Fensham, R.; Martinez-Vilalta, J.; Lloret, F. Structural overshoot of tree growth with climate variability and the global spectrum of drought-induced forest dieback. Glob. Chang. Biol. 2017, 23, 3742–3757. [Google Scholar] [CrossRef] [PubMed]
- Pilotto, I.L.; Rodriguez, D.A.; Chou, S.C.; Garofolo, L.; Gomes, J.L. Impacts of the land use and land-cover changes on local Hydroclimate in Southwestern Amazon. Clim. Dynam. 2023, 61, 5597–5612. [Google Scholar] [CrossRef]
- Sierra, J.P.; Espinoza, J.C.; Junquas, C.; Wongchuing, S.; Polcher, J.; Morons, V.; Fitas, L.; Arias, P.A.; Schrapffers, A.; Pennel, R. Impacts of land-surface heterogeneities and Amazonian deforestation on the wet season onset in southern Amazon. Clim. Dyn. 2023, 61, 4867–4898. [Google Scholar] [CrossRef]
- Tomaz, J.S.; Bezerra, C.S.; De Aguiar, A.V.; Wrege, M.S.; Lopes, M.T. Prediction of the natural distribution, habitat and conservation of Stryphnodendron pulcherrimum (Willd) Hochr. In response to global climate change. Pesq. Agropec. Trop. 2022, 52, e72422. [Google Scholar]
- Metzger, M.J.; Rounsevell, M.D.A.; Acosta-Michlik, R.; Leemans, R.; Schroter, D. The vulnerability of ecosystem services to land use change. Agric. Ecosyst. Environ. 2006, 114, 69–85. [Google Scholar] [CrossRef]
- Chishugi, D.U.; Sonwa, D.J.; Kahindo, J.M.; Itunda, D.; Chishugi, J.B.; Felix, F.L.; Sahani, M. How climate change and land use/land cover change affect domestic water vulnerability in Yangambi Watersheds. Land 2021, 10, 165. [Google Scholar] [CrossRef]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impactos of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.F.; Velazco, S.J.E.; Marco Junior, P. ENMTML: An R package for a straightforward construction of complex ecological niche models. Environ. Model. Softw. 2020, 125, 104615. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Tim. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Ma, Y.; You, X. A sustainable conservation strategy of wildlife in urban ecosystems: Case of Gallinua chloropus in Beijing-Tianjin-Hebei region. Ecol. Inform. 2022, 68, 101571. [Google Scholar] [CrossRef]
- Aksoy, Ö.K. Predicting the Potential Distribution Area of the Platanus orientalis L. in Turkey Today and in the Future. Sustainability 2022, 14, 11706. [Google Scholar] [CrossRef]
- Muylaert, R.; Kingston, T.; Luo, J.; Vancine, M.H.; Galli, K.; Carlson, C.J.; John, R.S.; Rulli, M.C.; Hayman, D.T.S. Present and future distribution of bat hosts of sarbecoviruses: Implications for conservation and public health. Proc. R. Soc. Lond. Ser. B. Biol. Sci. 2022, 289, 20220397. [Google Scholar] [CrossRef]
- Maitner, B.S.; Boyle, B.; Casler, N.; Condit, R.; Donoghue, J.; Durán, S.M.; Guaderrama, D.; Hinchliff, C.E.; Jorgensen, P.M.; Kraft, N.J.B.; et al. The Bien R package: A tool to access the Botanical Information and Ecology Network (BIEN) database. Methods Ecol. Evol. 2018, 9, 373–379. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WordClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Firpo, M.A.F.; Guimarães, B.S.; Dantas, L.G.; Da Silva, M.G.B.; Alvez, L.M.; Chadwick, R.; Llopart, M.P.; De Oliveira, G.S. Assessment of CPIP6 models performance in simulating present day climate in Brazil. Front. Clim. 2022, 4, 1–27. [Google Scholar] [CrossRef]
- Monteverde, C.; De Sales, F.; Jones, C. Evaluation of the CMIP6 Performance in Simulating Precipitation in the Amazon River Basin. Climate 2022, 10, 122. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2021: The Physical Science Basis. In Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V.P., Zhai, A., Pirani, S.L., Connors, C., Péan, S., Berger, N., Caud, Y., Chen, L., Goldfarb, M.I., Gomis, M., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; In press. [Google Scholar]
- FAO; IIASA. Harmonized World Soil Database Version 2.0. Rome and Laxenburg. 2023, 69p. Available online: https://www.fao.org/documents/card/en/c/cc3823en (accessed on 30 March 2023).
- Alvarez, F.; Morandi, P.S.; Marimon-Junior, B.H.; Exavier, R.; Araújo, I.; Mariano, L.H.; Muller, A.O.; Felpausch, T.R.; Marimon, B.S. Climate defined but not soil-restricted: The distribution of a Neotropical tree through space and time. Plant Soil 2022, 471, 175–191. [Google Scholar] [CrossRef]
- Collevatti, R.; Terribile, L.C.; Lima-Ribeiro, M.S.; Nabout, J.C.; Oliveira, G.; Rangel, T.F.; Rabelo, S.G.; Diniz-Filho, J.F. A coupled phylogeographical and species distribution modelling approach recovers the demographical history of Neotropical seasonally dry forest tree species. Mol. Ecol. 2012, 21, 5845–5863. [Google Scholar] [CrossRef]
- Oliveira, G.; Rangel, T.F.; Lima-Ribeiro, M.S.; Terribile, L.C.; Diniz-Filho, A.F. Evaluating, partitioning, and mapping the spatial autocorrelation component in ecological niche modeling: A new approach based on environmentally equidistant records. Ecography 2014, 37, 637–647. [Google Scholar] [CrossRef]
- Velazco, S.J.E.; Galvão, F.; Villalobos, F.; Marco-Junior, P. Using worldwide edaphic data to model plant species niches: Na assessment at a continental extent. PLoS ONE 2017, 12, e0186025. [Google Scholar] [CrossRef] [PubMed]
- Aiello-Lammens, M.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R. SpThin: Na the R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence model. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- De Marco Junior, P.; Villen, S.; Mendes, P.; Nobrega, C.; Cortes, L.; Castro, T.; Souza, R. Vulnerability of Cerrado threatened mammals: Na the integrative landscape and climate modeling approach. Biodivers. Conserv. 2020, 29, 1637–1658. [Google Scholar] [CrossRef]
- Lobo, J.; Jiménez-Valverde, A.; Hortal, J. The uncertain nature of absences and their importance in species distribution modelling. Ecography 2010, 33, 103–114. [Google Scholar] [CrossRef]
- Senay, S.D.; Worner, S.P.; Ikeda, T. Novel Three-Step Pseudo-Adsence Selection Technique for Improved Species Distribution Modelling. PLoS ONE 2013, 8, e71218. [Google Scholar] [CrossRef] [PubMed]
- Zaniewski, A.E.; Lehmann, A.; Overton, J.M. Predicting species spatial distributions using presence-only data: A case study of native New Zealand ferns. Ecol. Modell. 2002, 157, 261–280. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assuming the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Thuiller, W.; Guéguen, M.; Renaud, J.; Karge, D.N.; Zimmermann, N.E. Uncertainty in ensembles of global biodiversity scenarios. Nat. Commun. 2019, 10, 1446. [Google Scholar] [CrossRef]
- Leroy, B.; Delsol, R.; Hugueny, B.; Meynard, C.N.; Barhoumi, C.; Massin, M.B.; Bellard, C. Without quality presence-absence data, discrimination metrics such as TSS can be misleading measures of model performance. J. Biogeogr. 2018, 45, 1994–2002. [Google Scholar]
- Boria, R.; Olson, L.; Goodman, S.M.; Anderson, R.P. Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol. Modell. 2014, 275, 73–77. [Google Scholar] [CrossRef]
- Sillero, N.; Barbosa, A.M. Common mistakes in ecological niche models. Int. J. Geogr. Inf. Sci. 2020, 35, 213–226. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Principal component analysis. Wiley Interdiscip. Rev. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Mcmeans, B.C.; Mccann, K.S.; Guzzo, M.M.; Vartley, T.J.; Bieg, C.; Blanchfield, P.J.; Fernandes, T.; Giacomini, H.C.; Middel, T.; Rennie, M.D.; et al. Winter in water: Differential responses and the maintenance of biodiversity. Ecol. Lett. 2020, 23, 922–938. [Google Scholar] [CrossRef] [PubMed]
- Bordoloi, R.; Das, B.; Yam, G.; Pandey, P.K.; Tripathi, O.P. Modeling of water holding capacity using readily available soil characteristics. Agric. Res. 2019, 8, 347–355. [Google Scholar] [CrossRef]
- Gomes, L.M.; Bezerra, C.S.; Aguiar, A.V.; Wrege, M.S.; Lopes, M.T.G. Prediction of the natural distribution and conservation of Urena lobata L. in Brazil. Pesq. Agropec. Trop. 2022, 52, e72594. [Google Scholar] [CrossRef]
- Brandão, D.O.; Barata, L.E.S.; Nobre, C.A. The effects of environmental changes on plant species and forest dependent communities in the Amazon region. Forests 2022, 13, 466. [Google Scholar] [CrossRef]
- Gomes, V.H.F.; Vieira, I.C.G.; Salomão, R.P. Amazonian tree species threatened by deforestation an climate change. Nat. Clim. Change 2019, 9, 547–553. [Google Scholar] [CrossRef]
- Naranjo, A.; Melton, A.E.; Soltis, D.E.; Soltis, P.S. Endemism, projected climate change, and identifying species of critical concern in the Scrub Mint clade (Lamiaceae). Conserv. Sci. Pract. 2022, 4, e621. [Google Scholar] [CrossRef]
- Yang, Y.; Saatchi, S.S.; Xu, L.; Choi, S.; Philips, N.; Kennedy, R.; Keller, M.; Knyazikhin, Y.; Myneni, R.B. Post-drought decline of the Amazon carbon sink. Nat. Commun. 2018, 9, 3172–3181. [Google Scholar] [CrossRef]
- Kramer, A.T.; Ison, J.L.; Ashley, M.V.; Howe, H.F. The genetic paradox of forest fragmentation. Conserv. Biol. 2008, 22, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Van Dyck, H.; Baguette, M. Dispersal behaviour in fragmented landscape: Routine or special movements? Basic Appl. Ecol. 2005, 5, 535–545. [Google Scholar] [CrossRef]
- Jump, A.S.; Peñuelas, J. Running to stand still: Adaptation and the response of plants to rapid climate change. Ecol. Lett. 2005, 8, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
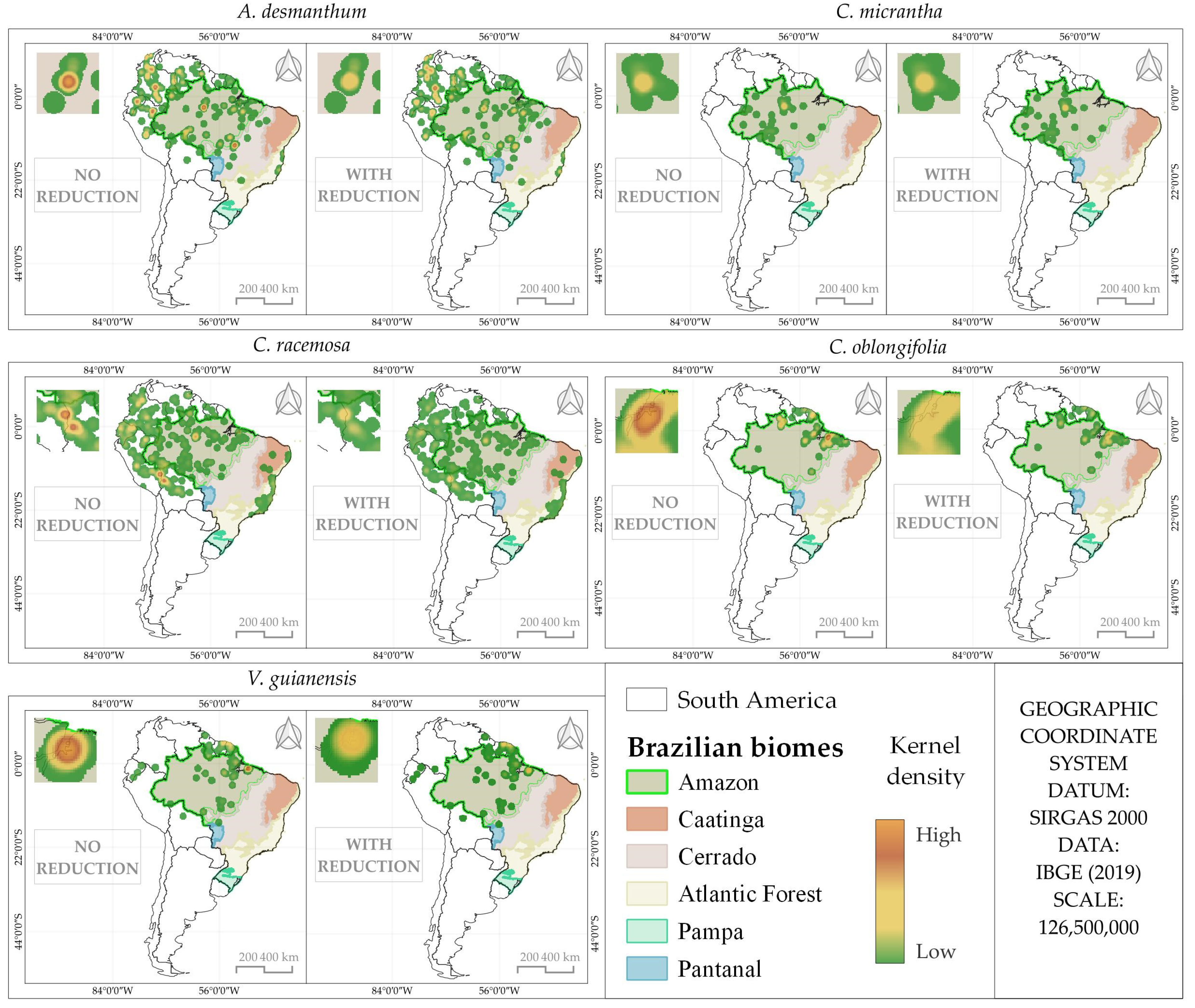
| Species | Number of Points | |
|---|---|---|
| Without Spatial Reduction | With Spatial Reduction | |
| A. desmanthum | 249 | 203 |
| C. micrantha | 120 | 58 |
| C. racemosa | 852 | 455 |
| C. oblongifolia | 110 | 71 |
| V. guianensis | 115 | 75 |
| Species | Base Period | SSP2–4.5 | |||
|---|---|---|---|---|---|
| 2021–2040 | % | 2041–2060 | % | ||
| A. desmanthum | 4,136,188 | 4,102,344 | –1 | 4,067,493 | –2 |
| C. micranta | 2,465,926 | 2,628,901 | 7 | 2,448,709 | −1 |
| C. racemosa | 4,093,816 | 3,544,000 | −13 | 2,889,697 | −29 |
| C. oblongifolia | 2,224,906 | 2,484,873 | 12 | 2,472,128 | 11 |
| V. guianensis | 2,999,466 | 2,892,412 | −4 | 2,596,902 | −13 |
| Species | Base Period | SSP5–8.5 | |||
| 2021–2040 | % | 2041–2060 | % | ||
| A. desmanthum | 4,136,188 | 4,087,326 | −1 | 2,450,481 | −41 |
| C. micranta | 2,465,926 | 2,599,843 | 5 | 2,393,141 | −3 |
| C. racemosa | 4,093,816 | 3,454,992 | −16 | 1,548,902 | −62 |
| C. oblongifolia | 2,224,906 | 2,431,419 | 9 | 1,803,522 | −19 |
| V. guianensis | 2,999,466 | 2,796,509 | −7 | 1,886,705 | −37 |
| Species | AUC | TSS | Sorensen |
|---|---|---|---|
| A. desmanthum | 0.995 ± 0.001 | 0.961 ± 0.016 | 0.980 ± 0.008 |
| C. micranta | 0.965 ± 0.019 | 0.847 ± 0.053 | 0.927 ± 0.024 |
| C. racemosa | 0.989 ± 0.007 | 0.932 ± 0.029 | 0.966 ± 0.014 |
| C. oblongifolia | 0.986 ± 0.009 | 0.916 ± 0.055 | 0.956 ± 0.031 |
| V. guianensis | 0.981 ± 0.018 | 0.906 ± 0.090 | 0.951 ± 0.050 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Morais, I.L.L.; de Lima, A.A.; Santos, I.N.L.d.; Meneses, C.; da Silva, R.F.; Lopes, R.; Ramos, S.L.F.; de Aguiar, A.V.; Wrege, M.S.; Lopes, M.T.G. Climate Change Impact on the Distribution of Forest Species in the Brazilian Amazon. Sustainability 2024, 16, 3458. https://doi.org/10.3390/su16083458
de Morais ILL, de Lima AA, Santos INLd, Meneses C, da Silva RF, Lopes R, Ramos SLF, de Aguiar AV, Wrege MS, Lopes MTG. Climate Change Impact on the Distribution of Forest Species in the Brazilian Amazon. Sustainability. 2024; 16(8):3458. https://doi.org/10.3390/su16083458
Chicago/Turabian Stylede Morais, Ingrid Lana Lima, Alexandra Amaro de Lima, Ivinne Nara Lobato dos Santos, Carlos Meneses, Rogério Freire da Silva, Ricardo Lopes, Santiago Linorio Ferreyra Ramos, Ananda Virginia de Aguiar, Marcos Silveira Wrege, and Maria Teresa Gomes Lopes. 2024. "Climate Change Impact on the Distribution of Forest Species in the Brazilian Amazon" Sustainability 16, no. 8: 3458. https://doi.org/10.3390/su16083458








