1. Introduction
Non-alcoholic fatty liver disease (NAFLD) refers to a spectrum of diseases, ranging from asymptomatic steatosis, to non-alcoholic steatohepatitis (NASH), to cirrhosis [
1]. NAFLD is defined as the accumulation of lipids, mainly triacylglycerol, in hepatocytes of individuals who do not consume significant amounts of alcohol (≤2 drinks/day for women, ≤3 drinks/day for men) [
2] and in whom other known causes of steatosis, such as certain chronic liver disease (hepatitis A, B, and C, Wilson’s disease) or medications have been excluded. NAFLD is mainly associated with clinical features of metabolic syndrome mainly type 2 diabetes and dyslipidemia [
3]. NAFLD patients have more than one feature of the Metabolic Syndrome, and now they are considered the hepatic components of the MS. Several scientific advances in understanding the association between NAFLD and MS have identified insulin resistance (IR) as the key aspect in the pathophysiology of both diseases [
4].
Diet composition is an environmental factor that might influence NAFLD severity [
5]. Over-nutrition or inappropriate diet, such as high carbohydrate or excessive fat intake, are thought to lead to chronically-elevated glucose, insulin, and free fatty acid concentrations in the blood [
6]. Diets with a high glycemic index and glycemic load were found to be positively associated with insulin resistance [
7] and the risk of type 2 diabetes, breast cancer, and heart disease [
8]. Nutrients, such as monounsaturated fatty acids (MUFAs), are well known to decrease oxidized Low-density lipoprotein -cholesterol (HDL-C) and triacylglycerol concentrations without a decrease in High-density lipoprotein (HDL)-cholesterol [
9]. Alanine aminotransferase, triglyceride, serum tumor necrosis factor-alpha levels, as well as fatty liver and fibrosis stage, improved after omega-3, eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) supplementation [
10,
11]. Zinc, ascorbic acid (vitamin C), and α-tocopherol (vitamin E) protect from NAFLD by decreasing intestinal permeability and oxidative stress [
12,
13].
The consumption of fructose, due to the intake of carbonated-beverages, honey, molasses, candies, and baked food, is increasing significantly. Animal testing has shown fructose-enriched diets to induce the parameters of metabolic syndrome and an increase in the percentage of fat-enriched cells, as well as an increase in fibrosis and in inflammatory markers [
14,
15].
Studies on the dietary pattern of NAFLD patients are scarce. One prospective study reported a Western-like dietary pattern trend in this population [
16] and another one studied the effect of a Mediterranean diet intervention versus a control diet on intrahepatic lipids reduction [
17]. The use of dietary pattern in establishing an association between dietary intake and NAFLD is of importance. This will enable the investigation and measure of the overall diet, which is more plausible to be associated with NAFLD than each studied nutrient or single foods [
18].
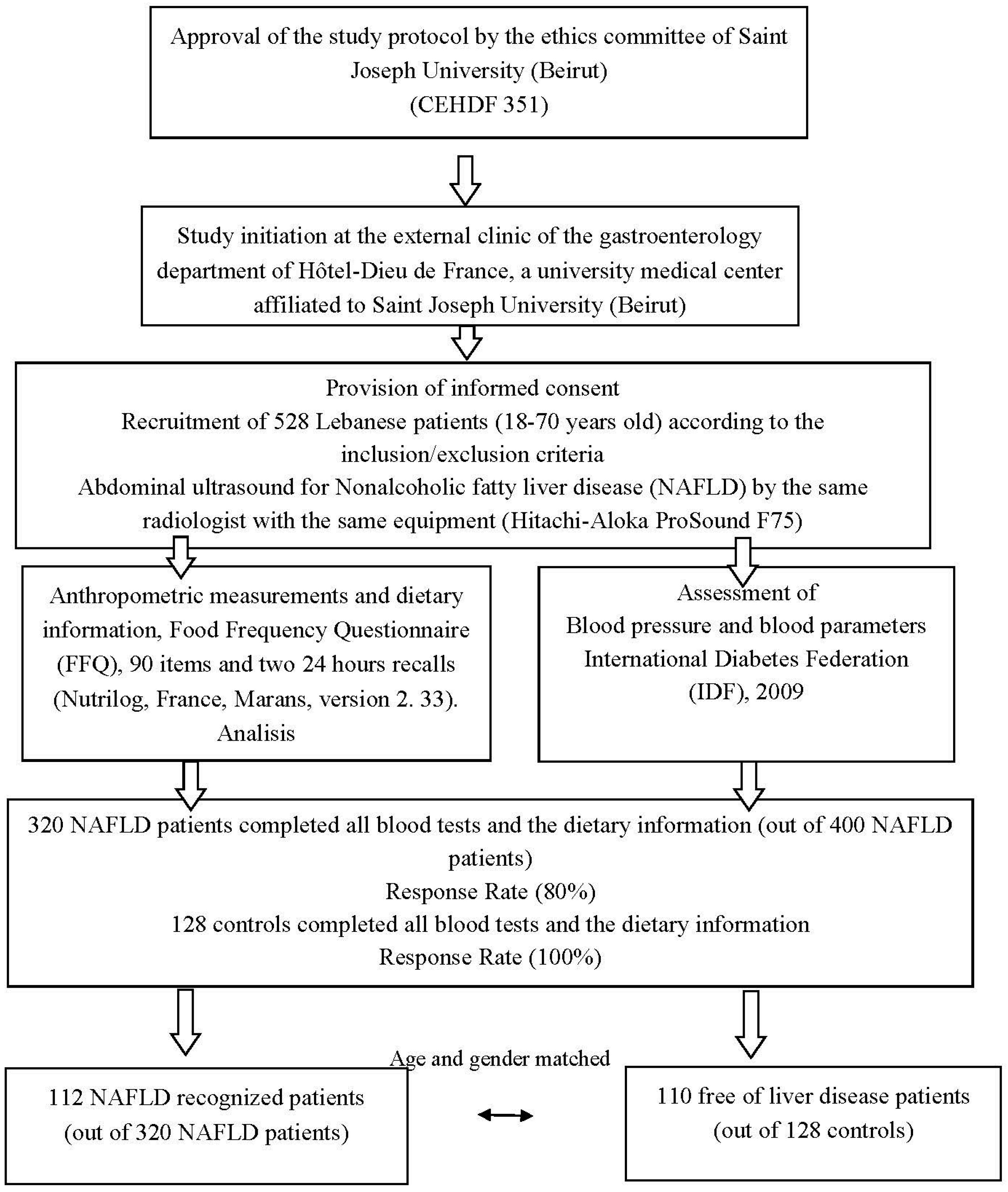
To our knowledge, no previous study was conducted in Lebanon to investigate the dietary patterns that predict NAFLD in Lebanese patients. The primary objective of the present study was to assess the nutritional profile of a sample of NAFLD patients (112 subjects) and compare it with controls (110 subjects). A secondary objective was to compare the dietary patterns between the two groups.
We hypothesized that there is a strong association of the high meat, fast food (Western-like dietary pattern) with NAFLD patients and an inverse association between traditional dietary pattern and the disease.
4. Discussion
This study confirmed the high prevalence of metabolic syndrome (62.5%) in cases with significant difference in its parameters as compared to controls (12.7%) (
Table 2). Marchesini et al., considered NAFLD as an additional feature of the metabolic syndrome [
37]. The latter is usually associated with central obesity, dyslipidemia, and type 2 diabetes [
38]. In our study, 55.3% of our cases were obese (BMI ≥ 30 kg/m
2) as compared to 8.2% for controls, (
p = 0.0001). The percentage of cases (males, females) having a waist circumference (cm) higher than the International Diabetes Federation (IDF) 2009 criteria was significantly higher in NAFLD cases in comparison with controls (
p = 0.02,
p = 0.001). These are typical findings for NAFLD patients, knowing that an increase in the waist circumference (cm) or in the waist/hip ratio indicates an accumulation of intra-abdominal adipose tissue responsible for the development of metabolic syndrome and liver steatosis [
38]. Furthermore, 18.8% of cases were associated with type 2 diabetes, 0.9% with cardiovascular diseases, and nearly 50.9% with hypertension (mmHg) (
Table 2). In the control group, 10% were hypertensive and none had CVD with almost an absence of type 2 diabetes. This is not a new finding knowing that type 2 diabetes and NAFLD are particularly closely related. The latter is associated with dysfunctional adipose tissue and associated with insulin resistance and pancreatic beta cell dysfunction [
39]. In addition, family history of cardiovascular disorders, diabetes, and hypertension was more prevalent in cases than in controls. Chehreh et al., reported positive family history for CVD, diabetes, dyslipidemia and hypertension among patients with NAFLD suggesting that the disease is multifactorial, involving either hereditary or environmental factors with possible interaction [
40].
Elevated HOMA-IR and serum triglycerides were characteristics of NAFLD patients (
Table 2). This may be explained by the increase in triglycerides and free fatty acids released from adipose tissues, mainly visceral ones. These interfere with insulin signaling by modulating insulin receptor substrate-2 (IRS-2) phosphorylation [
2]. Insulin resistance status is generally accompanied by impaired serum clearance of Very-low-density lipoprotein (VLDL) and intestinally-derived chylomicrons which result in elevated serum triglycerides [
41].
Regarding environmental factors, significant differences in physical activity and smoking were found between the two groups (
Table 2). Smoking is well known to be a source of oxidative stress, while exercise is known to reduce metabolic syndrome [
42]. A large-scale study by Church et al., (
n = 218 men) reported an inverse association between cardio respiratory fitness and the prevalence of NAFLD [
43]. Hannukainen et al. and Tamura et al. demonstrated that hepatic triglycerides accumulation decreases with exercise and increase delivery of glucose and insulin to the muscles [
44,
45].
Several studies reported an association between food patterns and metabolic diseases, such as T2D [
46] and obesity or cardiovascular diseases [
47]. Few reported on NAFLD [
16,
48]. According to food dietary patterns between the two groups, the results obtained were in line with other studies [
16,
48]. However, our study generated a new dietary pattern, the high fruit group. This confirmed the fact that fruits are an important part of the Lebanese diet and constituted a whole independent entity.
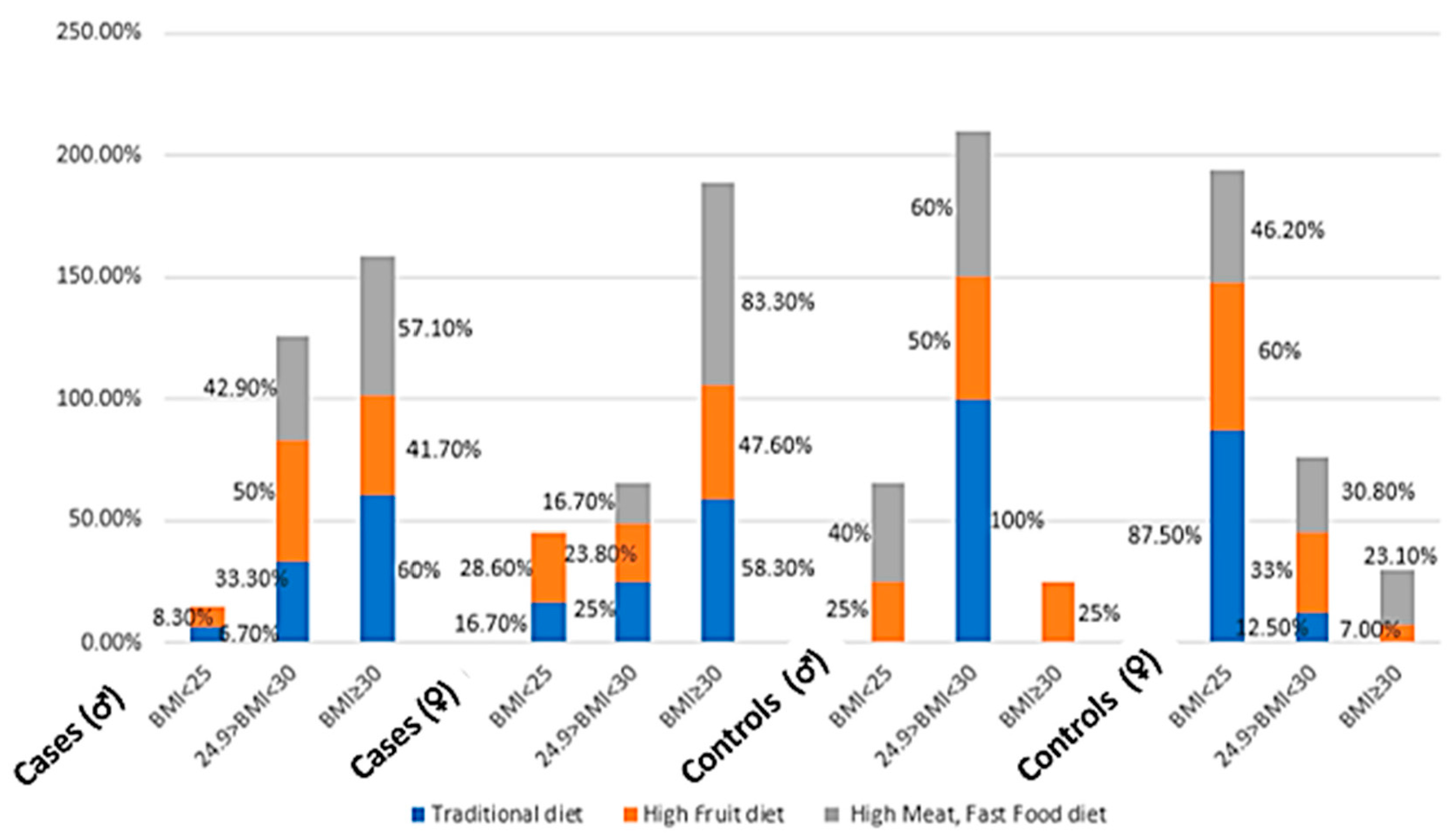
Twenty-eight percent of cases belonged to this group as compared to 12.1% of controls (
Figure 2). The high consumption of fruits was significantly different between cases and controls (women) after adjustment for energy intake/day, (
p < 0.05) (
Table 7). The fruits consumed were mainly plums, raisins, apples, and figs. These kinds of fruits are highly available on the Lebanese market for a relatively low cost, besides being a characteristic of the traditional diet. Other studies have found this high consumption of fruits, comparable to the results obtained, in other Mediterranean populations [
49,
50]. Fruits are well known to be rich in free fructose levels, as well as in simple carbohydrates. This result was confirmed by the significant correlation between fructose and sucrose intake/day and scores of the high fruit group pattern (
r = 0.307,
r = 0.252), respectively, (
p < 0.05). Molasses and honey are also rich in these monosaccharides and disaccharides. Honey is usually used as a replacement for sugar in most hot beverages, mainly in tea, while molasses is mainly consumed in desserts. Nevertheless, these two items showed small loading in the three components (less than 0.3). Carbonated beverages enriched in these monosaccharides and disaccharides loaded very low in the fruit group (<0.3), but loaded high in the fast food group (>0.3). The novelty in our study is that the high fruit group pattern increased the odds of NAFLD by four-fold,
p < 0.05, after adjustment with its covariables, and the mechanism behind this may be through the provision of large amounts of sugars, such as fructose [
51]. The consumption of fructose has been linked to NAFLD, as well as various aspects of the metabolic syndrome, including dyslipidemia, visceral adiposity, insulin resistance, and high blood pressure [
52].
Meat such as pork, chicken, beef meat and hotdog loaded high in the high meat, fast food group. Both groups consumed largely beef, chicken, or lamb meat (
Table 7) with almost the same percentage adhering to the high meat, fast food group. The significant difference in energy intake (kcal/day) and the frequency of red meat consumed between cases and controls could justify the fact that the control group is NAFLD-free. A study done by Zelber Sagi et al. [
20] showed that all types of meat were significantly associated with an increased risk for NAFLD. Other items, such as pizza, fries, cream, ketchup, and other condiments also loaded high in this diet group, confirmed by the significant correlation between sucrose intake/day and scores of this diet group. This kind of diet characterized by high intake of pasta, red meat, desserts, and pizzas triggered an increase in weight, a higher postprandial insulin secretion and, ultimately, an insulin resistance. This will increase liver fat storage via the de novo lipogenesis pathway [
16].
Food, such as fish, sardine, salmon, and tuna, loaded low (factor loading matrix < 0.2) in both the traditional and the high meat, fast food pattern groups. These items were rarely eaten among the Lebanese populations. The average consumption of fish and other seafood was well below the recommended servings/week (
Table 7). Although Lebanon is a coastal country, the Lebanese population avoids eating seafood for cultural, economic, and public health reasons. The general belief is that the coastline is polluted, and consumable fish are unavailable or very expensive. A study done by Nasreddine et al., reported this low consumption of seafood in Lebanese subjects with 73.6% of Lebanese adult participants consuming less than two servings of fish per week [
53].
Milk, cheese, labneh (a local soft creamy cheese), coffee, and tea also loaded weakly in the three groups. This can be explained by the fact that these items are equally consumed by participants, except for milk, which is considered as a flatulent item, and is consequently avoided by the general population. Vegetable oils and olives loaded equally in the first two components (traditional and high fruits groups). This reflects the main feature of the Lebanese traditional diet which is enriched in oil, mainly olive oil.
Our three groups’ results confirmed the statement that the Lebanese traditional dietary pattern is still present in a subgroup of our population and is highly representative of the Mediterranean diet, composed mainly of vegetables, seeds, olive oil, and legumes. However, the presence of the high meat, fast food group reveals a trend towards an animal-based westernized food pattern rich in proteins and refined cereals. The presence of the high fruit dietary pattern group is in line with the dietary culture trend and tradition of the people living around the Mediterranean basin.
Some limitations and bias were present in the study. The first limitation was the difficulty in matching BMI (kg/m
2) between controls and cases. For a period of two and a half years, only 8.2% of controls were classified as obese (BMI ≥ 30 kg/m
2). The second limitation was the ultrasound used to assess the presence or not of fatty liver. The limitation is its low sensitivity for mild steatosis (<33%) and to accurately quantify fatty infiltration [
54]. However, this technique still represents the first-line diagnostic tool for simple liver steatosis. It had been widely used in different studies, since it is a non-invasive method with a sensitivity of 60–94% and a specificity of 66–95% [
48,
55]. The third limitation stems from recall bias among patients. They may over-report physical activities, as well as under-report dietary intake, provide incorrect estimations of portion sizes, or have memory loss. To overcome these limitations, patients were interviewed and reported their dietary intake prior the disease diagnosis or prior to any diet change due to medical advice or drugs used. Another limitation came from the use of factor analysis which requires subjective decisions for grouping food for analysis or in choosing the method of rotation or for determining dietary patterns according to their loading factors.