Vitamin D and Immune Function
Abstract
:1. Introduction
2. Vitamin D and Immune Function
2.1. Vitamin D Sources
Seasonality of Vitamin D Status
2.2. Vitamin D Metabolism
2.3. Definition of Vitamin D Status
2.4. Safety of Supplementation in Humans
| typical daily dose | indication and side effects | costs | |
|---|---|---|---|
| NATIVE vitamin D | |||
| unhydroxylated, inactive from of vitamin D3 cholecalciferol calciol | 400–4000 IU (max 10,000 IU) |
| inexpensive |
| unhydroxylated, inactive form of vitamin D2 ergocalciferol vitamin D2 | 400–4000 IU (max 10,000 IU) | inexpensive | |
| ACTIVE vitamin D | |||
| hydroxylated, active form of vitamin D 1,25(OH)2D calcitriol 1,25-dihydroxyvitamin D3, 1,25-dihydroxycholecalciferol analog: alfacalcidol | 0.25–1.0 μg |
| expensive |
| other active vitamin D analogs: paricalcitol, doxercalciferol (vitamin D2 analogs) falecalcitriol, maxacalcitol (vitamin D3 analogs) |
| very expensive | |
2.5. Vitamin D and the Innate Immune System
2.6. Vitamin D and the Adaptive Immune System
- direct, endocrine effects on T cells mediated via systemic calcitriol.
- direct, intracrine conversion of 25(OH)D to calcitriol by T cells.
- direct, paracrine effects of calcitriol on T cells following conversion of 25(OH)D to calcitriol by monocytes or dendritic cells.
- indirect effects on antigen presentation to T cells mediated via localized APC affected by calcitriol.
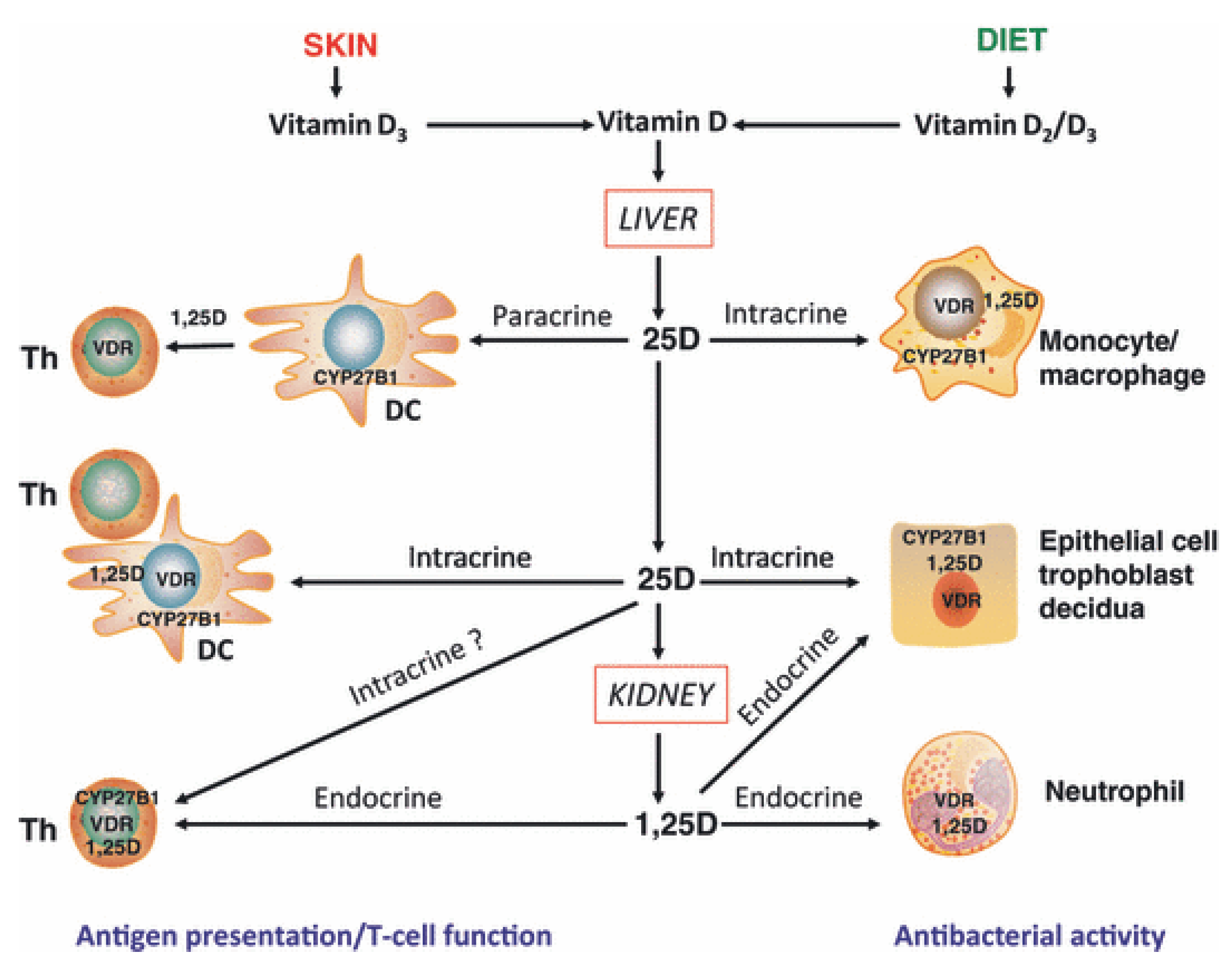
2.7. Vitamin D and Autoimmune Diseases
2.7.1. Type 1 Diabetes Mellitus
| author, year, country [reference] | sample size included (completed) | Subjects | Intervention (type, dose, duration) | Study results |
|---|---|---|---|---|
| Gabbay 2012, Brazil [93] | 38 (35) | new onset T1D (≤6 months) fasting C-peptide ≥ 0.6 ng/mL age 7–30 years | cholecalciferol (vitamin D3) oral, 2000 IU daily 18 months | =insulin needs, HbA1c slower decline of residual beta -cell function, protective immunologic effect including higher number of regulatory T-cells |
| Bizzarri 2010, Italy [111] | 34 (27) | new onset T1D < 12 weeks basal C-peptide 0.25 nmol/L age 11–35 years | calcitriol (1,25(OH)2D3) oral, 0.25 μg/day 2 years | =insulin needs =C-peptide levels =HbA1c |
| Walter 2010, Germany [110] | 40 (38) | new onset T1D < 2 months age 18–39 years | calcitriol (1,25(OH)2D3) oral, 0.25 μg/day 9 months | =insulin needs =C-peptide levels =HbA1c |
| Li 2009, China [113] | 35 (35) | LADA, diagnosis < 5 years age > 20 years | alphacalcidol 1α(OH)D3 oral, 0.25 μg 2×/day 1 year | slower decline of residual beta-cell function (diagnosis < 1 year) |
| Pitocco 2006, Italy [114] | 70 (67) | new onset T1D < 4 weeks age > 5 years | calcitriol (1,25(OH)2D3) oral, 0.25 μg on alternate days 1 year | ↓ insulin needs (at 3 and 6 months only) =C-peptide levels =HbA1c |
2.7.2. Multiple Sclerosis
3. Conclusion and Future Perspectives
Acknowledgments
Conflict of Interest
References
- Bouillon, R.; Carmeliet, G.; Verlinden, L.; van Etten, E.; Verstuyf, A.; Luderer, H.F.; Lieben, L.; Mathieu, C.; Demay, M. Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr. Rev. 2008, 29, 726–776. [Google Scholar] [CrossRef]
- Hewison, M. An update on vitamin D and human immunity. Clin. Endocrinol. 2012, 76, 315–325. [Google Scholar] [CrossRef]
- Weick, M.T. A history of rickets in the United States. Am. J. Clin. Nutr. 1967, 20, 1234–1241. [Google Scholar]
- Özkan, B. Nutritional rickets. J. Clin. Res. Pediatr. Endocrinol. 2010, 2, 137–143. [Google Scholar] [CrossRef]
- Walker, V.P.; Modlin, R.L. The vitamin D connection to pediatric infections and immune function. Pediatr. Res. 2009, 65, 106R–113R. [Google Scholar] [CrossRef]
- Mughal, M.Z. Rickets. Curr. Osteoporos. Rep. 2011, 9, 291–299. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Battault, S.; Whiting, S.J.; Peltier, S.L.; Sadrin, S.; Gerber, G.; Maixent, J.M. Vitamin D metabolism, functions and needs: From science to health claims. Eur. J. Nutr. 2013, 52, 429–441. [Google Scholar] [CrossRef]
- Hewison, M.; Gacad, M.A.; Lemire, J.; Adams, J.S. Vitamin D as a cytokine and hematopoetic factor. Rev. Endocr. Metab. Disord. 2001, 2, 217–227. [Google Scholar] [CrossRef]
- Adams, J.S.; Hewison, M. Update in vitamin D. J. Clin. Endocrinol. Metab. 2010, 95, 471–478. [Google Scholar] [CrossRef]
- Lamberg-Allardt, C. Vitamin D in foods and as supplements. Prog. Biophys. Mol. Biol. 2006, 92, 33–38. [Google Scholar] [CrossRef]
- Wacker, M.; Holick, M.F. Vitamin D—Effects on skeletal and extraskeletal health and the need for supplementation. Nutrients. 2013, 5, 111–148. [Google Scholar] [CrossRef]
- Calvo, M.S.; Whiting, S.J. Overview of the proceedings from Experimental Biology 2004 symposium: Vitamin D insufficiency: A significant risk factor in chronic diseases and potential disease-specific biomarkers of vitamin D sufficiency. J. Nutr. 2005, 135, 301–303. [Google Scholar]
- Tripkovic, L.; Lambert, H.; Hart, K.; Smith, C.P.; Bucca, G.; Penson, S.; Chope, G.; Hyppönen, E.; Berry, J.; Vieth, R.; et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 95, 1357–1364. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.J.; Zhang, F.; Richards, J.B.; Kestenbaum, B.; van Meurs, J.B.; Berry, D.; Kiel, D.P.; Streeten, E.A.; Ohlsson, C.; Koller, D.L.; et al. Common genetic determinants of vitamin D insufficiency: A genome-wide association study. Lancet 2010, 376, 180–188. [Google Scholar] [CrossRef]
- Maxwell, J.D. Seasonal variation in vitamin D. Proc. Nutr. Soc. 1994, 53, 533–543. [Google Scholar] [CrossRef]
- Van der Mei, I.A.; Ponsonby, A.L.; Engelsen, O.; Pasco, J.A.; McGrath, J.J.; Eyles, D.W.; Blizzard, L.; Dwyer, T.; Lucas, R.; Jones, G. The high prevalence of vitamin D insufficiency across Australian populations is only partly explained by season and latitude. Environ. Health Perspect. 2007, 115, 1132–1139. [Google Scholar] [CrossRef]
- Andersen, R.; Brot, C.; Jakobsen, J.; Mejborn, H.; Mølgaard, C.; Skovgaard, L.T.; Trolle, E.; Tetens, I.; Ovesen, L. Seasonal changes in vitamin D status among Danish adolescent girls and elderly women: The influence of sun exposure and vitamin D intake. Eur. J. Clin. Nutr. 2013, 67, 270–274. [Google Scholar] [CrossRef]
- Pittaway, J.K.; Ahuja, K.D.K.; Beckett, J.M.; Bird, M.-L.; Robertson, I.K.; Ball, M.J. Make vitamin D while the sun shines, take supplements when it doesn’t: A longitudinal, observational study of older adults in Tasmania, Australia. PLoS One 2013, 8, e59063. [Google Scholar]
- Danai, P.A.; Sinha, S.; Moss, M.; Haber, M.J.; Martin, G.S. Seasonal variation in the epidemiology of sepsis. Crit. Care Med. 2007, 35, 410–415. [Google Scholar] [CrossRef]
- White, A.N.J.; Ng, V.; Spain, C.V.; Johnson, C.C.; Kinlin, L.M.; Fisman, D.N. Let the sun shine in: Effects of ultraviolet radiation on invasive pneumococcal disease risk in Philadelphia, Pennsylvania. BMC Infect. Dis. 2009, 9, 196. [Google Scholar] [CrossRef]
- Heaney, R.P. Vitamin D-baseline status and effective dose. N. Engl. J. Med. 2012, 367, 77–78. [Google Scholar] [CrossRef]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the immune system. Curr. Opin. Pharmacol. 2010, 10, 482–496. [Google Scholar] [CrossRef]
- Heaney, R.P. Assessing vitamin D status. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 440–444. [Google Scholar] [CrossRef]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. JCEM 2011, 96, 53–58. [Google Scholar]
- Institute of Medicine, Dietary Reference Intakes for Calcium and Vitamin D; Institute of Medicine: Washington, DC, USA, 2010.
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, H.M.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. JCEM 2011, 96, 1911–1930. [Google Scholar]
- EFSA Panel on Dietetic Products Nutrition and Allergies Scientific Opinion on the Tolerable Upper Intake Level of vitamin D1. EFSA J. 2012, 10, 1–45.
- Vieth, R. Vitamin D toxicity, policy, and science. J. Bone Miner. Res. 2007, 22, 64–68. [Google Scholar] [CrossRef]
- Zittermann, A.; Prokop, S.; Gummert, J.F.; Borgermann, J. Safety issues of vitamin D supplementation. Anticancer Agents Med. Chem. 2013, 13, 4–10. [Google Scholar] [CrossRef]
- Vieth, R. The mechanisms of vitamin D toxicity. Bone Miner. 1990, 11, 267–272. [Google Scholar] [CrossRef]
- Vieth, R. Critique of the considerations for establishing the tolerable upper intake level for vitamin D: Critical need for revision upwards. J. Nutr. 2006, 136, 1117–1122. [Google Scholar]
- Jones, G. Pharmacokinetics of vitamin D toxicity. Am. J. Clin. Nutr. 2008, 88, 582–586. [Google Scholar]
- Lowe, H.; Cusano, N.E.; Binkley, N.; Blaner, W.S.; Bilezikian, J.P. Vitamin D toxicity due to a commonly available “over the counter” remedy from the Dominican Republic. JCEM 2011, 96, 291–295. [Google Scholar]
- KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. 2009, S1–S130. [CrossRef]
- Shoback, D. Clinical practice. Hypoparathyroidism. N. Engl. J. Med. 2008, 359, 391–403. [Google Scholar] [CrossRef]
- Bilezikian, J.P.; Khan, A.; Potts, J.T.; Brandi, M.L.; Clarke, B.L.; Shoback, D.; Jüppner, H.; D’Amour, P.; Fox, J.; Rejnmark, L.; et al. Hypoparathyroidism in the adult: Epidemiology, diagnosis, pathophysiology, target-organ involvement, treatment, and challenges for future reseach. J. Bone Miner. Res. 2011, 26, 2317–2337. [Google Scholar] [CrossRef]
- Mantovani, G. Clinical review: Pseudohypoparathyroidism: Diagnosis and treatment. JCEM 2011, 96, 3020–3030. [Google Scholar]
- Grad, R. Cod and the consumptive: A brief history of cod-liver oil in the treatment of pulmonary tuberculosis. Pharm. Hist. 2004, 46, 106–120. [Google Scholar]
- Wang, T.; Nestel, F.P.; Bourdeau, V.; Nagai, Y.; Wang, Q.; Liao, J.; Lin, R.; Hanrahan, J.H.; White, J.H. Cutting edge: 1,25-Dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 2004, 173, 2909–2912. [Google Scholar]
- Gombart, A.F.; Borregaard, N.; Koeffler, H.P. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005, 19, 1067–1077. [Google Scholar] [CrossRef]
- White, J.H. Vitamin D metabolism and signaling in the immune system. Rev. Endocr. Metab. Disord. 2012, 13, 21–29. [Google Scholar] [CrossRef]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef]
- Edfeldt, K.; Liu, P.T.; Chun, R.; Fabri, M.; Schenk, M.; Wheelwright, M.; Keegan, C.; Krutzik, S.R.; Adams, J.S.; Hewison, M.; et al. T-cell cytokines differentially control human monocyte antimicrobial responses by regulating vitamin D metabolism. Proc. Natl. Acad. Sci. USA 2010, 107, 22593–22598. [Google Scholar] [CrossRef]
- Ramanathan, B.; Davis, E.G.; Ross, C.R.; Blecha, F. Cathelicidins: Microbicidal activity, mechanisms of action, and roles in innate immunity. Microbes Infect. 2002, 4, 361–372. [Google Scholar] [CrossRef]
- Sørensen, O.; Cowland, J.B.; Askaa, J.; Borregaard, N. An ELISA for hCAP-18, the cathelicidin present in human neutrophils and plasma. J. Immunol. Methods 1997, 206, 53–59. [Google Scholar] [CrossRef]
- Jeng, L.; Yamshchikov, A.V; Judd, S.E.; Blumberg, H.M.; Martin, G.S.; Ziegler, T.R.; Tangpricha, V. Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. J. Transl. Med. 2009, 7, 28. [Google Scholar] [CrossRef]
- Gombart, A.F.; Bhan, I.; Borregaard, N.; Tamez, H.; Camargo, C.A.; Koeffler, H.P.; Thadhani, R. Low plasma level of cathelicidin antimicrobial peptide (hCAP18) predicts increased infectious disease mortality in patients undergoing hemodialysis. Clin. Infect. Dis. 2009, 48, 418–424. [Google Scholar] [CrossRef]
- Laaksi, I.; Ruohola, J.-P.; Tuohimaa, P.; Auvinen, A.; Haataja, R.; Pihlajamäki, H.; Ylikomi, T. An association of serum vitamin D concentrations <40 nmol/L with acute respiratory tract infection in young Finnish men. Am. J. Clin. Nutr. 2007, 86, 714–717. [Google Scholar]
- Ginde, A.A.; Mansbach, J.M.; Camargo, C.A. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch. Intern. Med. 2009, 169, 384–390. [Google Scholar] [CrossRef]
- Cannell, J.J.; Vieth, R.; Willett, W.; Zasloff, M.; Hathcock, J.N.; White, J.H.; Tanumihardjo, S.A.; Larson-Meyer, D.E.; Bischoff-Ferrari, H.A.; Lamberg-Allardt, C.J.; et al. Cod liver oil, vitamin A toxicity, frequent respiratory infections, and the vitamin D deficiency epidemic. Ann. Otol. Rhinol. Laryngol. 2008, 117, 864–870. [Google Scholar]
- Cannell, J.J.; Vieth, R.; Umhau, J.C.; Holick, M.F.; Grant, W.B.; Madronich, S.; Garland, C.F.; Giovannucci, E. Epidemic influenza and vitamin D. Epidemiol. Infect. 2006, 134, 1129–1140. [Google Scholar] [CrossRef]
- Black, P.N.; Scragg, R. Relationship between serum 25-hydroxyvitamin d and pulmonary function in the third national health and nutrition examination survey. Chest 2005, 128, 3792–3798. [Google Scholar]
- Janssens, W.; Bouillon, R.; Claes, B.; Carremans, C.; Lehouck, A.; Buysschaert, I.; Coolen, J.; Mathieu, C.; Decramer, M.; Lambrechts, D. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax 2010, 65, 215–220. [Google Scholar] [CrossRef]
- Litonjua, A.A.; Weiss, S.T. Is vitamin D deficiency to blame for the asthma epidemic? J. Allergy Clin. Immunol. 2007, 120, 1031–1035. [Google Scholar] [CrossRef]
- Dimeloe, S.; Nanzer, A.; Ryanna, K.; Hawrylowicz, C. Regulatory T cells, inflammation and the allergic response: The role of glucocorticoids and vitamin D. J. Steroid Biochem. Mol. Biol. 2010, 120, 86–95. [Google Scholar] [CrossRef]
- Murdoch, D.R.; Slow, S.; Chambers, S.T.; Jennings, L.C.; Stewart, A.W.; Priest, P.C.; Florkowski, C.M.; Livesey, J.H.; Camargo, C.A.; Scragg, R. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: The VIDARIS randomized controlled trial. JAMA 2012, 308, 1333–1339. [Google Scholar] [CrossRef]
- Camargo, C.A.; Ganmaa, D.; Frazier, A.L.; Kirchberg, F.F.; Stuart, J.J.; Kleinman, K.; Sumberzul, N.; Rich-Edwards, J.W. Randomized trial of vitamin D supplementation and risk of acute respiratory infection in Mongolia. Pediatrics 2012, 130, e561–e567. [Google Scholar] [CrossRef]
- Bergman, P.; Norlin, A.-C.; Hansen, S.; Rekha, R.S.; Agerberth, B.; Björkhem-Bergman, L.; Ekström, L.; Lindh, J.D.; Andersson, J. Vitamin D3 supplementation in patients with frequent respiratory tract infections: A randomised and double-blind intervention study. BMJ Open 2012, 2. [Google Scholar] [CrossRef]
- Rigby, W.F.; Waugh, M.G. Decreased accessory cell function and costimulatory activity by 1,25-dihydroxyvitamin D3-treated monocytes. Arthritis Rheum. 1992, 35, 110–119. [Google Scholar] [CrossRef]
- Steinman, R.M.; Banchereau, J. Taking dendritic cells into medicine. Nature 2007, 449, 419–426. [Google Scholar] [CrossRef]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef]
- Mora, J.R.; Iwata, M.; von Andrian, U.H. Vitamin effects on the immune system: Vitamins A and D take centre stage. Nat. Rev. Immunol. 2008, 8, 685–698. [Google Scholar] [CrossRef]
- Penna, G.; Amuchastegui, S.; Giarratana, N.; Daniel, K.C.; Vulcano, M.; Sozzani, S.; Adorini, L. 1,25-Dihydroxyvitamin D3 selectively modulates tolerogenic properties in myeloid but not plasmacytoid dendritic cells. J. Immunol. 2007, 178, 145–153. [Google Scholar]
- Veldman, C.M.; Cantorna, M.T.; DeLuca, H.F. Expression of 1,25-dihydroxyvitamin D3 receptor in the immune system. Arch. Biochem. Biophys. 2000, 374, 334–338. [Google Scholar]
- Ferreira, G.B.; van Etten, E.; Verstuyf, A.; Waer, M.; Overbergh, L.; Gysemans, C.; Mathieu, C. 1,25-Dihydroxyvitamin D3 alters murine dendritic cell behaviour in vitro and in vivo. Diabetes Metab. Res. Rev. 2011, 27, 933–941. [Google Scholar] [CrossRef]
- Müller, K.; Diamant, M.; Bendtzen, K. Inhibition of production and function of interleukin-6 by 1,25-dihydroxyvitamin D3. Immunol. Lett. 1991, 28, 115–120. [Google Scholar] [CrossRef]
- Adorini, L.; Penna, G.; Giarratana, N.; Uskokovic, M. Tolerogenic dendritic cells induced by vitamin D receptor ligands enhance regulatory T cells inhibiting allograft rejection and autoimmune diseases. J. Cell. Biochem. 2003, 88, 227–233. [Google Scholar] [CrossRef]
- Griffin, M.D.; Lutz, W.; Phan, V.A.; Bachman, L.A.; McKean, D.J.; Kumar, R. Dendritic cell modulation by 1α,25 dihydroxyvitamin D3 and its analogs: A vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2001, 98, 6800–6805. [Google Scholar]
- Panda, D.K.; Miao, D.; Tremblay, M.L.; Sirois, J.; Farookhi, R.; Hendy, G.N.; Goltzman, D. Targeted ablation of the 25-hydroxyvitamin D 1a-hydroxylase enzyme: Evidence for skeletal, reproductive , and immune dysfunction. Proc. Natl. Acad. Sci. USA 2001, 98, 7498–7503. [Google Scholar]
- Enioutina, E.Y.; Bareyan, D.; Raymond, A. TLR-induced local metabolism of vitamin D3 plays an important role in the diversification of adaptive immune responses. J. Immunol. 2009, 182, 4296–4305. [Google Scholar] [CrossRef]
- Coussens, A.K.; Wilkinson, R.J.; Hanifa, Y.; Nikolayevskyy, V.; Elkington, P.T.; Islam, K.; Timms, P.M.; Venton, T.R.; Bothamley, G.H.; Packe, G.E.; et al. Vitamin D accelerates resolution of inflammatory responses during tuberculosis treatment. Proc. Natl. Acad. Sci. USA 2012, 109, 15449–15454. [Google Scholar] [CrossRef] [Green Version]
- Provvedini, D.M.; Tsoukas, C.D.; Deftos, L.J.; Manolagas, S.C. 1,25-Dihydroxyvitamin D3 receptors in human leukocytes. Science 1983, 221, 1181–1183. [Google Scholar]
- Lemire, J.M.; Adams, J.S.; Sakai, R.; Jordan, S.C. 1a,25-dihydroxyvitamin D3 suppresses proliferation and immunoglobulin production by normal human peripheral blood mononuclear cells. J. Clin. Investig. 1984, 74, 657–661. [Google Scholar] [CrossRef]
- Chen, S.; Sims, G.P.; Chen, X.X.; Gu, Y.Y.; Chen, S.; Lipsky, P.E. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J. Immunol. 2007, 179, 1634–1647. [Google Scholar]
- Mahon, B.D.; Wittke, A.; Weaver, V.; Cantorna, M.T. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J. Cell. Biochem. 2003, 89, 922–932. [Google Scholar] [CrossRef]
- Lemire, J.M.; Adams, J.S.; Kermani-Arab, V.; Bakke, A.C.; Sakai, R.; Jordan, S.C. 1,25-Dihydroxyvitamin D3 suppresses human T helper/inducer lymphocyte activity in vitro. J. Immunol. 1985, 134, 3032–3035. [Google Scholar]
- Cantorna, M.T. Mechanisms underlying the effect of vitamin D on the immune system. Proc. Nutr. Soc. 2011, 69, 286–289. [Google Scholar] [CrossRef]
- Baeke, F.; Korf, H.; Overbergh, L.; Verstuyf, A.; Thorrez, L.; van Lommel, L.; Waer, M.; Schuit, F.; Gysemans, C.; Mathieu, C. The vitamin D analog, TX527, promotes a human CD4+CD25highCD127low regulatory T cell profile and induces a migratory signature specific for homing to sites of inflammation. J. Immunol. 2011, 186, 132–142. [Google Scholar] [CrossRef]
- Lemire, J.M.; Archer, D.C.; Beck, L.; Spiegelberg, H.L. Immunosuppressive actions of 1,25-dihydroxyvitamin D3: Preferential inhibition of Th1 functions. J. Nutr. 1995, 125, 1704S–1708S. [Google Scholar]
- Van Belle, T.L.; Gysemans, C.; Mathieu, C. Vitamin D in autoimmune, infectious and allergic diseases: A vital player? Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 617–632. [Google Scholar] [CrossRef]
- Palmer, M.T.; Lee, Y.K.; Maynard, C.L.; Oliver, J.R.; Bikle, D.D.; Jetten, A.M.; Weaver, C.T. Lineage-specific effects of 1,25-dihydroxyvitamin D3 on the development of effector CD4 T cells. J. Biol. Chem. 2011, 286, 997–1004. [Google Scholar]
- Giulietti, A.; Gysemans, C.; Stoffels, K.; van Etten, E.; Decallonne, B.; Overbergh, L.; Bouillon, R.; Mathieu, C. Vitamin D deficiency in early life accelerates Type 1 diabetes in non-obese diabetic mice. Diabetologia 2004, 47, 451–462. [Google Scholar] [CrossRef]
- Boonstra, A.; Barrat, F.J.; Crain, C.; Heath, V.L.; Savelkoul, H.F.J.; Garra, A.O. 1α,25-dihydroxyvitamin D3 has a direct effect on naive CD4+T cells to enhance the development of Th2 cells. J. Immunol. 2001, 167, 4974–4980. [Google Scholar]
- Penna, G.; Amuchastegui, S.; Cossetti, C.; Aquilano, F.; Mariani, R.; Sanvito, F.; Doglioni, C.; Adorini, L. Diabetic mice by the vitamin D receptor agonist elocalcitol 1. J. Immunol. 2006, 177, 8504–8511. [Google Scholar]
- Joshi, S.; Pantalena, L.; Liu, X.K.; Sarah, L.; Liu, H.; Rohowsky-kochan, C.; Yoshimura, A.; Steinman, L.; Gaffen, S.L.; Ichiyama, K.; et al. 1,25-Dihydroxyvitamin D3 ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A. Mol. Cell. Biol. 2011, 31, 3653–3669. [Google Scholar] [CrossRef]
- Jeffery, L.E.; Burke, F.; Mura, M.; Zheng, Y.; Qureshi, O.S.; Hewison, M.; Walker, L.S.K.; Lammas, D.A.; Raza, K.; Sansom, D.M. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J. Immunol. 2009, 183, 5458–5467. [Google Scholar] [CrossRef]
- Rudensky, A.Y. Regulatory T cells and foxP3. Immunol. Rev. 2011, 241, 260–268. [Google Scholar] [CrossRef]
- Barrat, F.J.; Cua, D.J.; Boonstra, A.; Richards, D.F.; Crain, C.; Savelkoul, H.F.; de Waal-malefyt, R.; Coffman, R.L.; Hawrylowicz, C.M.; Garra, A.O. In vitro generation of interleukin 10-producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1 )- and Th2-inducing cytokines. J. Exp. Med. 2002, 195, 603–616. [Google Scholar] [CrossRef]
- Ardalan, M.R.; Maljaei, H.; Shoja, M.M.; Piri, A.R.; Khosroshahi, H.T.; Noshad, H.; Argani, H. Calcitriol started in the donor, expands the population of CD4+ CD25+ T cells in renal transplant recipients. Transplant. Proc. 2007, 39, 951–953. [Google Scholar] [CrossRef]
- Prietl, B.; Pilz, S.; Wolf, M.; Tomaschitz, A.; Obermayer-Pietsch, B.; Graninger, W.; Pieber, T.R. Vitamin D supplementation and regulatory T cells in apparently healthy subjects: Vitamin D treatment for autoimmune diseases? Isr. Med. Assoc. J. 2010, 12, 136–139. [Google Scholar]
- Bock, G.; Prietl, B.; Mader, J.K.; Höller, E.; Wolf, M.; Pilz, S.; Graninger, W.B.; Obermayer-Pietsch, B.M. The effect of vitamin D supplementation on peripheral regulatory T cells and β cell function in healthy humans: A randomized controlled trial. Diabetes Metab. Res. Rev. 2011, 25, 942–945. [Google Scholar]
- Gabbay, M.A.L.; Sato, M.N.; Finazzo, C.; Duarte, A.J.S.; Dib, S.A. Effect of cholecalciferol as adjunctive therapy with insulin on protective immunologic profile and decline of residual β-cell function in new-onset type 1 diabetes mellitus. Arch. Pediatr. Adolesc. Med. 2012, 166, 601–607. [Google Scholar] [CrossRef]
- Zittermann, A.; Tenderich, G.; Koerfer, R. Vitamin D and the adaptive immune system with special emphasis to allergic reactions and allograft rejection. Inflamm. Allergy Drug Targets 2009, 8, 161–168. [Google Scholar] [CrossRef]
- Becker, K.G. The common genetic hypothesis of autoimmune/inflammatory disease. Curr. Opin. Allergy. Clin. Immunol. 2001, 1, 399–405. [Google Scholar]
- Moroni, L.; Bianchi, I.; Lleo, A. Geoepidemiology, gender and autoimmune disease. Autoimmun. Rev. 2012, 11, A386–A392. [Google Scholar] [CrossRef]
- Zittermann, A. Vitamin D in preventive medicine: Are we ignoring the evidence? Br. J. Nutr. 2003, 89, 552–572. [Google Scholar] [CrossRef]
- Cantorna, M.T.; Zhu, Y.; Froicu, M.; Wittke, A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am. J. Clin. Nutr. 2004, 80, 1717–1720. [Google Scholar]
- Bock, G.; Pieber, T.R.; Prietl, B. Vitamin D: Role in autoimmunity. CAB Rev. 2012, 7, 1–7. [Google Scholar]
- Antico, A.; Tampoia, M.; Tozzoli, R.; Bizzaro, N. Can supplementation with vitamin D reduce the risk or modify the course of autoimmune diseases? A systematic review of the literature. Autoimmun. Rev. 2012, 12, 127–136. [Google Scholar] [CrossRef]
- Hyppönen, E.; Läärä, E.; Reunanen, A.; Järvelin, M.R.; Virtanen, S.M. Intake of vitamin D and risk of type 1 diabetes: A birth-cohort study. Lancet 2001, 358, 1500–1503. [Google Scholar] [CrossRef]
- Hyppönen, E. Vitamin D and increasing incidence of type 1 diabetes-evidence for an association? Diabetes Obes. Metab. 2010, 12, 737–743. [Google Scholar] [CrossRef]
- Littorin, B.; Blom, P.; Schölin, A.; Arnqvist, H.J. Lower levels of plasma 25-hydroxyvitamin D among young adults at diagnosis of autoimmune type 1 diabetes compared with control subjects: Results from the nationwide Diabetes Incidence Study in Sweden (DISS). Diabetologia 2006, 49, 2847–2852. [Google Scholar] [CrossRef]
- Takiishi, T.; Gysemans, C.; Bouillon, R.; Mathieu, C. Vitamin D and diabetes. Endocrinol. Metab. Clin. North. Am. 2010, 39, 419–446. [Google Scholar] [CrossRef]
- Zipitis, C.S.; Akobeng, A.K. Vitamin D supplementation in early childhood and risk of type 1 diabetes: A systematic review and meta-analysis. Arch. Dis. Child. 2008, 93, 512–517. [Google Scholar] [CrossRef]
- Vitamin D supplement in early childhood and risk for Type I (insulin-dependent) diabetes mellitus. The EURODIAB Substudy 2 Study Group. Diabetologia 1999, 42, 51–54. [CrossRef]
- Gregori, S.; Giarratana, N.; Smiroldo, S.; Uskokovic, M.; Adorini, L. A 1α,25-dihydroxyvitamin D3 analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes 2002, 51, 1367–1374. [Google Scholar] [CrossRef]
- Mathieu, C.; Waer, M.; Laureys, J.; Rutgeerts, O.; Bouillon, R. Prevention of autoimmune diabetes in NOD mice by 1,25 dihydroxyvitamin D3. Diabetologia 1994, 37, 552–558. [Google Scholar] [CrossRef]
- Driver, J.P.; Foreman, O.; Mathieu, C.; van Etten, E.; Serreze, D.V. Comparative therapeutic effects of orally administered 1,25-dihydroxyvitamin D3 and 1α-hydroxyvitamin D3 on type-1 diabetes in non-obese diabetic mice fed a normal-calcaemic diet. Clin. Exp. Immunol. 2008, 151, 76–85. [Google Scholar]
- Walter, M.; Kaupper, T.; Adler, K.; Foersch, J.; Bonifacio, E.; Ziegler, A.G. No effect of the 1α,25-dihydroxyvitamin D3 on beta-cell residual function and insulin requirement in adults with new-onset type 1 diabetes. Diabetes Care 2010, 33, 1443–1448. [Google Scholar] [CrossRef]
- Bizzarri, C.; Pitocco, D.; Napoli, N.; di Stasio, E.; Maggi, D.; Manfrini, S.; Suraci, C.; Cavallo, M.G.; Cappa, M.; Ghirlanda, G.; et al. No protective effect of calcitriol on beta-cell function in recent-onset type 1 diabetes: The IMDIAB XIII trial. Diabetes Care 2010, 33, 1962–1963. [Google Scholar] [CrossRef]
- Bailey, R.; Cooper, J.D.; Zeitels, L.; Smyth, D.J.; Yang, J.H.M.; Walker, N.M.; Hyppönen, E.; Dunger, D.B.; Ramos-Lopez, E.; Badenhoop, K.; et al. Association of the vitamin D metabolism gene CYP27B1 with type 1 diabetes. Diabetes 2007, 56, 2616–2621. [Google Scholar] [CrossRef]
- Li, X.; Liao, L.; Yan, X.; Huang, G.; Lin, J.; Lei, M.; Wang, X.; Zhou, Z. Protective effects of 1-alpha-hydroxyvitamin D3 on residual beta-cell function in patients with adult-onset latent autoimmune diabetes (LADA). Diabetes Metab. Res. Rev. 2009, 25, 411–416. [Google Scholar] [CrossRef]
- Pitocco, D.; Crinò, A.; di Stasio, E.; Manfrini, S.; Guglielmi, C.; Spera, S.; Anguissola, G.B.; Visalli, N.; Suraci, C.; Matteoli, M.C.; et al. The effects of calcitriol and nicotinamide on residual pancreatic beta-cell function in patients with recent-onset Type 1 diabetes (IMDIAB XI). Diabet. Med. 2006, 23, 920–923. [Google Scholar] [CrossRef]
- Munger, K.L.; Levin, L.I.; Hollis, B.W.; Howard, N.S.; Ascherio, A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 2006, 296, 2832–2838. [Google Scholar] [CrossRef]
- Kragt, J.; van Amerongen, B.; Killestein, J.; Dijkstra, C.; Uitdehaag, B.; Polman, C.; Lips, P. Higher levels of 25-hydroxyvitamin D are associated with a lower incidence of multiple sclerosis only in women. Mult. Scler. 2009, 15, 9–15. [Google Scholar] [CrossRef]
- Munger, K.L.; Zhang, S.M.; O’Reilly, E.; Hernán, M.A.; Olek, M.J.; Willett, W.C.; Ascherio, A. Vitamin D intake and incidence of multiple sclerosis. Neurology 2004, 62, 60–65. [Google Scholar] [CrossRef]
- Soilu-Hänninen, M.; Aivo, J.; Lindström, B.-M.; Elovaara, I.; Sumelahti, M.-L.; Färkkilä, M.; Tienari, P.; Atula, S.; Sarasoja, T.; Herrala, L.; et al. A randomised, double blind, placebo controlled trial with vitamin D3 as an add on treatment to interferon β-1b in patients with multiple sclerosis. J. Neurol. Neurosurg. Psychiatr. 2012, 83, 565–571. [Google Scholar] [CrossRef]
- Stein, M.S.; Liu, Y.; Gray, O.M.; Baker, J.E.; Kolbe, S.C.; Ditchfield, M.R.; Egan, G.F.; Mitchell, P.J.; Harrison, L.C.; Butzkueven, H.; et al. A randomized trial of high-dose vitamin D2 in relapsing-remitting multiple sclerosis. Neurology 2011, 77, 1611–1618. [Google Scholar] [CrossRef]
- Kampman, M.T.; Steffensen, L.H.; Mellgren, S.I.; Jørgensen, L. Effect of vitamin D3 supplementation on relapses, disease progression, and measures of function in persons with multiple sclerosis: Exploratory outcomes from a double-blind randomised controlled trial. Mult. Scler. 2012, 18, 1144–1151. [Google Scholar] [CrossRef]
- Chang, J.-H.; Cha, H.-R.; Lee, D.-S.; Seo, K.Y.; Kweon, M.-N. 1,25-Dihydroxyvitamin D3 inhibits the differentiation and migration of T(H)17 cells to protect against experimental autoimmune encephalomyelitis. PLoS One 2010, 5, e12925. [Google Scholar]
- Smolders, J.; Thewissen, M.; Peelen, E.; Menheere, P.; Cohen, J.W.; Tervaert, J.W.C.; Damoiseaux, J.; Hupperts, R. Vitamin D status is positively correlated with regulatory T cell function in patients with multiple sclerosis. PLoS One 2009, 4, e6635. [Google Scholar] [CrossRef]
- Royal, W.; Mia, Y.; Li, H.; Naunton, K. Peripheral blood regulatory T cell measurements correlate with serum vitamin D levels in patients with multiple sclerosis. J. Neuroimmunol. 2009, 213, 135–141. [Google Scholar] [CrossRef]
- Smolders, J.; Schuurman, K.G.; van Strien, M.E.; Melief, J.; Hendrickx, D.; Hol, E.M.; van Eden, C.; Luchetti, S.; Huitinga, I. Expression of vitamin D receptor and metabolizing enzymes in multiple sclerosis-affected brain tissue. J. Neuropathol. Exp. Neurol. 2013, 72, 91–105. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Prietl, B.; Treiber, G.; Pieber, T.R.; Amrein, K. Vitamin D and Immune Function. Nutrients 2013, 5, 2502-2521. https://doi.org/10.3390/nu5072502
Prietl B, Treiber G, Pieber TR, Amrein K. Vitamin D and Immune Function. Nutrients. 2013; 5(7):2502-2521. https://doi.org/10.3390/nu5072502
Chicago/Turabian StylePrietl, Barbara, Gerlies Treiber, Thomas R. Pieber, and Karin Amrein. 2013. "Vitamin D and Immune Function" Nutrients 5, no. 7: 2502-2521. https://doi.org/10.3390/nu5072502




