Bird Pollinator Visitation is Equivalent in Island and Plantation Planting Designs in Tropical Forest Restoration Sites
Abstract
:1. Introduction
2. Methods
2.1. Sites
2.2. Observations
2.3. Mist Netting
2.4. Land Cover Measurements
2.5. Treatment of Data and Analyses
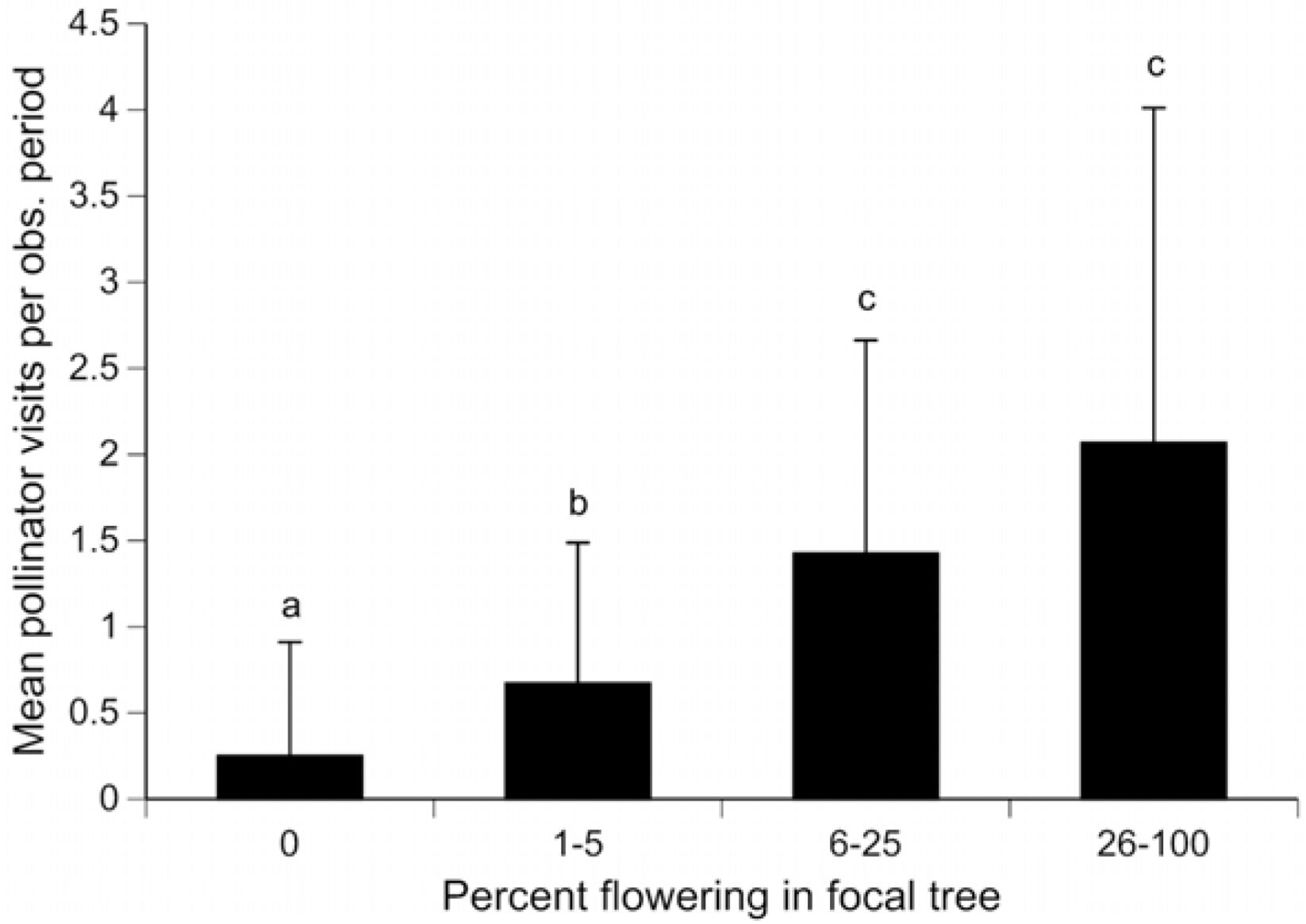
3. Results and Discussion
| Scientific Name | Common Name | Family | Number of visits observed at I. edulis trees | Number of visits where flowers were visited (Percent) | Percent all flower visits observed |
|---|---|---|---|---|---|
| Amazilia tzacatl* | Rufous-tailed Hummingbird | Trochilidae | 78 | 54 (69.2) | 53.5 |
| Oreothlypis peregrina | Tennessee Warbler** | Parulidae | 45 | 9 (20.0) | 8.9 |
| Coereba flaveola | Bananaquit | Thraupidae | 18 | 15 (83.3) | 14.9 |
| Amazilia edward* | Snowy-bellied Hummingbird | Trochilidae | 12 | 10 (83.3) | 9.9 |
| Setophaga fusca | Blackburnian Warbler** | Parulidae | 10 | 3 (30.0) | 3.0 |
| Chlorostilbon assimilis* | Garden Emerald | Trochilidae | 7 | 3 (42.9) | 3.0 |
| Elvira chionura* | White-tailed Emerald | Trochilidae | 3 | 2 (66.7) | 2.0 |
| Unidentified hummingbird* | Trochilidae | 3 | 2 (66.7) | 2.0 | |
| Phaethornis striigularis* | Stripe-throated Hermit | Trochilidae | 2 | 2 (100) | 2.0 |
| Phaethornis guy* | Green Hermit | Trochilidae | 1 | 1 (100) | 1.0 |
| English name | Scientific name | Number of captures | Open-country or Woody-associated |
|---|---|---|---|
| Green Hermit | Phaethornis guy | 23 | W |
| Stripe-throated Hermit | Phaethornis striigularis | 7 | W |
| Scaly-breasted Hummingbird | Phaeochroa cuvierii | 3 | W |
| Violet Sabrewing | Campylopterus hemileucurus | 7 | W |
| Garden Emerald | Chlorostilbon assimilis | 16 | O |
| Violet-crowned Woodnymph | Thalurania colombica | 3 | W |
| Blue-throated Goldentail | Hylocharis eliciae | 1 | W |
| Charming Hummingbird | Amazilia decora | 6 | W |
| Snowy-bellied Hummingbird | Amazilia edward | 9 | O |
| Rufous-tailed Hummingbird | Amazilia tzacatl | 90 | O |
| White-tailed Emerald | Elvira chionura | 8 | W |
| Green-crowned Brilliant | Heliodoxa jacula | 2 | W |
| Bananaquit | Coereba flaveola | 26 | W |
| Blackburnian Warbler | Setophaga fusca | 7 | W |
| Tennessee Warbler | Oreothlypis peregrina | 47 | W |
4. Conclusions
Acknowledgments
Conflict of Interest
References
- FAO (Food and Agriculture Organization of the United Nations). Global Forest Resources Assessment 2010. FAO Forestry Paper No. 163. Available online: http://www.fao.org/docrep/013/i1757e/i1757e.pdf (accessed on 4 October 2012).
- FAO (Food and Agriculture Organization of the United Nations). Global Forest Resources Assessment 2010. Global Tables, Trends in extent of Forest 1990–2010. Available online: http://www.fao.org/forestry/fra/fra2010/en/ (accessed 4 October 2012).
- Tinker, P.B.; Ingram, J.S.I.; Struwe, S. Effects of slash-and-burn agriculture and deforestation on climate change. Agric. Ecosyst. Environ. 1996, 58, 13–22. [Google Scholar] [CrossRef]
- Gentry, A.H. Tropical Forest Biodiversity: Distributional Patterns and Their Conservational Significance. Oikos 1992, 63, 19–28. [Google Scholar] [CrossRef]
- Asner, G.P.; Powell, G.V.N.; Mascaro, J.; Knapp, D.E.; Clark, J.K.; Jacobson, J.; Kennedy Bowdoin, T.; Balaji, A.; Paez-Acosta, G.; Victoria, E.; Secada, L.; Valqui, M.; FlintHughes, R. High-resolution forest carbon stocks and emissions in the Amazon. Proc. Nat. Acad. Sci. USA 2010, 107, 16738–16742. [Google Scholar] [CrossRef]
- Chazdon, R.L. Beyond deforestation: restoring forests and ecosystem services on degraded lands. Science 2008, 320, 1458–1460. [Google Scholar] [CrossRef]
- Harris, J.A.; Hobbs, R.J.; Higgs, E.; Aronson, J. Ecological restoration and global climate change. Restoration Ecol. 2006, 14, 170–176. [Google Scholar] [CrossRef]
- Corbin, J.D.; Holl, K.D. Applied nucleation as a forest restoration strategy. Forest Ecol. Manage. 2012, 265, 37–46. [Google Scholar] [CrossRef]
- Rey Benayas, J.M.; Bullock, J.M.; Newton, A.C. Creating woodland islets to reconcile ecological restoration, conservation, and agricultural land use. Front. Ecol. Environ. 2008, 6, 329–336. [Google Scholar] [CrossRef] [Green Version]
- Morrison, E.B.; Lindell, C.A.; Holl, K.D.; Zahawi, R.A. Patch size effects on avian foraging behaviour: implications for tropical forest restoration design. J. Appl. Ecol. 2010, 47, 130–138. [Google Scholar] [CrossRef]
- Lindell, C.A.; Reid, J.L.; Cole, R.J. Planting design and tree species effects on avian seed dispersers in a tropical forest restoration experiment. Restoration Ecol. 2013, in press. [Google Scholar]
- Dixon, K.W. Pollination and restoration. Science 2009, 325, 571–573. [Google Scholar] [CrossRef]
- Forup, M.L.; Henson, K.W.E.; Craze, P.G.; Memmott, J. The restoration of ecological interactions: plant-pollinator networks on ancient and restored heathlands. J. Appl. Ecol. 2008, 45, 745–752. [Google Scholar]
- Lomova, B.; Keith, D.A.; Hochulia, D.F. Pollination and plant reproductive success in restored urban landscapes dominated by a pervasive exotic pollinator. Landscape Urban Plan. 2010, 96, 232–239. [Google Scholar] [CrossRef]
- Ollerton, J.; Winfree, R.; Tarrant, S. How many flowering plants are pollinated my animals? Oikos 2011, 120, 321–326. [Google Scholar]
- Martén-Rodríguez, S.; Almarales-Castro, A.; Fenster, C.B. Evaluation of pollination syndromes in Antillean Gesneriaceae: evidence for bat, hummingbird and generalized flowers. J. Ecol. 2009, 97, 348–359. [Google Scholar] [CrossRef]
- Kaiser, C.N.; Hansen, D.M.; Müller, C.B. Habitat structure affects reproductive success of the rare endemic tree Syzygium mamillatum (Myrtaceae) in restored and unrestored sites in Mauritius. Biotropica 2008, 40, 86–94. [Google Scholar]
- Fink, R.D.; Lindell, C.A.; Morrison, E.B.; Zahawi, R.A.; Holl, K.D. Patch size and tree species influence the number and duration of bird visits in forest restoration plots in southern Costa Rica. Restoration Ecol. 2009, 17, 479–486. [Google Scholar] [CrossRef]
- Koptur, S. Outcrossing and pollinator limitation of fruit set: breeding systems of Neotropical Inga trees (Fabaceae: Mimosoideae). Evolution 1984, 38, 1130–1143. [Google Scholar] [CrossRef]
- Feinsinger, P.; Tiebout, H.M., III; Young, B.E. Do tropical bird-pollinated plants exhibit density-dependent interactions? Ecology 1991, 72, 1953–1963. [Google Scholar]
- Brody, A.K.; Mitchell, R.J. Effects of experimental manipulation of inflorescence size on pollination and pre-dispersal seed predation in the hummingbird-pollinated plant Ipomopsis aggregate. Oecologia 1997, 110, 89–93. [Google Scholar]
- Lindenmayer, D.B.; Knight, E.J.; Crane, M.J.; Montague-Drake, R.; Michael, D.R.; MacGregor, C.I. What makes an effective restoration planting for woodland birds? Biol. Conserv. 2010, 143, 289–301. [Google Scholar] [CrossRef]
- Stouffer, P.C.; Bierregaard, R.O. Effects of Forest Fragmentation on Understory Hummingbirds in Amazonian Brazil. Conserv. Biol. 1995, 9, 1085–1094. [Google Scholar] [CrossRef]
- Pearman, P.B. The scale of community structure: habitat variation and avian guilds in tropical forest understory. Ecol. Monogr. 2002, 72, 19–39. [Google Scholar] [CrossRef]
- Lindell, C.A.; Chomentowski, W.H.; Zook, J.R.; Kaiser, S.A. Generalizability of neotropical bird abundance and richness models. Animal Conserv. 2006, 9, 445–455. [Google Scholar] [CrossRef]
- Hadley, A.S.; Betts, M.G. Tropical deforestation alters hummingbird movement patterns. Biol. Lett.-UK 2009, 5, 207–210. [Google Scholar]
- Holl, K.D.; Zahawi, R.A.; Cole, R.J.; Ostertag, R.; Cordell, S. Planting seedlings in tree islands versus plantations as a large-scale tropical forest restoration strategy. Restoration Ecol. 2011, 19, 470–479. [Google Scholar] [CrossRef]
- FAO (Food and Agricultura Organization of the United Nations). 1983. Food and fruit bearing species 3: Examples from Latin America. FAO Forestry Paper. 44/3. Rome. Available online: http://www.fao.org/docrep/016/ap368e/ap368e00.pdf (accessed 18 December 2012).
- FAO (Food and Agriculture Organization of the United Nations). Appendix 1:Definitions as in Forest Resources Assessment Working Paper 1 and comments. 1998. Available online: http://www.fao.org/docrep/006/ad665e/ad665e06.htm (accessed 5 April 2009).
- SAS. Production Glimmix Procedure. 2011. Available online: http://support.sas.com/rnd/app/da/glimmix.html(accessed (accessed in March 2011).
- Stevens, J. Applied Multivariate Statistics for the Social Sciences, 4th ed; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 2002. [Google Scholar]
- Stiles, F.G.; Skutch, A.F. A Guide to the Birds of Costa Rica; Cornell University Press: Ithaca, NY, USA, 1989. [Google Scholar]
- Lindell, C.A.; Cole, R.J.; Holl, K.D.; Zahawi, R.A. Migratory bird species in young tropical forest restoration sites: effects of vegetation height, planting design, and season. Bird Conserv. Int. 2012, 22, 94–105. [Google Scholar] [CrossRef]
- Wunderle, J.M., Jr. Population characteristics of Black-throated Blue Warblers wintering in three sites on Puerto Rico. Auk 1995, 112, 931–946. [Google Scholar] [CrossRef]
- Brown, D.R.; Sherry, T.W. Alternative strategies of space use and response to resource change in a wintering migrant songbird. Behav. Ecol. 2008, 19, 1314–1325. [Google Scholar] [CrossRef]
- Pocock, M.J.O.; Evane, D.J.; Memmott, J. The robustness and restoration of a network of ecological networks. Science 2012, 335, 973–977. [Google Scholar] [CrossRef] [Green Version]
- Amorim, F.W.; Galetto, L.; Sazima, M. Beyond the pollination syndrome: nectar ecology and the role of diurnal and nocturnal pollinators in the reproductive success of Inga sessilis (Fabaceae). Plant Biol. 2012. [Google Scholar] [CrossRef]
- Fumero-Cabán, J.J.; Meléndez-Ackerman, E.J. Relative pollination effectiveness of floral visitors of Pitcairnea angustifolia (Bromeliaceae). Am. J. Bot. 2007, 94, 419–424. [Google Scholar] [CrossRef]
- Caraballo-Ortiz, M.A.; Santiago-Valentín, E. The breeding system and effectiveness of introduced and native pollinators of the endangered tropical tree Goetzea elegans (Solanaceae). J. Poll. Ecol. 2011, 4, 26–33. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lindell, C.A.; Thurston, G.M. Bird Pollinator Visitation is Equivalent in Island and Plantation Planting Designs in Tropical Forest Restoration Sites. Sustainability 2013, 5, 1177-1187. https://doi.org/10.3390/su5031177
Lindell CA, Thurston GM. Bird Pollinator Visitation is Equivalent in Island and Plantation Planting Designs in Tropical Forest Restoration Sites. Sustainability. 2013; 5(3):1177-1187. https://doi.org/10.3390/su5031177
Chicago/Turabian StyleLindell, Catherine A., and Ginger M. Thurston. 2013. "Bird Pollinator Visitation is Equivalent in Island and Plantation Planting Designs in Tropical Forest Restoration Sites" Sustainability 5, no. 3: 1177-1187. https://doi.org/10.3390/su5031177
APA StyleLindell, C. A., & Thurston, G. M. (2013). Bird Pollinator Visitation is Equivalent in Island and Plantation Planting Designs in Tropical Forest Restoration Sites. Sustainability, 5(3), 1177-1187. https://doi.org/10.3390/su5031177




