Detection of Flavescence dorée Grapevine Disease Using Unmanned Aerial Vehicle (UAV) Multispectral Imagery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Acquisition
2.1.1. Experimental Sites
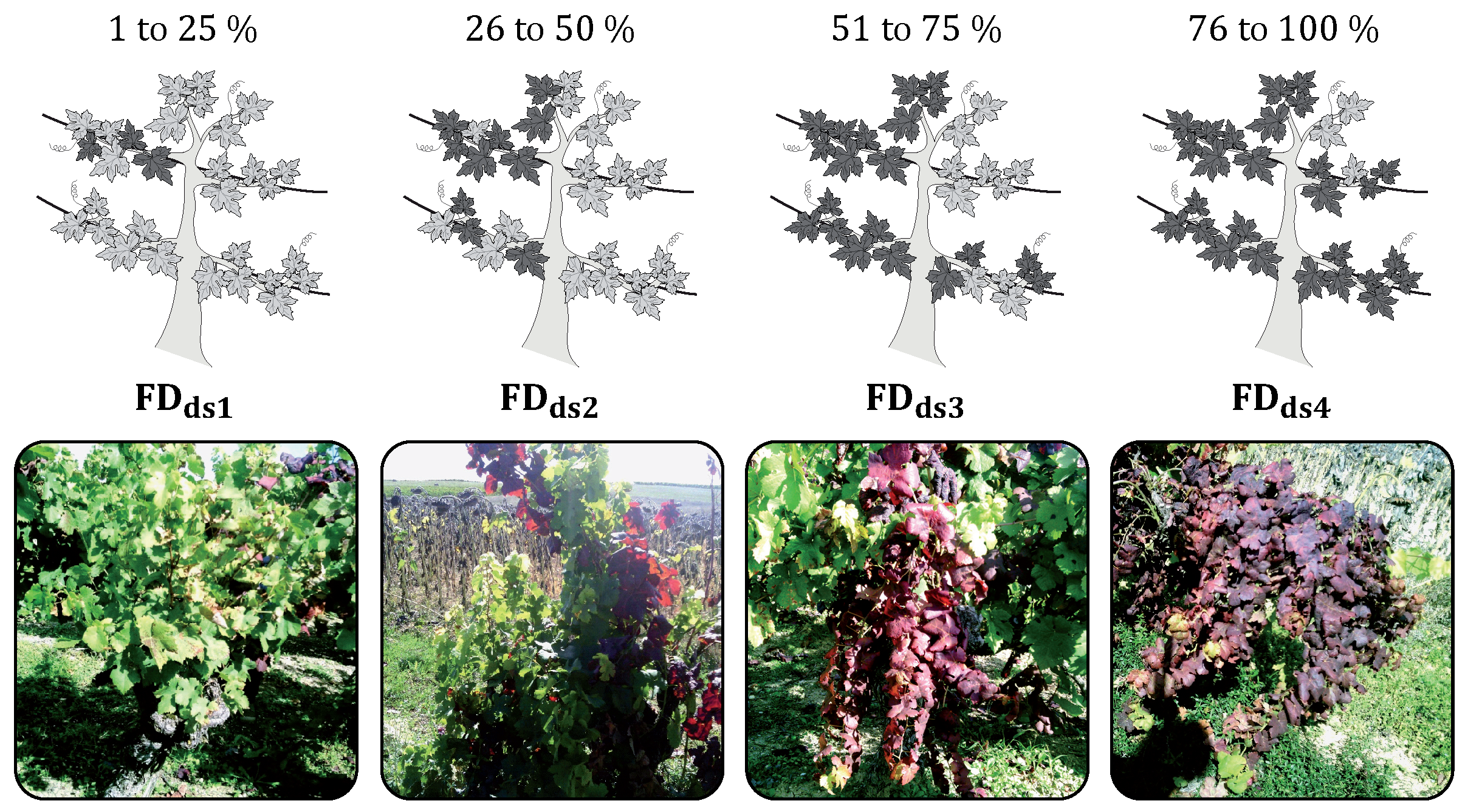
2.1.2. Field Data Acquisition
2.1.3. UAV Multispectral Imagery Acquisition and Pre-Processing
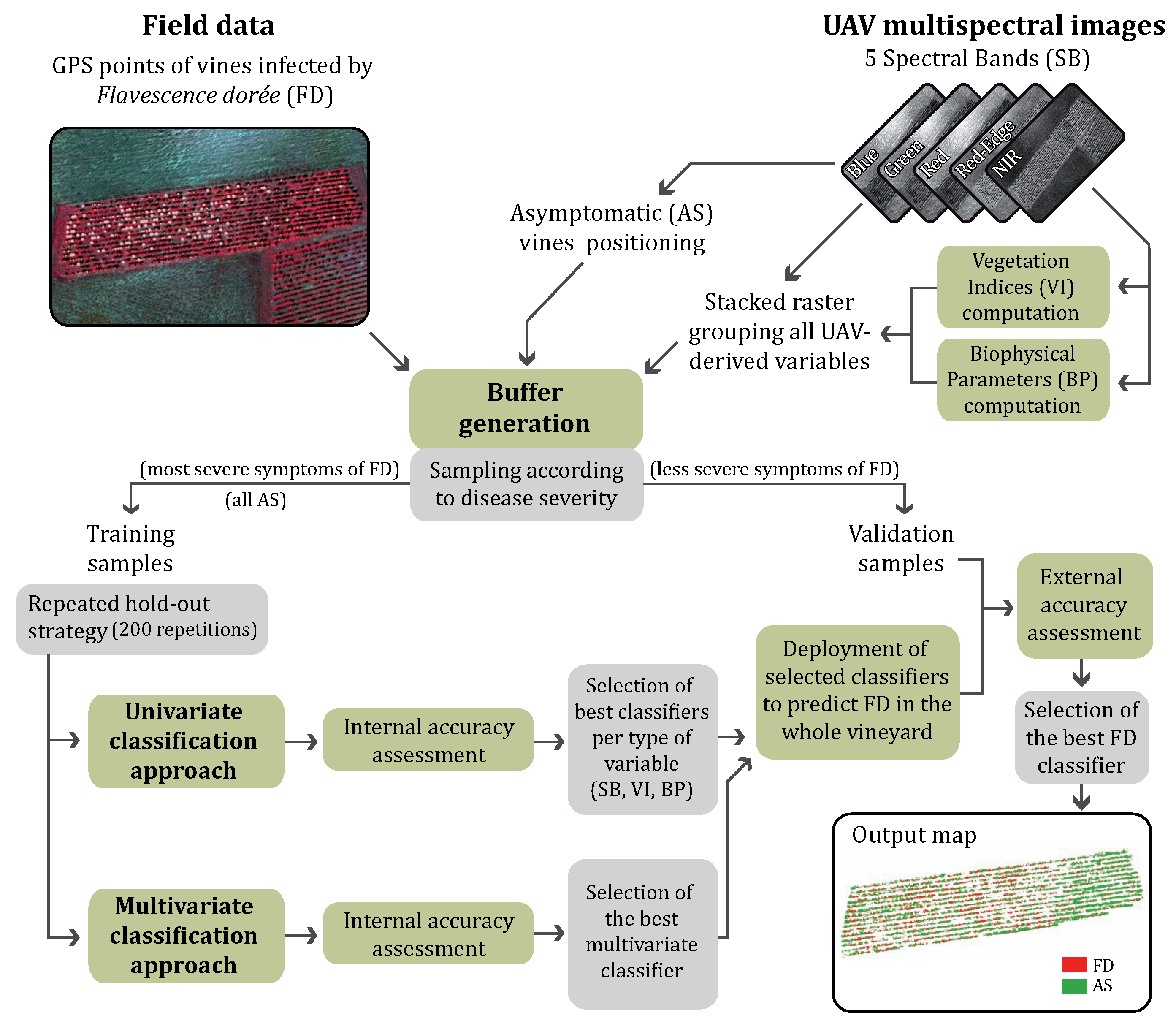
2.2. Data Processing and Analysis
2.2.1. Computing Vegetation Indices and Biophysical Parameters
2.2.2. Buffer Generation and Sampling Strategy
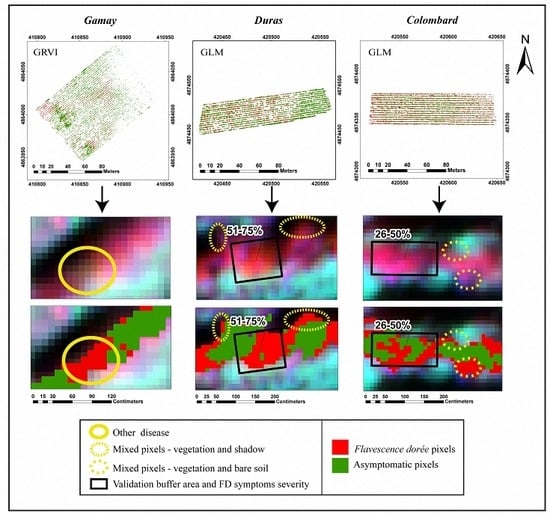
2.2.3. Detection of Flavescence dorée Symptoms on UAV Images
2.2.4. General Principle
2.2.5. Univariate and Multivariate Classification Approaches
2.2.6. External Accuracy Assessment
3. Results
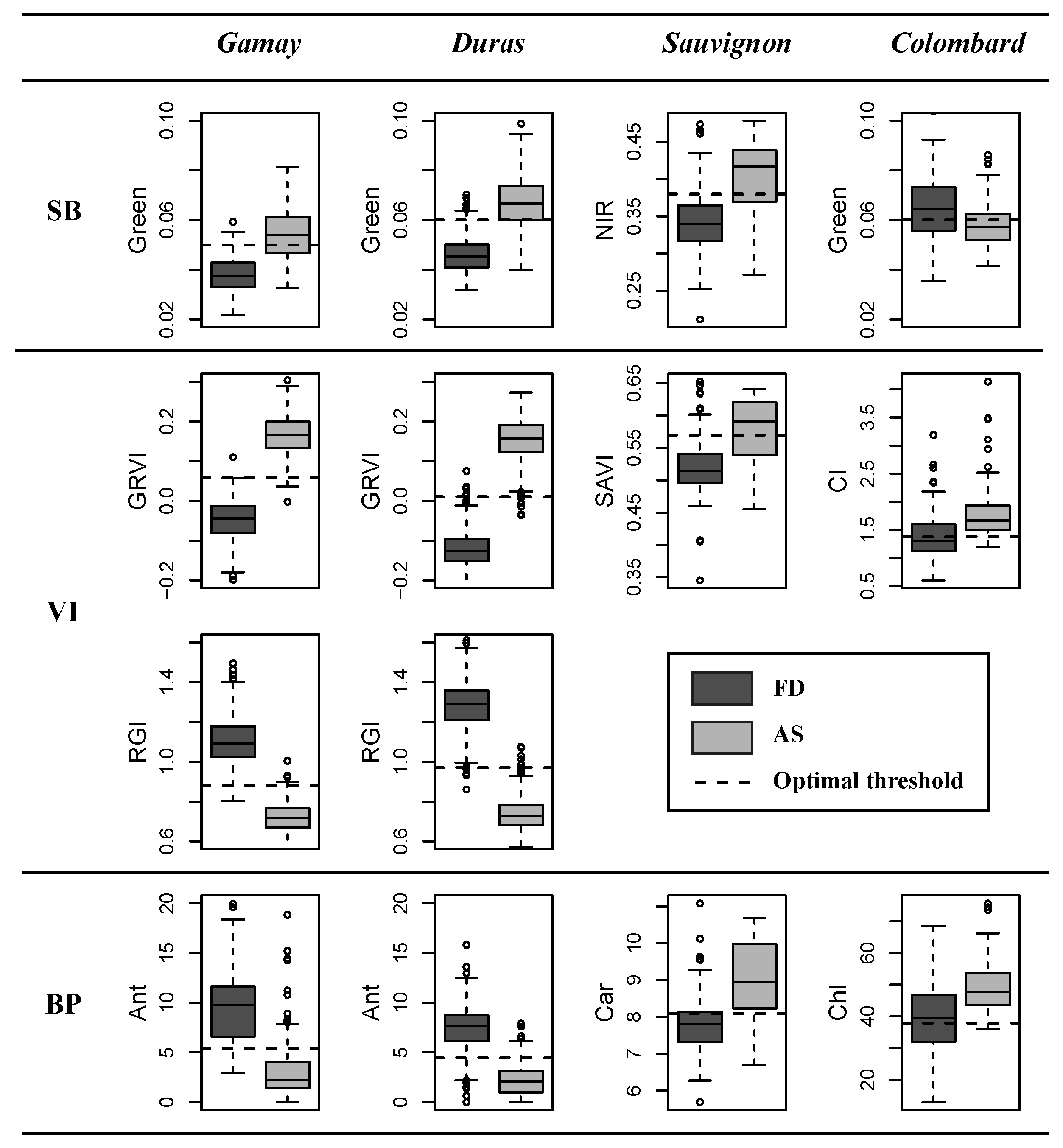
3.1. Univariate Accuracy Assessment
3.1.1. Spectral Bands
3.1.2. Vegetation Indices
3.1.3. Biophysical Parameters
3.2. Multivariate Accuracy Assessment
3.2.1. Features Selection
3.2.2. GLM Performance Assessment
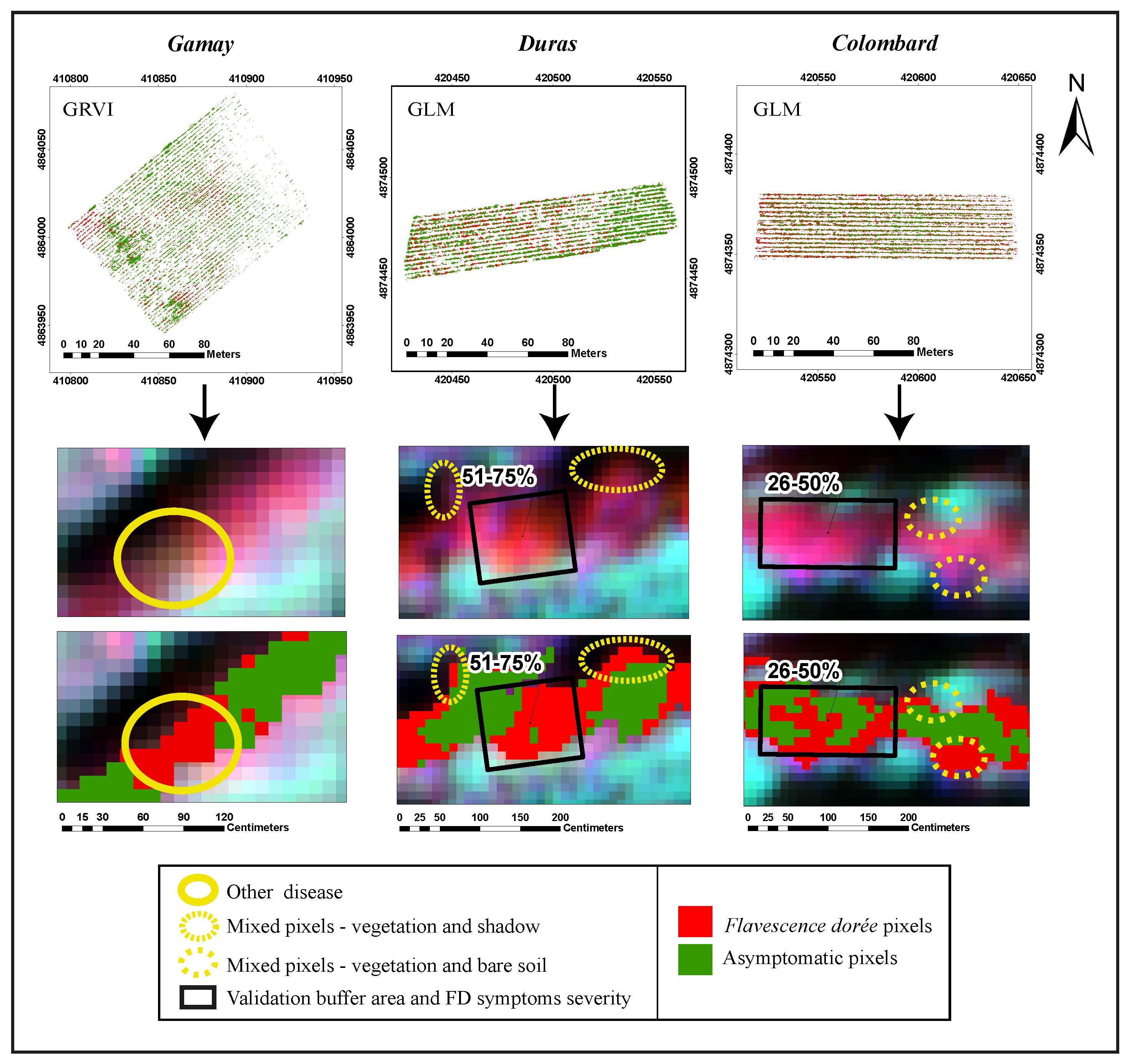
3.3. Application to Whole Vineyards and External Accuracy Assessment
4. Discussion
4.1. Univariate and Multivariate Analyses
4.2. Application to Whole Vineyards and External Accuracy Assessment
4.3. Reproductibility of the Method
5. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chuche, J.; Thiéry, D. Biology and ecology of the Flavescence dorée vector Scaphoideus titanus: A review. Agron. Sustain. Dev. 2014, 34, 381. [Google Scholar] [CrossRef]
- Schvester, D.; Carle, P.; Moutous, G. Sur la transmission de la Flavescence dorée des vignes par une cicadelle. Comptes Rendus des Séances de l’Académie d’Agriculture de France 1961, 47, 1021–1024. [Google Scholar]
- Mori, N.; Bressan, A.; Martin, M.; Guadagnini, M.; Girolami, V.; Bertaccini, A. Experimental transmission by Scaphoideus titanus Ball of two Flavescence doree—Type phytoplasmas. VITIS J. Grapevine Res. 2002, 41, 99. [Google Scholar]
- Bonfils, J.; Schvester, D. The leafhoppers (Homoptera: Auchenorrhyncha) and their relationship with vineyards in south-western France. Ann. Epiphyt. 1960, 11, 325–336. [Google Scholar]
- Caudwell, A. Identification D’une Nouvelle Maladie à Virus de la Vigne, “la Flavescence dorée”, étude des Phénomènes de Localisation des Symptômes et de Rétablissement. Ph.D. Thesis, Institut National de la Recherche Agronomique, Paris, France, 1964. [Google Scholar]
- Pavan, F.; Mori, N.; Bigot, G.; Zandigiacomo, P. Border effect in spatial distribution of Flavescence dorée affected grapevines and outside source of Scaphoideus titanus vectors. Bull. Insectol. 2012, 65, 281–290. [Google Scholar]
- Jaunisses à phytoplasmes de la vigne. 2006. Available online: http://www.vignevin.com/fileadmin/users/ifv/publications/A_telecharger/JaunissesPhytoplasmesVigne.pdf (accessed on 30 December 2016).
- GDON. Guide flavescence—Aide au diagnostic de la Flavescence dorée GDON du sauternais et des Graves; Technical Report; GDON du Sauternais et des Graves: Bordeaux, France, 2014. [Google Scholar]
- Kazmi, S.J.H.; Usery, E.L. Application of remote sensing and GIS for the monitoring of diseases: A unique research agenda for geographers. Remote Sens. Rev. 2001, 20, 45–70. [Google Scholar] [CrossRef]
- Franke, J.; Menz, G. Multi-temporal wheat disease detection by multi-spectral remote sensing. Precis. Agric. 2007, 8, 161–172. [Google Scholar] [CrossRef]
- Sankaran, S.; Mishra, A.; Ehsani, R.; Davis, C. A review of advanced techniques for detecting plant diseases. Comput. Electron. Agric. 2010, 72, 1–13. [Google Scholar] [CrossRef]
- Gennaro, S.F.D.; Battiston, E.; Marco, S.D.; Facini, O.; Matese, A.; Nocentini, M.; Palliotti, A.; Mugnai, L. Unmanned Aerial Vehicle (UAV)-based remote sensing to monitor grapevine leaf stripe disease within a vineyard affected by esca complex. Phytopathol. Mediterr. 2016, 55, 262–275. [Google Scholar]
- Carter, G.A.; Knapp, A.K. Leaf optical properties in higher plants: Linking spectral characteristics to stress and chlorophyll concentration. Am. J. Bot. 2001, 88, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Naidu, R.A.; Perry, E.M.; Pierce, F.J.; Mekuria, T. The potential of spectral reflectance technique for the detection of Grapevine leafroll-associated virus-3 in two red-berried wine grape cultivars. Comput. Electron. Agric. 2009, 66, 38–45. [Google Scholar] [CrossRef]
- Mahlein, A.K.; Oerke, E.C.; Steiner, U.; Dehne, H.W. Recent advances in sensing plant diseases for precision crop protection. Eur. J. Plant. Pathol. 2012, 133, 197–209. [Google Scholar] [CrossRef]
- Yang, C.M.; Cheng, C.H.; Chen, R.K. Changes in spectral characteristics of rice canopy infested with brown planthopper and leaffolder. Crop Sci. 2007, 47, 329–335. [Google Scholar] [CrossRef]
- Johnson, L.F.; Roczen, D.; Youkhana, S. Vineyard canopy density mapping with IKONOS satellite imagery. In Proceedings of the Third International Conference on Geospatial Information in Agriculture and Forestry, Denver, CO, USA, 5–7 November 2001. [Google Scholar]
- Mirik, M.; Jones, D.; Price, J.A. Satellite Remote sensing of wheat infected by wheat streak mosaic virus. Plant Dis. 2011, 95, 4–12. [Google Scholar] [CrossRef]
- Huang, W.; Lamb, D.W.; Niu, Z.; Zhang, Y.; Liu, L.; Wang, J. Identification of yellow rust in wheat using in-situ spectral reflectance measurements and airborne hyperspectral imaging. Precis. Agric. 2007, 8, 187–197. [Google Scholar] [CrossRef]
- Reynolds, G.J.; Windels, C.E.; MacRae, I.V.; Laguette, S. Remote sensing for assessing Rhizoctonia crown and root rot severity in sugar beet. Plant Dis. 2012, 96, 497–505. [Google Scholar] [CrossRef]
- Stilwell, A.R.; Hein, G.L.; Zygielbaum, A.I.; Rundquist, D.C. Proximal sensing to detect symptoms associated with wheat curl mite-vectored viruses. Int. J. Remote Sens. 2013, 34, 4951–4966. [Google Scholar] [CrossRef]
- Zhang, M.; Qin, Z.; Liu, X.; Ustin, S.L. Detection of stress in tomatoes induced by late blight disease in California, USA, using hyperspectral remote sensing. Int. J. Appl. Earth Obs. Geoinf. 2003, 4, 295–310. [Google Scholar] [CrossRef]
- MacDonald, S.L.; Staid, M.; Staid, M.; Cooper, M.L. Remote hyperspectral imaging of grapevine leafroll-associated virus 3 in cabernet sauvignon vineyards. Comput. Electron. Agric. 2016, 130, 109–117. [Google Scholar] [CrossRef]
- Mazzetto, F.; Calcante, A.; Mena, A.; Vercesi, A. Integration of optical and analogue sensors for monitoring canopy health and vigour in precision viticulture. Precis. Agric. 2010, 11, 636–649. [Google Scholar] [CrossRef]
- Colomina, I.; Molina, P. Unmanned aerial systems for photogrammetry and remote sensing: A review. ISPRS J. Photogramm. Remote Sens. 2014, 92, 79–97. [Google Scholar] [CrossRef]
- Matese, A.; Toscano, P.; Di Gennaro, S.F.; Genesio, L.; Vaccari, F.P.; Primicerio, J.; Belli, C.; Zaldei, A.; Bianconi, R.; Gioli, B. Intercomparison of UAV, aircraft and satellite remote sensing platforms for precision viticulture. Remote Sens. 2015, 7, 2971–2990. [Google Scholar] [CrossRef]
- Bock, C.H.; Nutter, F.W., Jr. Detection and measurement of plant disease symptoms using visible-wavelength photography and image analysis. CAB Rev. 2011, 6, 1–15. [Google Scholar] [CrossRef]
- Steele, M.R.; Gitelson, A.A.; Rundquist, D.C.; Merzlyak, M.N. Nondestructive estimation of anthocyanin content in grapevine leaves. Am. J. Enol. Viticult. 2009, 60, 87–92. [Google Scholar]
- Steele, M.R.; Gitelson, A.A.; Rundquist, D.C. A comparison of two techniques for nondestructive measurement of chlorophyll content in grapevine leaves. Agron. J. 2008, 100, 779–782. [Google Scholar] [CrossRef]
- Blondlot, A.; Gate, P.; Poilvé, H. Providing operational nitrogen recommendations to farmers using satellite imagery. In Proceedings of the 5th European Conference on Precision Agriculture, Uppsala, Sweden, 9–12 June 2005; pp. 345–352. [Google Scholar]
- Lacaze, R.; Baret, F.; Camacho, F.; d’Andrimont, R.; Freitas, S.C.; Pacholczyk, P.; Poilvé, H.; Smets, B.; Tansey, K.; Wagner, W. Geoland2—Towards an operational GMES land monitoring core service: The biogeophysical parameter core mapping service. In Proceedings of the 34th International Symposium on Remote Sensing of Environment, Sydney, Australia, 10–14 April 2011. [Google Scholar]
- Chen, F.; Weber, K.T.; Anderson, J.; Gokhale, B. Comparison of MODIS fPAR products with Landsat-5 TM-derived fPAR over semiarid rangelands of Idaho. GISci. Remote Sens. 2010, 47, 360–378. [Google Scholar] [CrossRef]
- Meroni, M.; Rembold, F.; Verstraete, M.M.; Gommes, R.; Schucknecht, A.; Beye, G. Investigating the relationship between the inter-annual variability of satellite-derived vegetation phenology and a proxy of biomass production in the Sahel. Remote Sens. 2014, 6, 5868–5884. [Google Scholar] [CrossRef]
- Roumiguié, A.; Jacquin, A.; Sigel, G.; Poilvé, H.; Hagolle, O.; Daydé, J. Validation of a forage production index (FPI) derived from MODIS fCover time-series using high-resolution satellite imagery: Methodology, results and opportunities. Remote Sens. 2015, 7, 11525–11550. [Google Scholar] [CrossRef]
- Poilvé, H. geoland2 BioPar Methods Compendium MERIS FR Biophysical Products; Technical Report BP-RP-BP038; VITO: Toulouse, France, 2010. [Google Scholar]
- Jacquemoud, S.; Verhoef, W.; Baret, F.; Bacour, C.; Zarco-Tejada, P.J.; Asner, G.P.; François, C.; Ustin, S.L. PROSPECT+ SAIL models: A review of use for vegetation characterization. Remote Sens. Environ. 2009, 113, S56–S66. [Google Scholar] [CrossRef]
- Jacquemoud, S.; Baret, F. PROSPECT: A model of leaf optical properties spectra. Remote Sens. Environ. 1990, 34, 75–91. [Google Scholar] [CrossRef]
- Féret, J.B.; François, C.; Asner, G.P.; Gitelson, A.A.; Martin, R.E.; Bidel, L.P.; Ustin, S.L.; le Maire, G.; Jacquemoud, S. PROSPECT-4 and 5: Advances in the leaf optical properties model separating photosynthetic pigments. Remote Sens. Environ. 2008, 112, 3030–3043. [Google Scholar] [CrossRef]
- Féret, J.B.; Noble, S.; Gitelson, A.; Jacquemoud, S. PROSPECT-Dynamic: Modeling leaf optical properties through a complete lifecycle. Remote Sens. Environ. 2017, 193, 204–215. [Google Scholar] [CrossRef]
- Verhoef, W. Light scattering by leaf layers with application to canopy reflectance modeling: The SAIL model. Remote Sens. Environ. 1984, 16, 125–141. [Google Scholar] [CrossRef]
- Rouse, J., Jr.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the Great Plains with ERTS. In Proceedings of the NASA Goddard Space Flight Center 3d ERTS-1 Symposium, Washington, DC, USA, 10–14 December 1973. [Google Scholar]
- Gitelson, A.A.; Merzlyak, M.N.; Chivkunova, O.B. Optical properties and nondestructive estimation of anthocyanin content in plant leaves. Photochem. Photobiol. 2001, 74, 38–45. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Keydan, G.P.; Merzlyak, M.N. Three-band model for noninvasive estimation of chlorophyll, carotenoids, and anthocyanin contents in higher plant leaves. Geophys. Res. Lett. 2006, 33, L11402. [Google Scholar] [CrossRef]
- Gamon, J.A.; Surfus, J.S. Assessing leaf pigment content and activity with a reflectometer. New Phytol. 1999, 143, 105–117. [Google Scholar] [CrossRef]
- Van den Berg, A.K.; Perkins, T.D. Nondestructive estimation of anthocyanin content in autumn sugar maple leaves. HortScience 2005, 40, 685–686. [Google Scholar]
- Gitelson, A.A.; Kaufman, Y.J.; Stark, R.; Rundquist, D. Novel algorithms for remote estimation of vegetation fraction. Remote Sens. Environ. 2002, 80, 76–87. [Google Scholar] [CrossRef]
- Motohka, T.; Nasahara, K.N.; Oguma, H.; Tsuchida, S. Applicability of green-red vegetation index for remote sensing of vegetation phenology. Remote Sens. 2010, 2, 2369–2387. [Google Scholar] [CrossRef]
- Huete, A.R. A soil-adjusted vegetation index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kaufman, Y.J.; Merzlyak, M.N. Use of a green channel in remote sensing of global vegetation from EOS-MODIS. Remote Sens. Environ. 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Richardson, A.J.; Wiegand, C.L. Distinguishing vegetation from soil background information. Photogramm. Eng. Remote Sens. 1977, 43, 1541–1552. [Google Scholar]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Huang, C.; Davis, L.S.; Townshend, J.R.G. An assessment of support vector machines for land cover classification. Int. J. Remote Sens. 2002, 23, 725–749. [Google Scholar] [CrossRef]
- Saatchi, S.; Buermann, W.; Ter Steege, H.; Mori, S.; Smith, T.B. Modeling distribution of Amazonian tree species and diversity using remote sensing measurements. Remote Sens. Environ. 2008, 112, 2000–2017. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Collett, D. Modelling Binary Data; Chapman and Hall: London, UK, 1991. [Google Scholar]
- Blackburn, G.A.; Steele, C.M. Towards the remote sensing of matorral vegetation physiology: Relationships between spectral reflectance, pigment, and biophysical characteristics of semiarid bushland canopies. Remote Sens. Environ. 1999, 70, 278–292. [Google Scholar] [CrossRef]
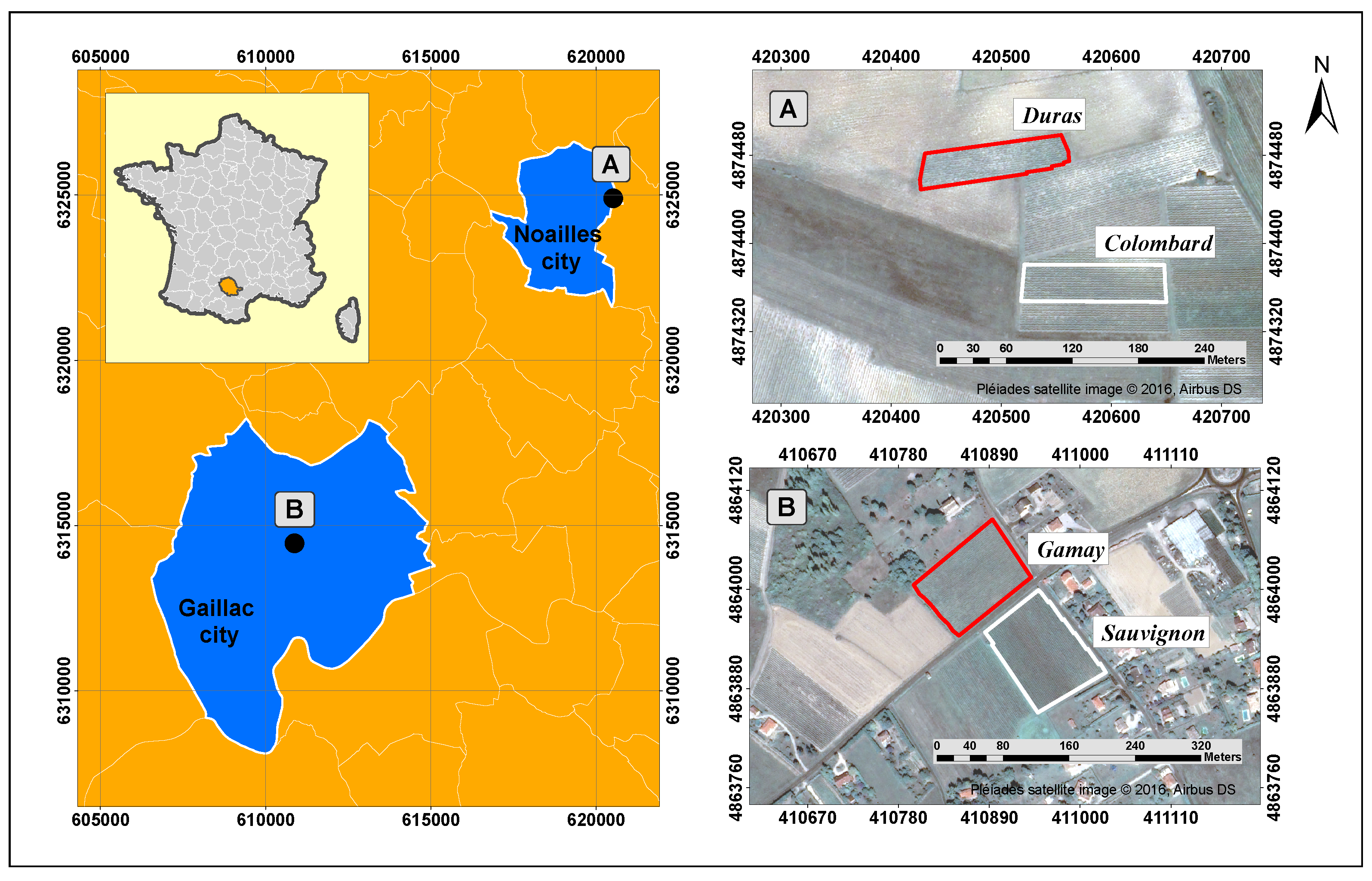

| Gamay | Sauvignon | Duras | Colombard | |
|---|---|---|---|---|
| Berry color | red | white | red | white |
| Vineyard size (ha) | 1.1 | 1.2 | 0.4 | 0.4 |
| Row orientation | 45.0° | 135.0° | 80.0° | 100.0° |
| Number of positioned vines | 389 | 9 | 264 | 40 |
| Mean plant length (m) | 1.34 | 1.40 | 1.15 | 1.68 |
| Mean plant width (m) | 0.48 | 0.92 | 1.02 | 0.84 |
| Number of FDds1 (1% to 25%) | 170 | 0 | 68 | 1 |
| Number of FDds2 (26% to 50%) | 103 | 7 | 44 | 3 |
| Number of FDds3 (51% to 75%) | 45 | 2 | 44 | 7 |
| Number of FDds4 (76% to 100%) | 71 | 0 | 108 | 29 |
| Characteristic Name | Description |
|---|---|
| Platform | Long range DT-18 |
| Sensor | DT-5Bands |
| Sensor type | Global shutter—distortion free |
| Number of bands | 5 |
| Spectral wavelengths | Blue (455–495 nm) |
| Green (540–580 nm) | |
| Red (658–678 nm) | |
| Red-Edge (707–727 nm) | |
| NIR (800–880 nm) | |
| Dimension | 3.6 mm × 4.8 mm |
| Automatic Gain Control | Yes |
| Resolution | 960 × 1280 pixels |
| Focal length | 5.5 mm |
| Field of view | 47.2° |
| Output data | 12-bit RAW |
| Image size | 1.8 MB |
| Flight altitude Above Ground Level (AGL) | 120 m |
| Image acquisition | 5 images (each band) |
| Image triggering | Controlled by the autopilot |
| Ground resolution | 0.08 m/pixel |
| Ground Picture Size | Width 105 m × Height 79 m at 120 m AGL |
| Surface area covered | 3 at 150 m AGL |
| Onboard storage | 32 GB micro Secure Digital (SD) card |
| Calibrated panel | Included (with reflectance data) |
| Index Name | Formula | References |
|---|---|---|
| Normalized difference vegetation index | [41] | |
| Anthocyanin reflectance index | [28,42] | |
| Modified anthocyanin reflectance index | [28,42,43] | |
| Red-green index | [28,44] | |
| Anthocyanin content index | [45] | |
| Modified anthocyanin content index | [28] | |
| Chlorophyll index | [29,43] | |
| Green-red vegetation index | [46,47] | |
| Soil-adjusted vegetation index | [48] | |
| Green normalized difference vegetation index | [49] | |
| Difference vegetation index | [50,51] |
| Parameter Name | Acronym | Description | Unit and Typical Range |
|---|---|---|---|
| fCover | fCov | Fractional cover of green vegetation | 0.0 to 1.0 |
| (interception in vertical view) | |||
| Leaf Chlorophyll content | Chl | Chlorophyll content in the leaves | 20 to 80 |
| (per leaf unit area) | |||
| Leaf Anthocyanin content | Ant | Anthocyanin content in the leaves | 0 to 12 |
| (per leaf unit area) | |||
| Leaf Carotenoid content | Car | Carotenoid content of the leaves | 0 to 15 |
| (per leaf unit area) |
| Gamay | Sauvignon | Duras | Colombard | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Train. | Val. | Train. | Val. | Train. | Val. | Train. | Val. | ||
| FDds1 | number of vines | 0 | 170 | 0 | - | 0 | 68 | 0 | 1 |
| (valid pixels) | (0) | (6243) | (0) | - | (0) | (5940) | (0) | (136) | |
| FDds2 | number of vines | 0 | 103 | 7 | - | 0 | 44 | 0 | 3 |
| (valid pixels) | (0) | (3960) | (53) | - | (0) | (3867) | (0) | (354) | |
| FDds3 | number of vines | 0 | 45 | 2 | - | 0 | 44 | 0 | 7 |
| (valid pixels) | (0) | (1709) | (14) | - | (0) | (4094) | (0) | (711) | |
| FDds4 | number of vines | 24 | 47 | 0 | - | 58 | 50 | 21 | 8 |
| (valid pixels) | (287) | (1914) | (0) | - | (698) | (3987) | (244) | (762) | |
| All FD | number of vines | 24 | 367 | 9 | - | 58 | 206 | 21 | 19 |
| (valid pixels) | (287) | (13,826) | (67) | - | (698) | (17,888) | (244) | (1963) | |
| AS | number of vines | 24 | - | 9 | - | 58 | - | 21 | - |
| (valid pixels) | (293) | - | (77) | - | (676) | - | (260) | - | |
| Reference Data | |||
|---|---|---|---|
| Flavescence dorée (FD) | Asymptomatic (AS) | ||
| Classification | Flavescence dorée (FD) | True Positive | False Positive |
| (FD pixel classified as FD) | (AS pixel classified as FD) | ||
| results | Asymptomatic (AS) | False Negative | True Negative |
| (FD pixel classified as AS) | (AS pixel classified as AS) | ||
| Red Cultivars | White Cultivars | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gamay | Duras | Sauvignon | Colombard | ||||||
| Mean AUC | Std | Mean AUC | Std | Mean AUC | Std | Mean AUC | Std | ||
| SB | Blue | 0.68 | 0.01 | 0.59 | 0.01 | 0.53 | 0.03 | 0.52 | 0.01 |
| Green | 0.91 | 0.01 | 0.96 | 0.00 | 0.67 | 0.03 | 0.68 | 0.01 | |
| Red | 0.65 | 0.01 | 0.82 | 0.01 | 0.50 | 0.03 | 0.50 | 0.01 | |
| RedEdge | 0.70 | 0.01 | 0.52 | 0.01 | 0.66 | 0.02 | 0.63 | 0.01 | |
| NIR | 0.54 | 0.01 | 0.66 | 0.01 | 0.76 | 0.02 | 0.63 | 0.01 | |
| VI | NDVI | 0.63 | 0.01 | 0.88 | 0.00 | 0.66 | 0.03 | 0.58 | 0.01 |
| ARI | 0.94 | 0.00 | 0.98 | 0.00 | 0.64 | 0.02 | 0.67 | 0.01 | |
| MARI | 0.97 | 0.00 | 0.96 | 0.00 | 0.50 | 0.03 | 0.72 | 0.01 | |
| RGI | 1.00 | 0.00 | 1.00 | 0.00 | 0.75 | 0.02 | 0.76 | 0.01 | |
| MACI | 0.95 | 0.00 | 0.94 | 0.00 | 0.54 | 0.03 | 0.75 | 0.01 | |
| ACI | 0.95 | 0.00 | 0.94 | 0.00 | 0.53 | 0.02 | 0.75 | 0.01 | |
| CI | 0.71 | 0.01 | 0.75 | 0.01 | 0.64 | 0.02 | 0.78 | 0.01 | |
| GRVI | 1.00 | 0.00 | 1.00 | 0.00 | 0.75 | 0.02 | 0.76 | 0.01 | |
| SAVI | 0.50 | 0.01 | 0.76 | 0.01 | 0.76 | 0.02 | 0.63 | 0.01 | |
| GNDVI | 0.95 | 0.00 | 0.94 | 0.00 | 0.53 | 0.02 | 0.75 | 0.01 | |
| DVI | 0.52 | 0.01 | 0.71 | 0.01 | 0.76 | 0.02 | 0.63 | 0.01 | |
| BP | fCov | 0.79 | 0.01 | 0.92 | 0.00 | 0.62 | 0.03 | 0.64 | 0.01 |
| Ant | 0.94 | 0.00 | 0.97 | 0.00 | 0.65 | 0.02 | 0.55 | 0.01 | |
| Car | 0.81 | 0.01 | 0.85 | 0.01 | 0.80 | 0.02 | 0.49 | 0.01 | |
| Chl | 0.58 | 0.01 | 0.55 | 0.01 | 0.54 | 0.03 | 0.76 | 0.01 | |
| Red Cultivars | White Cultivars | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gamay | Duras | Sauvignon | Colombard | ||||||
| Sens. | Spec. | Sens. | Spec. | Sens. | Spec. | Sens. | Spec. | ||
| SB | Green | 0.89 | 0.74 | 0.94 | 0.85 | - | - | 0.46 | 0.81 |
| NIR | - | - | - | - | 0.78 | 0.71 | - | - | |
| VI | RGI | 1.00 | 0.98 | 0.99 | 0.99 | - | - | - | - |
| CI | - | - | - | - | - | - | 0.58 | 0.89 | |
| GRVI | 0.99 | 0.98 | 0.99 | 0.99 | - | - | - | - | |
| SAVI | - | - | - | - | 0.81 | 0.67 | - | - | |
| DVI | - | - | - | - | 0.80 | 0.68 | - | - | |
| BP | Ant | 0.88 | 0.82 | 0.90 | 0.92 | - | - | - | - |
| Car | - | - | - | - | 0.72 | 0.74 | - | - | |
| Chl | - | - | - | - | - | - | 0.50 | 0.92 | |
| Red Cultivars | White Cultivars | ||||
|---|---|---|---|---|---|
| Gamay | Duras | Sauvignon | Colombard | ||
| Full model | Mean AIC | 38 | 81 | 113 | 334 |
| Num. of variables | 20 | 20 | 20 | 20 | |
| Simplified model | Mean AIC | 20 | 61 | 101 | 326 |
| Num. of variables | 9 | 7 | 10 | 12 | |
| Gain (% AIC reduction) | 47 | 26 | 11 | 2 | |
| Simplified model AUC | Mean | 1.00 | 1.00 | 0.95 | 0.95 |
| Std | 0.00 | 0.00 | 0.01 | 0.01 | |
| Simplified model sensitivity | Mean | 0.99 | 0.99 | 0.80 | 0.82 |
| Std | 0.01 | 0.01 | 0.11 | 0.06 | |
| Simplified model specificity | Mean | 0.97 | 0.99 | 0.85 | 0.90 |
| Std | 0.02 | 0.01 | 0.11 | 0.06 | |
| Type of Model | Cultivar | List of variables |
|---|---|---|
| Full model | (all) | [Blue + Green + RedEdge + NIR + NDVI + ARI + RGI + MACI + ACI |
| + CI + GRVI + SAVI + GNDVI + DVI + GLCV + Ant + Car + Chl] | ||
| Simplified models | Gamay | [Blue + NDVI + AVI + GNDVI + MACI + ACI + CI + GRVI + Car] |
| Sauvignon | [Green + RedEdge + NIR + NDVI + RGI + ACI + GRVI + DVI + fCov + Car] | |
| Duras | [Blue + NIR + RGI + GRVI + DVI + Chl + Car] | |
| Colombard | [RedEdge + NIR + NDVI + ARI + RGI + ACI + MACI + CI + GRVI + SAVI + GNDVI + Chl] |
| Gamay | Duras | Colombard | ||||
|---|---|---|---|---|---|---|
| Ranked Position | Classifier | RMSE (n = 365) | Classifier | RMSE (n = 206) | Classifier | RMSE (n = 19) |
| 1 | GRVI | 1.24 | GLM | 1.16 | GLM | 1.28 |
| 2 | RGI | 1.24 | GRVI | 1.21 | CI | 1.76 |
| 3 | GLM | 1.27 | RGI | 1.21 | Green | 1.99 |
| 4 | Green | 1.38 | Green | 1.25 | Chl | 2.28 |
| 5 | Ant | 1.50 | Ant | 1.26 | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albetis, J.; Duthoit, S.; Guttler, F.; Jacquin, A.; Goulard, M.; Poilvé, H.; Féret, J.-B.; Dedieu, G. Detection of Flavescence dorée Grapevine Disease Using Unmanned Aerial Vehicle (UAV) Multispectral Imagery. Remote Sens. 2017, 9, 308. https://doi.org/10.3390/rs9040308
Albetis J, Duthoit S, Guttler F, Jacquin A, Goulard M, Poilvé H, Féret J-B, Dedieu G. Detection of Flavescence dorée Grapevine Disease Using Unmanned Aerial Vehicle (UAV) Multispectral Imagery. Remote Sensing. 2017; 9(4):308. https://doi.org/10.3390/rs9040308
Chicago/Turabian StyleAlbetis, Johanna, Sylvie Duthoit, Fabio Guttler, Anne Jacquin, Michel Goulard, Hervé Poilvé, Jean-Baptiste Féret, and Gérard Dedieu. 2017. "Detection of Flavescence dorée Grapevine Disease Using Unmanned Aerial Vehicle (UAV) Multispectral Imagery" Remote Sensing 9, no. 4: 308. https://doi.org/10.3390/rs9040308
APA StyleAlbetis, J., Duthoit, S., Guttler, F., Jacquin, A., Goulard, M., Poilvé, H., Féret, J.-B., & Dedieu, G. (2017). Detection of Flavescence dorée Grapevine Disease Using Unmanned Aerial Vehicle (UAV) Multispectral Imagery. Remote Sensing, 9(4), 308. https://doi.org/10.3390/rs9040308