An Examination of Diet for the Maintenance of Remission in Inflammatory Bowel Disease
Abstract
:1. Introduction
2. Literature Search
3. Diet in the Etiology of IBD
3.1. The Western Diet Pattern
3.2. Carbohydrate Intake as a Risk Factor for IBD
3.3. Protein Intake as a Risk Factor for IBD
3.4. Dairy Intake as Risk Factor for IBD
3.5. Dietary Fat Intake as a Risk Factor for IBD
4. Diet Interventions and IBD
4.1. Low Residue Diet
4.2. Semi-Vegetarian Diet
4.3. FODMAPs Diet
4.4. Exclusion Diets
4.5. Novel Anti-Inflammatory Diet Therapies
4.6. Fiber Supplements
4.6.1. Oat Bran
4.6.2. Wheat Bran
4.6.3. Psyllium
4.6.4. Germinated Barley Foodstuff
4.7. Role of Fat in the Diet
4.8. Popular Diet Plans with Patients
5. Pharmaconutrition
5.1. Curcumin (Turmeric)
5.2. Probiotics
5.3. Vitamin D
6. Challenges in Creating Evidence-Based Guidelines
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baumgart, D.C.; Sandborn, W.J. Inflammatory bowel disease: Clinical aspects and established and evolving therapies. Lancet 2007, 369, 1641–1657. [Google Scholar] [CrossRef]
- Bhasin, S.; Singh, H.; Targownik, L.E.; Israeli, E.; Bernstein, C.N. Rates and reasons for nonuse of prescription medication for inflammatory bowel disease in a referral clinic. Inflamm. Bowel Dis. 2016, 22, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Whitten, K.E.; Rogers, P.; Ooi, C.K.Y.; Day, A.S. International survey of enteral nutrition protocols used in children with Crohn’s disease. J. Dig. Dis. 2012, 13, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Day, A.S.; Whitten, K.E.; Sidler, M.; Lemberg, D.A. Systematic review: Nutritional therapy in paediatric Crohn’s disease. Aliment. Pharmacol. Ther. 2008, 27, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Wall, C.L.; Day, A.S.; Gearry, R.B. Use of exclusive enteral nutrition in adults with Crohn’s disease: A review. World J. Gastroenterol. 2013, 19, 7652–7660. [Google Scholar] [CrossRef] [PubMed]
- Vidarsdottir, J.B.; Johannsdottir, S.E.; Thorsdottir, I.; Bjornsson, E.; Ramel, A. A cross-sectional study on nutrient intake and -status in inflammatory bowel disease patients. Nutr. J. 2016, 15, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piodi, L.P.; Poloni, A.; Ulivieri, F.M. Managing osteoporosis in ulcerative colitis: Something new? World J. Gastroenterol. 2014, 20, 14087–14098. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Peng, Y. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Weisshof, R.; Chermesh, I. Micronutrient deficiencies in inflammatory bowel disease. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Filippi, J.; Al-Jaouni, R.; Wiroth, J.; Hébuterne, X.; Schneider, S.M. Nutritional deficiencies in patients with Crohn’s disease in remission. Inflamm. Bowel Dis. 2006, 12, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Ballegaard, M.; Bjergstrøm, A.; Brøndum, S.; Hylander, E.; Jensen, L.; Ladefoged, K. Self-reported food intolerance in chronic inflammatory bowel disease. Scand. J. Gastroenterol. 1997, 32, 569–571. [Google Scholar] [CrossRef] [PubMed]
- Kinsey, L.; Burden, S. A survey of people with inflammatory bowel disease to investigate their views of food and nutritional issues. Eur. J. Clin. Nutr. 2016, 70, 852–854. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.B.; Lee, D.; Long, M.D.; Kappelman, M.D.; Martin, C.F.; Sandler, R.S.; Lewis, J.D. Dietary patterns and self-reported associations of diet with symptoms of inflammatory bowel disease. Dig. Dis. Sci. 2013, 58, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Holt, D.Q.; Strauss, B.J.; Moore, G.T. Patients with inflammatory bowel disease and their treating clinicians have different views regarding diet. J. Hum. Nutr. Diet. 2016, 30, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Owczarek, D.; Rodacki, T.; Domagała-Rodacka, R.; Cibor, D.; Mach, T. Diet and nutritional factors in inflammatory bowel diseases. World J. Gastroenterol. 2016, 22, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Walton, M.; Alaunyte, I. Do patients living with ulcerative colitis adhere to healthy eating guidelines? A cross-sectional study. Br. J. Nutr. 2014, 112, 1628–1635. [Google Scholar] [CrossRef] [PubMed]
- Sousa Guerreiro, C.; Cravo, M.; Costa, A.R.; Miranda, A.; Tavares, L.; Moura-Santos, P.; Leitão, C.N. A comprehensive approach to evaluate nutritional status in Crohn’s patients in the era of biologic therapy: A case-control study. Am. J. Gastroenterol. 2007, 102, 2551–2556. [Google Scholar] [CrossRef] [PubMed]
- Grimshaw, J.M.; Eccles, M.P.; Lavis, J.N.; Hill, S.J.; Squires, J.E. Knowledge translation of research findings. Implement. Sci. 2012, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C.; Rampertab, S.D.; Mullin, G.E. Existing dietary guidelines for Crohn’s disease and ulcerative colitis. Expert Rev. Gastroenterol. Hepatol. 2011, 5, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Richman, E.; Rhodes, J.M. Review article: Evidence-based dietary advice for patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2013, 38, 1156–1171. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Allen, R.; Ashley, S.; Becker, S.; Cummins, P.; Gbadamosi, A.; Wilson, S. British Dietetic Association evidence-based guidelines for the dietary management of Crohn’s disease in adults. J. Hum. Nutr. Diet. 2014, 27, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.K.; Abraham, B.; El-Serag, H. Dietary intake and risk of developing inflammatory bowel disease: A systematic review of the literature. Am. J. Gastroenterol. 2011, 106, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Hebert, J.R.; Rashvand, S.; Rashidkhani, B.; Hekmatdoost, A. Inflammatory Potential of Diet and Risk of Ulcerative Colitis in a Case-Control Study from Iran. Nutr. Cancer 2016, 68, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Khalili, H.; Konijeti, G.G.; Higuchi, L.M.; De Silva, P.; Korzenik, J.R.; Chan, A.T. A prospective study of long-term intake of dietary fiber and risk of Crohn’s disease and ulcerative colitis. Gastroenterology 2013, 145, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Racine, A.; Carbonnel, F.; Chan, S.S.M.; Hart, A.R.; Bueno-de-Mesquita, H.B.; Oldenburg, B.; Key, T. Dietary patterns and risk of inflammatory bowel disease in Europe. Inflamm. Bowel Dis. 2016, 22, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Khalili, H.; Konijeti, G.G.; Higuchi, L.M.; de Silva, P.; Fuchs, C.S.; Chan, A.T. Long-term intake of dietary fat and risk of ulcerative colitis and Crohn’s disease. Gut 2014, 63, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Tjonneland, A.; Overvad, K.; Bergmann, M.M.; Nagel, G.; Linseisen, J.; Hallmans, G.; Palmqvist, R.; Sjodin, H.; Hagglund, G.; Berglund, G.; et al. Linoleic acid, a dietary n-6 polyunsaturated fatty acid, and the aetiology of ulcerative colitis: A nested case-control study within a European prospective cohort study. Gut 2009, 58, 1606–1611. [Google Scholar] [PubMed]
- Opstelten, J.L.; Leenders, M.; Dik, V.K.; Chan, S.S.M.; van Schaik, F.D.M.; Khaw, K.-T.; Grip, O. Dairy Products, Dietary Calcium, and Risk of Inflammatory Bowel Disease: Results From a European Prospective Cohort Investigation. Inflamm. Bowel Dis. 2016, 22, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Amre, D.K.; D’Souza, S.; Morgan, K.; Seidman, G.; Lambrette, P.; Grimard, G.; Chotard, V. Imbalances in dietary consumption of fatty acids, vegetables, and fruits are associated with risk for crohn’s disease in children. Am. J. Gastroenterol. 2007, 102, 2016–2025. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, N.; Kono, S.; Wakai, K.; Fukuda, Y.; Satomi, M.; Shimoyama, T.; Kobashi, G. Dietary risk factors for inflammatory bowel disease: A multicenter case-control study in Japan. Inflamm. Bowel Dis. 2005, 11, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Jantchou, P.; Morois, S.; Clavel-Chapelon, F.; Boutron-Ruault, M.-C.; Carbonnel, F. Animal protein intake and risk of inflammatory bowel disease: The E3N prospective study. Am. J. Gastroenterol. 2010, 105, 2195–2201. [Google Scholar] [CrossRef] [PubMed]
- Gearry, R.B.; Irving, P.M.; Barrett, J.S.; Nathan, D.M.; Shepherd, S.J.; Gibson, P.R. Reduction of dietary poorly absorbed short-chain carbohydrates (FODMAPs) improves abdominal symptoms in patients with inflammatory bowel disease-a pilot study. J. Crohn’s Colitis 2009, 3, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Prince, A.C.; Myers, C.E.; Joyce, T.; Irving, P.; Lomer, M.C.E.; Whelan, K. Fermentable Carbohydrate Restriction (Low FODMAP Diet) in Clinical Practice Improves Functional Gastrointestinal Symptoms in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2016, 22, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Maagaard, L.; Ankersen, D.V.; Vegh, Z.; Burisch, J.; Jensen, L.; Pedersen, N.; Munkholm, P. Follow-up of patients with functional bowel symptoms treated with a low FODMAP diet. World J. Gastroenterol. 2016, 22, 4009–4019. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Turroni, S. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2015. [Google Scholar] [CrossRef] [PubMed]
- Strisciuglio, C.; Giugliano, F.P.; Martinelli, M.; Cenni, S.; Greco, L.; Staiano, A.; Miele, E. Impact of Environmental and Familial Factors in a Cohort of Pediatric Patients with Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Marlow, G.; Ellett, S.; Ferguson, I.R.; Zhu, S.; Karunasinghe, N.; Jesuthasan, A.C.; Ferguson, L.R. Transcriptomics to study the effect of a Mediterranean-inspired diet on inflammation in Crohn’s disease patients. Hum. Genom. 2013, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- Suskind, D.L.; Wahbeh, G.; Cohen, S.A.; Damman, C.J.; Klein, J.; Braly, K.; Lee, D. Patients Perceive Clinical Benefit with the Specific Carbohydrate Diet for Inflammatory Bowel Disease. Dig. Dis. Sci. 2016, 61, 3255–3260. [Google Scholar] [CrossRef] [PubMed]
- Obih, C.; Wahbeh, G.; Lee, D.; Braly, K.; Giefer, M.; Shaffer, M.L.; Suskind, D.L. Specific carbohydrate diet for pediatric inflammatory bowel disease in clinical practice within an academic IBD center. Nutrition 2015, 32, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Burgis, J.C.; Nguyen, K.; Park, K.T.; Cox, K. Response to strict and liberalized specific carbohydrate diet in pediatric Crohn’s disease. World J. Gastroenterol. 2016, 22, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Kakodkar, S.; Farooqui, A.J.; Mikolaitis, S.L.; Mutlu, E.A. The Specific Carbohydrate Diet for Inflammatory Bowel Disease: A Case Series. J. Acad. Nutr. Diet. 2015, 115, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Levenstein, S.; Prantera, C.; Luzi, C.; D’Ubaldi, A. Low residue or normal diet in Crohn’s disease: A prospective controlled study in Italian patients. Gut 1985, 26, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Chiba, M.; Abe, T.; Tsuda, H.; Sugawara, T.; Tsuda, S.; Tozawa, H.; Imai, H. Lifestyle-related disease in Crohn’s disease: Relapse prevention by a semi-vegetarian diet. World. J. Gastroenterol. 2010, 16, 2484–2495. [Google Scholar] [CrossRef] [PubMed]
- Gunasekeera, V.; Mendall, M.A.; Chan, D.; Kumar, D. Treatment of Crohn’s Disease with an IgG4-Guided Exclusion Diet: A Randomized Controlled Trial. Dig. Dis. Sci. 2016, 61, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Olendzki, B.C.; Silverstein, T.D.; Persuitte, G.M.; Ma, Y.; Baldwin, K.R.; Cave, D. An anti-inflammatory diet as treatment for inflammatory bowel disease: A case series report. Nutr. J. 2014, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Academy, A.; Pediatrics, O.F.; Recommendations, C.; Guidelines, C.P. Classifying recommendations for clinical practice guidelines. Pediatrics 2004, 114, 874–877. [Google Scholar]
- Del Pinto, R.; Pietropaoli, D.; Chandar, A.K.; Ferri, C.; Cominelli, F. Association Between Inflammatory Bowel Disease and Vitamin D Deficiency: A Systematic Review and Meta-analysis. Inflamm. Bowel Dis. 2015, 21, 2708–2717. [Google Scholar] [CrossRef] [PubMed]
- Meckel, K.; Li, Y.C.; Lim, J.; Kocherginsky, M.; Weber, C.; Almoghrabi, A.; Cohen, R.D. Serum 25-hydroxyvitamin D concentration is inversely associated with mucosal inflammation in patients with ulcerative colitis. Am. J. Clin. Nutr. 2016, 104, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Torki, M.; Gholamrezaei, A.; Mirbagher, L.; Danesh, M.; Kheiri, S.; Emami, M.H. Vitamin D Deficiency Associated with Disease Activity in Patients with Inflammatory Bowel Diseases. Dig. Dis. Sci. 2015, 60, 3085–3091. [Google Scholar] [CrossRef] [PubMed]
- Raftery, T.; Merrick, M.; Healy, M.; Mahmud, N.; O’Morain, C.; Smith, S.; O’Sullivan, M. Vitamin D Status Is Associated with Intestinal Inflammation as Measured by Fecal Calprotectin in Crohn’s Disease in Clinical Remission. Dig. Dis. Sci. 2015, 60, 2427–2435. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Cagan, A.; Gainer, V.S.; Cai, T.; Cheng, S.-C.; Savova, G.; Shaw, S.Y. Normalization of plasma 25-hydroxy vitamin D is associated with reduced risk of surgery in Crohn’s disease. Inflamm. Bowel Dis. 2013, 19, 1921–1927. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Banares, F.; Hinojosa, J.; Sanchez-Lombrana, J.L.; Navarro, E.; Martinez-Salmeron, J.F.; Garcia-Puges, A.; Gine, J.J. Randomized clinical trial of Plantago ovata seeds (dietary fiber) as compared with mesalamine in maintaining remission in ulcerative colitis. Spanish Group for the Study of Crohn’s Disease and Ulcerative Colitis (GETECCU). Am. J. Gastroenterol. 1999, 94, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Hallert, C.; Kaldma, M.; Petersson, B.G. Ispaghula husk may relieve gastrointestinal symptoms in ulcerative colitis in remission. Scand. J. Gastroenterol. 1991, 26, 747–750. [Google Scholar] [CrossRef] [PubMed]
- Hanai, H.; Iida, T.; Takeuchi, K.; Watanabe, F.; Maruyama, Y.; Andoh, A.; Yamada, M. Curcumin maintenance therapy for ulcerative colitis: Randomized, multicenter, double-blind, placebo-controlled trial. Clin. Gastroenterol. Hepatol. 2006, 4, 1502–1506. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.A.; Leonard, M.C. Curcumin for inflammatory bowel disease: A review of human studies. Altern. Med. Rev. 2011, 16, 152. [Google Scholar] [PubMed]
- Kumar, S.; Ahuja, V.; Sankar, M.J.; Kumar, A.; Moss, A.C. Curcumin for maintenance of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2012. [Google Scholar] [CrossRef]
- Hallert, C.; Bjorck, I.; Nyman, M.; Pousette, A.; Granno, C.; Svensson, H. Increasing fecal butyrate in ulcerative colitis patients by diet: Controlled pilot study. Inflamm. Bowel Dis. 2003, 9, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Hanai, H.; Kanauchi, O.; Mitsuyama, K.; Andoh, A.; Takeuchi, K.; Takayuki, I.; Bamba, T. Germinated barley foodstuff prolongs remission in patients with ulcerative colitis. Int. J. Mol. Med. 2004, 13, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Faghfoori, Z.; Shakerhosseini, R.; Navai, L.; Somi, M.H.; Nikniaz, Z.; Abadi, A. Effects of an Oral Supplementation of Germinated Barley Foodstuff on Serum CRP Level and Clinical Signs in Patients with Ulcerative Colitis. Health Promot. Perspect. 2014, 4, 116–121. [Google Scholar] [PubMed]
- Faghfoori, Z.; Navai, L.; Shakerhosseini, R.; Somi, M.H.; Nikniaz, Z.; Norouzi, M.F. Effects of an oral supplementation of germinated barley foodstuff on serum tumour necrosis factor-alpha, interleukin-6 and -8 in patients with ulcerative colitis. Ann. Clin. Biochem. 2011, 48, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Brotherton, C.S.; Taylor, A.G.; Bourguignon, C.; Anderson, J.G. A high-fiber diet may improve bowel function and health-related quality of life in patients with Crohn disease. Gastroenterol. Nurs. 2014, 37, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Tang, W.; Ching, J.Y.; Wong, M.; Chow, C.M.; Hui, A.J.; Li, M.F. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn’s and colitis epidemiology study. Gastroenterology 2013, 145, 158 e2–165 e2. [Google Scholar] [CrossRef] [PubMed]
- Lovasz, B.D.; Golovics, P.A.; Vegh, Z.; Lakatos, P.L. New trends in inflammatory bowel disease epidemiology and disease course in Eastern Europe. Dig. Liver Dis. 2013, 45, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Ukwenya, A.Y.; Ahmed, A.; Odigie, V.I.; Mohammed, A. Inflammatory bowel disease in Nigerians: Still a rare diagnosis? Ann. Afr. Med. 2011, 10, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Al-Mofarreh, M.A.; Al-Mofleh, I.A. Emerging inflammatory bowel disease in saudi outpatients: A report of 693 cases. Saudi J. Gastroenterol. 2013, 19, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Archampong, T.N.; Nkrumah, K.N. Inflammatory bowel disease in Accra: What new trends. West Afr. J. Med. 2013, 32, 40–44. [Google Scholar] [PubMed]
- Ng, S.C. Emerging leadership lecture: Inflammatory bowel disease in Asia: Emergence of a “Western” disease. J. Gastroenterol. Hepatol. 2015, 30, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Holmboe-Ottesen, G.; Wandel, M. Changes in dietary habits after migration and consequences for health: A focus on South Asians in Europe. Food Nutr. Res. 2012, 56, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pugazhendhi, S.; Sahu, M.K.; Subramanian, V.; Pulimood, A.; Ramakrishna, B.S. Environmental factors associated with Crohn’s disease in India. Indian J. Gastroenterol. 2011, 30, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Legaki, E.; Gazouli, M. Influence of environmental factors in the development of inflammatory bowel diseases. World J. Gastrointest. Pharmacol. Ther. 2016, 7, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Albenberg, L.; Compher, C.; Baldassano, R.; Piccoli, D.; Lewis, J.D.; Wu, G.D. Diet in the pathogenesis and treatment of inflammatory bowel diseases. Gastroenterology 2015, 148, 1087–1106. [Google Scholar] [CrossRef] [PubMed]
- Devereux, G. The increase in the prevalence of asthma and allergy: Food for thought. Nat. Rev. Immunol. 2006, 6, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.Y.; Devkota, S.; Moscoso, D.; Chang, E.B.; Leone, V.A. The role of diet in triggering human inflammatory disorders in the modern age. Microbes Infect. 2013, 15, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Hart, A.R.; Luben, R.; Olsen, A.; Tjonneland, A.; Linseisen, J.; Nagel, G.; Appleby, P. Diet in the aetiology of ulcerative colitis: A European prospective cohort study. Digestion 2008, 77, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Geerling, B.J.; Dagnelie, P.C.; Badart-Smook, A.; Russel, M.G.; Stockbrugger, R.W.; Brummer, R.J. Diet as a risk factor for the development of ulcerative colitis. Am. J. Gastroenterol. 2000, 95, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Reif, S.; Klein, I.; Lubin, F.; Farbstein, M.; Hallak, A.; Gilat, T. Pre-illness dietary factors in inflammatory bowel disease. Gut 1997, 40, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G. Adherence to Mediterranean diet and risk of cancer: A systematic review and meta-analysis of observational studies. Int. J. Cancer 2014, 135, 1884–1897. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Missbach, B.; König, J.; Hoffmann, G. Adherence to a Mediterranean diet and risk of diabetes: A systematic review and meta-analysis. Public Health Nutr. 2015, 18, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R. Anti-inflammatory effects of the Mediterranean diet: The experience of the PREDIMED study. Proc. Nutr. Soc. 2010, 69, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Serra-Majem, L.; Bes-Rastrollo, M.; Román-Viñas, B.; Pfrimer, K.; Sánchez-Villegas, A.; Martínez-González, M.A. Dietary patterns and nutritional adequacy in a Mediterranean country. Br. J. Nutr. 2009, 101, S21–S28. [Google Scholar] [CrossRef] [PubMed]
- Niewiadomski, O.; Studd, C.; Wilson, J.; Williams, J.; Hair, C.; Knight, R.; Dowling, D. Influence of food and lifestyle on the risk of developing inflammatory bowel disease. Intern. Med. J. 2016, 46, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Persson, P.G.; Ahlbom, A.; Hellers, G. Diet and inflammatory bowel disease: A case-control study. Epidemiology 1992, 3, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Feagan, B.G.; Sandborn, W.J.; Mittmann, U.; Bar-Meir, S.; D’Haens, G.; Bradette, M.; Hébuterne, X. Omega-3 free fatty acids for the maintenance of remission in Crohn disease: The EPIC Randomized Controlled Trials. JAMA 2008, 299, 1690–1697. [Google Scholar] [CrossRef] [PubMed]
- Lev-Tzion, R.; Griffiths, A.M.; Leder, O.; Turner, D. Omega 3 fatty acids (fish oil) for maintenance of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2014, 2, CD006320. [Google Scholar]
- Turner, D.; Shah, P.S.; Steinhart, A.H.; Zlotkin, S.; Griffiths, A.M. Maintenance of remission in inflammatory bowel disease using omega-3 fatty acids (fish oil): A systematic review and meta-analyses. Inflamm. Bowel Dis. 2011, 17, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Cabré, E.; Domènech, E. Impact of environmental and dietary factors on the course of inflammatory bowel disease. World J. Gastroenterol. 2012, 18, 3814–3822. [Google Scholar] [CrossRef] [PubMed]
- John, S.; Luben, R.; Shrestha, S.S.; Welch, A.; Khaw, K.-T.; Hart, A.R. Dietary n-3 polyunsaturated fatty acids and the aetiology of ulcerative colitis: A UK prospective cohort study. Eur. J. Gastroenterol. Hepatol. 2010, 22, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, K.; Nakamura, M.; Odahara, S.; Koido, S.; Katahira, K.; Shiraishi, H.; Tajiri, H. N-3 polyunsaturated fatty acid diet therapy for patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2010, 16, 1696–1707. [Google Scholar] [CrossRef] [PubMed]
- Vanhauwaert, E.; Matthys, C.; Verdonck, L.; De Preter, V. Low-Residue and Low-Fiber Diets in Gastrointestinal Disease Management. Adv. Nutr. 2015, 6, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.; Ross, V.; Mahadevan, U. Popular exclusionary diets for inflammatory bowel disease: The search for a dietary culprit. Inflamm. Bowel Dis. 2014, 20, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Asakura, H.; Suzuki, K.; Kitahora, T.; Morizane, T. Is there a link between food and intestinal microbes and the occurrence of Crohn’s disease and ulcerative colitis? J. Gastroenterol. Hepatol. 2008, 23, 1794–1801. [Google Scholar] [CrossRef] [PubMed]
- Packey, C.D.; Sartor, R.B. Commensal bacteria, traditional and opportunistic pathogens, dysbiosis and bacterial killing in inflammatory bowel diseases. Curr. Opin. Infect. Dis. 2009, 22, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Sartor, R.B. Microbial Influences in Inflammatory Bowel Diseases. Gastroenterology 2008, 134, 577–594. [Google Scholar] [CrossRef] [PubMed]
- Walters, S.S.; Quiros, A.; Rolston, M.; Grishina, I.; Li, J.; Fenton, A.; Nieves, R. Analysis of Gut Microbiome and Diet Modification in Patients with Crohn ’ s Disease. SOJ Microbiol. Infect. Dis. 2014, 2, 1–13. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Li, S.; Gan, R.-Y.; Zhou, T.; Xu, D.-P.; Li, H.-B. Impacts of gut bacteria on human health and diseases. Int. J. Mol. Sci. 2015, 16, 7493–7519. [Google Scholar] [CrossRef] [PubMed]
- Bartel, G.; Weiss, I.; Turetschek, K.; Schima, W.; Puspok, A.; Waldhoer, T.; Gasche, C. Ingested matter affects intestinal lesions in Crohn’s disease. Inflamm. Bowel Dis. 2008, 14, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.R.; Shepherd, S.J. Evidence-based dietary management of functional gastrointestinal symptoms: The FODMAP approach. J. Gastroenterol. Hepatol. 2010, 25, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.C.; Yu, S.; Fedewa, A. Systematic review: Dietary fibre and FODMAP-restricted diet in the management of constipation and irritable bowel syndrome. Aliment. Pharmacol. Ther. 2015, 41, 1256–1270. [Google Scholar] [CrossRef] [PubMed]
- Marsh, A.; Eslick, E.M.; Eslick, G.D. Does a diet low in FODMAPs reduce symptoms associated with functional gastrointestinal disorders? A comprehensive systematic review and meta-analysis. Eur. J. Nutr. 2016, 55, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Simrén, M.; Axelsson, J.; Gillberg, R.; Abrahamsson, H.; Svedlund, J.; Björnsson, E.S. Quality of life in inflammatory bowel disease in remission: The impact of IBS-like symptoms and associated psychological factors. Am. J. Gastroenterol. 2002, 97, 389–396. [Google Scholar] [CrossRef]
- Takagi, S.; Utsunomiya, K.; Kuriyama, S.; Yokoyama, H.; Takahashi, S.; Iwabuchi, M.; Sasaki, I. Effectiveness of an “half elemental diet” as maintenance therapy for Crohn’s disease: A randomized-controlled trial. Aliment. Pharmacol. Ther. 2006, 24, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Takagi, S.; Utsunomiya, K.; Kuriyama, S.; Yokoyama, H.; Takahashi, S.; Umemura, K.; Funayama, Y. Quality of life of patients and medical cost of “half elemental diet” as maintenance therapy for Crohn’s disease: Secondary outcomes of a randomised controlled trial. Dig. Liver Dis. 2009, 41, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Gophna, U.; Sommerfeld, K.; Gophna, S.; Doolittle, W.F.; Van Zanten, S.J.O. Differences between tissue-associated intestinal microfloras of patients with Crohn’s disease and ulcerative colitis. J. Clin. Microbiol. 2006, 44, 4136–4141. [Google Scholar] [CrossRef] [PubMed]
- Ott, S.J.; Musfeldt, M.; Wenderoth, D.F.; Hampe, J.; Brant, O.; Fölsch, U.R.; Schreiber, S. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut 2004, 53, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Wedlake, L.; Slack, N.; Andreyev, H.J.; Whelan, K. Fiber in the treatment and maintenance of inflammatory bowel disease: A systematic review of randomized controlled trials. Inflamm. Bowel Dis. 2014, 20, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Roediger, W.E. Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology 1982, 83, 424–429. [Google Scholar] [PubMed]
- Vernia, P.; Marcheggiano, A.; Caprilli, R.; Frieri, G.; Corrao, G.; Valpiani, D.; Torsoli, A. Short-chain fatty acid topical treatment in distal ulcerative colitis. Aliment. Pharmacol. Ther. 1995, 9, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Kanauchi, O.; Suga, T.; Tochihara, M.; Hibi, T.; Naganuma, M.; Homma, T.; Andoh, A. Treatment of ulcerative colitis by feeding with germinated barley foodstuff: First report of a multicenter open control trial. J. Gastroenterol. 2002, 37, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Steinhart, A.H.; Griffiths, A.M. Omega 3 fatty acids (fish oil) for maintenance of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2007. [Google Scholar] [CrossRef]
- Shivananda, S.; Lennard-Jones, J.; Logan, R.; Fear, N.; Price, A.; Carpenter, L.; Van Blankenstein, M. Incidence of inflammatory bowel disease across Europe: Is there a difference between north and south? Results of the European Collaborative Study on Inflammatory Bowel Disease (EC-IBD). Gut 1996, 39, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Serra-Majem, L. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef] [PubMed]
- Gottschall, E. Breaking the Viscous Cycle; Kirkton Press: Baltimore, MD, USA, 1994. [Google Scholar]
- Nieves, R.; Jackson, R.T. Specific carbohydrate diet in treatment of inflammatory bowel disease. Tenn. Med. 2004, 97, 407. [Google Scholar] [PubMed]
- Cordain, L. The Paleo Diet. 2016. Available online: http://thepaleodiet.com/ (accessed on 30 September 2016).
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef] [PubMed]
- Losurdo, G.; Iannone, A.; Contaldo, A.; Ierardi, E.; Di Leo, A.; Principi, M. Escherichia coli Nissle 1917 in Ulcerative Colitis Treatment: Systematic Review and Meta-analysis. J. Gastrointest. Liver Dis. 2015, 24, 499–505. [Google Scholar]
- Fujiya, M.; Ueno, N. Kohgo, Probiotics for maintenance of remission in inflammatory bowel diseases: A meta-anlysis of randomized controlled trials. Clin. J.Gastroenterol. 2014, 1, 1–13. [Google Scholar] [CrossRef]
- Shen, J.; Ran, H.Z.; Yin, M.H.; Zhou, T.X.; Xiao, D.S. Meta-analysis: The effect and adverse events of Lactobacilli versus placebo in maintenance therapy for Crohn disease. Intern. Med. J. 2009, 39, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, K.; Gordon, M.; Fagbemi, A.O.; Thomas, A.G.; Akobeng, A.K. Probiotics for maintenance of remission in ulcerative colitis. Cochrane Database Syst. Rev. 2011. [Google Scholar] [CrossRef]
- Shen, J.; Zuo, Z.X.; Mao, A.P. Effect of probiotics on inducing remission and maintaining therapy in ulcerative colitis, Crohn’s disease, and pouchitis: Meta-analysis of randomized controlled trials. Inflamm. Bowel Dis. 2014, 20, 21–35. [Google Scholar] [CrossRef]
- Mouli, V.P.; Ananthakrishnan, A.N. Review article: Vitamin D and inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2014, 39, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Kabbani, T.A.; Koutroubakis, I.E.; Schoen, R.E.; Ramos-Rivers, C.; Shah, N.; Swoger, J.; Baidoo, L. Association of Vitamin D Level With Clinical Status in Inflammatory Bowel Disease: A 5-Year Longitudinal Study. Am. J. Gastroenterol. 2016, 111, 712–719. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N.; Cheng, S.-C.; Cai, T.; Cagan, A.; Gainer, V.S.; Szolovits, P.; Kohane, I. Association between reduced plasma 25-hydroxy vitamin D and increased risk of cancer in patients with inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 2014, 12, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Gubatan, J.; Mitsuhashi, S.; Zenlea, T.; Rosenberg, L.; Robson, S.; Moss, A.C. Low Serum Vitamin D During Remission Increases Risk of Clinical Relapse in Patients With Ulcerative Colitis. Clin. Gastroenterol Hepatol. 2017, 15, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Hlavaty, T.; Krajcovicova, A.; Payer, J. Vitamin D therapy in inflammatory bowel diseases: Who, in what form, and how much? J. Crohns Colitis 2015, 9, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, S.P.; Agnholt, J.; Glerup, H.; Lyhne, S.; Villadsen, G.E.; Hvas, C.L.; Dahlerup, J.F. Clinical trial: Vitamin D3 treatment in Crohn’s disease—A randomized double-blind placebo-controlled study. Aliment. Pharmacol. Ther. 2010, 32, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Suez, J. Personalized Nutrition by Prediction of Glycemic Responses. Cell 2015, 163, 1079–1095. [Google Scholar] [CrossRef] [PubMed]
- Dolan, K.T.; Chang, E.B. Diet, gut microbes, and the pathogenesis of inflammatory bowel diseases. Mol. Nutr. Food Res. 2016. [Google Scholar] [CrossRef] [PubMed]
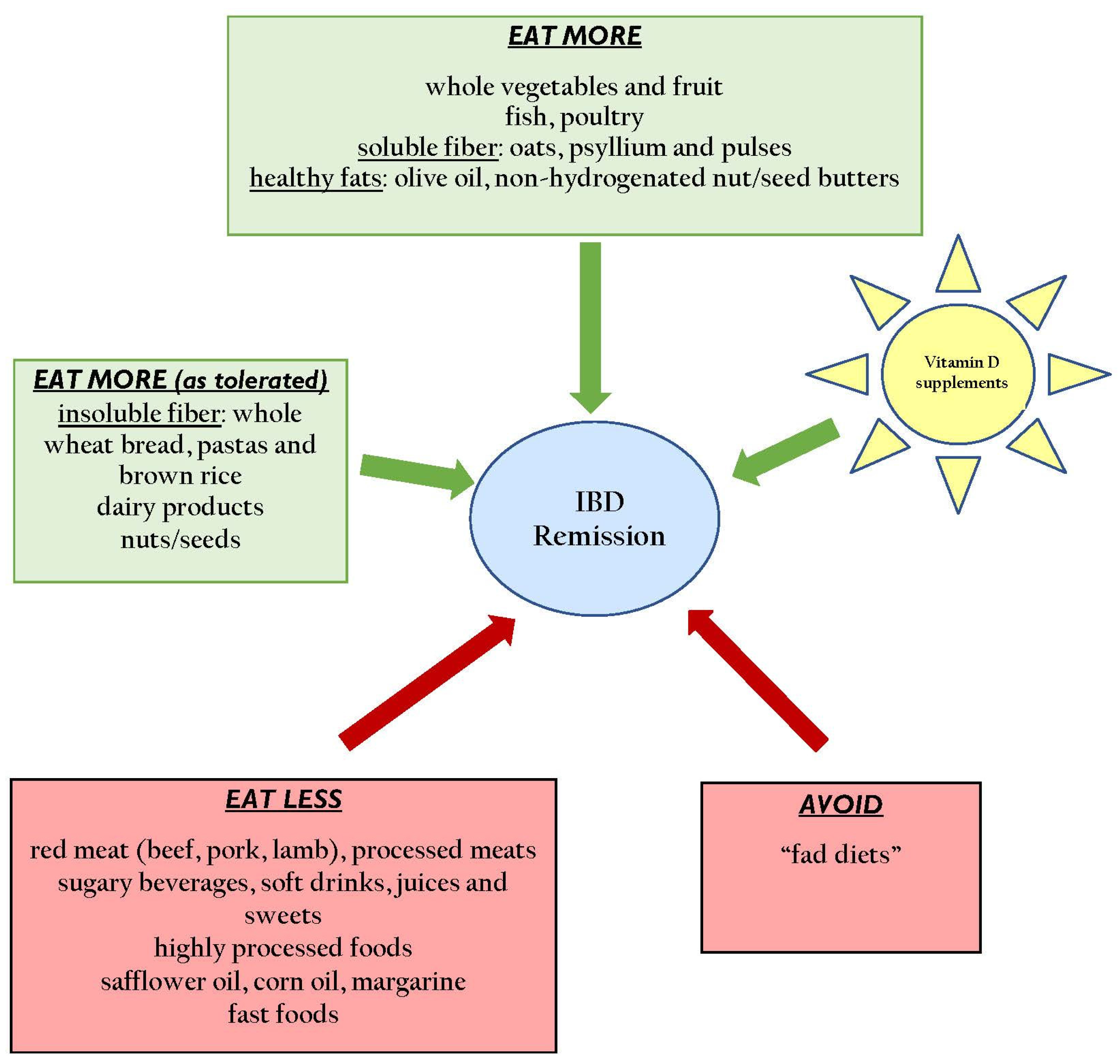
| Statement | Recommendation |
|---|---|
| Encourage high dietary fiber intake from foods, especially from fruits and vegetables [22,23,24,25] | Strongly Recommend * |
| Avoid n-6 PUFA (safflower oils, corn oils, margarine) and trans-unsaturated fatty acid consumption [23,24,26,27] | Strongly Recommend |
| Encourage consumption of dairy products [28,29] | Recommend † (if tolerated) |
| Limit/avoid refined carbohydrates, especially sweetened beverages and soft drinks [30] | Recommend |
| Limit red meat consumption, especially from beef, pork, lamb and processed meats [22,31] | Recommend |
| FODMAP Diet [32,33,34] | Optional ‡ (for the management of IBS-overlay) |
| Mediterranean Diet Pattern [35,36,37] | Optional |
| Specific Carbohydrate Diet [38,39,40,41] | No Recommendation β |
| Low Residue Diet [42] | |
| Semi-vegetarian Diet [43] | |
| IgG4-guided Elimination Diet [44] | |
| IBD-AID [45] | |
| Paleo Diet | No Recommendation |
| Statement | Type of IBD | Recommendation |
|---|---|---|
| Vitamin D (minimum 1200 IU/day) [47,48,49,50,51] | Both | Strongly Recommend * (aim for levels of serum 25 (OHD) >75 nmol/L |
| Psyllium (minimum 4 grams/day) [52,53] | UC | Recommend † |
| Curcumin (1-gram bid) [54,55,56] | UC | Optional ‡ |
| Oat bran supplementation (20 grams/day) [57] | UC | Optional |
| Germinated Barley Foodstuff (minimum 20 grams/day) [58,59,60] | UC | Optional |
| Wheat bran (1/2 cup daily) [61] | CD | Optional |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haskey, N.; Gibson, D.L. An Examination of Diet for the Maintenance of Remission in Inflammatory Bowel Disease. Nutrients 2017, 9, 259. https://doi.org/10.3390/nu9030259
Haskey N, Gibson DL. An Examination of Diet for the Maintenance of Remission in Inflammatory Bowel Disease. Nutrients. 2017; 9(3):259. https://doi.org/10.3390/nu9030259
Chicago/Turabian StyleHaskey, Natasha, and Deanna L. Gibson. 2017. "An Examination of Diet for the Maintenance of Remission in Inflammatory Bowel Disease" Nutrients 9, no. 3: 259. https://doi.org/10.3390/nu9030259






