By-Product Valorization as a Means for the Brewing Industry to Move toward a Circular Bioeconomy
Abstract
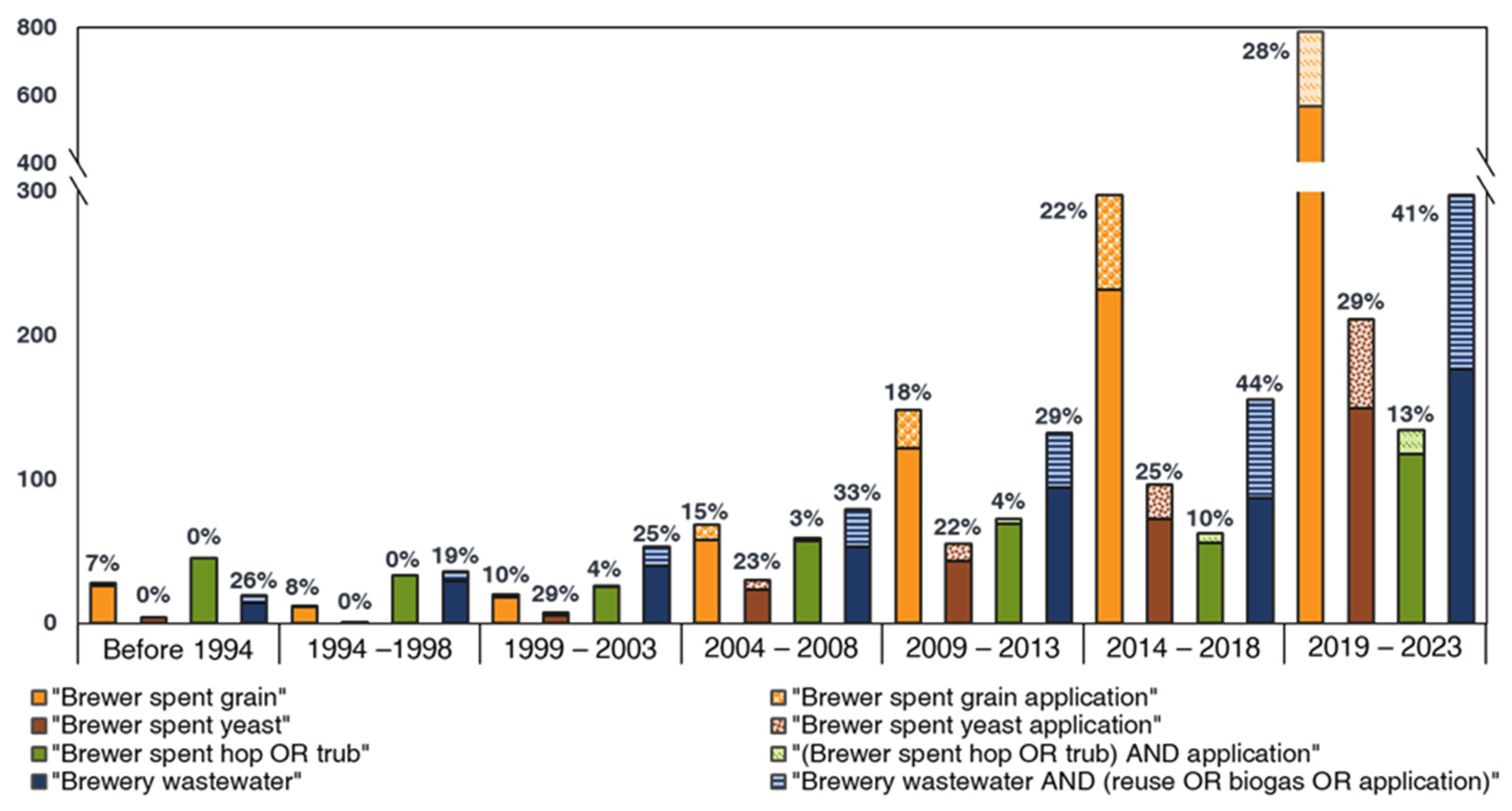
:1. Introduction
2. Raw Materials, Beer Production Process and Related By-Products
2.1. Main Constituents for Beer Production
2.2. Beer Production and By-Product Generation
3. Resource Conservation: Closing the Loop
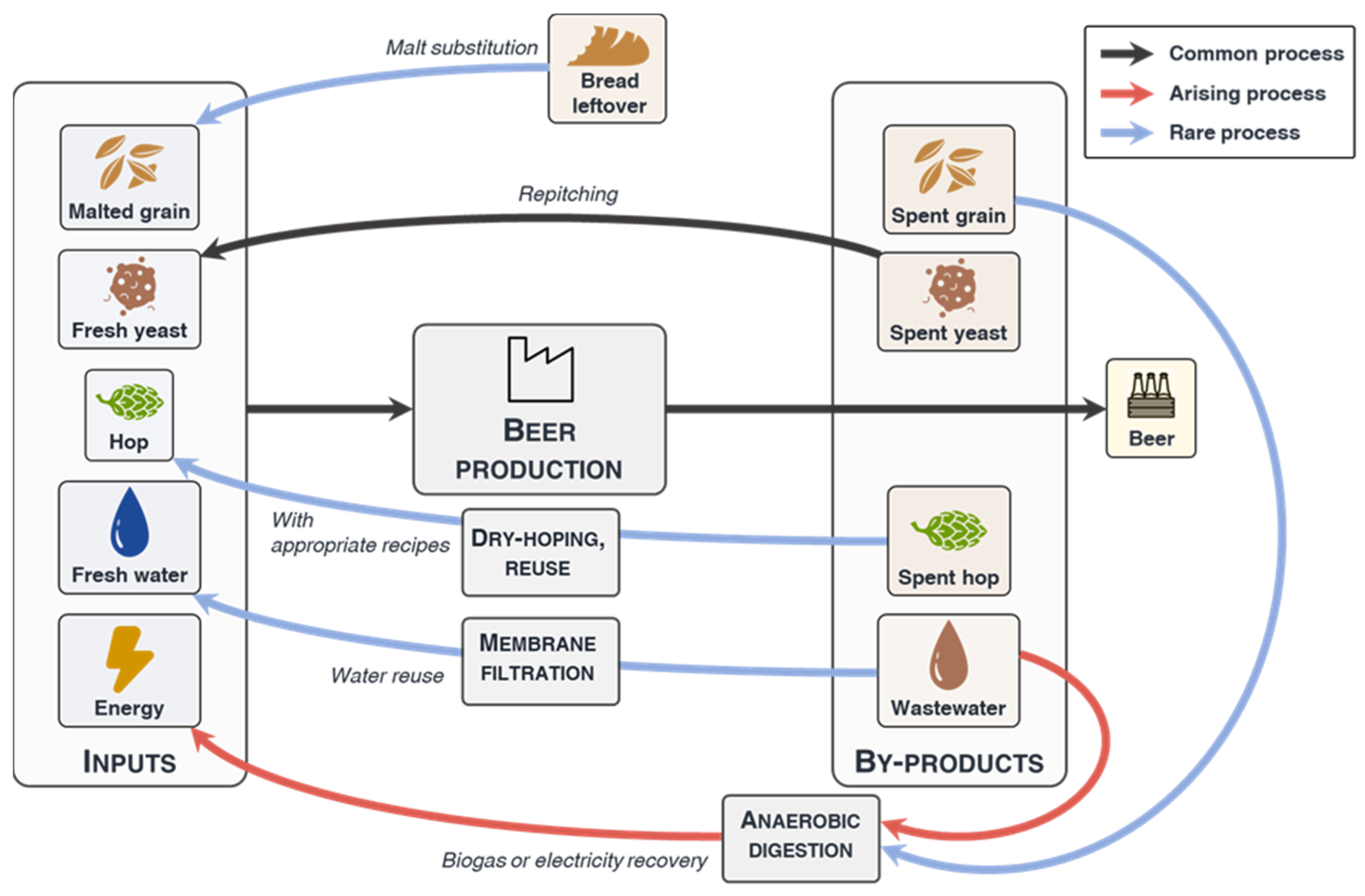
3.1. Raw Materials Substitution or Reuse
3.2. Energy Recovery
3.3. Water Reclamation
4. Direct Applications of Brewery By-Products
4.1. Current Use of Brewery By-Products
4.2. Solid By-Products
4.3. Brewery Wastewater
5. Brewery as Biorefinery
5.1. Non-Starch Polysaccharides
5.2. Proteins and Peptides
5.3. Lipids and Fatty Acids
5.4. Phenolic Compounds
5.5. PHA and Related Biopolymers
5.6. Other Biomolecules
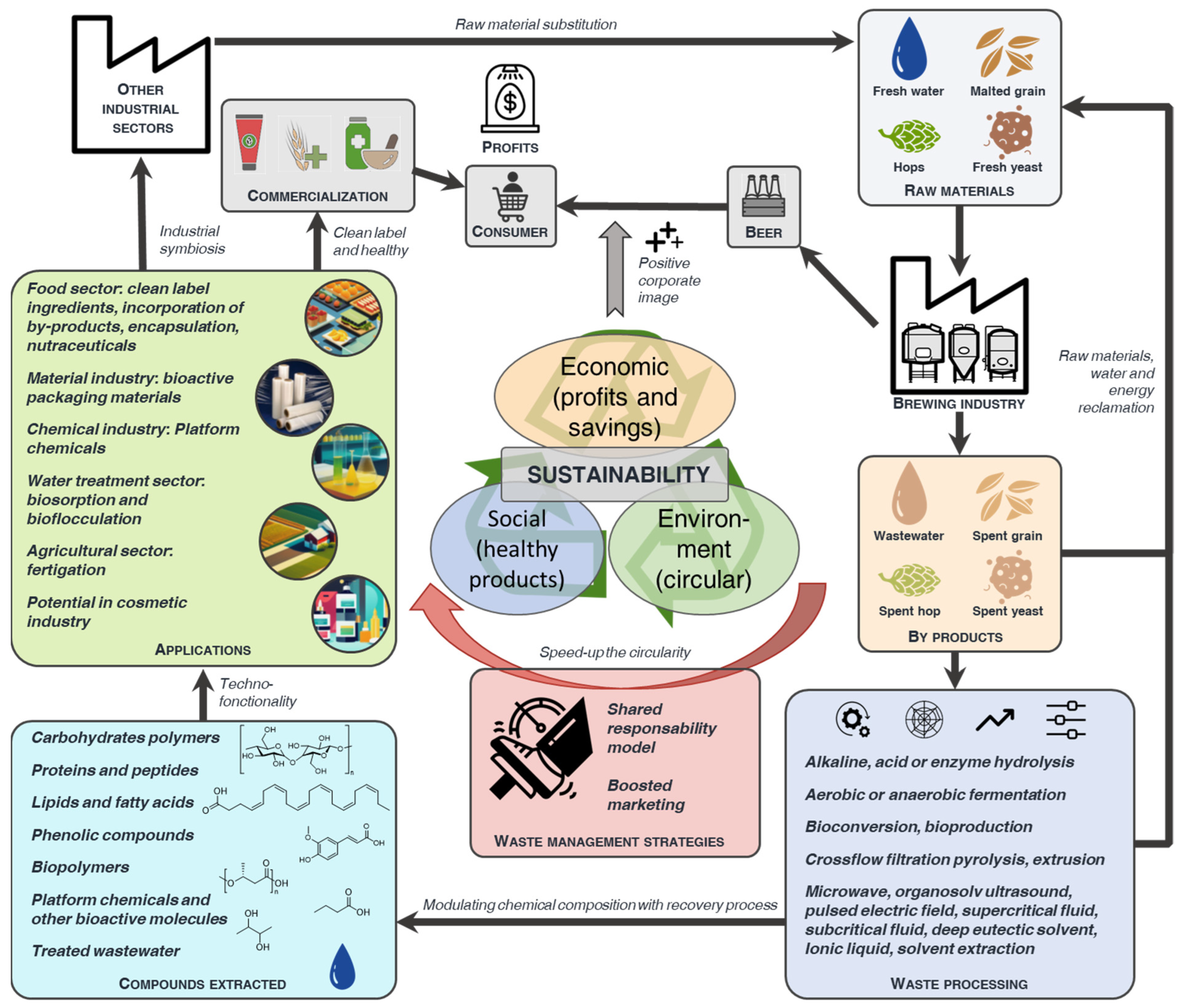
6. By-Product Extract Applications
7. Strategy for Efficient By-Product Management
7.1. Sustainable Economic Model Implementation Strategies
7.2. Sustainable Practices as a Marketing Tool
7.3. Global Strategy for an Environmentally Friendly Brewing Industry
8. Concluding Remarks and Perspectives for Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leisner, C.P. Climate Change Impacts on Food Security-Focus on Perennial Cropping Systems and Nutritional Value. Plant Sci. 2020, 293, 110412. [Google Scholar] [CrossRef] [PubMed]
- Cansino-Loeza, B.; Ponce-Ortega, J.M. Sustainable Assessment of Water-Energy-Food Nexus at Regional Level through a Multi-Stakeholder Optimization Approach. J. Clean. Prod. 2021, 290, 125194. [Google Scholar] [CrossRef]
- Wang, W.; Xiong, P.; Zhang, H.; Zhu, Q.; Liao, C.; Jiang, G. Analysis, Occurrence, Toxicity and Environmental Health Risks of Synthetic Phenolic Antioxidants: A Review. Environ. Res. 2021, 201, 111531. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Dagestani, A.A. What Lies about Circular Economy Practices and Performance? Fresh Insights from China. J. Clean. Prod. 2023, 416, 137893. [Google Scholar] [CrossRef]
- Rodríguez-Espíndola, O.; Cuevas-Romo, A.; Chowdhury, S.; Díaz-Acevedo, N.; Albores, P.; Despoudi, S.; Malesios, C.; Dey, P. The Role of Circular Economy Principles and Sustainable-Oriented Innovation to Enhance Social, Economic and Environmental Performance: Evidence from Mexican SMEs. Int. J. Prod. Econ. 2022, 248, 108495. [Google Scholar] [CrossRef]
- Keller, F.; Lee, R.P.; Meyer, B. Life Cycle Assessment of Global Warming Potential, Resource Depletion and Acidification Potential of Fossil, Renewable and Secondary Feedstock for Olefin Production in Germany. J. Clean. Prod. 2020, 250, 119484. [Google Scholar] [CrossRef]
- Valta, K.; Kosanovic, T.; Malamis, D.; Moustakas, K.; Loizidou, M. Overview of Water Usage and Wastewater Management in the Food and Beverage Industry. Desalin. Water Treat. 2015, 53, 3335–3347. [Google Scholar] [CrossRef]
- Bonato, S.V.; Augusto de Jesus Pacheco, D.; Schwengber ten Caten, C.; Caro, D. The Missing Link of Circularity in Small Breweries’ Value Chains: Unveiling Strategies for Waste Management and Biomass Valorization. J. Clean. Prod. 2022, 336, 130275. [Google Scholar] [CrossRef]
- Vieira, E.; Cunha, S.C.; Ferreira, I.M.P.L.V.O. Characterization of a Potential Bioactive Food Ingredient from Inner Cellular Content of Brewer’s Spent Yeast. Waste Biomass Valorization 2019, 10, 3235–3242. [Google Scholar] [CrossRef]
- Moran-Aguilar, M.G.; Costa-Trigo, I.; Calderón-Santoyo, M.; Domínguez, J.M.; Aguilar-Uscanga, M.G. Production of Cellulases and Xylanases in Solid-State Fermentation by Different Strains of Aspergillus niger Using Sugarcane Bagasse and Brewery Spent Grain. Biochem. Eng. J. 2021, 172, 108060. [Google Scholar] [CrossRef]
- Cerqueira e Silva, K.F.; Rabelo, R.S.; Feltre, G.; Hubinger, M. Bitter Substances Recovery from Hot Trub: A Study of Polymeric Membranes Performance in a Sequential Mode with Fouling Investigation. Sep. Purif. Technol. 2022, 303, 122241. [Google Scholar] [CrossRef]
- BarthHaas Group. Report 2022/2023; BarthHaas: Nuremberg, Germany, 2023. [Google Scholar]
- Ashraf, A.; Ramamurthy, R.; Rene, E.R. Wastewater Treatment and Resource Recovery Technologies in the Brewery Industry: Current Trends and Emerging Practices. Sustain. Energy Technol. Assess. 2021, 47, 101432. [Google Scholar] [CrossRef]
- Karlović, A.; Jurić, A.; Ćorić, N.; Habschied, K.; Krstanović, V.; Mastanjević, K. By-Products in the Malting and Brewing Industries—Re-Usage Possibilities. Fermentation 2020, 6, 82. [Google Scholar] [CrossRef]
- Mitri, S.; Salameh, S.-J.; Khelfa, A.; Leonard, E.; Maroun, R.G.; Louka, N.; Koubaa, M. Valorization of Brewers’ Spent Grains: Pretreatments and Fermentation, a Review. Fermentation 2022, 8, 50. [Google Scholar] [CrossRef]
- Umego, E.C.; Barry-Ryan, C. Review of the Valorization Initiatives of Brewing and Distilling By-Products. Crit. Rev. Food Sci. Nutr. 2023, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Sganzerla, W.G.; Ampese, L.C.; Mussatto, S.I.; Forster-Carneiro, T. A Bibliometric Analysis on Potential Uses of Brewer’s Spent Grains in a Biorefinery for the Circular Economy Transition of the Beer Industry. Biofuels Bioprod. Biorefin. 2021, 15, 1965–1988. [Google Scholar] [CrossRef]
- Rani, H.; Bhardwaj, R.D. Quality Attributes for Barley Malt: “The Backbone of Beer”. J. Food Sci. 2021, 86, 3322–3340. [Google Scholar] [CrossRef] [PubMed]
- Wannenmacher, J.; Gastl, M.; Becker, T. Phenolic Substances in Beer: Structural Diversity, Reactive Potential and Relevance for Brewing Process and Beer Quality. Compr. Rev. Food Sci. Food Saf. 2018, 17, 953–988. [Google Scholar] [CrossRef] [PubMed]
- Gyurchev, N.Y.; Coral-Medina, Á.; Weening, S.M.; Almayouf, S.; Kuijpers, N.G.A.; Nevoigt, E.; Louis, E.J. Beyond Saccharomyces Pastorianus for Modern Lager Brews: Exploring Non-Cerevisiae Saccharomyces Hybrids with Heterotic Maltotriose Consumption and Novel Aroma Profile. Front. Microbiol. 2022, 13, 1025132. [Google Scholar] [CrossRef]
- Langstaff, S.A.; Lewis, M.J. The Mouthfeel of Beer—A Review. J. Inst. Brew. 1993, 99, 31–37. [Google Scholar] [CrossRef]
- Capece, A.; Romaniello, R.; Siesto, G.; Romano, P. Conventional and Non-Conventional Yeasts in Beer Production. Fermentation 2018, 4, 38. [Google Scholar] [CrossRef]
- Kahle, E.-M.; Zarnkow, M.; Jacob, F. Beer Turbidity Part 1: A Review of Factors and Solutions. J. Am. Soc. Brew. Chem. 2021, 79, 99–114. [Google Scholar] [CrossRef]
- BIER. Beverage Industry Continues to Drive Improvement in Water, Energy, and Emissions Efficiency; 2021 Benchmarking Study Trends & Observations; Beverage Industry Environmental Roundtable: St. Paul, MN, USA, 2022; Available online: https://www.bieroundtable.com/wp-content/uploads/2021-BIER-Executive-Summary-Report.pdf (accessed on 23 February 2024).
- Carlsberg Group. ESG Report 2022; Carlsberg: Copenhagen, Denmark, 2022. [Google Scholar]
- Garcia-Garcia, G.; Stone, J.; Rahimifard, S. Opportunities for Waste Valorisation in the Food Industry—A Case Study with Four UK Food Manufacturers. J. Clean. Prod. 2019, 211, 1339–1356. [Google Scholar] [CrossRef]
- Krausová, I.; Cejnar, R.; Kučera, J.; Dostálek, P. Impact of the Brewing Process on the Concentration of Silicon in Lager Beer. J. Inst. Brew. 2014, 120, 433–437. [Google Scholar] [CrossRef]
- Vieira, E.; Brandão, T.; Ferreira, I.M.P.L.V.O. Evaluation of Brewer’s Spent Yeast To Produce Flavor Enhancer Nucleotides: Influence of Serial Repitching. J. Agric. Food Chem. 2013, 61, 8724–8729. [Google Scholar] [CrossRef] [PubMed]
- Kalayu, G. Serial Re-Pitching: Its Effect on Yeast Physiology, Fermentation Performance, and Product Quality. Ann. Microbiol. 2019, 69, 787–796. [Google Scholar] [CrossRef]
- Reis, S.F.; Messias, S.; Bastos, R.; Martins, V.J.; Correia, V.G.; Pinheiro, B.A.; Silva, L.M.; Palma, A.S.; Coimbra, M.A.; Coelho, E. Structural Differences on Cell Wall Polysaccharides of Brewer’s Spent Saccharomyces and Microarray Binding Profiles with Immune Receptors. Carbohydr. Polym. 2023, 301, 120325. [Google Scholar] [CrossRef]
- Martin-Lobera, C.; Aranda, F.; Lozano-Martinez, P.; Caballero, I.; Blanco, C.A. Bread as a Valuable Raw Material in Craft Ale Beer Brewing. Foods 2022, 11, 3013. [Google Scholar] [CrossRef] [PubMed]
- Dymchenko, A.; Geršl, M.; Gregor, T. The perspective of circular food waste management in the combined case of bakery and brewery. In Zero Waste Management and Circular Economy; Mendelova Univerzita v Brně: Brno, Czech Republic, 2021; pp. 129–135. [Google Scholar]
- Maina, S.; Kachrimanidou, V.; Koutinas, A. A Roadmap towards a Circular and Sustainable Bioeconomy through Waste Valorization. Curr. Opin. Green Sustain. Chem. 2017, 8, 18–23. [Google Scholar] [CrossRef]
- Brancoli, P.; Bolton, K.; Eriksson, M. Environmental Impacts of Waste Management and Valorisation Pathways for Surplus Bread in Sweden. Waste Manag. 2020, 117, 136–145. [Google Scholar] [CrossRef]
- Nunes, C.d.S.O.; de Carvalho, G.B.M.; da Silva, M.L.C.; da Silva, G.P.; Machado, B.A.S.; Uetanabaro, A.P.T. Cocoa Pulp in Beer Production: Applicability and Fermentative Process Performance. PLoS ONE 2017, 12, e0175677. [Google Scholar] [CrossRef]
- Hrabia, O.; Ditrych, M.; Ciosek, A.; Fulara, K.; Andersen, M.L.; Poreda, A. Effect of Dry Hopping on the Oxidative Stability of Beer. Food Chem. 2022, 394, 133480. [Google Scholar] [CrossRef]
- Hauser, D.G.; Lafontaine, S.R.; Shellhammer, T.H. Extraction Efficiency of Dry-Hopping. J. Am. Soc. Brew. Chem. 2019, 77, 188–198. [Google Scholar] [CrossRef]
- Gasiński, A.; Kawa-Rygielska, J.; Paszkot, J.; Pietrzak, W.; Śniegowska, J.; Szumny, A. Second Life of Hops: Analysis of Beer Hopped with Hop Pellets Previously Used to Dry-Hop a Beer. LWT 2022, 159, 113186. [Google Scholar] [CrossRef]
- Pinto, M.B.C.; Vardanega, R.; Náthia-Neves, G.; de França, P.R.L.; Kurozawa, L.E.; Meireles, M.A.A.; Schmidt, F.L. Novel Brazilian Hop (Humulus lupulus L.) Extracts through Supercritical CO2 Extraction: Enhancing Hop Processing for Greater Sustainability. Food Res. Int. 2023, 172, 113169. [Google Scholar] [CrossRef]
- Lamberti, L.; Boffa, L.; Grillo, G.; Concari, S.; Cavani, F.; Cravotto, G. Industrial Multiple-Effect Fractional Condensation under Vacuum for the Recovery of Hop Terpene Fractions in Water. Foods 2023, 12, 1716. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Sillero, L.; Forster-Carneiro, T.; Solera, R.; Perez, M. Determination of Anaerobic Co-Fermentation of Brewery Wastewater and Brewer’s Spent Grains for Bio-Hydrogen Production. BioEnergy Res. 2023, 16, 1073–1083. [Google Scholar] [CrossRef]
- Hakobyan, L.; Gabrielyan, L.; Blbulyan, S.; Trchounian, A. The Prospects of Brewery Waste Application in Biohydrogen Production by Photofermentation of Rhodobacter Sphaeroides. Int. J. Hydrogen Energy 2021, 46, 289–296. [Google Scholar] [CrossRef]
- Sarkar, O.; Rova, U.; Christakopoulos, P.; Matsakas, L. Influence of Initial Uncontrolled PH on Acidogenic Fermentation of Brewery Spent Grains to Biohydrogen and Volatile Fatty Acids Production: Optimization and Scale-Up. Bioresour. Technol. 2021, 319, 124233. [Google Scholar] [CrossRef]
- Gomes, M.M.; Sakamoto, I.K.; Silva Rabelo, C.A.B.; Silva, E.L.; Varesche, M.B.A. Statistical Optimization of Methane Production from Brewery Spent Grain: Interaction Effects of Temperature and Substrate Concentration. J. Environ. Manag. 2021, 288, 112363. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Costa, J.M.; Tena-Villares, M.; Buller, L.S.; Mussatto, S.I.; Forster-Carneiro, T. Dry Anaerobic Digestion of Brewer’s Spent Grains toward a More Sustainable Brewery: Operational Performance, Kinetic Analysis, and Bioenergy Potential. Fermentation 2022, 9, 2. [Google Scholar] [CrossRef]
- Mainardis, M.; Flaibani, S.; Mazzolini, F.; Peressotti, A.; Goi, D. Techno-Economic Analysis of Anaerobic Digestion Implementation in Small Italian Breweries and Evaluation of Biochar and Granular Activated Carbon Addition Effect on Methane Yield. J. Environ. Chem. Eng. 2019, 7, 103184. [Google Scholar] [CrossRef]
- Rojas-Chamorro, J.A.; Romero, I.; López-Linares, J.C.; Castro, E. Brewer’s Spent Grain as a Source of Renewable Fuel through Optimized Dilute Acid Pretreatment. Renew. Energy 2020, 148, 81–90. [Google Scholar] [CrossRef]
- Rojas-Chamorro, J.A.; Romero-García, J.M.; Cara, C.; Romero, I.; Castro, E. Improved Ethanol Production from the Slurry of Pretreated Brewers’ Spent Grain through Different Co-Fermentation Strategies. Bioresour. Technol. 2020, 296, 122367. [Google Scholar] [CrossRef]
- Wagner, E.; Sierra-Ibarra, E.; Rojas, N.L.; Martinez, A. One-Pot Bioethanol Production from Brewery Spent Grain Using the Ethanologenic Escherichia coli MS04. Renew. Energy 2022, 189, 717–725. [Google Scholar] [CrossRef]
- Alonso-Riaño, P.; Sanz Diez, M.T.; Blanco, B.; Beltrán, S.; Trigueros, E.; Benito-Román, O. Water Ultrasound-Assisted Extraction of Polyphenol Compounds from Brewer’s Spent Grain: Kinetic Study, Extract Characterization, and Concentration. Antioxidants 2020, 9, 265. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Ribeiro, B.; Ferreira, A.F.; Tavares, M.L.A.; Vladic, J.; Vidović, S.; Cvetkovic, D.; Melkonyan, L.; Avetisova, G.; Goginyan, V.; et al. Scenedesmus obliquus Microalga-Based Biorefinery—From Brewery Effluent to Bioactive Compounds, Biofuels and Biofertilizers—Aiming at a Circular Bioeconomy. Biofuels Bioprod. Biorefin. 2019, 13, 1169–1186. [Google Scholar] [CrossRef]
- Taylor, R.P.; Jones, C.L.W.; Laubscher, R.K. Empirical Comparison of Activated Sludge and High Rate Algal Ponding Technologies Used to Recover Water, Nitrogen and Carbon from Brewery Effluent. J. Water Process Eng. 2021, 40, 101840. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, Z.; Lv, M.; Liu, G.; Feng, Y. Enhancing Anaerobic Digestion Performance of Synthetic Brewery Wastewater with Direct Voltage. Bioresour. Technol. 2020, 315, 123764. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial Fuel Cells: Methodology and Technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef]
- Negassa, L.W.; Mohiuddin, M.; Tiruye, G.A. Treatment of Brewery Industrial Wastewater and Generation of Sustainable Bioelectricity by Microbial Fuel Cell Inoculated with Locally Isolated Microorganisms. J. Water Process Eng. 2021, 41, 102018. [Google Scholar] [CrossRef]
- Asensio, Y.; Llorente, M.; Tejedor-Sanz, S.; Fernández-Labrador, P.; Manchon, C.; Ortiz, J.M.; Ciriza, J.F.; Monsalvo, V.; Rogalla, F.; Esteve-Núñez, A. Microbial Electrochemical Fluidized Bed Reactor (ME-FBR): An Energy-Efficient Advanced Solution for Treating Real Brewery Wastewater with Different Initial Organic Loading Rates. J. Environ. Chem. Eng. 2021, 9, 106619. [Google Scholar] [CrossRef]
- Lu, M.; Chen, S.; Babanova, S.; Phadke, S.; Salvacion, M.; Mirhosseini, A.; Chan, S.; Carpenter, K.; Cortese, R.; Bretschger, O. Long-Term Performance of a 20-L Continuous Flow Microbial Fuel Cell for Treatment of Brewery Wastewater. J. Power Sources 2017, 356, 274–287. [Google Scholar] [CrossRef]
- Anwar, N.; Rahaman, M.S. Membrane Desalination Processes for Water Recovery from Pre-Treated Brewery Wastewater: Performance and Fouling. Sep. Purif. Technol. 2020, 252, 117420. [Google Scholar] [CrossRef]
- Toran, M.S.; Labrador, P.F.; Ciriza, J.F.; Asensio, Y.; Reigersman, A.; Arevalo, J.; Rogalla, F.; Monsalvo, V.M. Membrane-Based Processes to Obtain High-Quality Water from Brewery Wastewater. Front. Chem. Eng. 2021, 3, 734233. [Google Scholar] [CrossRef]
- Götz, G.; Geißen, S.-U.; Ahrens, A.; Reimann, S. Adjustment of the Wastewater Matrix for Optimization of Membrane Systems Applied for Water Reuse in Breweries. J. Membr. Sci. 2014, 465, 68–77. [Google Scholar] [CrossRef]
- Verhuelsdonk, M.; Glas, K.; Parlar, H. Economic Evaluation of the Reuse of Brewery Wastewater. J. Environ. Manag. 2021, 281, 111804. [Google Scholar] [CrossRef]
- Tatullo, M.; Simone, G.M.; Tarullo, F.; Irlandese, G.; Vito, D.D.; Marrelli, M.; Santacroce, L.; Cocco, T.; Ballini, A.; Scacco, S. Antioxidant and Antitumor Activity of a Bioactive Polyphenolic Fraction Isolated from the Brewing Process. Sci. Rep. 2016, 6, 36042. [Google Scholar] [CrossRef]
- Colpo, I.; Funck, V.M.; Martins, M.E.S. Waste Management in Craft Beer Production: Study of Industrial Symbiosis in the Southern Brazilian Context. Environ. Eng. Sci. 2022, 39, 418–430. [Google Scholar] [CrossRef]
- Rahman, I.; Nanu, L.; Sozen, E. The Adoption of Environmental Practices in Craft Breweries: The Role of Owner-Managers’ Consumption Values, Motivation, and Perceived Business Challenges. J. Clean. Prod. 2023, 416, 137948. [Google Scholar] [CrossRef]
- Abbas, C.A. Production of Antioxidants, Aromas, Colours, Flavours, and Vitamins by Yeasts. In Yeasts in Food and Beverages; Querol, A., Fleet, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 285–334. ISBN 978-3-540-28398-0. [Google Scholar]
- Tonk, S.; Rápó, E. Linear and Nonlinear Regression Analysis for the Adsorption of Remazol Dye by Romanian Brewery Waste By-Product, Saccharomyces cerevisiae. Int. J. Mol. Sci. 2022, 23, 11827. [Google Scholar] [CrossRef] [PubMed]
- Soh, E.Y.S.; Lim, S.S.; Chew, K.W.; Phuang, X.W.; Ho, V.M.V.; Chu, K.Y.H.; Wong, R.R.; Lee, L.Y.; Tiong, T.J. Valorization of Spent Brewery Yeast Biosorbent with Sonication-Assisted Adsorption for Dye Removal in Wastewater Treatment. Environ. Res. 2022, 204, 112385. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.M.; Vidya Shetty, K.; Srinikethan, G. Biosorption of Nickel (II) and Cadmium (II). In Methods for Bioremediation of Water and Wastewater Pollution; Inamuddin, M.I.A., Asiri, A.M., Lichtfouse, E., Eds.; Environmental Chemistry for a Sustainable World; Springer International Publishing: Cham, Switzerland, 2020; pp. 373–391. ISBN 978-3-030-48985-4. [Google Scholar]
- Mosai, A.K. Simultaneous Recovery of Pd(II), Ir(III) and Rh(III) from Aqueous Solutions by Spent Brewer’s Yeast-Functionalised Zeolite Using Flow-through Column Mode. Miner. Eng. 2021, 163, 106770. [Google Scholar] [CrossRef]
- Rubio, F.T.V.; Haminiuk, C.W.I.; de Freitas Santos, P.D.; Martelli-Tosi, M.; Thomazini, M.; de Carvalho Balieiro, J.C.; Makimori, G.Y.F.; Fávaro-Trindade, C.S. Investigation on Brewer’s Spent Yeast as a Bio-Vehicle for Encapsulation of Natural Colorants from Pumpkin (Cucurbita moschata) Peels. Food Funct. 2022, 13, 10096–10109. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Fu, J.; Li, J.; Tang, Y.; Shao, Z.; Zhou, D.; Song, L. Efficient Encapsulation of Curcumin into Spent Brewer’s Yeast Using a PH-Driven Method. Food Chem. 2022, 394, 133537. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.J.; Nielsen, S.H.M.; Johansen, M.; Nielsen, F.K.; Dragsbæk, F.B.; Sørensen, O.S.B.; Eriksen, N.T. Metabolic Performance of Black Soldier Fly Larvae during Entomoremediation of Brewery Waste. J. Appl. Entomol. 2023, 147, 423–431. [Google Scholar] [CrossRef]
- Lucas, E.; Guo, M.; Guillén-Gosálbez, G. Low-Carbon Diets Can Reduce Global Ecological and Health Costs. Nat. Food 2023, 4, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Anisha, A.; Kaushik, D.; Kumar, M.; Kumar, A.; Esatbeyoglu, T.; Proestos, C.; Khan, M.R.; Elobeid, T.; Kaur, J.; Oz, F. Volarisation of Brewer’s Spent Grain for Noodles Preparation and Its Potential Assessment against Obesity. Int. J. Food Sci. Technol. 2023, 58, 3154–3179. [Google Scholar] [CrossRef]
- Lalić, A.; Karlović, A.; Marić, M. Use of Brewers’ Spent Grains as a Potential Functional Ingredient for the Production of Traditional Herzegovinian Product Ćupter. Fermentation 2023, 9, 123. [Google Scholar] [CrossRef]
- Riera-Vila, I.; Anderson, N.O.; Flavin Hodge, C.; Rogers, M. Anaerobically-Digested Brewery Wastewater as a Nutrient Solution for Substrate-Based Food Production. Horticulturae 2019, 5, 43. [Google Scholar] [CrossRef]
- Alayu, E.; Leta, S. Evaluation of Irrigation Suitability Potential of Brewery Effluent Post Treated in a Pilot Horizontal Subsurface Flow Constructed Wetland System: Implications for Sustainable Urban Agriculture. Heliyon 2021, 7, e07129. [Google Scholar] [CrossRef] [PubMed]
- Mabasa, N.C.; Jones, C.L.W.; Laing, M. The Use of Treated Brewery Effluent for Salt Tolerant Crop Irrigation. Agric. Water Manag. 2021, 245, 106590. [Google Scholar] [CrossRef]
- del Río, J.C.; Prinsen, P.; Gutiérrez, A. Chemical Composition of Lipids in Brewer’s Spent Grain: A Promising Source of Valuable Phytochemicals. J. Cereal Sci. 2013, 58, 248–254. [Google Scholar] [CrossRef]
- Ikram, S.; Huang, L.; Zhang, H.; Wang, J.; Yin, M. Composition and Nutrient Value Proposition of Brewers Spent Grain. J. Food Sci. 2017, 82, 2232–2242. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, A.; Arendt, E.K.; Zannini, E.; Sahin, A.W. Brewer’s Spent Yeast (BSY), an Underutilized Brewing By-Product. Fermentation 2020, 6, 123. [Google Scholar] [CrossRef]
- Outeiriño, D.; Costa-Trigo, I.; Pinheiro de Souza Oliveira, R.; Pérez Guerra, N.; Domínguez, J.M. A Novel Approach to the Biorefinery of Brewery Spent Grain. Process Biochem. 2019, 85, 135–142. [Google Scholar] [CrossRef]
- Outeiriño, D.; Costa-Trigo, I.; Rodríguez, A.; Pérez Guerra, N.; Domínguez, J.M. Recovery and Reuse of Ionic Liquid Cholinium Glycinate in the Treatment of Brewery Spent Grain. Sep. Purif. Technol. 2021, 254, 117651. [Google Scholar] [CrossRef]
- Leite, P.; Silva, C.; Salgado, J.M.; Belo, I. Simultaneous Production of Lignocellulolytic Enzymes and Extraction of Antioxidant Compounds by Solid-State Fermentation of Agro-Industrial Wastes. Ind. Crops Prod. 2019, 137, 315–322. [Google Scholar] [CrossRef]
- Cassoni, A.C.; Costa, P.; Mota, I.; Vasconcelos, M.W.; Pintado, M. Recovery of Lignins with Antioxidant Activity from Brewer’s Spent Grain and Olive Tree Pruning Using Deep Eutectic Solvents. Chem. Eng. Res. Des. 2023, 192, 34–43. [Google Scholar] [CrossRef]
- Provost, V.; Dumarcay, S.; Ziegler-Devin, I.; Boltoeva, M.; Trébouet, D.; Villain-Gambier, M. Deep Eutectic Solvent Pretreatment of Biomass: Influence of Hydrogen Bond Donor and Temperature on Lignin Extraction with High β-O-4 Content. Bioresour. Technol. 2022, 349, 126837. [Google Scholar] [CrossRef]
- Parchami, M.; Agnihotri, S.; Taherzadeh, M.J. Aqueous Ethanol Organosolv Process for the Valorization of Brewer’s Spent Grain (BSG). Bioresour. Technol. 2022, 362, 127764. [Google Scholar] [CrossRef] [PubMed]
- Sibhatu, H.K.; Anuradha Jabasingh, S.; Yimam, A.; Ahmed, S. Ferulic Acid Production from Brewery Spent Grains, an Agro-Industrial Waste. LWT 2021, 135, 110009. [Google Scholar] [CrossRef]
- Zago, E.; Tillier, C.; De Leener, G.; Nandasiri, R.; Delporte, C.; Bernaerts, K.V.; Shavandi, A. Sustainable Production of Low Molecular Weight Phenolic Compounds from Belgian Brewers’ Spent Grain. Bioresour. Technol. Rep. 2022, 17, 100964. [Google Scholar] [CrossRef]
- Sobek, S.; Zeng, K.; Werle, S.; Junga, R.; Sajdak, M. Brewer’s Spent Grain Pyrolysis Kinetics and Evolved Gas Analysis for the Sustainable Phenolic Compounds and Fatty Acids Recovery Potential. Renew. Energy 2022, 199, 157–168. [Google Scholar] [CrossRef]
- Amraoui, Y.; Prabhu, A.A.; Narisetty, V.; Coulon, F.; Kumar Chandel, A.; Willoughby, N.; Jacob, S.; Koutinas, A.; Kumar, V. Enhanced 2,3-Butanediol Production by Mutant Enterobacter Ludwigii Using Brewers’ Spent Grain Hydrolysate: Process Optimization for a Pragmatic Biorefinery Loom. Chem. Eng. J. 2022, 427, 130851. [Google Scholar] [CrossRef]
- Barroso, T.L.C.T.; Sganzerla, W.G.; Castro, L.E.N.; Freiria, N.L.M.; Barbero, G.F.; Lovillo, M.P.; Rostagno, M.A.; Forster-Carneiro, T. Removal of 5-Hydroxymethylfurfural from Brewer’s Spent Grains Hydrolysates Obtained by Subcritical Water Hydrolysis: An Approach Using Liquid-Liquid Extraction. J. Supercrit. Fluids 2023, 201, 106004. [Google Scholar] [CrossRef]
- Weiermüller, J.; Akermann, A.; Laudensack, W.; Chodorski, J.; Blank, L.M.; Ulber, R. Brewers’ Spent Grain as Carbon Source for Itaconate Production with Engineered Ustilago maydis. Bioresour. Technol. 2021, 336, 125262. [Google Scholar] [CrossRef] [PubMed]
- Lojananan, N.; Cheirsilp, B.; Intasit, R.; Billateh, A.; Srinuanpan, S.; Suyotha, W.; Boonsawang, P. Successive Process for Efficient Biovalorization of Brewers’ Spent Grain to Lignocellulolytic Enzymes and Lactic Acid Production through Simultaneous Saccharification and Fermentation. Bioresour. Technol. 2024, 397, 130490. [Google Scholar] [CrossRef]
- Imandi, S.B.; Karanam, S.K.; Nagumantri, R.; Srivastava, R.K.; Sarangi, P.K. Neural Networks and Genetic Algorithm as Robust Optimization Tools for Modeling the Microbial Production of Poly-β-Hydroxybutyrate (PHB) from Brewers’ Spent Grain. Biotechnol. Appl. Biochem. 2023, 70, 962–978. [Google Scholar] [CrossRef]
- Carvalheira, M.; Amorim, C.L.; Oliveira, A.C.; Guarda, E.C.; Costa, E.; Ribau Teixeira, M.; Castro, P.M.L.; Duque, A.F.; Reis, M.A.M. Valorization of Brewery Waste through Polyhydroxyalkanoates Production Supported by a Metabolic Specialized Microbiome. Life 2022, 12, 1347. [Google Scholar] [CrossRef]
- da Silva, A.M.M.; Almeida, F.S.; da Silva, M.F.; Goldbeck, R.; Sato, A.C.K. How Do PH and Temperature Influence Extraction Yield, Physicochemical, Functional, and Rheological Characteristics of Brewer Spent Grain Protein Concentrates? Food Bioprod. Process. 2023, 139, 34–45. [Google Scholar] [CrossRef]
- da Fonseca, Y.A.; da Silva Barreto, E.; Lomar, P.F.; de Queiroz Silva, S.; Gurgel, L.V.A.; Baêta, B.E.L. Biobased Production of Volatile Fatty Acids from Brewer’s Spent Grain: Optimization and Insights into the Impact of Protein Extraction on Process Performance. Biochem. Eng. J. 2024, 203, 109218. [Google Scholar] [CrossRef]
- He, Y.; Kuhn, D.D.; Ogejo, J.A.; O’Keefe, S.F.; Fraguas, C.F.; Wiersema, B.D.; Jin, Q.; Yu, D.; Huang, H. Wet Fractionation Process to Produce High Protein and High Fiber Products from Brewer’s Spent Grain. Food Bioprod. Process. 2019, 117, 266–274. [Google Scholar] [CrossRef]
- Grudniewska, A.; Popłoński, J. Simple and Green Method for the Extraction of Xanthohumol from Spent Hops Using Deep Eutectic Solvents. Sep. Purif. Technol. 2020, 250, 117196. [Google Scholar] [CrossRef]
- Klimek, K.; Tyśkiewicz, K.; Miazga-Karska, M.; Dębczak, A.; Rój, E.; Ginalska, G. Bioactive Compounds Obtained from Polish “Marynka” Hop Variety Using Efficient Two-Step Supercritical Fluid Extraction and Comparison of Their Antibacterial, Cytotoxic, and Anti-Proliferative Activities In Vitro. Molecules 2021, 26, 2366. [Google Scholar] [CrossRef] [PubMed]
- Nazareth, T.C.; Zanutto, C.P.; Maass, D.; Ulson de Souza, A.A.; de Arruda Guelli Ulson de Souza, S.M. A Low-Cost Brewery Waste as a Carbon Source in Bio-Surfactant Production. Bioprocess Biosyst. Eng. 2021, 44, 2269–2276. [Google Scholar] [CrossRef] [PubMed]
- Berzosa, A.; Delso, C.; Sanz, J.; Sánchez-Gimeno, C.; Raso, J. Sequential Extraction of Compounds of Interest from Yeast Biomass Assisted by Pulsed Electric Fields. Front. Bioeng. Biotechnol. 2023, 11, 1197710. [Google Scholar] [CrossRef] [PubMed]
- Artifon, W.; Mazur, L.P.; de Souza, A.A.U.; de Oliveira, D. Production of Bioflocculants from Spent Brewer’s Yeast and Its Application in the Treatment of Effluents with Textile Dyes. J. Water Process Eng. 2022, 49, 102997. [Google Scholar] [CrossRef]
- Russo, G.L.; Langellotti, A.L.; Verardo, V.; Martín-García, B.; Di Pierro, P.; Sorrentino, A.; Baselice, M.; Oliviero, M.; Sacchi, R.; Masi, P. Formulation of New Media from Dairy and Brewery Wastes for a Sustainable Production of DHA-Rich Oil by Aurantiochytrium mangrovei. Mar. Drugs 2022, 20, 39. [Google Scholar] [CrossRef]
- Boukid, F.; Pera, J.; Parladé, J.; Castellari, M. Fungal Bioconversion of Brewery By-Products: Assessment of Fatty Acids and Sterols Profiles. Qual. Assur. Saf. Crops Foods 2022, 14, 202–211. [Google Scholar] [CrossRef]
- Amorim, M.; Pinheiro, H.; Pintado, M. Valorization of Spent Brewer’s Yeast: Optimization of Hydrolysis Process towards the Generation of Stable ACE-Inhibitory Peptides. LWT 2019, 111, 77–84. [Google Scholar] [CrossRef]
- Marson, G.V.; Pereira, D.T.V.; da Costa Machado, M.T.; Di Luccio, M.; Martínez, J.; Belleville, M.-P.; Hubinger, M.D. Ultrafiltration Performance of Spent Brewer’s Yeast Protein Hydrolysate: Impact of PH and Membrane Material on Fouling. J. Food Eng. 2021, 302, 110569. [Google Scholar] [CrossRef]
- Thakkar, A.; Barbera, E.; Sforza, E.; Bertucco, A.; Davis, R.; Kumar, S. Flash Hydrolysis of Yeast (Saccharomyces cerevisiae) for Protein Recovery. J. Supercrit. Fluids 2021, 173, 105240. [Google Scholar] [CrossRef]
- Pejin, J.; Radosavljević, M.; Kocić-Tanackov, S.; Marković, R.; Djukić-Vuković, A.; Mojović, L. Use of Spent Brewer’s Yeast in L-(+) Lactic Acid Fermentation. J. Inst. Brew. 2019, 125, 357–363. [Google Scholar] [CrossRef]
- Pasquet, P.L.; Villain-Gambier, M.; Ziegler-Devin, I.; Julien-David, D.; Trébouet, D. Valorization of Phenolic Compounds from Brewery Wastewater: Performances Assessment of Ultrafiltration and Nanofiltration Process with Application of HPLC Coupled with Antioxidant Analysis Tool. Chem. Eng. J. 2023, 476, 146696. [Google Scholar] [CrossRef]
- Pasquet, P.L.; Bertagnolli, C.; Villain-Gambier, M.; Trébouet, D. Investigation of Phenolic Compounds Recovery from Brewery Wastewater with Coupled Membrane and Adsorption Process. J. Environ. Chem. Eng. 2024, 12, 112478. [Google Scholar] [CrossRef]
- Wu, H.; Dalke, R.; Mai, J.; Holtzapple, M.; Urgun-Demirtas, M. Arrested Methanogenesis Digestion of High-Strength Cheese Whey and Brewery Wastewater with Carboxylic Acid Production. Bioresour. Technol. 2021, 332, 125044. [Google Scholar] [CrossRef]
- Amini, M.; Yousefi-Massumabad, H.; Younesi, H.; Abyar, H.; Bahramifar, N. Production of the Polyhydroxyalkanoate Biopolymer by Cupriavidus necator Using Beer Brewery Wastewater Containing Maltose as a Primary Carbon Source. J. Environ. Chem. Eng. 2020, 8, 103588. [Google Scholar] [CrossRef]
- Tamang, P.; Banerjee, R.; Köster, S.; Nogueira, R. Comparative Study of Polyhydroxyalkanoates Production from Acidified and Anaerobically Treated Brewery Wastewater Using Enriched Mixed Microbial Culture. J. Environ. Sci. 2019, 78, 137–146. [Google Scholar] [CrossRef]
- Lu, H.; Peng, M.; Zhang, G.; Li, B.; Li, Y. Brewery Wastewater Treatment and Resource Recovery through Long Term Continuous-Mode Operation in Pilot Photosynthetic Bacteria-Membrane Bioreactor. Sci. Total Environ. 2019, 646, 196–205. [Google Scholar] [CrossRef]
- Caruso, M.A.; Piermaria, J.A.; Abraham, A.G.; Medrano, M. β-Glucans Obtained from Beer Spent Yeasts as Functional Food Grade Additive: Focus on Biological Activity. Food Hydrocoll. 2022, 133, 107963. [Google Scholar] [CrossRef]
- Su, X.; Jin, Q.; Xu, Y.; Wang, H.; Huang, H. Subcritical Water Treatment to Modify Insoluble Dietary Fibers from Brewer’s Spent Grain for Improved Functionality and Gut Fermentability. Food Chem. 2024, 435, 137654. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.M.; Camina, J.L.; Borroni, V.; Pérez, E.E. Protein Recovery from Brewery Solid Wastes. Food Chem. 2023, 407, 134810. [Google Scholar] [CrossRef]
- Grudniewska, A.; Pastyrczyk, N. New Insight for Spent Hops Utilization: Simultaneous Extraction of Protein and Xanthohumol Using Deep Eutectic Solvents. Biomass Convers. Biorefin. 2023, 13, 14975–14986. [Google Scholar] [CrossRef]
- Naibaho, J.; Korzeniowska, M.; Wojdyło, A.; Muchdatul Ayunda, H.; Foste, M.; Yang, B. Techno-Functional Properties of Protein from Protease-Treated Brewers’ Spent Grain (BSG) and Investigation of Antioxidant Activity of Extracted Proteins and BSG Residues. J. Cereal Sci. 2022, 107, 103524. [Google Scholar] [CrossRef]
- Kruk, M.; Varmanen, P.; Edelmann, M.; Chamlagain, B.; Trząskowska, M. Food By-Product Valorisation in Nutrients through Spent Brewer’s Yeast Bioprocessing with Propionibacterium freudenreichii. J. Clean. Prod. 2024, 434, 140102. [Google Scholar] [CrossRef]
- Hashemi Gahruie, H.; Mostaghimi, M.; Ghiasi, F.; Tavakoli, S.; Naseri, M.; Hosseini, S.M.H. The Effects of Fatty Acids Chain Length on the Techno-Functional Properties of Basil Seed Gum-Based Edible Films. Int. J. Biol. Macromol. 2020, 160, 245–251. [Google Scholar] [CrossRef]
- Song, C.; Hu, X.; Liu, Z.; Li, S.; Kitamura, Y. Combination of Brewery Wastewater Purification and CO2 Fixation with Potential Value-Added Ingredients Production via Different Microalgae Strains Cultivation. J. Clean. Prod. 2020, 268, 122332. [Google Scholar] [CrossRef]
- Tsimogiannis, D.; Oreopoulou, V. Classification of Phenolic Compounds in Plants. In Polyphenols in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 263–284. ISBN 978-0-12-813768-0. [Google Scholar]
- Fernandes, J.; Fialho, M.; Santos, R.; Peixoto-Plácido, C.; Madeira, T.; Sousa-Santos, N.; Virgolino, A.; Santos, O.; Vaz Carneiro, A. Is Olive Oil Good for You? A Systematic Review and Meta-Analysis on Anti-Inflammatory Benefits from Regular Dietary Intake. Nutrition 2020, 69, 110559. [Google Scholar] [CrossRef]
- Martinez-Gomez, A.; Caballero, I.; Blanco, C.A. Phenols and Melanoidins as Natural Antioxidants in Beer. Structure, Reactivity and Antioxidant Activity. Biomolecules 2020, 10, 400. [Google Scholar] [CrossRef]
- Potì, F.; Santi, D.; Spaggiari, G.; Zimetti, F.; Zanotti, I. Polyphenol Health Effects on Cardiovascular and Neurodegenerative Disorders: A Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 351. [Google Scholar] [CrossRef] [PubMed]
- Cortese, M.; Gigliobianco, M.R.; Peregrina, D.V.; Sagratini, G.; Censi, R.; Di Martino, P. Quantification of Phenolic Compounds in Different Types of Crafts Beers, Worts, Starting and Spent Ingredients by Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2020, 1612, 460622. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Molnar Jazić, J.; Gouveia, L.; Maletić, S.; Tomić, M.; Agbaba, J.; Vladić, J. Valorisation of Microalga Tetradesmus Obliquus Grown in Brewery Wastewater Using Subcritical Water Extraction towards Zero Waste. Chem. Eng. J. 2022, 437, 135324. [Google Scholar] [CrossRef]
- Kabir, E.; Kaur, R.; Lee, J.; Kim, K.-H.; Kwon, E.E. Prospects of Biopolymer Technology as an Alternative Option for Non-Degradable Plastics and Sustainable Management of Plastic Wastes. J. Clean. Prod. 2020, 258, 120536. [Google Scholar] [CrossRef]
- Mannina, G.; Presti, D.; Montiel-Jarillo, G.; Carrera, J.; Suárez-Ojeda, M.E. Recovery of Polyhydroxyalkanoates (PHAs) from Wastewater: A Review. Bioresour. Technol. 2020, 297, 122478. [Google Scholar] [CrossRef] [PubMed]
- Moll, E.; González-Martínez, C.; Chiralt, A. Release and Antibacterial Action of Phenolic Acids Incorporated into PHBV Films. Food Packag. Shelf Life 2023, 38, 101112. [Google Scholar] [CrossRef]
- Saavedra del Oso, M.; Mauricio-Iglesias, M.; Hospido, A. Evaluation and Optimization of the Environmental Performance of PHA Downstream Processing. Chem. Eng. J. 2021, 412, 127687. [Google Scholar] [CrossRef]
- Maina, S.; Prabhu, A.A.; Vivek, N.; Vlysidis, A.; Koutinas, A.; Kumar, V. Prospects on Bio-Based 2,3-Butanediol and Acetoin Production: Recent Progress and Advances. Biotechnol. Adv. 2022, 54, 107783. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.K.; Rahayu, F.; Yustina, I.; Fatah, G.S.A.; Kariada, I.K.; Antarlina, S.S.; Jufri, A.; Pamungkas, D. Contribution of Yeast and Its Biomass for the Preparation of Industrially Essential Materials: A Boon to Circular Economy. Bioresour. Technol. Rep. 2023, 23, 101508. [Google Scholar] [CrossRef]
- de Melo, A.N.F.; de Souza, E.L.; da Silva Araujo, V.B.; Magnani, M. Stability, Nutritional and Sensory Characteristics of French Salad Dressing Made with Mannoprotein from Spent Brewer’s Yeast. LWT-Food Sci. Technol. 2015, 62, 771–774. [Google Scholar] [CrossRef]
- Martins, Z.E.; Pinho, O.; Ferreira, I.M.P.L.V.O. Impact of New Ingredients Obtained from Brewer’s Spent Yeast on Bread Characteristics. J. Food Sci. Technol. 2018, 55, 1966–1971. [Google Scholar] [CrossRef] [PubMed]
- Vriesekoop, F.; Haynes, A.; van der Heijden, N.; Liang, H.; Paximada, P.; Zuidberg, A. Incorporation of Fermented Brewers Spent Grain in the Production of Sourdough Bread. Fermentation 2021, 7, 96. [Google Scholar] [CrossRef]
- Lamas, D.L.; Gende, L.B. Valorisation of Brewers’ Spent Grain for the Development of Novel Beverage and Food Products. Appl. Food Res. 2023, 3, 100314. [Google Scholar] [CrossRef]
- Spinelli, S.; Conte, A.; Del Nobile, M.A. Microencapsulation of Extracted Bioactive Compounds from Brewer’s Spent Grain to Enrich Fish-Burgers. Food Bioprod. Process. 2016, 100, 450–456. [Google Scholar] [CrossRef]
- Gutiérrez-Barrutia, M.B.; del Castillo, M.D.; Arcia, P.; Cozzano, S. Feasibility of Extruded Brewer’s Spent Grain as a Food Ingredient for a Healthy, Safe, and Sustainable Human Diet. Foods 2022, 11, 1403. [Google Scholar] [CrossRef]
- Gutierrez-Barrutia, M.B.; Cozzano, S.; Arcia, P.; del Castillo, M.D. Assessment of in Vitro Digestion of Reduced Sugar Biscuits with Extruded Brewers’ Spent Grain. Food Res. Int. 2023, 172, 113160. [Google Scholar] [CrossRef]
- Lomuscio, E.; Bianchi, F.; Cervini, M.; Giuberti, G.; Simonato, B.; Rizzi, C. Durum Wheat Fresh Pasta Fortification with Trub, a Beer Industry By-Product. Foods 2022, 11, 2496. [Google Scholar] [CrossRef]
- Barbosa-Pereira, L.; Aurrekoetxea, G.P.; Angulo, I.; Paseiro-Losada, P.; Cruz, J.M. Development of New Active Packaging Films Coated with Natural Phenolic Compounds to Improve the Oxidative Stability of Beef. Meat Sci. 2014, 97, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Moreirinha, C.; Vilela, C.; Silva, N.H.C.S.; Pinto, R.J.B.; Almeida, A.; Rocha, M.A.M.; Coelho, E.; Coimbra, M.A.; Silvestre, A.J.D.; Freire, C.S.R. Antioxidant and Antimicrobial Films Based on Brewers Spent Grain Arabinoxylans, Nanocellulose and Feruloylated Compounds for Active Packaging. Food Hydrocoll. 2020, 108, 105836. [Google Scholar] [CrossRef]
- Paredes-Ramos, M.; Conde Piñeiro, E.; Lopez Vilariño, J.M. Brewers’ Spent Hop Revalorization for the Production of High Added-Value Cosmetics Ingredients with Elastase Inhibition Capacity. Sci. Rep. 2022, 12, 22074. [Google Scholar] [CrossRef]
- Almendinger, M.; Rohn, S.; Pleissner, D. Malt and Beer-Related by-Products as Potential Antioxidant Skin-Lightening Agents for Cosmetics. Sustain. Chem. Pharm. 2020, 17, 100282. [Google Scholar] [CrossRef]
- Costa, E.M.; Oliveira, A.S.; Silva, S.; Ribeiro, A.B.; Pereira, C.F.; Ferreira, C.; Casanova, F.; Pereira, J.O.; Freixo, R.; Pintado, M.E.; et al. Spent Yeast Waste Streams as a Sustainable Source of Bioactive Peptides for Skin Applications. Int. J. Mol. Sci. 2023, 24, 2253. [Google Scholar] [CrossRef] [PubMed]
- Censi, R.; Vargas Peregrina, D.; Gigliobianco, M.R.; Lupidi, G.; Angeloni, C.; Pruccoli, L.; Tarozzi, A.; Di Martino, P. New Antioxidant Ingredients from Brewery By-Products for Cosmetic Formulations. Cosmetics 2021, 8, 96. [Google Scholar] [CrossRef]
- Navarro-López, E.; Ruíz-Nieto, A.; Ferreira, A.; Acién, F.G.; Gouveia, L. Biostimulant Potential of Scenedesmus obliquus Grown in Brewery Wastewater. Molecules 2020, 25, 664. [Google Scholar] [CrossRef] [PubMed]
- Nkeumaleu, A.T.; Benetti, D.; Haddadou, I.; Di Mare, M.; Ouellet-Plamondon, C.M.; Rosei, F. Brewery Spent Grain Derived Carbon Dots for Metal Sensing. RSC Adv. 2022, 12, 11621–11627. [Google Scholar] [CrossRef] [PubMed]
- Wakayama, N.J.; Park, Y.W. The Dynamics of Fine-Grained Firm–Stakeholder Contentions and Synergies in the Process of Sustainable Development: The Case of Cassava-Based Beer Production in Africa. Sustainability 2024, 16, 1618. [Google Scholar] [CrossRef]
- Oliveira Silva, W.D.; Morais, D.C. Transitioning to a Circular Economy in Developing Countries: A Collaborative Approach for Sharing Responsibilities in Solid Waste Management of a Brazilian Craft Brewery. J. Clean. Prod. 2021, 319, 128703. [Google Scholar] [CrossRef]
- Merga, B.; Mohammed, M.; Ahmed, A. Socio-Economic Impacts of Possible Brewery Waste Recycling in Agriculture. Cogent Environ. Sci. 2020, 6, 1732112. [Google Scholar] [CrossRef]
- Lee, N.M.; Callison, C.; Seltzer, T. Sustainable Beer: Testing the Effects of Water Conservation Messages and Brewery Type on Consumer Perceptions. J. Food Prod. Mark. 2020, 26, 619–638. [Google Scholar] [CrossRef]
- Stelick, A.; Sogari, G.; Rodolfi, M.; Dando, R.; Paciulli, M. Impact of Sustainability and Nutritional Messaging on Italian Consumers’ Purchase Intent of Cereal Bars Made with Brewery Spent Grains. J. Food Sci. 2021, 86, 531–539. [Google Scholar] [CrossRef]



| By-Product | Target Compounds | Method | Operating Conditions | Outcome | Reference |
|---|---|---|---|---|---|
| BSG | Carbohydrates | Fungal solid-state fermentation, ionic liquid extraction and enzymatic hydrolysis | Fermentation: 30 °C; 1 × 106 gspore·gBSG−1 of Aspergillus brasiliensis; 5 d Extraction: 90 °C; 10 g of cholinium glycinate; 16 h Enzymatic hydrolysis: 50 °C; 160 rpm; 4 h | 24.12 ± 2.07 glucan, 54.37 ± 2.34 xylan and 57.04 ± 2.22% arabinan recovery yield Ionic liquid recovery with similar performances up to 5 cycles | [82] |
| BSG | Carbohydrates | Ionic liquid extraction | Extraction: 90 °C; 120 rpm; 5:100 BSG:IL (w:w) with cholinium glycinate ionic liquid; 16 h Antisolvent extraction: room temperature; 1:1 acetone:water (v:v); 30 min + centrifugation at 2755× g; 30 min + filtration 0.45 µm | 71% delignification with high glucan, xylan and arabinan recovery | [83] |
| BSG | Enzymes | Fungal solid-state fermentation | Fermentation: 25 °C; 75% relative humidity; 2 × 106 spore inoculate; 7 d Extraction: 1:5 BSG:water (w:v) | Aspergillus ibericus: 300–313 Uxylanase·gdryBSG−1 and 51–62 Ucellulase·gdryBSG−1 Aspergillus niger CECT2088: 94 ± 4 Uβ-glucosidase·gdryBSG−1 | [84] |
| BSG | Enzymes | Fungal solid-state fermentation | Pretreatment: 121 °C; 1% NaOH:water (w:v); 1 h + 98–100 °C in water; 1 h Fermentation: 30 °C; 80% relative humidity; 5 × 106 spore inoculate of A. niger CECT2070; 7 d Extraction: 1:10 BSG:water (w:v) with 50 mmol·L−1 citrate buffer at pH 4.8 | 1400.8 Uxylanase·gdryBSG−1 and 6.23 Ucellulase·gdryBSG−1 | [10] |
| BSG | Lignin | Deep eutectic solvent extraction | 120 °C; 5:1 lactic acid: choline chloride (mol:mol); 5 h | Lignin extraction yield of 54.4 ± 2.6% with >75% purity | [85] |
| BSG | Lignin | Deep eutectic solvent extraction | 80 °C; 1:20 biomass:DES (m:m); 1:10 choline chloride:lactic acid (mol:mol); 24 h | Lignin extraction yield of 39.3% with 53% of β-O-4 inter-unit bonds | [86] |
| BSG | Lignin and carbohydrates | Organosolv extraction and centrifugation | Organosolv pretreatment: 180 °C; 1:1 ethanol:water (v:v); 120 min Centrifugation: 4500× g; 5 min | Lignin fraction: 95% (w:w) with 58% yield Glucan (cellulose) fraction: 39% (w:w) with 60% yield | [87] |
| BSG | Phenolic compounds | Acid pretreatment and alkaline hydrolysis | Acid pretreatment: 120 °C; 0.2% sulfuric acid; 17 min Alkaline hydrolysis: 120 °C; 1:20 BSG:NaOH solution (w:v); 2% NaOH (w:v); 90 min Extraction: 35.4% HCl addition; 80% ethanol addition and evaporation | 1.185 mgGAE·gdryBSG−1 including 0.46 mg·gdryBSG−1 of ferulic acid extract | [88] |
| BSG | Phenolic compounds | Microwave assisted extraction | Microwave pretreatment: 600 W; 30 min Extraction: 80 °C; 1:30 BSG:solvent (w:v); 70:30 ethanol:water (v:v); 60 min | 13.23 mgferulicacidequivalent·gdryBSG−1 extract rich in ferulic and p-Coumaric acids | [89] |
| BSG | Phenolic compounds and proteins | Ultrasound assisted extraction | 47 °C; 20 kHz; 21.7 mLwater·gBSG−1; pulse mode (5 s/5 s); 0.5 h | Phenolic compounds: 3.28 ± 0.12 mgGAE·gdryBSG−1 Proteins: 82 ± 1 mgprotein·gdryBSG−1 | [50] |
| BSG | Phenolic compounds, fatty acids and nitrogen compounds | Pyrolysis | 25–700 °C; 1, 2 and 4 °C·min−1 heating rate; 50 mL·min−1 nitrogen flow | 13.94% phenolic compounds, 23.95% fatty acids and 25.10% nitrogen-compounds release | [90] |
| BSG | Platform chemical—2,3-Butanediol | Bacterial bioproduction | Pretreatment: 400 W; 10% BSG:water (w:v); 0.5% NaOH:water (v:v); 60 s Enzymatic hydrolysis: pH 6.0; 50 °C; 10% BSG:water (w:v); 2% enzyme:water (v:v) Culture: 30 °C; 180 rpm; Enterobacter ludwigii mutant strain inoculate; 12 h | batch: 16.4 gBDO·L−1 with 0.41 gBDO·gglucose−1 fed-batch: 118.5 gBDO·L−1 with 0.43 gBDO·gglucose−1 | [91] |
| BSG | Platform chemical—5-hydroxymethylfurfural | Subcritical water hydrolysis and liquid-liquid extraction | Subcritical water hydrolysis: 180 °C; 15 MPa; 22.5 gwater·gBSG−1; 5 mL·min−1 water flow Liquid-liquid extraction: pH 6.5; 35 °C; 1:2 hydrolysate:ethyl acetate (v:v) | 73.85% of dry BSG 5-hydroxymethylfurfural recovery | [92] |
| BSG | Platform chemical—itaconate | Fugal bioconversion | Hydroloysis pretreatment: 180 °C; 600 rpm; 1:8 BSG:water (w:v); 15 min Enzymatic hydrolysis: pH 7.5; 50 °C; 70 gdry matter·L−1 BSG:water (w:v); 25 rpm; 72 h Culture: pH 6.5; 30 °C; engineered Ustilago maydis; 120 rpm | 0.38 g·gcarbohydrates−1 itaconate productivity with 0.11 g·L−1·h−1 yield | [93] |
| BSG | Platform chemical—VFAs | Fermentation | pH 9.0; 35 °C; 1:5 anerobic sludge:BSG (volatile solids:volatile solids) inoculate; 10 d | 9258 mgVFA·L−1 with high acetic and butyric acids content | [43] |
| BSG | Platform chemical—Lactic acid | Simultaneous saccharification and fermentation | pH 5.5; 37 °C; 100 rpm; Lactobacillus pentosus TISTR 920 inoculate; cellulase and xylanase enzyme cocktail converted from BSG; 72 h | 59.3 ± 1.0 g·L−1 lactic acid production with 0.297 g·gBSG−1 yield | [94] |
| BSG | Polyhydroxyalkanoates or related biopolymer | Bacterial bioproduction | 50.12 gBSG·L−1; 0.22 gyeast extract·L−1; 24.06% (v:v) salt concentration; Bacillus sphaericus NCIM 2478 inoculate | 916.31 mg·L−1 of PHB | [95] |
| BSG | Polyhydroxyalkanoates or related biopolymer | Bacterial bioproduction | pH 8.0–8.8; room temperature; microbial mixed culture enriched with PHA accumulating organisms | 35.2 ± 5.5% (w:w) of PHA accumulated | [96] |
| BSG | Proteins | Alkaline extraction | pH 11.0; 60 °C; 1:17 BSG:water (w:v); 3 h with sequential centrifugation | 87% protein yield | [97] |
| BSG | Proteins and plateform chemical—VFAs | Alkaline pretreatment and fermentation | Alkaline pretreatment: 70 °C; 455 mbar; 10 gdryBSG in 100 mL of 0.18 molNaOH·L−1; 1 h Fermentation: pH 8.0; 20 °C; 0.67 gvolatilesolids,substrate·gvolatilesolids,inoculum−1 inoculate; 10 d | Amino acids recovery along with 6448.9 ± 328.9 mg·L−1 of VFA production with 54.0 ± 0.1 of long chain volatile fatty acids | [98] |
| BSG | Proteins and fibers | Enzymatic hydrolysis and sieving | Enzymatic hydrolysis: pH 8.0; 60 °C; 20 µLenzyme·gBSG−1; 4 h | Protein fraction: 42.8% (w:w) with 43.7% yield Fiber fraction: 80.4% (w:w) with 56.4% yield | [99] |
| BSH | Xanthohumol | Deep eutectic solvent extraction | Extraction:60 °C; BSH:DES 1:50 (w:w); choline chloride:propylene glycol 1:2 (mol:mol); 1 h Antisolvent extraction: water:DES 3:1 (v:w) | 2.30 mg·gBSH−1 of xanthohumol | [100] |
| BSH | Xanthohumol, terpene derivatives, α- and β-acids | Supercritical fluid extraction | 50 °C; 300 bar; 50 kgCO2·kgBSH−1 | Concentrate of xanthohumol, terpene derivatives, α- and β-acids with 11.4% yield with antibacterial, antiproliferative towards cancer cells | [101] |
| Trub | Biosurfactant | Bacterial bioconversion | 30 °C; 200 rpm; 4.18% (v:v) trub; 6 g·L−1 yeast extract; 0.8 g·L−1 peptone; Bacillus subtilis ATCC 6051 inoculate; 72 h | 62.74 ± 2.09 mg·L−1 of surfactin | [102] |
| Trub | Bitter substances | Cross-flow filtration | Microfiltration in diafiltration mode: 25 °C; 4–5 bar; 0.2 µm PVDF membrane Nanofiltration: 25 °C; 20 bar; 150–300 and 300–500 Da PIP membrane | Concentration of bitters substances: permeation of 81% with microfiltration and retention of 90% with nanofiltration | [11] |
| BSY | Amino acids, glutathione, protein, and β-glucans | Pulsed electric field assisted extraction, incubation and autolysis | Pulse electric field: 15 kV·cm−1; 39.8 to 159.3 µs Incubation: 25 °C; 24 h Autolysis: pH 7.0; 11 d | Amino acids: 114.91 ± 2.86; gluthatione: 7.08 ± 0.64; proteins 187.82 ± 3.75 in mg·gdryBSY−1 After incubation, β-glucans: 275–300 mg·gdryBSY−1; After autolysis, manose: 1.7–2.2 g·L−1 | [103] |
| BSY | Bioflocculant | Alkaline hydrolysis | 100 °C; 2 mol·L−1 NaOH; 2 times; 1 h | Fraction with 46% of proteins and 29% of carbohydrates used as bioflocculant: treatment of recalcitrant dyes from textile wastewater | [104] |
| BSY | Fatty acids | Algal bioproduction with dairy wastewater | Pretreatment: lactase and protease hydrolysis of dairy wastewater and mix with BSY Culture: 28 °C; 400 mgdry weight·L−1 Aurantiochytrium mangrovei inoculate; 15.34 gglucose·L−1; 3.22 gBSY·L−1 | Biomass production of 3.35 ± 0.08 g·L−1·d−1 with 38.9 ± 0.88% lipid and 29.8% docosahexaenoic acid of dry weight | [105] |
| BSY | Fatty acids and sterols | Fungal bioconversion | Incubation: 22–25 °C; 1:30 (v:v) Pleurotus ostreatus inoculate; 5–7 weeks Fruiting: 15 °C, 80–90% relative humidity | Lipids content: 89.5–173.5 µg·gbiomass−1 with high polyinsatured fatty acid content Phytosterols: 257–416 µg·gbiomass−1 | [106] |
| BSY | Peptides | Autolysis and enzymatic hydrolysis | Autolysis: 70 °C; 5 h Enzymatic hydrolysis: 70 °C; 4% (v:v) enzyme; 4.5 h | Antihypertensive biological activity peptides which can be concentrated by NF; final IC50: 75.1 ± 10.5 µgprotein·mL−1 | [107] |
| BSY | Peptides | Cross-flow filtration | pH 8.0; 50 °C; 5 bar; 30 kDa RC membrane | 66% peptide retention with limited fouling | [108] |
| BSY | Yeast extract | Flash hydrolysis | 240 °C; 10% dry yeast:water (w:v); 10 s | Yeast extract: 66.5% carbon, 70.4% nitrogen and 61.0% yeast recovery | [109] |
| BSY + BSG | Platform chemical—Lactic acid | BSY and BSG co-fermentation | Enzymatic pretreatment: hydrolysis at acid pH, addition of 50 gBSY·L−1 Fermentation: pH 6.2; 37 °C; 5% (v:v) of Lactobacillus rhamnosus ATCC 7469 inoculate; 36 h | 0.89 g·L−1·h−1 lactic acid productivity with 89% yield | [110] |
| BWW | Phenolic compounds | Algal bioproduction | Culture: 23–25 °C; 43.2 µmolphotons·m−2·s−1 fluorescent light; Scenedesmus obliquus Subcritical waster extraction: 200 °C; 35 bar; 1000 rpm; 1:10 biomass:water (w:v); 10 min | 1.016 ± 0.005 mgGAE·mL−1 of total phenolic content including 0.167 ± 0.003 mgcatechin equivalent·mL−1 of total flavonoids | [51] |
| BWW | Phenolic compounds | Cross-flow filtration | Ultrafiltration: variable pH; 60 °C; 2.2–2.3 bar; 5 m·s−1; 15 kDa TiO2-ZrO2 membrane Nanofiltration: variable pH; 50 °C; 23 bar; 600 L·h−1; 200 Da TFC membrane | Total phenolic content rejection: 8% and 0% during ultrafiltration and 84% and 88% during nanofiltration, for pH 6.5 and 13.5 respectively—mainly flavonoids compounds 0.167 ± 0.003 mgcatechin equivalent·mL−1 of total flavonoids | [111] |
| BWW | Phenolic compounds | Cross-flow filtration and ion-exchange process | Ultrafiltration: pH 6.5; 60 °C; 2.3 bar; 5 m·s−1; 15 kDa TiO2-ZrO2 membrane Ion-exchange: pH 6.5; 25 °C; 160 rpm; SCAV4 resin with OH− as counter ion; 50 gdryresin·L−1; 24 h | Adsorption capacity: 35.4 ± 0.8 mg mggallicacid equivalent·L−1 (corresponding to 84% of initial phenolic compounds) with a selectivity factor of 5.86 ± 0.97 compared to carbohydrates | [112] |
| BWW | Platform chemical—Volatile fatty acids | Co-fermentation with cheese whey | pH 6.0; 40 °C; 100 gCOD·L−1; 4 d hydraulic residence time | 16 g·L−1·d−1 of total carboxylic acids with 0.66 gCODdigested·gCODfed−1 yield | [113] |
| BWW | Polyhydroxyalkanoates or related biopolymer | Bacterial bioproduction | 30 °C; 200 rpm; Cupriavidus necator inoculate; 5 d | P3HB-co-P3HV copolymer with 2.3 and 0.65 g·L−1 of P3HB and P3HV respectively | [114] |
| BWW | Polyhydroxyalkanoates or related biopolymer | Bacterial bioproduction | Fed batch of acidified or anaerobically treated brewery wastewater: 30 °C; microbial mixed culture enriched with PHA accumulating organisms; 105 d | 44% (w:w) of PHA accumulated | [115] |
| BWW | Proteins, carbohydrates and bioactive compounds | Photosynthetic bacterial bioproduction | 5–32 °C; 125 W·m−2; 1.0 g·L−1·d−1 organic loading rate; 1200 mg·L−1 inoculate size; 72 h HRT; 440 d | 483.5 mg·Leffluent−1·d−1 biomass production with 420.9 protein, 177.6 polysaccharides, 2.53 carotenoid, 10.75 bacteriochlorophyll and 38.6 mg gbiomass−1 coenzyme Q10 | [116] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasquet, P.-L.; Villain-Gambier, M.; Trébouet, D. By-Product Valorization as a Means for the Brewing Industry to Move toward a Circular Bioeconomy. Sustainability 2024, 16, 3472. https://doi.org/10.3390/su16083472
Pasquet P-L, Villain-Gambier M, Trébouet D. By-Product Valorization as a Means for the Brewing Industry to Move toward a Circular Bioeconomy. Sustainability. 2024; 16(8):3472. https://doi.org/10.3390/su16083472
Chicago/Turabian StylePasquet, Paul-Loup, Maud Villain-Gambier, and Dominique Trébouet. 2024. "By-Product Valorization as a Means for the Brewing Industry to Move toward a Circular Bioeconomy" Sustainability 16, no. 8: 3472. https://doi.org/10.3390/su16083472






