CO2 Emission Compensation by Tree Species in Some Urban Green Areas
Abstract
:1. Introduction
- Calculation of the CO2 storage of four Italian urban parks to assess their contribution to the heat island effect and climate change mitigation.
- Calculation of the CO2 compensated by the four parks, based on the trees’ potential heat reduction (Q) and evapotranspiration (ETP).
- Comparison of the trees’ CO2 storage with the CO2 compensated by each park.
2. Material and Methods
2.1. Study Areas
2.2. Data Collection
2.3. Potential CO2 Storage Assessment
2.4. Potential Heat Reduction (Q) and Potential Evapotranspiration (ETP) Assessment
2.4.1. Estimation of Evapotranspiration
- Tm = average air temperature (°C)
- Tmax = daily maximum temperature (°C)
- Tmin = daily minimum temperature (°C)
- Rse = daily extraterrestrial solar radiation (mm/d)
2.4.2. Heat Reduction Potential (Q) Calculation
- Qw = air heat reduction due to water consumption W/m2
- ETR = amount of water consumed by the plant (mm/day)
- A = canopy surface area (m2)
- mw = rate of water consumption by the plant (kg/second)
- hfg = latent heat of evaporation of water (J/kg)
- Ta = average temperature (°C)
- ρw = water density (kg/m3)
2.5. Radiative Forcing and CO2 Compensated by the CIRIAF Model
3. Results
3.1. Heat Reduction Effect and Stored CO2 Results
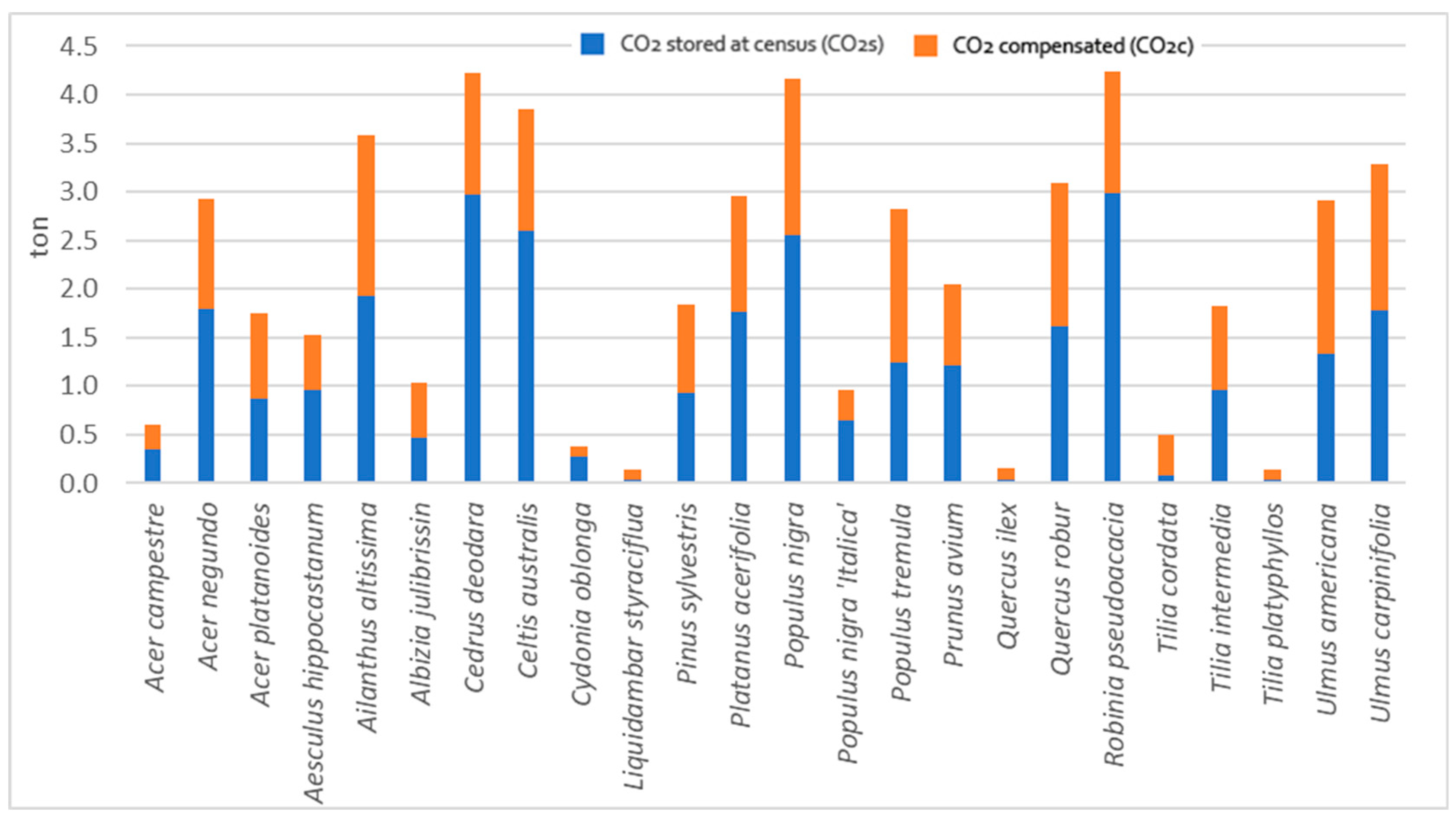
3.2. Compensated CO2 and Stored CO2 Comparison
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baglivo, C.; Albanese, P.M.; Congedo, P.M. Relationship between Shape and Energy Performance of Buildings under Long-Term Climate Change. J. Build. Eng. 2024, 84, 108544. [Google Scholar] [CrossRef]
- Yue, X.L.; Gao, Q.X. Contributions of Natural Systems and Human Activity to Greenhouse Gas Emissions. Adv. Clim. Chang. Res. 2018, 9, 243–252. [Google Scholar] [CrossRef]
- Carter, J.G.; Cavan, G.; Connelly, A.; Guy, S.; Handley, J.; Kazmierczak, A. Climate Change and the City: Building Capacity for Urban Adaptation. Prog. Plann. 2015, 95, 1–66. [Google Scholar] [CrossRef]
- Kabisch, N.; Korn, H.; Stadler, J.; Bonn, A. Nature-Based Solutions to Climate Change Adaptation in Urban Areas. Linkages between Science, Policy and Practice; Springer Nature: New York, NY, USA, 2017; ISBN 9783319537504. [Google Scholar]
- European Commission. The European Green Deal; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- Gill, S.E.; Handley, J.F.; Ennos, A.R.; Pauleit, S. Adapting Cities for Climate Change: The Role of the Green Infrastructure LK. Built Environ. 2007, 33, 115–132. Available online: https://Slc.on.Worldcat.Org/Oclc/123729317 (accessed on 26 September 2019). [CrossRef]
- Nowak, D.J.; Dwyer, J.F. Understanding the Benefits and Costs of Urban Forest Ecosystems. In Urban and Community Forestry in the Northeast; Springer: Dordrecht, The Netherlands, 2010; pp. 11–25. [Google Scholar] [CrossRef]
- Ning, T.; Wang, J.; Elgered, G.; Dick, G.; Wickert, J.; Bradke, M.; Sommer, M.; Querel, R.; Smale, D. The Uncertainty of the Atmospheric Integrated Water Vapour Estimated from GNSS Observations. Atmos. Meas. Tech. 2016, 9, 79–92. [Google Scholar] [CrossRef]
- Carrus, G.; Scopelliti, M.; Lafortezza, R.; Colangelo, G.; Ferrini, F.; Salbitano, F.; Agrimi, M.; Portoghesi, L.; Semenzato, P.; Sanesi, G. Go Greener, Feel Better? The Positive Effects of Biodiversity on the Well-Being of Individuals Visiting Urban and Peri-Urban Green Areas. Landsc. Urban Plan. 2015, 134, 221–228. [Google Scholar] [CrossRef]
- Shashua-Bar, L.; Hoffman, M.E. Vegetation as a Climatic Component in the Design of an Urban Street. Energy Build. 2000, 31, 221–235. [Google Scholar] [CrossRef]
- Cao, X.; Onishi, A.; Chen, J.; Imura, H. Quantifying the Cool Island Intensity of Urban Parks Using ASTER and IKONOS Data. Landsc. Urban Plan. 2010, 96, 224–231. [Google Scholar] [CrossRef]
- Nowak, D.J.; Crane, D.E.; Stevens, J.C. Air Pollution Removal by Urban Trees and Shrubs in the United States. Urban For. Urban Green. 2006, 4, 115–123. [Google Scholar] [CrossRef]
- Nowak, D.J.; Greenfield, E.J.; Hoehn, R.E.; Lapoint, E. Carbon Storage and Sequestration by Trees in Urban and Community Areas of the United States. Environ. Pollut. 2013, 178, 229–236. [Google Scholar] [CrossRef]
- Massetti, L.; Petralli, M.; Brandani, G.; Napoli, M.; Ferrini, F.; Fini, A.; Pearlmutter, D.; Orlandini, S.; Giuntoli, A. Modelling the Effect of Urban Design on Thermal Comfort and Air Quality: The SMARTUrban Project. Build. Simul. 2019, 12, 169–175. [Google Scholar] [CrossRef]
- Lin, B.S.; Lin, Y.J. Cooling Effect of Shade Trees with Different Characteristics in a Subtropical Urban Park. HortScience 2010, 45, 83–86. [Google Scholar] [CrossRef]
- Zardo, L.; Geneletti, D.; Pérez-Soba, M.; Van Eupen, M. Estimating the Cooling Capacity of Green Infrastructures to Support Urban Planning. Ecosyst. Serv. 2017, 26, 225–235. [Google Scholar] [CrossRef]
- Orlandi, F.; Fornaciari, M.; Ranfa, A.; Proietti, C.; Ruga, L.; Meloni, G.; Burnelli, M.; Ventura, F. LIFE-CLIVUT, Ecosystem Benefits of Urban Green Areas: A Pilot Case Study in Perugia (Italy). iFor. Biogeosci. For. 2022, 15, 133–140. [Google Scholar] [CrossRef]
- Orlandi, F.; Ranfa, A.; Proietti, C.; Ruga, L.; Ventura, F.; Fornaciari, M. Dynamic Estimates of Tree Carbon Storage and Shade in Mediterranean Urban Areas. Int. For. Rev. 2022, 24, 225–239. [Google Scholar] [CrossRef]
- Cvejić, R.; Eler, K.; Pintar, M.; Železnikar, Š.; Haase, D.; Kabisch, N.; Strohbach, M. Green Surge—A Typology of Urban Green Spaces, Eco-System Services Provisioning Services and Demands. 2015. Available online: https://assets.centralparknyc.org/pdfs/institute/p2p-upelp/1.004_Greensurge_A+Typology+of+Urban+Green+Spaces.pdf (accessed on 18 April 2024).
- Muscas, D.; Fornaciari, M.; Proietti, C.; Ruga, L.; Orlandi, F. Tree Growth Rate under Urban Limiting Conditions. Eur. J. For. Res. 2023, 142, 1423–1437. [Google Scholar] [CrossRef]
- Muscas, D.; Orlandi, F.; Petrucci, R.; Proietti, C.; Ruga, L.; Fornaciari, M. Effects of Urban Tree Pruning on Ecosystem Services Performance. Trees For. People J. 2024, 15, 100503. [Google Scholar] [CrossRef]
- Zianis, D.; Muukkonen, P.; Mäkipää, R.; Mencuccini, M. Biomass and Stem Volume Equations for Tree Species in Europe. In Silva Fennica Monographs; The Finnish Society of Forest Science, The Finnish Forest Research Institute: Helsinki, Finland, 2005; ISBN 9514019830. [Google Scholar]
- McPherson, E.G.; van Doorn, N.; Peper, P.J. Urban Tree Database and Allometric Equations; General Technical Report PSW-GTR-253; US Department of Agriculture, Forest Service, Pacific Southwest Research Station: Albany, CA, USA, 2016; 86p.
- Nowak, D.J. Air Pollution Removal by Chicago’s Urban Forest. In Chicago’s Urban Forest Ecosystem: Results of the Chicago Urban Forest Climate Project; General Technical Report NE-186; United States Department of Agriculture: Washington, DC, USA, 1994; Volume 201. [Google Scholar]
- Nowak, D.; Crane, D. The Urban Forest Effects (UFORE) Model: Quantifying Urban Forest Structure and Functions. In Integrated Tools for Natural Resources Inventories in the 21st Century; General Technical Report NC-212; U.S. Department of Agriculture, Forest Service, North Central Research Station: St. Paul, MN, USA, 1998; Volume 714–720. [Google Scholar]
- Nowak, D.J. Understanding i-Tree: Summary of Programs and Methods; General Technical Report NRS-200; U.S. Department of Agriculture, Forest Service, Northern Research Station: Madison, WI, USA, 2020; 100p. [CrossRef]
- Bianchi, M. Il Progetto “Ri.Selv.Italia”: Programma Comune Di Ricerca Sulla Selvicoltura in Italia. Forest 2004, 1, 109–111. [Google Scholar] [CrossRef]
- Secco, L.; Agnoloni, S.; Cantiani, P.; De Meo, I.; Ferretti, F.; Pettenella, D. A Methodology to Integrate SFM Standards on Forest Cultural Heritage into Meso-Scale Forest Planning: Preliminary Results of the Ri.Selv.Italia 4.2 Research Project. In Proceedings of the International Conference on Cultural Heritage and Sustainable Forest Management: The Role of Traditional Knowledge, Florence, Italy, 8–11 June 2006; Parrotta, J., Agnoletti, M., Johann, E., Eds.; 2006; Volume 2, ISBN 9788392239642. Available online: https://www.research.unipd.it/handle/11577/1557801 (accessed on 18 April 2024).
- Tritton, L.M.; Hornbeck, J.W. Biomass Equations for Major Tree Species of the Northeast; U.S. Department of Agriculture, Forest Service, Northeastern Forest Experimental Station: Broomall, PA, USA, 1982; 46p. [CrossRef]
- Sinacore, K.; Hall, J.S.; Potvin, C.; Royo, A.A.; Ducey, M.J.; Ashton, M.S. Unearthing the Hidden World of Roots: Root Biomass and Architecture Differ among Species within the Same Guild. PLoS ONE 2017, 12, e0185934. [Google Scholar] [CrossRef]
- Lamlom, S.H.; Savidge, R.A. A Reassessment of Carbon Content in Wood: Variation within and between 41 North American Species. Biomass Bioenergy 2003, 25, 381–388. [Google Scholar] [CrossRef]
- Dekić, J.P.; Mitković, P.B.; Dinic Branković, M.M.; Igić, M.Z.; Dekić, P.S.; Mitković, M.P. The Study of Effects of Greenery on Temperature Reduction in Urban Areas. Therm. Sci. 2018, 2018, 988–1000. [Google Scholar] [CrossRef]
- Hargreaves, G.H.; Samani, Z.A. Reference Crop Evapotranspiration from Temperature. Appl. Eng. Agric. 1985, 1, 96–99. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration—Guidelines for Computing Crop Water Requirements. Available online: http://www.fao.org/docrep/x0490e/x0490e00.htm (accessed on 26 September 2019).
- Dussadee, N.; Ramaraj, R.; Sutassanamarlee, N. Effect of Plant Shading and Water Consumption on Heat Reduction of Ambient Air. Chiang Mai J. Sci. 2018, 45, 188–197. [Google Scholar]
- Houghton, J.T.; Ding, Y.; Griggs, D.J.; Noguer, M.; van der Linden, P.J.; Dai, X.; Maskell, K.; Johnson, C.A. Climate Change 2001: The Scientific Basis. Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2001; pp. 16–17. ISBN 0521 80767 0. [Google Scholar]
- Myhre, G.; Shindell, D.; Bréon, F.-M.F.-M.; Collins, W.; Fuglestvedt, J.; Huang, J.; Koch, D.; Lamarque, J.-F.J.-F.; Lee, D.; Mendoza, B.; et al. Anthropogenic and Natural Radiative Forcing. In Climate Change 2013—The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2013; pp. 659–740. [Google Scholar]
- Myhre, G.; Highwood, E.J.; Shine, K.P.; Stordal, F. New Estimates of Radiative Forcing Due to Well Mixed Greenhouse Gases. Geophys. Res. Lett. 1998, 25, 2715–2718. [Google Scholar] [CrossRef]
- Forster, P.; Ramaswamy, V.; Artaxo, P.; Berntsen, T.; Betts, R.; Fahey, D.W.; Haywood, J.; Lean, J.; Lowe, D.C.; Myhre, G.; et al. Changes in Atmospheric Constituents and in Radiative Forcing. Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- Vanninen, P.; Mäkelä, A. Carbon Budget for Scots Pine Trees: Effects of Size, Competition and Site Fertility on Growth Allocation and Production. Tree Physiol. 2005, 25, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Pretzsch, H.; Biber, P.; Uhl, E.; Dahlhausen, J.; Rötzer, T.; Caldentey, J.; Koike, T.; van Con, T.; Chavanne, A.; Seifert, T.; et al. Crown Size and Growing Space Requirement of Common Tree Species in Urban Centres, Parks, and Forests. Urban For. Urban Green. 2015, 14, 466–479. [Google Scholar] [CrossRef]
- Hynynen, J. Predicting Tree Crown Ratio for Unthinned and Thinned Scots Pine Stands. Can. J. For. Res. 1995, 25, 57–62. [Google Scholar] [CrossRef]
- Mäkinen, H.; Isomäki, A. Thinning Intensity and Long-Term Changes in Increment and Stem Form of Scots Pine Trees. For. Ecol. Manag. 2004, 203, 21–34. [Google Scholar] [CrossRef]
- Suchocka, M.; Swoczyna, T.; Kosno-Jończy, J.; Kalaji, H.M. Impact of heavy pruning on development and photosynthesis of Tilia cordata Mill. trees. PLoS ONE 2021, 16, e0256465. [Google Scholar] [CrossRef]





| Species | N° Plants | Mean Age | Q/Day Mean Value (W) | Mean CO2 Stored at Census (t) |
|---|---|---|---|---|
| Acer campestre | 70 | 13 (±6.28) | 457.90 | 0.46 |
| Acer opalus | 2 | 20 (±2.35) | 458.39 | 0.21 |
| Acer platanoides | 3 | 20 (±8.45) | 471.11 | 0.91 |
| Aesculus x carnea | 10 | 26 (±7.27) | 558.50 | 0.25 |
| Cupressus arizonica | 79 | 19 (±26.3) | 632.76 | 0.40 |
| Juglans regia | 3 | 36 (±19.38) | 967.28 | 1.26 |
| Ligustrum vulgare | 1 | 16 | 267.86 | 0.24 |
| Pinus halepensis | 5 | 26 (±4.47) | 948.21 | 0.93 |
| Pinus pinea | 2 | 33 (±3.15) | 840.66 | 1.71 |
| Populus alba | 9 | 13 (±6.46) | 607.00 | 0.50 |
| Populus nigra | 115 | 13 (±15.44) | 359.69 | 0.64 |
| Populus nigra I‘talica’ | 17 | 18 (±4.02) | 162.23 | 0.37 |
| Prunus cerasifera | 2 | 78 (±71.65) | 438.87 | 0.53 |
| Quercus crenata | 6 | 13 (±2.52) | 479.01 | 0.35 |
| Quercus ilex | 137 | 18 (±6.17) | 956.81 | 0.72 |
| Quercus petraea | 3 | 17 (±11.60) | 1255.51 | 0.74 |
| Quercus pubescens | 17 | 29 (±11.64) | 874.37 | 0.54 |
| Quercus robur | 8 | 14 (±4.14) | 640.98 | 0.34 |
| Robinia pseudoacacia | 38 | 17 (±3.69) | 301.83 | 0.35 |
| Salix alba | 9 | 14 (±5.31) | 651.45 | 0.86 |
| Salix babylonica | 8 | 14 (±6.47) | 771.39 | 0.51 |
| Salix viminalis | 10 | 41 (±35.09) | 1100.23 | 3.19 |
| Tilia platyphyllos | 5 | 19 (±1.81) | 478.15 | 0.62 |
| Ulmus carpinifolia | 14 | 9 (±3.14) | 656.04 | 0.25 |
| Ulmus laevis | 15 | 13 (±2.24) | 771.37 | 0.37 |
| Ulmus pumila | 3 | 16 (±0.57) | 1493.46 | 0.67 |
| Total | 592 | 22 | 664.27 | 0.67 |
| Species | N° Plants | Mean Age | Q/Day Mean Value (W) | Mean CO2 Stored at Census (t) |
|---|---|---|---|---|
| Acer opalus | 7 | 28 (±6.45) | 959.83 | 0.51 |
| Acer rubrum | 4 | 19 (±6.14) | 863.65 | 0.66 |
| Acer saccharum | 4 | 21 (±13.45) | 810.46 | 0.42 |
| Aesculus hippocastanum | 6 | 41 | 789.77 | 0.78 |
| Cercis siliquastrum | 40 | 11 (±6.80) | 426.42 | 0.29 |
| Cupressus arizonica | 41 | 13 (±1.45) | 464.51 | 0.32 |
| Cupressus sempervirens | 42 | 11 (±0.65) | 418.35 | 0.22 |
| C. sempervirens ‘pyramidalis’ | 21 | 16 (±1.53) | 498.87 | 0.56 |
| Ficus carica | 2 | 9 (±0.70) | 164.62 | 0.22 |
| Fraxinus ornus | 12 | 15 (±2.74) | 893.44 | 0.32 |
| Hibiscus sp. | 6 | 6 | 35.71 | 0.20 |
| Olea europaea | 63 | 28 (±26.42) | 539.66 | 1.28 |
| Pinus pinaster | 4 | 34 (±1.12) | 1132.99 | 1.17 |
| Pinus pinea | 38 | 34 (±7.43) | 1285.60 | 2.67 |
| Populus nigra I‘talica’ | 6 | 26 (±13.31) | 1354.51 | 0.89 |
| Quercus ilex | 63 | 21 (±3.79) | 1078.37 | 1.08 |
| Robinia pseudoacacia | 8 | 19 (±3.42) | 566.39 | 0.41 |
| Ulmus carpinifolia | 43 | 17 (±5.68) | 736.12 | 0.76 |
| Ulmus pumila | 3 | 15 (±1.90) | 671.41 | 0.53 |
| Total | 413 | 20 | 720.56 | 0.70 |
| Species | N° Plants | Mean Age | Q/Day Mean Value (W) | Mean CO2 Stored at Census (t) |
|---|---|---|---|---|
| Acer campestre | 3 | 12 (±7.33) | 343.72 | 0.35 |
| Acer negundo | 4 | 31 (±2.75) | 1513.35 | 1.79 |
| Acer platanoides | 2 | 16 (±15.75) | 1177.14 | 0.87 |
| Aesculus hippocastanum | 4 | 44 (±4.35) | 765.18 | 0.96 |
| Ailanthus altissima | 3 | 38 (±3.35) | 2203.99 | 1.93 |
| Albizia julibrissin | 1 | 31 | 765.18 | 0.46 |
| Cedrus deodara | 1 | 52 | 1691.51 | 2.97 |
| Celtis australis | 13 | 34 (±15.14) | 1667.56 | 2.60 |
| Cydonia oblonga | 1 | 39 | 148.15 | 0.27 |
| Liquidambar styraciflua | 2 | 7 (±5.70) | 148.15 | 0.03 |
| Pinus sylvestris | 3 | 32 (±8.43) | 1215.38 | 0.93 |
| Platanus acerifolia | 7 | 34 (±7.32) | 1592.22 | 1.77 |
| Populus nigra | 4 | 26 (±5.55) | 2147.49 | 2.55 |
| Populus nigra I‘talica’ | 1 | 22 | 421.47 | 0.65 |
| Populus tremula | 3 | 16 (±2.30) | 2122.12 | 1.24 |
| Prunus avium | 3 | 101 (±10.56) | 1131.74 | 1.21 |
| Quercus ilex | 1 | 5 | 168.47 | 0.03 |
| Quercus robur | 1 | 29 | 1966.95 | 1.62 |
| Robinia pseudoacacia | 1 | 35 | 1682.30 | 2.99 |
| Tilia cordata | 3 | 6 | 559.50 | 0.08 |
| Tilia intermedia | 4 | 19 (±6.38) | 1142.47 | 0.96 |
| Tilia platyphyllos | 2 | 4 (±1.35) | 148.15 | 0.04 |
| Ulmus americana | 1 | 22 | 2108.50 | 1.34 |
| Ulmus carpinifolia | 2 | 24 | 2008.12 | 1.79 |
| Total | 70 | 28 | 1201.62 | 1.23 |
| Species | N° Plants | Mean Age | Q/Day Mean Value (W) | Mean CO2 Stored at Census (t) |
|---|---|---|---|---|
| Acer campestre | 7 | 31 (±15.34) | 409.38 | 1.04 |
| Acer opalus | 15 | 32 (±9.99) | 682.80 | 0.74 |
| Acer spp. | 1 | 70 | 1586.65 | 6.34 |
| Cedrus deodara | 4 | 32 (±10.08) | 1449.56 | 1.91 |
| Celtis australis | 20 | 31 (±9.37) | 1456.08 | 2.49 |
| Cupressus sempervirens | 1 | 150 | 1604.57 | 2.74 |
| Diospyros lotus | 1 | 15 | 827.61 | 0.44 |
| Fraxinus angustifolia | 1 | 24 | 2337.98 | 2.00 |
| Juglans regia | 8 | 23 (±8.47) | 889.93 | 0.49 |
| Ligustrum japonicum | 1 | 23 | 870.12 | 0.57 |
| Morus spp. | 2 | 33 (±5.05) | 539.91 | 0.73 |
| Picea abies | 1 | 27 | 1443.40 | 0.71 |
| Pinus sylvestris | 1 | 37 | 453.59 | 0.86 |
| Prunus domestica | 1 | 116 | 892.49 | 1.11 |
| Pyrus communis | 1 | 113 | 737.30 | 0.66 |
| Quercus pubescens | 7 | 88 (±13.82) | 2583.18 | 5.29 |
| Taxus baccata | 2 | 24 (±7.35) | 1379.09 | 0.49 |
| Tilia cordata | 12 | 24 (±5.74) | 1684.37 | 1.71 |
| Tilia platyphyllos | 3 | 32 (±17.39) | 2427.30 | 3.06 |
| Tilia tomentosa | 11 | 35 (±13.77) | 1618.10 | 3.06 |
| Ulmus carpinifolia | 1 | 14 | 454.51 | 0.57 |
| Ulmus laevis | 1 | 17 | 619.77 | 0.69 |
| Ulmus minor | 1 | 13 | 1016.44 | 0.50 |
| Total | 103 | 44 | 1215.83 | 1.66 |
| Park | N° Trees | Mean Tree Age | Tot. CO2s (t) | Tot. CO2c (t) | CO2c–CO2s | Leaf Area/DBH |
|---|---|---|---|---|---|---|
| Chico Mendez Park | 1145 | 16.71 | 431.22 | 403.39 | −27.84 | 7.36 |
| Pescaia Park | 450 | 16.72 | 366.35 | 233.40 | −132.96 | 5.87 |
| 11 September Park | 106 | 20.82 | 104.80 | 74.37 | −30.43 | 7.59 |
| Villa Ghigi Park | 144 | 30.58 | 206.28 | 151.28 | −55.00 | 7.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fornaciari, M.; Muscas, D.; Rossi, F.; Filipponi, M.; Castellani, B.; Di Giuseppe, A.; Proietti, C.; Ruga, L.; Orlandi, F. CO2 Emission Compensation by Tree Species in Some Urban Green Areas. Sustainability 2024, 16, 3515. https://doi.org/10.3390/su16093515
Fornaciari M, Muscas D, Rossi F, Filipponi M, Castellani B, Di Giuseppe A, Proietti C, Ruga L, Orlandi F. CO2 Emission Compensation by Tree Species in Some Urban Green Areas. Sustainability. 2024; 16(9):3515. https://doi.org/10.3390/su16093515
Chicago/Turabian StyleFornaciari, Marco, Desirée Muscas, Federico Rossi, Mirko Filipponi, Beatrice Castellani, Alessia Di Giuseppe, Chiara Proietti, Luigia Ruga, and Fabio Orlandi. 2024. "CO2 Emission Compensation by Tree Species in Some Urban Green Areas" Sustainability 16, no. 9: 3515. https://doi.org/10.3390/su16093515






