Maternal Fructose Intake Affects Transcriptome Changes and Programmed Hypertension in Offspring in Later Life
Abstract
:1. Introduction
2. Metabolic Effects of Fructose on Renal Biology and Hypertension
3. Effect of Maternal Fructose Consumption on Programmed Hypertension
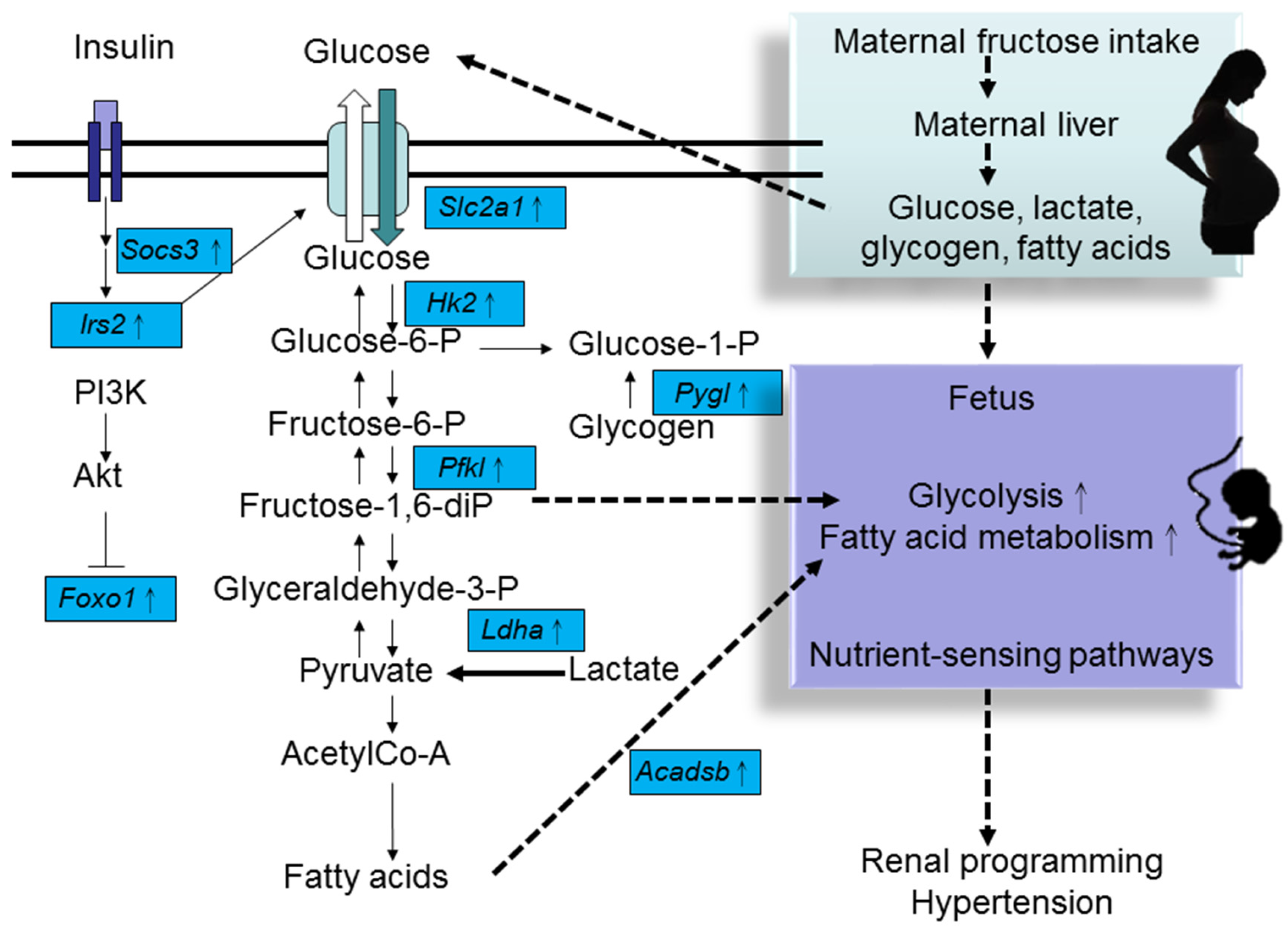
4. HF Consumption Induces Renal Transcriptome Changes
5. Reprogramming Strategy to Prevent Maternal HF Consumption-Induced Programmed Hypertension
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Johnson, R.J.; Segal, M.S.; Sautin, Y.; Nakagawa, T.; Feig, D.I.; Kang, D.H.; Gersch, M.S.; Benner, S.; Sánchez-Lozada, L.G. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am. J. Clin. Nutr. 2007, 86, 899–906. [Google Scholar] [PubMed]
- Marriott, B.P.; Cole, N.; Lee, E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J. Nutr. 2009, 139, 1228S–1235S. [Google Scholar] [CrossRef] [PubMed]
- Egan, B.M.; Zhao, Y.; Axon, R.N. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA 2010, 303, 2043–2050. [Google Scholar] [CrossRef] [PubMed]
- Haugen, A.C.; Schug, T.T.; Collman, G.; Heindel, J.J. Evolution of DOHaD: The impact of environmental health sciences. J. Dev. Orig. Health Dis. 2015, 6, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Bagby, S.P. Maternal nutrition, low nephron number, and hypertension in later life: Pathways of nutritional programming. J. Nutr. 2007, 137, 1066–1072. [Google Scholar] [PubMed]
- Kett, M.M.; Denton, K.M. Renal programming: Cause for concern? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R791–R803. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Joles, J.A. Reprogramming: A preventive strategy in hypertension focusing on the kidney. Int. J. Mol. Sci. 2015, 17, E23. [Google Scholar] [CrossRef] [PubMed]
- Tappy, L.; Lê, K.A. Metabolic effects of fructose and the worldwide increase in obesity. Physiol. Rev. 2010, 90, 23–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Caballero, B.; Mitchell, D.C.; Loria, C.; Lin, P.H.; Champagne, C.M.; Elmer, P.J.; Ard, J.D.; Batch, B.C.; Anderson, C.A.; et al. Reducing consumption of sugar-sweetened beverages is associated with reduced blood pressure: A prospective study among United States adults. Circulation 2010, 121, 2398–2406. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.J.; Stamler, J.; van Horn, L.; Robertson, C.E.; Chan, Q.; Dyer, A.R.; Huang, C.C.; Rodriguez, B.L.; Zhao, L.; Daviglus, M.L.; et al. Sugar-sweetened beverage, sugar intake of individuals, and their blood pressure: International study of macro/micronutrients and blood pressure. Hypertension 2011, 57, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.M.; Dulloo, A.G.; Yepuri, G.; Montani, J.P. Fructose ingestion acutely elevates blood pressure in healthy young humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R730–R737. [Google Scholar] [CrossRef] [PubMed]
- Perez-Pozo, S.E.; Schold, J.; Nakagawa, T.; Sánchez-Lozada, L.G.; Johnson, R.J.; Lillo, J.L. Excessive fructose intake induces the features of metabolic syndrome in healthy adult men: Role of uric acid in the hypertensive response. Int. J. Obes. 2010, 34, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Le, M.T.; Frye, R.F.; Rivard, C.J.; Cheng, J.; McFann, K.K.; Segal, M.S.; Johnson, R.J.; Johnson, J.A. Effects of high-fructose corn syrup and sucrose on the pharmacokinetics of fructose and acute metabolic and hemodynamic responses in healthy subjects. Metabolism 2012, 61, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Sanchez-Lozada, L.G.; Nakagawa, T. The effect of fructose on renal biology and disease. J. Am. Soc. Nephrol. 2010, 21, 2036–2039. [Google Scholar] [CrossRef] [PubMed]
- Karalius, V.P.; Shoham, D.A. Dietary sugar and artificial sweetener intake and chronic kidney disease: A review. Adv. Chronic Kidney Dis. 2013, 20, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.T.; Yuen, V.G.; McNeill, J.H. The fructose-fed rat: A review on the mechanisms of fructose-induced insulin resistance and hypertension. Mol. Cell Biochem. 2009, 332, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Kelishadi, R.; Mansourian, M.; Heidari-Beni, M. Association of fructose consumption and components of metabolic syndrome in human studies: A systematic review and meta-analysis. Nutrition 2014, 30, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Jayalath, V.H.; de Souza, R.J.; Ha, V.; Mirrahimi, A.; Blanco-Mejia, S.; Di Buono, M.; Jenkins, A.L.; Leiter, L.A.; Wolever, T.M.; Beyene, J.; et al. Sugar-sweetened beverage consumption and incident hypertension: A systematic review and meta-analysis of prospective cohorts. Am. J. Clin. Nutr. 2015, 102, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Toop, C.R.; Gentili, S. Fructose beverage consumption induces a metabolic syndrome phenotype in the rat: A systematic review and meta-analysis. Nutrients 2016, 8, E577. [Google Scholar] [CrossRef] [PubMed]
- Glushakova, O.; Kosugi, T.; Roncal, C.; Mu, W.; Heinig, M.; Cirillo, P.; Sánchez-Lozada, L.G.; Johnson, R.J.; Nakagawa, T. Fructose induces the inflammatory molecule ICAM-1 in endothelial cells. J. Am. Soc. Nephrol. 2008, 19, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Lozada, L.G.; Tapia, E.; Jiménez, A.; Bautista, P.; Cristóbal, M.; Nepomuceno, T.; Soto, V.; Avila-Casado, C.; Nakagawa, T.; Johnson, R.J.; et al. Fructose-induced metabolic syndrome is associated with glomerular hypertension and renal microvascular damage in rats. Am. J. Physiol. Ren. Physiol. 2007, 292, F423–F429. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.V.; Kiat, H. The mechanisms underlying fructose-induced hypertension: A review. J. Hypertens. 2015, 33, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Madero, M.; Perez-Pozo, S.E.; Jalal, D.; Johnson, R.J.; Sánchez-Lozada, L.G. Dietary fructose and hypertension. Curr. Hypertens. Rep. 2011, 13, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Regnault, T.R.; Gentili, S.; Sarr, O.; Toop, C.R.; Sloboda, D.M. Fructose, pregnancy and later life impacts. Clin. Exp. Pharmacol. Physiol. 2013, 40, 824–837. [Google Scholar] [CrossRef] [PubMed]
- Dornas, W.C.; de Lima, W.G.; Pedrosa, M.L.; Silva, M.E. Health implications of high-fructose intake and current research. Adv. Nutr. 2015, 6, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.; Gardiner, S.M.; Elmes, M.; Gardner, D.S. Excess maternal salt or fructose intake programmes sex-specific, stress- and fructose-sensitive hypertension in the offspring. Br. J. Nutr. 2016, 115, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Leu, S.; Wu, K.L.; Lee, W.C.; Chan, J.Y. Melatonin prevents maternal fructose intake-induced programmed hypertension in the offspring: Roles of nitric oxide and arachidonic acid metabolites. J. Pineal Res. 2014, 57, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Wu, K.L.; Lee, W.C.; Leu, S.; Chan, J.Y. Maternal fructose-intake-induced renal programming in adult male offspring. J. Nutr. Biochem. 2015, 26, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Lee, W.C.; Wu, K.L.; Leu, S.; Chan, J.Y. Targeting arachidonic acid pathway to prevent programmed hypertension in maternal fructose-fed male adult rat offspring. J. Nutr. Biochem. 2016, 38, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Wu, K.L.; Lee, W.C.; Leu, S.; Chan, J.Y.; Tain, Y.L. Aliskiren administration during early postnatal life sex-specifically alleviates hypertension programmed by maternal high fructose consumption. Front. Physiol. 2016, 7, 299. [Google Scholar] [CrossRef] [PubMed]
- Yamada-Obara, N.; Yamagishi, S.I.; Taguchi, K.; Kaida, Y.; Yokoro, M.; Nakayama, Y.; Ando, R.; Asanuma, K.; Matsui, T.; Ueda, S.; et al. Maternal exposure to high-fat and high-fructose diet evokes hypoadiponectinemia and kidney injury in rat offspring. Clin. Exp. Nephrol. 2016, in press. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Lee, W.C.; Leu, S.; Wu, K.; Chan, J. High salt exacerbates programmed hypertension in maternal fructose-fed male offspring. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.F.; Dickerson, J.; Kechichian, T.B.; Yin, H.; Gamble, P.; Salazar, A.; Patrikeev, I.; Motamedi, M.; Saade, G.R.; Costantine, M.M. High-fructose diet in pregnancy leads to fetal programming of hypertension, insulin resistance, and obesity in adult offspring. Am. J. Obstet. Gynecol. 2016, 215, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, N.G.; Kaplan, B.; Karvonen, M.J.; Lind, J.; Malm, M. Permeability of human placenta to glucose, fructose, and xylose. Acta Physiol. Scand. 1956, 36, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, D.D.; Roux, J.; Villee, C.A. Studies of the mechanism of fructose production by human placenta. J. Physiol. 1959, 146, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Norheim, F.; Gjelstad, I.M.; Hjorth, M.; Vinknes, K.J.; Langleite, T.M.; Holen, T.; Jensen, J.; Dalen, K.T.; Karlsen, A.S.; Kielland, A.; et al. Molecular nutrition research: The modern way of performing nutritional science. Nutrients 2012, 4, 1898–1944. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Ying, Z.; Noble, E.; Zhao, Y.; Agrawal, R.; Mikhail, A.; Zhuang, Y.; Tyagi, E.; Zhang, Q.; Lee, J.H.; et al. Systems nutrigenomics reveals brain gene networks linking metabolic and brain disorders. EBioMedicine 2016, 7, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.M.; Tain, Y.L.; Leu, S.; Wu, K.L.; Lee, W.C.; Chan, J.Y. Developmental programming of the metabolic syndrome: Next-generation sequencing analysis of transcriptome expression in a rat model of maternal high fructose intake. Sheng Li Xue Bao 2016, 68, 557–567. [Google Scholar] [PubMed]
- Efeyan, A.; Comb, W.C.; Sabatini, D.M. Nutrient-sensing mechanisms and pathways. Nature 2015, 517, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsu, C.N.; Chan, J.Y. PPARs link early life nutritional insults to later programmed hypertension and metabolic syndrome. Int. J. Mol. Sci. 2015, 17, E20. [Google Scholar] [CrossRef] [PubMed]
- Buffat, C.; Boubred, F.; Mondon, F.; Chelbi, S.T.; Feuerstein, J.M.; Lelièvre-Pégorier, M.; Vaiman, D.; Simeoni, U. Kidney gene expression analysis in a rat model of intrauterine growth restriction reveals massive alterations of coagulation genes. Endocrinology 2007, 148, 5549–5557. [Google Scholar] [CrossRef] [PubMed]
- Campbell, W.B.; Falck, J.R. Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors. Hypertension 2007, 49, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Huang, L.T.; Chan, J.Y.; Lee, C.T. Transcriptome analysis in rat kidneys: Importance of genes involved in programmed hypertension. Int. J. Mol. Sci. 2015, 16, 4744–4758. [Google Scholar] [CrossRef] [PubMed]
- Sloboda, D.M.; Li, M.; Patel, R.; Clayton, Z.E.; Yap, C.; Vickers, M.H. Early life exposure to fructose and offspring phenotype: Implications for long term metabolic homeostasis. J. Obes. 2014, 2014, 203474. [Google Scholar] [CrossRef] [PubMed]
- Paixão, A.D.; Alexander, B.T. How the kidney is impacted by the perinatal maternal environment to develop hypertension. Biol. Reprod. 2013, 89, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Huang, L.T.; Chan, J.Y. Transcriptional regulation of programmed hypertension by melatonin: An epigenetic perspective. Int. J. Mol. Sci. 2014, 15, 18484–18495. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.C.; Sheen, J.M.; Yu, H.R.; Lin, Y.J.; Chen, C.C.; Tiao, M.M.; Tsai, C.C.; Huang, L.T.; Tain, Y.L. Early postnatal treatment with soluble epoxide hydrolase inhibitor or 15-deoxy-Δ(12,14)-prostagandin J2 prevents prenatal dexamethasone and postnatal high saturated fat diet induced programmed hypertension in adult rat offspring. Prostaglandins Other Lipid Mediat. 2016, 124, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Koeners, M.P.; Wesseling, S.; Ulu, A.; Sepúlveda, R.L.; Morisseau, C.; Braam, B.; Hammock, B.D.; Joles, J.A. Soluble epoxide hydrolase in the generation and maintenance of high blood pressure in spontaneously hypertensive rats. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E691–E698. [Google Scholar] [CrossRef] [PubMed]
- Sherman, R.C.; Langley-Evans, S.C. Early administration of angiotensin-converting enzyme inhibitor captopril, prevents the development of hypertension programmed by intrauterine exposure to a maternal low-protein diet in the rat. Clin. Sci. 1998, 94, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Sherman, R.C.; Langley-Evans, S.C. Antihypertensive treatment in early postnatal life modulates prenatal dietary influences upon blood pressure in the rat. Clin. Sci. 2000, 98, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Lee, C.T.; Huang, L.T.; Tain, Y.L. Aliskiren in early postnatal life prevents hypertension and reduces asymmetric dimethylarginine in offspring exposed to maternal caloric restriction. J. Renin Angiotensin Aldosterone Syst. 2015, 16, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, K.; Ji, H. Sex differences in primary hypertension. Biol. Sex Differ. 2012, 3, 7. [Google Scholar] [CrossRef] [PubMed]
| Types of Fructose Intake | Strain | Programming Effects | Age at Which the Effects Were Measured | References |
|---|---|---|---|---|
| 10% w/v fructose plus 4% NaCl in drinking water 28 days before conception and throughout gestation and lactation | Male Sprague–Dawley rats | ↑ systolic BP, ↑ mean arterial BP | At 9 weeks of age | [26] |
| 60% HF diet throughout pregnancy and lactation | Male Sprague–Dawley rats | ↑ systolic BP, ↑ mean arterial BP | At 12 weeks of age | [27,28,29] |
| 60% HF diet throughout pregnancy and lactation | Male and female Sprague–Dawley rats | ↑ systolic BP | At 12 weeks of age | [30] |
| 60% HF diet throughout pregnancy and lactation plus 1% NaCl in drinking water from weaning to 3 months of age | Male Sprague–Dawley rats | ↑ systolic BP, ↑ mean arterial BP; postnatal high-salt aggravates prenatal HF-induced programmed hypertension | At 12 weeks of age | [31] |
| 56.7% HF/high-fat diet throughout pregnancy and lactation | Male Sprague–Dawley rats | ↑ mean arterial BP | At 16 weeks of age | [32] |
| 10% w/v fructose in drinking water throughout pregnancy and lactation | C57BL/6J mice | ↑ mean arterial BP, obesity, metabolic dysfunction | At 12 months of age | [33] |
| Gene ID | Symbol | Kidney | Brain | Heart |
|---|---|---|---|---|
| Fructose and mannose metabolism | ||||
| ENSRNOG00000001214 | Pfkl | 2.3 | 1.5 | 2.2 |
| ENSRNOG00000006116 | Hk2 | 1.8 | ND | 2.1 |
| ENSRNOG00000018911 | Pfkfb3 | 1.8 | ND | 4.5 |
| Adipocytokine signaling pathway | ||||
| ENSRNOG00000002946 | Socs3 | 1.6 | 0.5 | 3.9 |
| ENSRNOG00000007390 | Nfkbia | 1.9 | 1.9 | 3.5 |
| ENSRNOG00000004473 | Ppargc1a | 2.3 | 1.6 | 2.7 |
| ENSRNOG00000007284 | Slc2a1 | 3.0 | 2.3 | ND |
| ENSRNOG00000023509 | Irs2 | 2.1 | ND | 1.6 |
| Glycolysis/Gluconeogenesis | ||||
| ENSRNOG00000001214 | Pfkl | 2.3 | 1.5 | 2.2 |
| ENSRNOG00000006116 | Hk2 | 1.8 | ND | 2.1 |
| ENSRNOG00000013009 | Ldha | 2.2 | ND | 1.6 |
| Fatty acid metabolism | ||||
| ENSRNOG00000020624 | Acadsb | 1.9 | 2.0 | ND |
| Insulin signaling pathway | ||||
| ENSRNOG00000002946 | Socs3 | 1.6 | 0.5 | 3.9 |
| ENSRNOG00000004473 | Ppargc1a | 2.3 | 1.6 | 2.7 |
| ENSRNOG00000006388 | Pygl | 1.9 | ND | 3.2 |
| ENSRNOG00000006116 | Hk2 | 1.8 | ND | 2.1 |
| ENSRNOG00000023509 | Irs2 | 2.1 | ND | 1.6 |
| ENSRNOG00000003463 | Srebf1 | 2.1 | ND | 1.6 |
| ENSRNOG00000013397 | Foxo1 | 1.8 | ND | 2.2 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tain, Y.-L.; Chan, J.Y.H.; Hsu, C.-N. Maternal Fructose Intake Affects Transcriptome Changes and Programmed Hypertension in Offspring in Later Life. Nutrients 2016, 8, 757. https://doi.org/10.3390/nu8120757
Tain Y-L, Chan JYH, Hsu C-N. Maternal Fructose Intake Affects Transcriptome Changes and Programmed Hypertension in Offspring in Later Life. Nutrients. 2016; 8(12):757. https://doi.org/10.3390/nu8120757
Chicago/Turabian StyleTain, You-Lin, Julie Y. H. Chan, and Chien-Ning Hsu. 2016. "Maternal Fructose Intake Affects Transcriptome Changes and Programmed Hypertension in Offspring in Later Life" Nutrients 8, no. 12: 757. https://doi.org/10.3390/nu8120757
APA StyleTain, Y. -L., Chan, J. Y. H., & Hsu, C. -N. (2016). Maternal Fructose Intake Affects Transcriptome Changes and Programmed Hypertension in Offspring in Later Life. Nutrients, 8(12), 757. https://doi.org/10.3390/nu8120757







