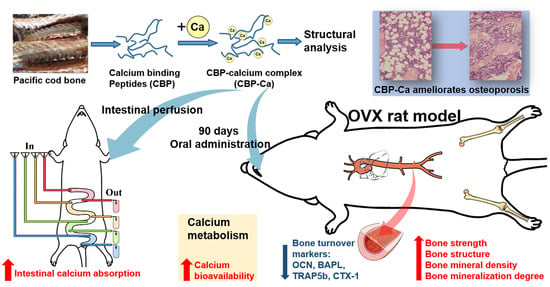
Functional Calcium Binding Peptides from Pacific Cod (Gadus macrocephalus) Bone: Calcium Bioavailability Enhancing Activity and Anti-Osteoporosis Effects in the Ovariectomy-Induced Osteoporosis Rat Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CBP
2.3. Amino Acid Profile
2.4. Preparation of CBP-Calcium Complex (CBP-Ca)
2.5. Structural Characterization of CBP and CBP-Ca in Solution
2.5.1. UV Absorption
2.5.2. Circular Dichroism Analysis
2.5.3. Fourier Transform Infrared (FTIR) Spectroscopy Measurement
2.6. In Situ Single-Pass Intestinal Perfusion (SPIP) Study
2.6.1. Animals
2.6.2. Single-Pass Intestinal Perfusion (SPIP) Experiment
2.6.3. Data Analysis and Equations
2.7. Calcium Bioavailability and Anti-Osteoporosis Activity of CBP-Ca
2.7.1. Animals and Treatments
2.7.2. Calcium Bioavailability of CBP-Ca
2.7.3. Sampling and Analytical Methods
2.8. Statistical Analysis
3. Results and Discussion
3.1. Calcium Binding Activity and Amino Acid Composition of CBP
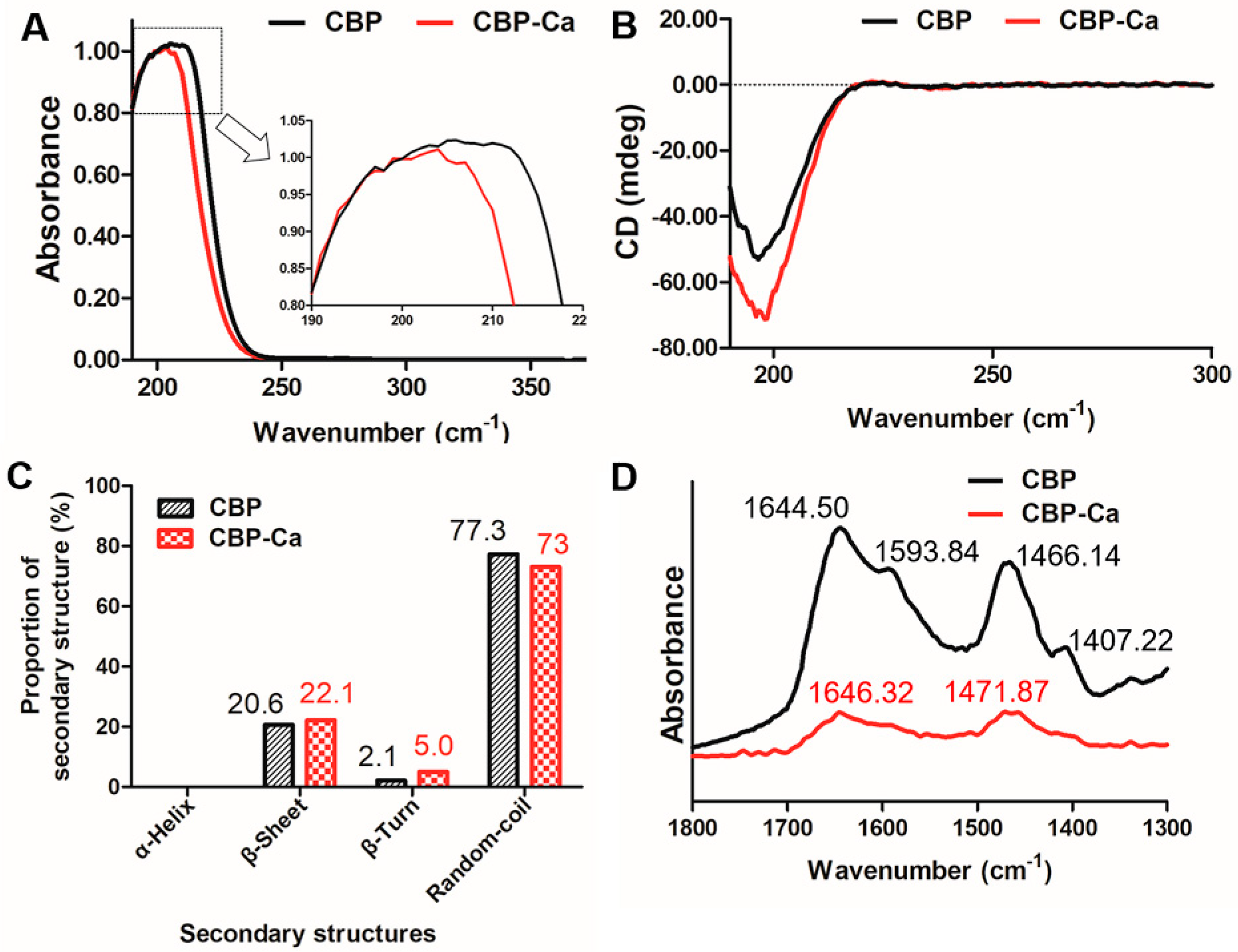
3.2. Structural Differences between CBP and CBP-Ca in Solution
3.2.1. UV Absorption Spectrum of CBP and CBP-Ca
3.2.2. Secondary Structure of CBP and CBP-Ca in Solution
3.2.3. Fourier Transform Infrared (FTIR) Spectra of CBP and CBP-Ca
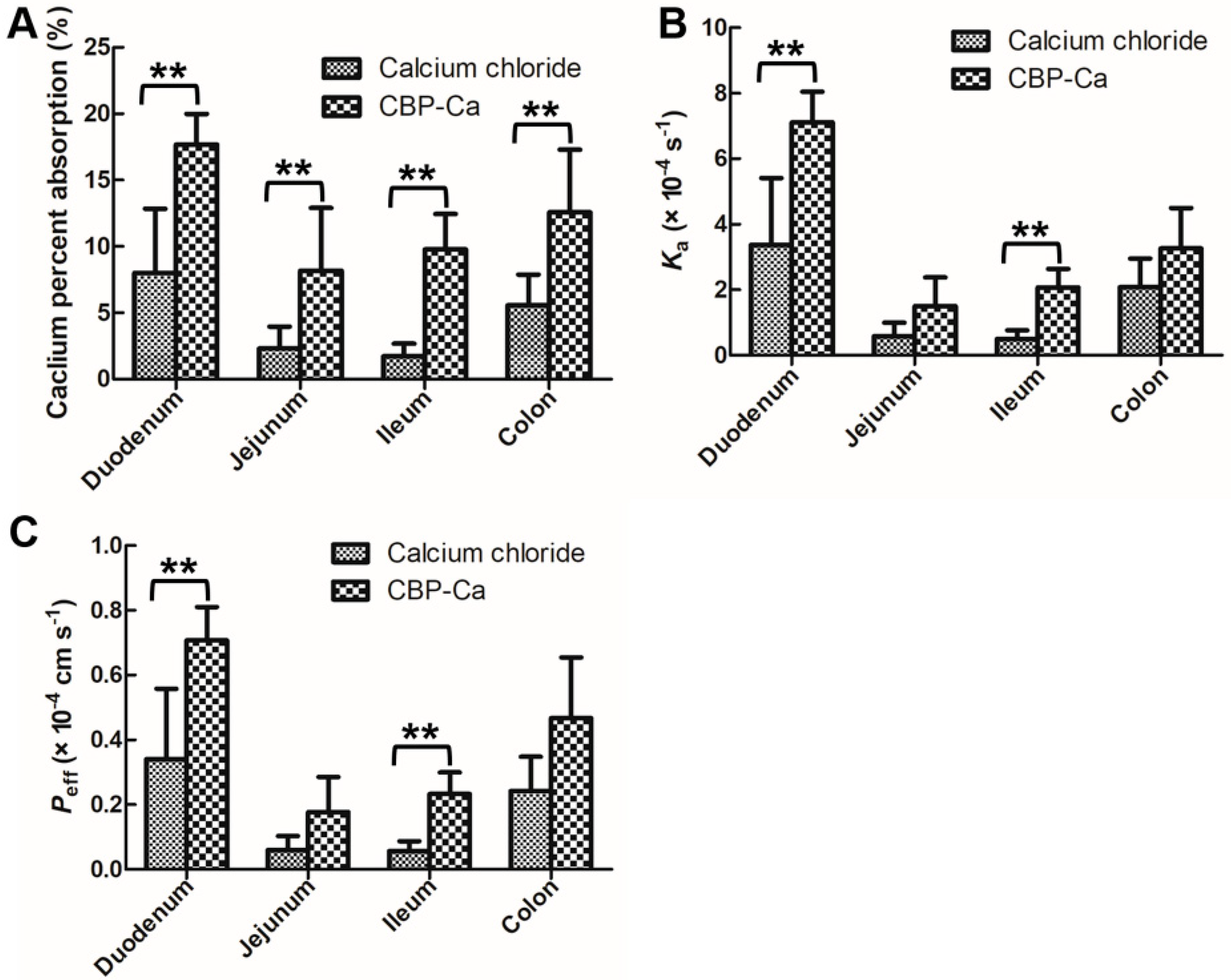
3.3. CBP-Ca Improved the Rat Intestinal Calcium Absorption
3.4. CBP-Ca Improved the Calcium Bioavailability of OVX Rats
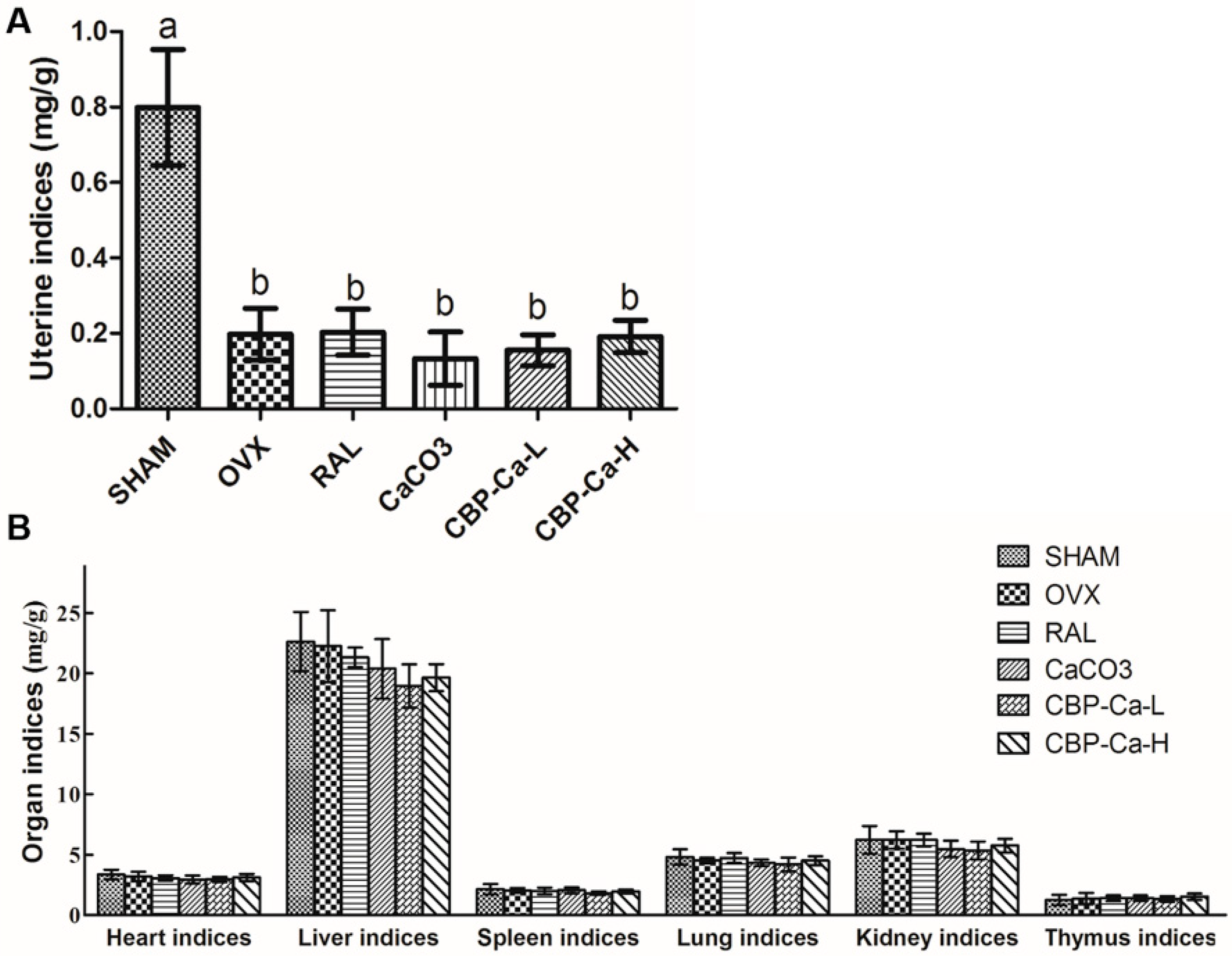
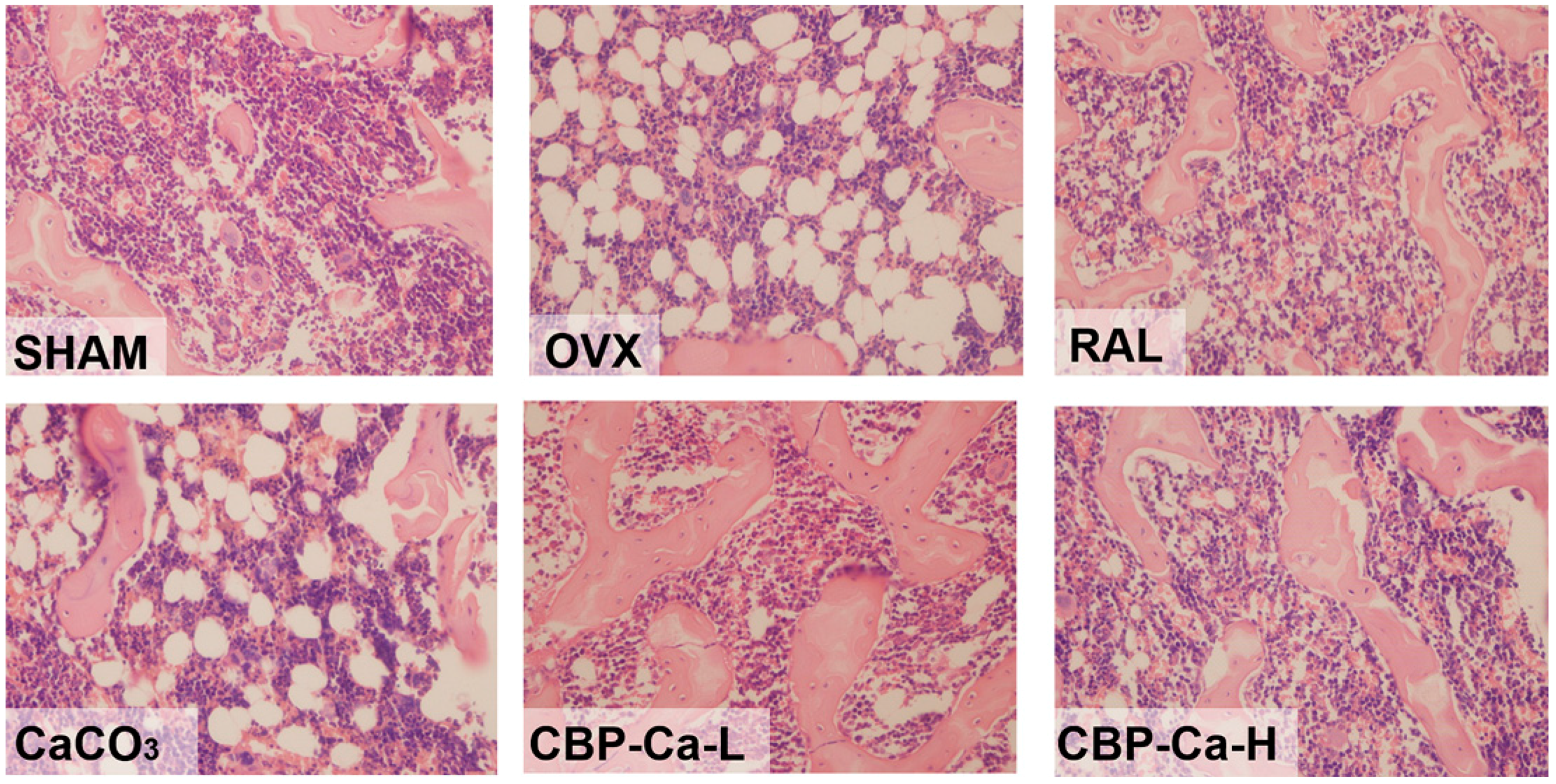
3.5. CBP-Ca Has Protective Effects in Bone Microarchitecture of OVX Rats
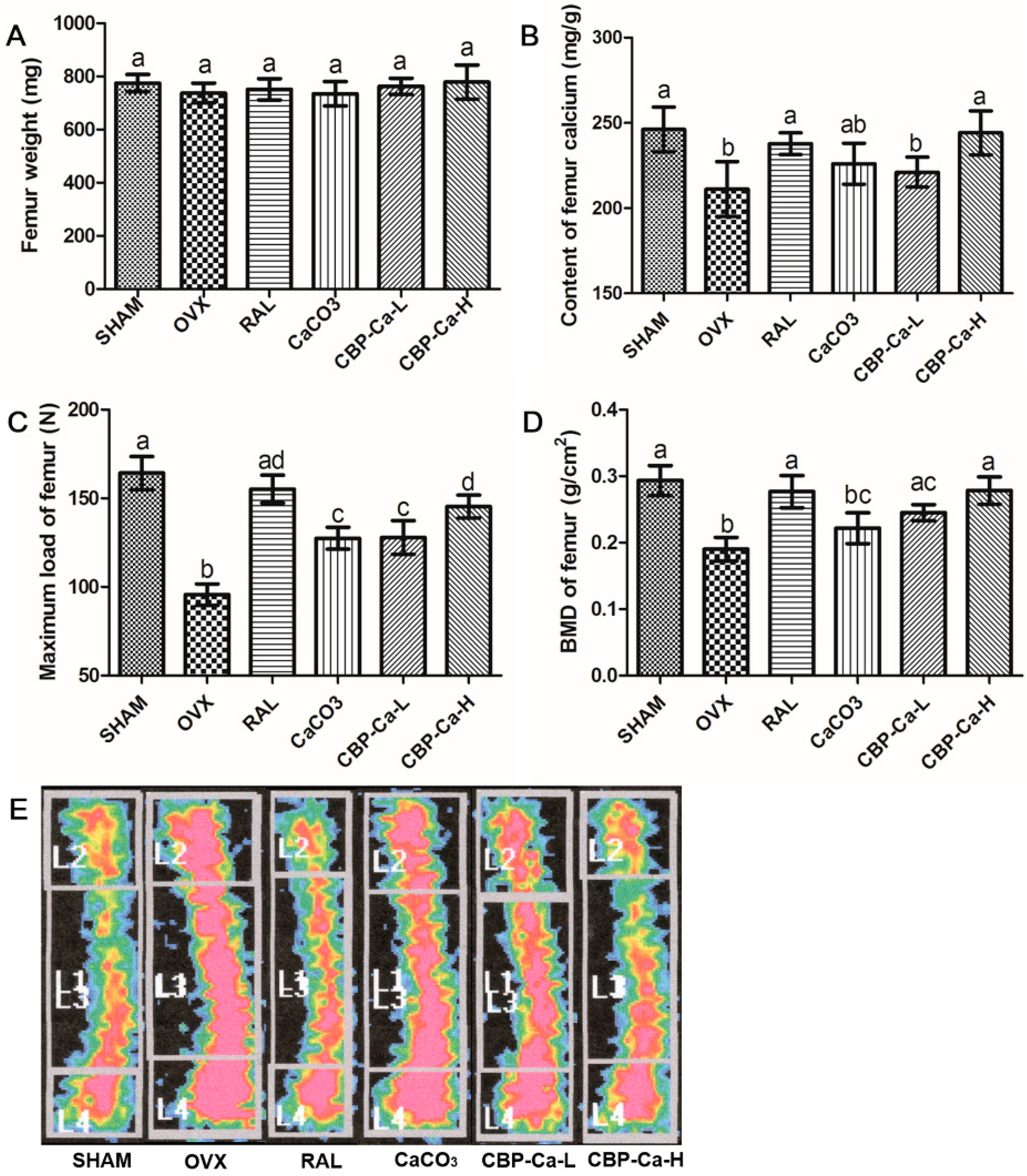
3.6. CBP-Ca Enhanced Bone Properties in OVX Rats
3.7. CBP-Ca Reduces the Bone Turnover Rate of OVX Rats
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hwang, Y.-H.; Ha, H.; Kim, R.; Cho, C.-W.; Song, Y.-R.; Hong, H.-D.; Kim, T. Anti-osteoporotic effects of polysaccharides isolated from persimmon leaves via osteoclastogenesis inhibition. Nutrients 2018, 10, 901. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Hofbauer, L.C. Osteoporosis treatment: Recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017, 5, 898–907. [Google Scholar] [CrossRef]
- Eastell, R.; Szulc, P. Use of bone turnover markers in postmenopausal osteoporosis. Lancet Diabetes Endocrinol. 2017, 5, 908–923. [Google Scholar] [CrossRef]
- Areco, V.; Rivoira, M.A.; Rodriguez, V.; Marchionatti, A.M.; Carpentieri, A.; Tolosa de Talamoni, N. Dietary and pharmacological compounds altering intestinal calcium absorption in humans and animals. Nutr. Res. Rev. 2015, 28, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Naylor, K.; Eastell, R. Bone turnover markers: Use in osteoporosis. Nat. Rev. Rheumatol. 2012, 8, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Binkley, N.; Blank, R.D.; Leslie, W.D.; Lewiecki, E.M.; Eisman, J.A.; Bilezikian, J.P. Osteoporosis in crisis: It’s time to focus on fracture. J. Bone Mine. Res. 2017, 32, 1391–1394. [Google Scholar] [CrossRef] [PubMed]
- Bone, H.G.; Wagman, R.B.; Brandi, M.L.; Brown, J.P.; Chapurlat, R.; Cummings, S.R.; Czerwiński, E.; Fahrleitner-Pammer, A.; Kendler, D.L.; Lippuner, K.; et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: Results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017, 5, 513–523. [Google Scholar] [CrossRef]
- Guo, L.; Harnedy, P.A.; Li, B.; Hou, H.; Zhang, Z.; Zhao, X.; FitzGerald, R.J. Food protein-derived chelating peptides: Biofunctional ingredients for dietary mineral bioavailability enhancement. Trends Food Sci. Technol. 2014, 37, 92–105. [Google Scholar] [CrossRef]
- Sun, N.; Wu, H.; Du, M.; Tang, Y.; Liu, H.; Fu, Y.; Zhu, B. Food protein-derived calcium chelating peptides: A review. Trends Food Sci. Technol. 2016, 58, 140–148. [Google Scholar] [CrossRef]
- Charoenphun, N.; Cheirsilp, B.; Sirinupong, N.; Youravong, W. Calcium-binding peptides derived from tilapia (Oreochromis niloticus) protein hydrolysate. Eur. Food Res. Technol. 2013, 236, 57–63. [Google Scholar] [CrossRef]
- Hou, H.; Wang, S.; Zhu, X.; Li, Q.; Fan, Y.; Cheng, D.; Li, B. A novel calcium-binding peptide from Antarctic krill protein hydrolysates and identification of binding sites of calcium-peptide complex. Food Chem. 2018, 243, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, B.; Hou, H.; Zhang, H.; Zhao, X. Isolation and identification of calcium-chelating peptides from Pacific cod skin gelatin and their binding properties with calcium. Food Funct. 2017, 8, 4441–4448. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-R.; Wang, L.; Wang, R.; Chen, Z.-X. Calcium-Binding Capacity of Wheat Germ Protein Hydrolysate and Characterization of Peptide–Calcium Complex. J. Agric. Food Chem. 2013, 61, 7537–7544. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Liu, Y.; Kolba, N.; Guo, D.; He, H. Desalted Duck Egg White Peptides Promote Calcium Uptake and Modulate Bone Formation in the Retinoic Acid-Induced Bone Loss Rat and Caco-2 Cell Model. Nutrients 2017, 9, 490. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.-K.; Kim, S.-K. Calcium-binding peptide derived from pepsinolytic hydrolysates of hoki (Johnius belengerii) frame. Eur. Food Res. Technol. 2007, 224, 763–767. [Google Scholar] [CrossRef]
- Peng, Z.; Hou, H.; Zhang, K.; Li, B. Effect of calcium-binding peptide from Pacific cod (Gadus macrocephalus) bone on calcium bioavailability in rats. Food Chem. 2017, 221, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.-Y.; Ko, S.-C.; Nam, S.Y.; Oh, J.; Kim, Y.-M.; Kim, J.-I.; Kim, N.; Yi, M.; Jung, W.-K. Fish bone peptide promotes osteogenic differentiation of MC3T3-E1 pre-osteoblasts through upregulation of MAPKs and Smad pathways activated BMP-2 receptor. Cell Biochem. Funct. 2018, 36, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive peptides from marine processing waste and shellfish: A review. J. Funct. Foods 2012, 4, 6–24. [Google Scholar] [CrossRef]
- Toppe, J.; Albrektsen, S.; Hope, B.; Aksnes, A. Chemical composition, mineral content and amino acid and lipid profiles in bones from various fish species. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007, 146, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Harnedy, P.A.; O’Keeffe, M.B.; Zhang, L.; Li, B.; Hou, H.; FitzGerald, R.J. Fractionation and identification of Alaska pollock skin collagen-derived mineral chelating peptides. Food Chem. 2015, 173, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Nara, M.; Tanokura, M. Infrared spectroscopic study of the metal-coordination structures of calcium-binding proteins. Biochem. Biophys. Res. Commun. 2008, 369, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, Q.; Fan, J.; Li, X.; Zhuang, Y. Purification and characterization of peptides inhibiting MMP-1 activity with C terminate of Gly-Leu from simulated gastrointestinal digestion hydrolysates of tilapia (Oreochromis niloticus) skin gelatin. J. Agric. Food Chem. 2018, 66, 593–601. [Google Scholar] [CrossRef]
- Ma, H.; Chen, H.; Sun, L.; Tong, L.; Zhang, T. Improving permeability and oral absorption of mangiferin by phospholipid complexation. Fitoterapia 2014, 93, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.; Zhao, Y.; Yu, Z.; Tian, Y.; Wang, Y.; Wang, S.; Wang, J.; Xue, C. Phosphorylated peptides from antarctic krill (Euphausia superba) prevent estrogen deficiency induced osteoporosis by inhibiting bone resorption in ovariectomized rats. J. Agric. Food Chem. 2015, 63, 9550–9557. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Mu, X.; Huang, H.; Nie, R.; Liu, Z.; Zeng, M. Isolation of a calcium-binding peptide from tilapia scale protein hydrolysate and its calcium bioavailability in rats. J. Funct. Foods 2014, 6, 575–584. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Wang, J.; Xue, C.; Chang, Y.; Xue, Y. Preparation and anti-osteoporotic activities in vivo of phosphorylated peptides from Antarctic krill (Euphausia superba). Peptides 2015, 68, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Akagündüz, Y.; Mosquera, M.; Giménez, B.; Alemán, A.; Montero, P.; Gómez-Guillén, M.C. Sea bream bones and scales as a source of gelatin and ACE inhibitory peptides. LWT Food Sci. Technol. 2014, 55, 579–585. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Huang, Q.; Huang, S.; Lin, J.; Wang, S.; Huang, Y.; Hong, J.; Rao, P. Novel Peptide with a Specific Calcium-Binding Capacity from Whey Protein Hydrolysate and the Possible Chelating Mode. J. Agric. Food Chem. 2014, 62, 10274–10282. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, N.J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 2007, 1, 2876–2890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nara, M.; Morii, H.; Tanokura, M. Coordination to divalent cations by calcium-binding proteins studied by FTIR spectroscopy. Biochim. Biophys. Acta 2013, 1828, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
- Barth, A. The infrared absorption of amino acid side chains. Prog. Biophys. Mol. Biol. 2000, 74, 141–173. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, S.; Cai, X.; Hong, J.; Wang, S. A specific peptide with calcium chelating capacity isolated from whey protein hydrolysate. J. Funct. Foods 2014, 10, 46–53. [Google Scholar] [CrossRef]
- Dezani, T.M.; Dezani, A.B.; da Silva Junior, J.B.; dos Reis Serra, C.H. Single-Pass Intestinal Perfusion (SPIP) and prediction of fraction absorbed and permeability in humans: A study with antiretroviral drugs. Eur. J. Pharm. Biopharm. 2016, 104, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Khanal, R.C.; Nemere, I. Regulation of intestinal calcium transport. Annu. Rev. Nutr. 2008, 28, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Pu, F.; Chen, N.; Xue, S. Calcium intake, calcium homeostasis and health. Food Sci. Hum. Wellness 2016, 5, 8–16. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Wei, X.; Gou, J.; Tang, X.; He, H.; Xu, H. Influence of lipid composition on the oral bioavailability of cinnarizine sub-microemulsions: Oral bioavailability of cinnarizine sub-microemulsions. Eur. J. Lipid Sci. Technol. 2017, 119, 1600184. [Google Scholar] [CrossRef]
- Alkhamees, O.A.; Al-Roujayee, A.S.; Abuohashish, H.M.; Ahmed, M.M. Anti-osteoporotic effects of an antidepressant tianeptine on ovariectomized rats. Biomed. Pharmacother. 2017, 87, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Johnston, B.D.; Ward, W.E. The Ovariectomized Rat as a model for studying alveolar bone loss in postmenopausal women. BioMed Res. Int. 2015, 2015, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Furuta, S.; Nagata, T.; Ohnuki, K.; Akasaka, T.; Shirouchi, B.; Sato, M.; Kondo, R.; Shimizu, K. Inhibitory effects of the leaves of loquat (Eriobotrya japonica) on bone mineral density loss in ovariectomized mice and osteoclast differentiation. J. Agric. Food Chem. 2014, 62, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Granton, P.V.; Holdsworth, D.W.; Turley, E.A. Oral administration of hyaluronan reduces bone turnover in ovariectomized rats. J. Agric. Food Chem. 2013, 61, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Drissi, H.; Sanjay, A. The multifaceted osteoclast; far and beyond bone resorption. J. Cell. Biochem. 2016, 117, 1753–1756. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Iki, M.; Fujita, Y.; Tamaki, J.; Kouda, K.; Yura, A.; Moon, J.-S.; Winzenrieth, R.; Iwaki, H.; Ishizuka, R.; et al. Greater milk intake is associated with lower bone turnover, higher bone density, and higher bone microarchitecture index in a population of elderly Japanese men with relatively low dietary calcium intake: Fujiwara-kyo Osteoporosis Risk in Men (FORMEN) Study. Osteoporos. Int. 2015, 26, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Duggan, S.N.; Purcell, C.; Kilbane, M.; O’Keane, M.; McKenna, M.; Gaffney, P.; Ridgway, P.F.; Boran, G.; Conlon, K.C. An association between abnormal bone turnover, systemic inflammation, and osteoporosis in patients with chronic pancreatitis: A case-matched study. Am. J. Gastroenterol. 2015, 110, 336–345. [Google Scholar] [CrossRef] [PubMed]


| Amino Acid | CBP | Amino Acid | CBP |
|---|---|---|---|
| Aspartic acid (Asp) | 84 | Leucine (Leu) | 11 |
| Threonine (Thr) | 22 | Tyrosine (Tyr) | 3 |
| Serine (Ser) | 59 | Phenylalanine (Phe) | 10 |
| Glutamic acid (Glu) | 97 | Lysine (Lys) | 14 |
| Glycine (Gly) | 322 | Histamine (His) | 29 |
| Alanine (Ala) | 86 | Arginine (Arg) | 46 |
| Cysteine (Cys) | 15 | Proline (Pro) | 106 |
| Valine (Val) | 10 | Hydroxyproline (Hyp) | 72 |
| Methionine (Met) | 2 | Imino acid (Pro + Hyp) | 178 |
| Isoleucine (Ile) | 12 | Total | 1000 |
| Ca Intake (mg/day) | Urinary Ca (mg/day) | Fecal Ca (mg/day) | Ca Apparent Absorption (mg/day) | Ca Apparent Absorption Rate (%) | Ca Retention (mg/day) | Ca Retention Rate (%) | |
|---|---|---|---|---|---|---|---|
| SHAM | 57.74 ± 4.34 a | 1.48 ± 0.28 ac | 19.57 ± 2.90 a | 38.17 ± 5.37 ac | 65.90 ± 5.97 a | 36.69 ± 5.37 a | 63.33 ± 6.15 a |
| OVX | 58.95 ± 4.15 a | 1.43 ± 0.28 ac | 34.63 ± 3.07 b | 24.32 ± 4.84 b | 41.02 ± 6.41 b | 22.96 ± 4.76 b | 38.72 ± 6.41 b |
| RAL | 58.18 ± 3.58 a | 1.26 ± 0.21 ac | 19.43 ± 2.08 a | 38.75 ± 2.53 a | 66.64 ± 2.56 a | 37.49 ± 2.58 a | 64.46 ± 2.64 a |
| CaCO3 | 57.14 ± 3.80 a | 1.53 ± 0.09 a | 29.13 ± 3.48 b | 28.01 ± 5.16 bc | 48.79 ± 7.30 b | 26.48 ± 5.13 b | 46.11 ± 7.32 b |
| CBP-Ca-L | 29.53 ± 2.11 b | 0.62 ± 0.10 b | 6.39 ± 2.10 c | 23.13 ± 2.25 b | 78.44 ± 6.64 c | 22.51 ± 2.26 b | 76.34 ± 6.68 c |
| CBP-Ca-H | 61.70 ± 3.99 a | 1.10 ± 0.16 c | 18.16 ± 3.34 a | 43.54 ± 5.60 a | 70.41 ± 5.65 c | 42.44 ± 5.65 a | 68.62 ± 5.77 ac |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Li, B.; Chen, Q.; Zhang, Z.; Zhao, X.; Hou, H. Functional Calcium Binding Peptides from Pacific Cod (Gadus macrocephalus) Bone: Calcium Bioavailability Enhancing Activity and Anti-Osteoporosis Effects in the Ovariectomy-Induced Osteoporosis Rat Model. Nutrients 2018, 10, 1325. https://doi.org/10.3390/nu10091325
Zhang K, Li B, Chen Q, Zhang Z, Zhao X, Hou H. Functional Calcium Binding Peptides from Pacific Cod (Gadus macrocephalus) Bone: Calcium Bioavailability Enhancing Activity and Anti-Osteoporosis Effects in the Ovariectomy-Induced Osteoporosis Rat Model. Nutrients. 2018; 10(9):1325. https://doi.org/10.3390/nu10091325
Chicago/Turabian StyleZhang, Kai, Bafang Li, Qianru Chen, Zhaohui Zhang, Xue Zhao, and Hu Hou. 2018. "Functional Calcium Binding Peptides from Pacific Cod (Gadus macrocephalus) Bone: Calcium Bioavailability Enhancing Activity and Anti-Osteoporosis Effects in the Ovariectomy-Induced Osteoporosis Rat Model" Nutrients 10, no. 9: 1325. https://doi.org/10.3390/nu10091325