Abstract
Inflammatory bowel disease (IBD) is a chronic disease mediated by the immune system and characterized by the inflammation of the gastrointestinal tract. This study is to understand how the use of parenteral nutrition (PN) can affect the adult population diagnosed with IBD. We conducted a systematic review, meta-analysis, and meta-regression. From the different databases (MEDLINE, Scopus, Cochrane, LILACS, CINAHL, WOS), we found 119 registers with an accuracy of 16% (19 registers). After a full-text review, only 15 research studies were selected for qualitative synthesis and 10 for meta-analysis and meta-regression. The variables used were Crohn’s Disease Activity Index (CDAI), albumin, body weight (BW), and postoperative complications (COM). PN has shown to have efficacy for the treatment of IBD and is compatible with other medicines. The CDAI and albumin improve, although the effect of PN is greater after a while. However, the effect on the albumin could be less than the observed value in the meta-analysis due to possible publication bias. The BW does not change after intervention. COM utilizing PN has been observed, although the proportion is low. More studies specifically referring to ulcerative colitis (UC) and Crohn’s disease (CD) are needed to develop more concrete clinical results.
1. Introduction
During the last decades, the prevalence of inflammatory bowel disease (IBD) has increased in the U.S. and Europe [1]. Moreover, it has also increased in developing countries [2,3]; thus, IBD can be considered a common disease in wide areas of the world.
IBD is a chronic inflammatory disease mediated by the immune system. IBD includes Crohn’s disease (CD) and ulcerative colitis (UC). The altered response of the immune system leads to the inflammation of the gastrointestinal tract clinically defined by relapsing and remitting episodes [4,5]. The inflammatory process is characterized by a long-term overproduction of pro-inflammatory factors and an enhanced intestinal permeability [6]. IBD involves an inflammatory process of the intestinal layers and could cause abdominal swelling, fever, fatigue, weight loss, abdominal pain, diarrhea, bloody feces, etc. [7].
The European Society for Clinical Nutrition and Metabolism (ESPEN) has presented the guidelines and recommendations on clinical nutrition for the IBD [8]. The etiology of the IBD is not still completely understood [9,10]. Many studies have indicated that the genetic predisposition, diet, the environment, the intestinal microbial flora, and the immune responses are involved in the pathogenesis of IBD [4,5,11,12].
Diet and intestinal microbial flora could change the inflammatory response of the gastrointestinal tract [13,14]. Diet may reduce the symptoms and prevent the degenerative process of the IBD [15]. Therefore, it is considered a therapy for IBD [13]. Among the dietetic therapies for IBD, intestinal rest by parenteral nutrition (PN) is considered a strategy to reduce the inflammatory response of intestinal layers [16] and to recover from nutritional impairment [17]. The American Society for Parenteral and Enteral Nutrition (ASPEN) and ESPEN have described the use of PN [18,19,20]. PN could be considered a third way for human nutrition after oral intake and enteral nutrition. However, a combination of them has been studied [21]. PN could not advantage in IBD compared to other nutrition therapies. However, when the IBD patients are temporarily unable to receive significant oral or enteral nutrients, PN could be used as a nutritional treatment [21,22]. Also, in severe cases of IBD with surgical resection or bowel severe complications, PN could provide a supply of nutrients to maintain good nutritional status and reduce inflammatory reactions [23].
The aim of this review was to understand the use of PN and its effects on adults diagnosed with IBD.
2. Materials and Methods
To achieve this objective, a systematic review was conducted in agreement with the procedures and verification list described by PRISMA [24]. Afterward, a meta-analysis on the more common results, and a meta-regression with the co-variables, surgery (Yes/no), observed moment (days), and period of treatment (days), were conducted.
2.1. Systematic Review
A search of scientific works was conducted in the MEDLINE database, through the system of open retrieval system on the Internet such as PubMed, Cochrane, Scopus, Web of Science, CINAHL, and LILACS. The studies conducted over time, up to 8 July 2019, were compiled.
2.1.1. Inclusion and Exclusion Criteria
The studies selected had to comply with the following inclusion criteria: refer to an adult population (older than 18) diagnosed with IBD; study the effect of PN within IBD; be clinical trials; in English, Spanish, Portuguese, French, or German languages.
The following articles were excluded: those that referred to the infant population; to animals, to the use of PN in a healthy adult population; those that sought the effect of oral exclusion diets on IBD; that were observational studies; that were based on secondary sources.
2.1.2. Search Equation
To include content linked to the intervention, PN, a specific descriptor was used (MeSH), such as “Parenteral Nutrition, Total”, and the term “Parenteral Nutrition” in the title or abstract.
For the content linked to the population, we utilized the descriptor that referred to the disease “Inflammatory bowel diseases”, and its equivalent term in the title or abstract.
Also, the filters “Humans”, “Adult”, and “Clinical Trial” were utilized to achieve our objective.
Therefore, the main search equation designed for this study was:
((“Inflammatory Bowel Diseases” [Mesh] OR “Inflammatory Bowel Diseases” [Title/Abstract]) AND (“Parenteral Nutrition, Total” [Mesh] OR “Parenteral Nutrition” [Title/Abstract])) AND (Clinical Trial [ptyp] AND Humans [Mesh] AND adult [MeSH])
The search equation was adapted to each, and all of the databases described previously. The process was conducted between the months of June and July 2019.
2.1.3. Selection Process
After eliminating duplicate records, the process of selection was conducted in two phases. The first consisted of reviewing the titles and abstracts of all the article records resulting from the adapted search equations and shown by the databases by using the inclusion and exclusion criteria and the objective of the study as the screening measure. The screening and selection of the records/articles were conducted independently by the two researchers, both experts in the fields of nutrition. These researchers agreed on the discrepancies found in order to define the final suitability of the records/articles found in the databases. The precision of the search was calculated based on the ratio of the full-text articles selected for the review divided by the number of records found by the search equation, multiplied by one hundred.
The second phase was conducted by applying the inclusion/exclusion criteria to the complete text of all the scientific studies selected in the first phase, thus ensuring the relevance of each one of them.
2.1.4. Evaluation of the Quality of the Studies
The evaluation of the methodological quality of the included studies was performed by two independent researchers, using the CONSORT (Consolidated Standards of Reporting Trials) guide for clinical trials. This guide contains a list of 25 essential aspects that should be described in the publication of these studies. For each selected study, one point was assigned for each item present (if not applicable, it did not score). When an item was composed of several points, these were evaluated independently, giving the same value to each of them and subsequently an average was made (being the final result of that item), so that in no case could it beat the score of one point per item [25,26].
2.2. Meta-Analysis and Meta-Regression
To calculate the effect size of the enteral nutrition on the variables, Crohn’s Disease Activity Index (CDAI), albumin, postoperative complications (COM), and body weight (BW), a meta-analysis was performed. For this, the model of fixed effects and the model of random effects were utilized. The results were presented as a forest-plot, along with the percent Heterogeneity and its confidence interval at 95%, the T-value, and the heterogeneity test.
To explore the influence of each study over the effect size, we used a leave-one-out method; pooled estimates were calculated omitting one study at a time. In addition, we plotted a scatter plot introduced by Baujat et al. [27]. On the x-axis, the contribution of each study to the overall heterogeneity statistic is plotted. On the y-axis, the standardized difference of the overall treatment effect with and without each study is plotted; this quantity describes the influence of each study on the overall treatment effect. Therefore, studies that fall in the top right quadrant of the Baujat plot have the most influence.
Publication bias occurs when only favorable results are published, and this could have consequences on the results of the meta-analyses if these were included. To analyze the publication bias, a non-parametric analysis was conducted as proposed by Duval and Tweedie [28] based on the funnel-plot, estimating, and adjusting for the number and outcomes of missing studies in the meta-analysis. Another less-conservative proposal to estimate the number and outcomes of missing studies is the proposal by Copas et al. [29].
The meta-regression was utilized to understand if the duration of the intervention (days) or the surgery (yes/no) or observed moment (days) modified the effect size of the resulting variables CDAI, albumin, and BW. The effect size of COM was only related to the duration of the intervention. All the calculations were conducted within an R programming environment utilizing the packages meta version 4.9-6 [30] and metasens version 0.4-0 [31].
3. Results
3.1. Systematic Review
As a result of the specific search equations used on the different databases, a total of 145 records were found of scientific articles. A total of 26 records were duplicated, leaving a total of 119 records without duplication. In the first phase of the study, exactly 100 study records were discarded, leaving 19 full-text studies to review, so that the accuracy was 16%. The reasons for not including them were that 51 records showed that the study utilized a design that was not adequate, 15 did not use an adult population, 16 did not study the effect of PN, three were written in another language other than the ones cited above, (one in Japanese, one in Chinese, and one in German), 11 did not refer to the IBD, and four were still being conducted without showing results (Figure 1).
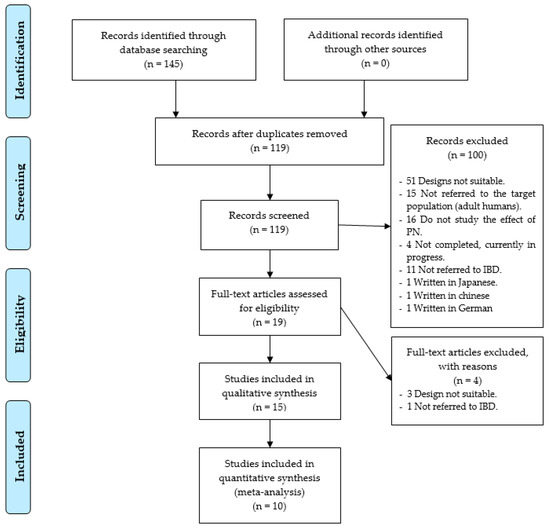
Figure 1.
Identification and selection of studies/records in the databases.
In the second phase, four trials were removed, three due to defects in its design, and one, because the patients studied, were not diagnosed with IBD. Therefore, only 15 research studies [32,33,34,35,36,37,38,39,40,41,42,43,44,45,46] were selected, as shown in Figure 1.
As for the designs of the studies included, 11were controlled and randomized clinical studies (73.4%), two non-randomized, controlled clinical trials (13.3%), and two non-randomized, non-controlled clinical trials (13.3%) were found. In addition, six of the studies found showed results that specifically referred to CD, one study to UC, and 11 studies had results on UC and CD, under the category of IBD. Also, 10 studies mentioned the results of the disease in its active form and five studies report disease outcomes in patients under surgery. Figure 2 shows this information in a chronological manner.
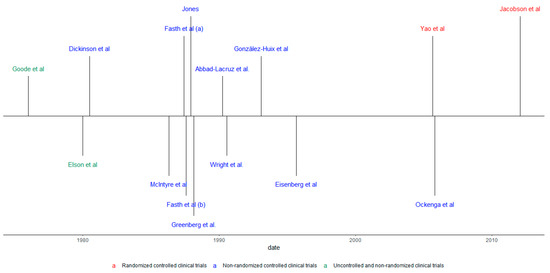
Figure 2.
Chronological review according to the type of study and population.
As for the variety of the components of formulas employed, each study used its own formulas, normally supplemented with vitamins, minerals, and electrolytes. However, some studies employed the following commercial components/complements: “Freamine®”, “Amigen®”, “Uniasa®”, “Dipeptamin®”, “Aminoplasmal®”, “Vamin®”, “Addamel®”, “Soluvit®”, and “Vitalipid®”.
In addition, a total of four types of objectives were found: six studies sought to compare the administration of PN with other techniques such as dextrose and electrolyte solutions, intravenous transfusions or oral diet, as long as possible; five studies compared PN with EN, among which, three with elemental formulas, and two with polymeric formulas; two studies sought to experiment with PN and two studies compared the same PN, but with some different component/form of withdrawal.
As for the manner of administration of the PN, the research studies generally employed a central venous catheter with the aid of an infusion pump.
The total population analyzed in the research studies found included a total of 557 individuals with IBD, with 382 diagnosed with CD, and 152 with UC.
The main tools utilized by the researchers to obtain the results were the scores, biomarkers, and tests to measure the activity of the disease—the CDAI, the Van Hees activity index (VHAI), the Truelove and Witts index; biomarkers such as CRP, ESR, the white blood cell count (WBC), levels of albumin, pre-albumin, transferrin, hemoglobin, platelet count, total bilirubin, alkaline phosphatase, etc.; and medical tests, such as the ileocolonoscopy. Complementary tests, such as urine and feces samples. Tests to measure the body’s composition, such as anthropometries and bioimpedance, to obtain parameters such as body weight (BW), triceps skinfold thickness (TSF), mid-arm muscle circumference (MAMC), etc. The follow-up of complications by health professionals, whether postoperative or during experimentation, such as infections, septicemias, cases of pneumonia, intestinal obstructions, pancreatitis, fever, hypoglycemia, hyperglycemia, etc.
Table 1 shows the main results schematically, found in the selected articles and Table 2 shows the scores obtained by the studies for their methodological quality according to the CONSORT guide.

Table 1.
Main results of the systematic review.

Table 2.
Methodological quality analysis according to the CONSORT guide [25] for reporting clinical trials.
3.2. Meta-Analysis and Meta-Regression
Only 10 clinical trials had common quality and variables needed to be used in the meta-analysis. These 10 trials worked with a total of 26 groups. The final size of the sample was comprised by 298 observed moments for 164 individuals, all with IBD, to which a PN treatment had been given. The common variables were the CDAI, albumin, BW, and COM, and the co-variables; duration of the intervention, surgery, and observed moment. Figure 3 shows the effect size of the use of PN. For the CDAI and albumin, the effects are positive when comparing the situation at the start and finish of the treatment with PN, independently of whether the situation with fixed effects (less probable) or random effects (more acceptable) was considered. However, for BW, the use of PN was not significant. For COM, the effect size was significantly different from zero but the 95% confidence interval was close to zero ([0.02; 0.63]).
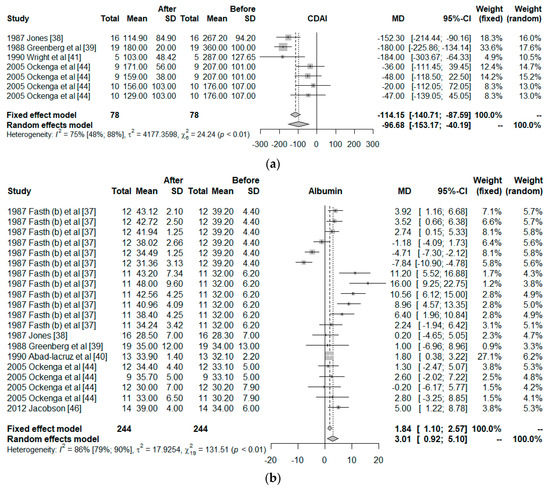
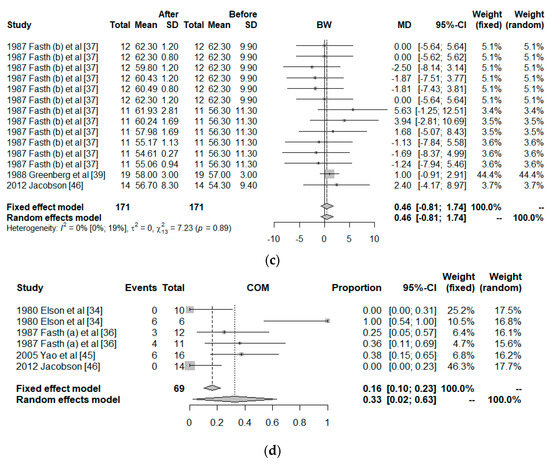
Figure 3.
Forest plot for: (a) Crohn’s Disease Activity Index (CDAI), (b) Albumin, (c) Body weight (BW), and (d) Postoperative complications (COM).
The influence of each study on the results of the meta-analysis are shown in Table 3, considering a model of random effects. For CDAI, the study of Greenberg et al. has a strong effect on the results, increasing the effect size of the PN on the CDAI. The outcomes of Fasth et al. study has the strongest influence on the albumin, but it is only 5%. All studies are homogeneous for the BW. Elson et al. worked with two groups, one showed 0/10 of COM but the other showed 6/6; therefore, their study has a strong influence on the results.

Table 3.
Influence analysis in a meta-analysis using the leave-one-out method (Random effect).
Figure 4 shows this influence through the Baujat plot. The numbers shown in the figure correspond to the articles shown in the table in the ID column. Notice that the studies 18, 10, and 2 correspond to Greenberg et al., Fasth (b) et al., and Elson et al.
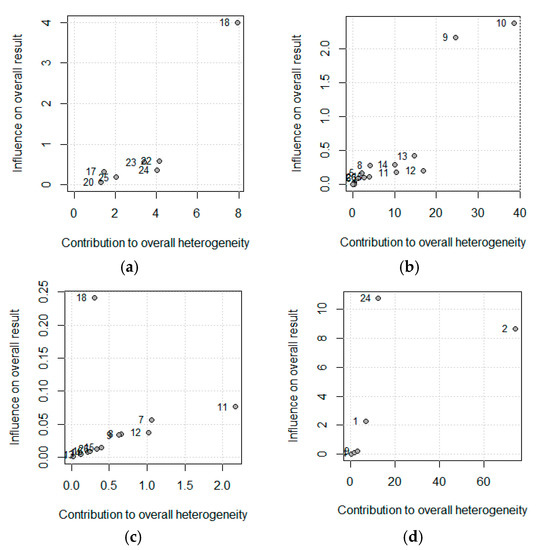
Figure 4.
Baujat plot for (a) Crohn’s Disease Activity Index (CDAI), (b) Albumin (c) Body Weight, and (d) Postoperative complications. The correspondence between the study and the number is shown in Table 2 (Id, Omitting).
A Funnel Plot represents the effects observed in the different studies (x-axis), and the standard error (y-axis). In the absence of heterogeneity and publication bias, the dots shown in the funnel plot should jointly adopt the aspect of a funnel, with the wider part corresponding to the smaller and more precise studies. A lack of symmetry could be due to this publication bias. The funnel plot is shown in Figure 5, and a lack of symmetry can be observed. Therefore, the non-parametric analysis proposed by Duval and Tweedie to analyze this asymmetry should show a lack of articles, and therefore, a publication bias. The results of this non-parametric analysis for the fixed-effects model and the random-effects model are shown in Table 4. These results show a possible publication bias in the albumin if a fixed-effects model is assumed; however, the random-effects models do not show this bias. The Copas analysis shows a possible publication bias and suggests that the benefits of PN on albumin could decrease from 3.01 to 2.0.
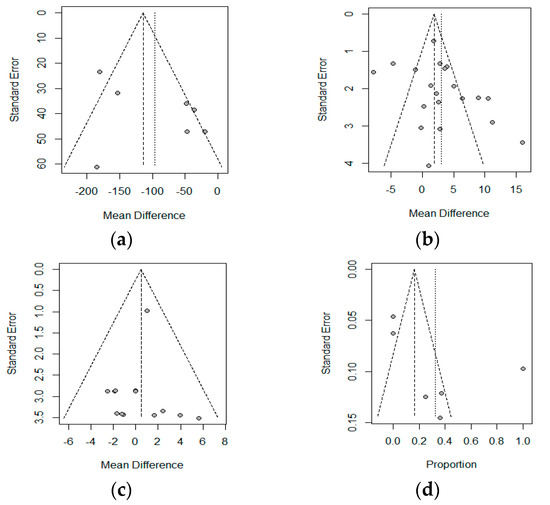
Figure 5.
Funnel plot for: (a) Crohn’s Disease Activity Index (CDAI), (b) Albumin (c) Body Weight, and (d) Postoperative complications. The correspondence between the study and the number is shown in Table 2 (Id, Omitting).

Table 4.
Number of studies that should be added and the estimated effect size.
With respect to the meta-regression, the results are shown in Table 5. There is a dependence of the CDAI score and albumin levels with the observed moment; we have to wait some days for confirming the effects of the PN (p < 0.01).

Table 5.
Meta-regression.
4. Discussion
Our systematic review included a total of 15 clinical trials, which compiled information from 557 individuals with IBD, and who had an intervention with PN. All the studies had a broad reach, and within the diverse effects found, BW, albumin, COM, and CDAI were the most common, allowing us to conduct a meta-analysis to arrive at more complete conclusions.
PN implies the intravenous administration of a mix of macronutrients, micronutrients, and electrolytes [47], and its main objective for IBD is to achieve bowel rest, correct nutritional deficits, and the elimination of antigenic stimuli in the mucosa [48]. PN is commonly used during the acute inflammatory phase in patients who are experiencing malnutrition, such as undernourishment [49]. This undernourishment could be a factor that affects micronutrient deficiency [50,51]. The results from the systematic review show that the administration of PN significantly improved the levels of ESR [35,38], cholesterol [46], total phospholipids [46], and serum albumin [37,38,39,40,44,46], without producing clinical symptoms of hypoglycemia, independent of the method of interruption [43]. This improvement of the albumin is mirrored in the results of the meta-analysis. The meta-regression performed showed that the improvement could be greater a few days after the intervention.
The most common type of under-nourishment in patients with IBD was protein-energetic malnutrition, mainly shown with weight loss [52,53]. This malnutrition could worsen due to diverse surgical interventions that are necessary for emerging situations or when the medical treatment fails [54]. Therefore, the nutritional support should be carefully chosen during the treatment and before the surgery, based on a plan that is customized according to the patient [55]. Some authors declare that PN results in an increase in BMI, helping to correct the individual’s malnutrition [23,56]. We have not collected the BMI; however we have identified the BW, which are equivalent terms in adults, and the meta-analysis did not show the existence of a change in BW in patients with IBD when administering PN.
The CDAI, developed by Best et al. [57], measures the activity of the disease in patients with CD, with high values indicating a high activity of this pathology. Therefore, a reduction of this index indicates an improvement. The clinical trials conducted showed improvements in this index, but while these were significant in Jones [38], Greenberg et al. [39], Wright et al. [41], the results in Okenga [44] were not. The meta-analysis shows a significant reduction of the values found for the CDAI, and this decrease is accentuated days after the application of PN. These results are in agreement with diverse expert researchers, who declare that PN could provide, along with a possible administration of drugs such as infliximab, an improvement in this pathology [54,58,59].
Despite the accessibility to immunosuppressive drugs, antibiotic treatments, and fecal microbial transplantation, patients experience a high rate of relapse of malabsorption due to intestinal insufficiency [60]. In the case of individuals affected with CD, more than half are subjected to some surgery, such as bowel resection within 10 years after the diagnosis, and a third of them require a resection within the following five years [60]. This is the reason why PN could be fundamental with respect to the survival of the patient, as its management has drastically improved in the last 10 years, and the rate of related complications has notably decreased [60].
Likewise, the role of PN in postoperative complications is controversial. A recent meta-analysis has shown that the pre-surgery nutritional supplementation reduced posterior complications after the surgery in patients with CD, and more specifically, the TPN showed a tendency of being higher than the standard of care without nutritional support, but without statistical significance [61]. Hypoalbuminemia is associated with more postoperative complications, and it is sometimes a contraindication for surgery that requires anastomosis without a protective ileostomy [62]. In our qualitative synthesis with respect to the TPN, the results by Jacobson [46] concluded that it could be recommended for reducing the risk of suffering from postoperative complications until achieving clinical remission, and Yao et al. [45] declare that the perioperative PN may improve humoral immunity, reverse malnutrition and facilitate the rehabilitation of the patient. However, Fasth et al. [36] indicate that the administration of postoperative NPT does not result in a reduction of the complication rate after the surgery, although this difference could be due to the small sample utilized in this study. Our meta-analysis showed that the postoperative complications utilizing PN exist, although the proportion is low.
The term bowel rest has been frequently linked to the use of PN with active IBD or important complications such as the control of sepsis or imminent surgical procedures, and it is also theoretically attractive because of the expectation that it could improve bowel inflammation by alleviating mechanical trauma, bowel secretions, and antigenic challenge of the foods [35,63]. On the contrary, the results by Jones [38] and Dickinson et al. [33] show that there are no differences in patients with CD treated with either EN or TPN, and in patients with IBD treated solely with hydration or TPN. According to Abad-Lacruz et al. [40], and Wright et al. [41], Gonzalez-Huix et al. [42], and Greenberg et al. [39], EN results in significantly less frequent abnormalities in the LFT than TPN in patients with IBD, the PN with bowel rest does not show evidence of having a better impact on the remission than EN in patients with active CD, likewise, the EN is safer, cheaper and nutritionally effective in severe attacks as compared with TPN, and there were no differences in the remission and activity of patients with active CD.
All of this coincides with diverse studies and clinical practice guides, which indicate that bowel rest is not necessary when nutritional therapy is utilized for managing the patients [48,64,65]. Therefore, they should be allowed to eat “ad libitum” when medical therapy is prescribed and when different nutritional regimes exist through which clinical remission and repair of the mucosa can be achieved [15,48,64,65,66].
Also, it has recently been shown that there is a high load of underfeeding, orders of “nil per os” or a diet with clear liquids, which is unjustified for patients who are hospitalized with CU, especially for patients admitted without evidence of an objective flare of the disease that could be provoking iatrogenic malnutrition, so that bowel rest and the nutritional treatment should be given special attention [67].
Despite being the first systematic review that deals with the general effects of PN on adult patients with IBD, this article is not exempt from limitations. It is possible that the CONSORT questionnaire was not the best for evaluating the NRCCT, and UNRCT reviewed; however, this limitation has been tried to be avoided by adjusting the items of this tool to the type of study, as no questionnaire was found that evaluated the RCCT, the NRCCT and the UNRCT [26,68]. Also, most of the studies were somewhat old, with the most current one being from 2012, which could have reduced the score of this tool on the methodological quality due to the lack of standard criteria at the time the clinical trials were conducted. The UC and CD data have been combined to develop the meta-analysis for the variables BW, albumin, and postoperative complications due to the low number of studies that separated these diseases to elaborate their results. However, these clinical entities have different clinical courses.
The results derived from this work could help in clinical practice, to help the health professionals with the creation of a guide oriented towards evaluating the addition of TPN within the set of medical therapies for an adult patient diagnosed with IBD. However, as future lines of research, the use of TPN with the said patients should be addressed, having in mind their quality of life, the manner of administration, and the composition of the nutritional therapy in all the surgical procedures possible.
5. Conclusions
PN has shown to have efficacy for the treatment of IBD and is compatible with other medicines. The CDAI and albumin improve, although the effect of PN is greater after a while. However, the effect on the albumin could be less than the observed value in the meta-analysis, due to possible publication bias. The body weight does not change after intervention. Postoperative complications utilizing PN has been observed, although the proportion is low. More studies specifically referred to UC and CD are needed to develop more concrete clinical results.
Author Contributions
Conceptualization—J.M.C., A.G.-H., and C.A.; methodology—J.M.C., C.A. and P.C. formal analysis—J.M.C., I.C. and P.C.; investigation—J.M.C., A.G.-H., and I.C.; writing—original draft preparation—J.M.C. and P.C.; writing—review and editing—A.G.-H., I.C., J.T.; visualization—J.T.; supervision—J.T. and P.C.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54. [Google Scholar] [CrossRef]
- Desai, H.G.; Gupte, P.A. Increasing incidence of Crohn’s disease in India: Is it related to improved sanitation? Indian J. Gastroenterol. 2005, 24, 23–24. [Google Scholar]
- Zheng, J.J.; Zhu, X.S.; Zhao, H.; Gao, Z.X.; Guo, Z.R.; Wang, Z. Crohn’s disease in mainland China: A systematic analysis of 50 years of research. Chin. J. Dig. Dis. 2005, 6, 175–181. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Chapman-Kiddell, C.A.; Davies, P.S.W.; Gillen, L.; Radford-Smith, G.L. Role of diet in the development of inflammatory bowel disease. Inflamm. Bowel Dis. 2010, 16, 137–151. [Google Scholar] [CrossRef]
- MacDonald, T.T.; Hutchings, P.; Choy, M.Y.; Murch, S.; Cooke, A. Tumour necrosis factor-alpha and interferon-gamma production measured at the single cell level in normal and inflamed human intestine. Clin. Exp. Immunol. 1990, 81, 301–305. [Google Scholar] [CrossRef]
- Eom, T.; Kim, Y.S.; Choi, C.H.; Sadowsky, M.J.; Unno, T. Current understanding of microbiota- and dietary-therapies for treating inflammatory bowel disease. J. Microbiol. 2018, 56, 189–198. [Google Scholar] [CrossRef]
- Forbes, A.; Escher, J.; Hébuterne, X.; Kłęk, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN guideline: Clinical nutrition in inflammatory bowel disease. Clin. Nutr. 2017, 36, 321–347. [Google Scholar] [CrossRef]
- Lowe, A.-M.; Roy, P.-O.; B.-Poulin, M.; Michel, P.; Bitton, A.; St-Onge, L.; Brassard, P. Epidemiology of Crohn’s Disease in QuéBec, Canada. Inflamm. Bowel Dis. 2009, 15, 429–435. [Google Scholar] [CrossRef]
- Loftus, C.G.; Loftus, E.V.; Harmsen, W.S.; Zinsmeister, A.R.; Tremaine, W.J.; Melton, L.J.; Sandborn, W.J. Update on the incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota, 1940–2000. Inflamm. Bowel Dis. 2007, 13, 254–261. [Google Scholar] [CrossRef]
- Abraham, C.; Medzhitov, R. Interactions Between the Host Innate Immune System and Microbes in Inflammatory Bowel Disease. Gastroenterology 2011, 140, 1729–1737. [Google Scholar] [CrossRef]
- Reddavide, R.; Rotolo, O.; Caruso, M.G.; Stasi, E.; Notarnicola, M.; Miraglia, C.; Nouvenne, A.; Meschi, T.; De’ Angelis, G.L.; Di Mario, F.; et al. The role of diet in the prevention and treatment of inflammatory bowel diseases. Acta Biomed. 2018, 89, 60–75. [Google Scholar]
- Ruemmele, F.M. Role of Diet in Inflammatory Bowel Disease. Ann. Nutr. Metab. 2016, 68, 33–41. [Google Scholar] [CrossRef]
- Aleksandrova, K.; Romero-Mosquera, B.; Hernandez, V. Diet, Gut Microbiome and Epigenetics: Emerging Links with Inflammatory Bowel Diseases and Prospects for Management and Prevention. Nutrients 2017, 9, 962. [Google Scholar] [CrossRef]
- Wedlake, L.; Slack, N.; Andreyev, H.J.N.; Whelan, K. Fiber in the treatment and maintenance of inflammatory bowel disease: A systematic review of randomized controlled trials. Inflamm. Bowel Dis. 2014, 20, 576–586. [Google Scholar] [CrossRef]
- Mirtallo, J.M. Parenteral nutrition: Can outcomes be improved? J. Parenter. Enter. Nutr. 2013, 37, 181–189. [Google Scholar] [CrossRef]
- Sen, A.; Ren, J.; Ruffin, M.T.; Turgeon, D.K.; Brenner, D.E.; Sidahmed, E.; Rapai, M.E.; Cornellier, M.L.; Djuric, Z. Relationships between serum and colon concentrations of carotenoids and fatty acids in randomized dietary intervention trial. Cancer Prev. Res. 2013, 6, 558–565. [Google Scholar] [CrossRef]
- Boullata, J.I.; Gilbert, K.; Sacks, G.; Labossiere, R.J.; Crill, C.; Goday, P.; Kumpf, V.J.; Mattox, T.W.; Plogsted, S.; Holcombe, B.; et al. ASPEN Clinical guidelines: Parenteral nutrition ordering, order review, compounding, labeling, and dispensing. J. Parenter. Enter. Nutr. 2014, 38, 334–377. [Google Scholar] [CrossRef]
- Staun, M.; Pironi, L.; Bozzetti, F.; Baxter, J.; Forbes, A.; Joly, F.; Jeppesen, P.; Moreno, J.; Hébuterne, X.; Pertkiewicz, M.; et al. ESPEN Guidelines on Parenteral Nutrition: Home Parenteral Nutrition (HPN) in adult patients. Clin. Nutr. 2009, 28, 467–479. [Google Scholar] [CrossRef]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.; et al. ESPEN guideline: Clinical nutrition in surgery. Clin. Nutr. 2017, 36, 623–650. [Google Scholar] [CrossRef]
- Russell, M.K.; Wischmeyer, P.E. Supplemental Parenteral Nutrition: Review of the Literature and Current Nutrition Guidelines. Nutr. Clin. Pract. 2018, 33, 359–369. [Google Scholar] [CrossRef]
- Semrad, C.E. Use of parenteral nutrition in patients with inflammatory bowel disease. Gastroenterol. Hepatol. 2012, 8, 393–395. [Google Scholar]
- Triantafillidis, J.K.; Papalois, A.E. The role of total parenteral nutrition in inflammatory bowel disease: Current aspects. Scand. J. Gastroenterol. 2014, 49, 3–14. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, T.P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Cobos-Carbó, A.; Augustovski, F. Declaración CONSORT 2010: Actualización de la lista de comprobación para informar ensayos clínicos aleatorizados de grupos paralelos. Med. Clin. 2011, 137, 213–215. [Google Scholar] [CrossRef]
- Cañuelo, D.C.; Sanz-Valero, J.; Wanden-Berghe, C. Consecuencias de la nutrición parenteral domiciliaria en adultos con síndrome de intestino corto: Revisión exploratoria. Hosp. Domic. 2019, 3, 149–162. [Google Scholar] [CrossRef]
- Baujat, B.; Mahé, C.; Pignon, J.P.; Hill, C. A graphical method for exploring heterogeneity in meta-analyses: Application to a meta-analysis of 65 trials. Stat. Med. 2002, 21, 2641–2652. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. A Nonparametric “Trim and Fill” Method of Accounting for Publication Bias in Meta-Analysis. J. Am. Stat. Assoc. 2000, 95, 89–98. [Google Scholar]
- Copas, J.B.; Shi, J.Q. A sensitivity analysis for publication bias in systematic reviews. Stat. Methods Med. Res. 2001, 10, 251–265. [Google Scholar] [CrossRef]
- Schwarzer, G. meta: An R package for meta-analysis. R News 2007, 7, 40–45. [Google Scholar]
- Metasens: Advanced Statistical Methods to Model and Adjust for Bias in Meta-Analysis. Available online: https://CRAN.R-project.org/package=metasens (accessed on 21 November 2019).
- Goode, A.; Hawkines, T.; Feggetter, J.G.; Johnston, I.D. Use of an elemental diet for long-term nutritional support in Crohn’s disease. Lancet 1976, 1, 122–124. [Google Scholar] [CrossRef]
- Dickinson, R.J.; Ashton, M.G.; Axon, A.T.; Smith, R.C.; Yeung, C.K.; Hill, G.L. Controlled trial of intravenous hyperalimentation and total bowel rest as an adjunct to the routine therapy of acute colitis. Gastroenterology 1980, 79, 1199–1204. [Google Scholar] [CrossRef]
- Elson, C.O.; Layden, T.J.; Nemchausky, B.A.; Rosenberg, J.L.; Rosenberg, I.H. An evaluation of total parenteral nutrition in the management of inflammatory bowel disease. Dig. Dis. Sci. 1980, 25, 42–48. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, P.B.; Powell-Tuck, J.; Wood, S.R.; Lennard-Jones, J.E.; Lerebours, E.; Hecketsweiler, P.; Galmiche, J.P.; Colin, R. Controlled trial of bowel rest in the treatment of severe acute colitis. Gut 1986, 27, 481–485. [Google Scholar] [CrossRef]
- Fasth, S.; Hultén, L.; Magnusson, O.; Nordgren, S.; Warnold, I. Postoperative complications in colorectal surgery in relation to preoperative clinical and nutritional state and postoperative nutritional treatment. Int. J. Colorectal Dis. 1987, 2, 87–92. [Google Scholar] [CrossRef]
- Fasth, S.; Hultén, L.; Magnusson, O.; Nordgren, S.; Warnold, I. The immediate and long-term effects of postoperative total parenteral nutrition on body composition. Int. J. Colorectal Dis. 1987, 2, 139–145. [Google Scholar] [CrossRef]
- Jones, V.A. Comparison of total parenteral nutrition and elemental diet in induction of remission of Crohn’s disease. Dig. Dis. Sci. 1987, 32, S100–S107. [Google Scholar] [CrossRef]
- Greenberg, G.R.; Fleming, C.R.; Jeejeebhoy, K.N.; Rosenberg, I.H.; Sales, D.; Tremaine, W.J. Controlled trial of bowel rest and nutritional support in the management of Crohn’s disease. Gut 1988, 29, 1309–1315. [Google Scholar] [CrossRef]
- Abad-Lacruz, A.; González-Huix, F.; Esteve, M.; Fernández-Bañares, F.; Cabré, E.; Boix, J.; Acero, D.; Humbert, P.; Gassull, M.A. Liver function tests abnormalities in patients with inflammatory bowel disease receiving artificial nutrition: A prospective randomized study of total enteral nutrition vs total parenteral nutrition. J. Parenter. Enter. Nutr. 1990, 14, 618–621. [Google Scholar] [CrossRef]
- Wright, R.A.; Adler, E.C. Peripheral parenteral nutrition is no better than enteral nutrition in acute exacerbation of Crohn’s disease: A prospective trial. J. Clin. Gastroenterol. 1990, 12, 396–399. [Google Scholar] [CrossRef]
- González-Huix, F.; Fernández-Bañares, F.; Esteve-Comas, M.; Abad-Lacruz, A.; Cabré, E.; Acero, D.; Figa, M.; Guilera, M.; Humbert, P.; de León, R.; et al. Enteral versus parenteral nutrition as adjunct therapy in acute ulcerative colitis. Am. J. Gastroenterol. 1993, 88, 227–232. [Google Scholar]
- Eisenberg, P.G.; Gianino, S.; Clutter, W.E.; Fleshman, J.W. Abrupt discontinuation of cycled parenteral nutrition is safe. Dis. Colon Rectum 1995, 38, 933–939. [Google Scholar] [CrossRef]
- Ockenga, J.; Borchert, K.; Stüber, E.; Lochs, H.; Manns, M.P.; Bischoff, S.C. Glutamine-enriched total parenteral nutrition in patients with inflammatory bowel disease. Eur. J. Clin. Nutr. 2005, 59, 1302–1309. [Google Scholar] [CrossRef]
- Yao, G.X.; Wang, X.R.; Jiang, Z.M.; Zhang, S.Y.; Ni, A.P. Role of perioperative parenteral nutrition in severely malnourished patients with Crohn’s disease. World J. Gastroenterol. 2005, 11, 5732–5734. [Google Scholar] [CrossRef]
- Jacobson, S. Early postoperative complications in patients with Crohn’s disease given and not given preoperative total parenteral nutrition. Scand. J. Gastroenterol. 2012, 47, 170–177. [Google Scholar] [CrossRef]
- Limketkai, B.N.; Wolf, A.; Parian, A.M. Nutritional Interventions in the Patient with Inflammatory Bowel Disease. Gastroenterol. Clin. 2018, 47, 155–177. [Google Scholar] [CrossRef]
- Durchschein, F.; Petritsch, W.; Hammer, H.F. Diet therapy for inflammatory bowel diseases: The established and the new. World J. Gastroenterol. 2016, 22, 2179–2194. [Google Scholar] [CrossRef]
- Wędrychowicz, A.; Zając, A.; Tomasik, P. Advances in nutritional therapy in inflammatory bowel diseases: Review. World J. Gastroenterol. 2016, 22, 1045–1066. [Google Scholar] [CrossRef]
- Poursadegh, F.; Ahadi, M.; Vosoughinia, H.; Salehi, M.; Namdar, A.B.; Farzanehfar, M.R.; Memar, B.; Ziaolhagh, R. A STROBE compliant observational study on trace elements in patients with ulcerative colitis and their relationship with disease activity. Medicine 2018. [Google Scholar] [CrossRef]
- Su, Q.; Li, X.; Mo, W.; Yang, Z. Low serum bilirubin, albumin, and uric acid levels in patients with Crohn’s disease. Medicine 2019, 98, e15664. [Google Scholar] [CrossRef]
- Guagnozzi, D.; González-Castillo, S.; Olveira, A.; Lucendo, A.J. Nutritional treatment in inflammatory bowel disease. An update. Rev. Esp. Enferm. Dig. 2012, 104, 479–488. [Google Scholar] [CrossRef]
- Hengstermann, S.; Valentini, L.; Schaper, L.; Buning, C.; Koernicke, T.; Maritschnegg, M.; Buhner, S.; Tillinger, W.; Regano, N.; Guglielmi, F.; et al. Altered status of antioxidant vitamins and fatty acids in patients with inactive inflammatory bowel disease. Clin. Nutr. 2008, 27, 571–578. [Google Scholar] [CrossRef]
- Schwartz, E. Perioperative Parenteral Nutrition in Adults with Inflammatory Bowel Disease: A Review of the Literature. Nutr. Clin. Pract. 2016, 31, 159–170. [Google Scholar] [CrossRef]
- Dreznik, Y.; Horesh, N.; Gutman, M.; Gravetz, A.; Amiel, I.; Jacobi, H.; Zmora, O.; Rosin, D. Preoperative Nutritional Optimization for Crohn’s Disease Patients Can Improve Surgical Outcome. Dig. Surg. 2018, 35, 442–447. [Google Scholar] [CrossRef]
- Turkot, M.; Sobocki, J. Results of home parenteral nutrition in patients with severe inflammatory bowel disease—An alternative for surgery of malnourished patients. Pol. Prz. Chir. 2017, 89, 23–27. [Google Scholar] [CrossRef]
- Best, W.R.; Becktel, J.M.; Singleton, J.W.; Kern, F.J. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology 1976, 70, 439–444. [Google Scholar] [CrossRef]
- Matsumoto, T.; Iida, M.; Kohgo, Y.; Imamura, A.; Kusugami, K.; Nakano, H.; Fujiyama, Y.; Matsui, T.; Hibi, T. Therapeutic efficacy of infliximab on active Crohn’s disease under nutritional therapy. Scand. J. Gastroenterol. 2005, 40, 1423–1430. [Google Scholar] [CrossRef]
- Furukawa, H.; Yamada, M.; Sakurai, T.; Takenaka, K.; Matsui, T.; Yao, T. Enteral nutrition and total parenteral nutrition in Crohn’s disease; factors influencing induction of remission. Nihon Shokakibyo Gakkai Zasshi 1997, 94, 813–825. [Google Scholar]
- Lauro, A.; D’Amico, F.; Gondolesi, G. The current therapeutic options for Crohn’s disease: From medical therapy to intestinal transplantation. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 1105–1117. [Google Scholar] [CrossRef]
- Brennan, G.T.; Ha, I.; Hogan, C.; Nguyen, E.; Jamal, M.M.; Bechtold, M.L.; Nguyen, D.L. Does preoperative enteral or parenteral nutrition reduce postoperative complications in Crohn’s disease patients: A meta-analysis. Eur. J. Gastroenterol. Hepatol. 2018, 30, 997–1002. [Google Scholar] [CrossRef]
- Nisar, P.J.; Appau, K.A.; Remzi, F.H.; Kiran, R.P.; Eng, F.; Glas, F. Preoperative Hypoalbuminemia Is Associated with Adverse Outcomes After Ileoanal Pouch Surgery. Inflamm. Bowel Dis. 2011, 18, 1034–1041. [Google Scholar] [CrossRef]
- Patil, S.A.; Cross, R.K. Medical versus surgical management of penetrating Crohn’s disease: The current situation and future perspectives situation and future perspectives. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 843–848. [Google Scholar] [CrossRef]
- Bitton, A.; Buie, D.; Enns, R.; Feagan, B.G.; Jones, J.L.; Marshall, J.K.; Whittaker, S.; Griffi, A.M. Treatment of Hospitalized Adult Patients With Severe Ulcerative Colitis: Toronto Consensus Statements. Am. J. Gastroenterol. 2012, 107, 179–194. [Google Scholar] [CrossRef]
- Wiese, D.M.; Rivera, R.; Seidner, D.L. Is There a Role for Bowel Rest in Nutrition Management of Crohn’s Disease? Nutr. Clin. Pract. 2008, 23, 309–317. [Google Scholar] [CrossRef]
- Storck, L.J.; Imoberdorf, R.; Ballmer, P.E. Nutrition in Gastrointestinal Disease: Liver, Pancreatic, and Inflammatory Bowel Disease. J. Clin. Med. 2019, 8, 1098. [Google Scholar] [CrossRef]
- Gallinger, Z.R.; Rumman, A.; Pivovarov, K.; Fortinsky, K.J.; Steinhart, A.H.; Weizman, A.V. Frequency and Variables Associated with Fasting Orders in Inpatients with Ulcerative Colitis: The Audit of Diet Orders—Ulcerative Colitis (ADORE-UC) Study. Inflamm. Bowel Dis. 2017, 23, 1790–1795. [Google Scholar] [CrossRef]
- Wanden-Berghe, C.; Sanz-Valero, J. Systematic reviews in nutrition: Standardized methodology. Br. J. Nutr. 2012, 107, S3–S7. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).