Advising Consumption of Green Vegetables, Beef, and Full-Fat Dairy Products Has No Adverse Effects on the Lipid Profiles in Children
Abstract
:1. Introduction
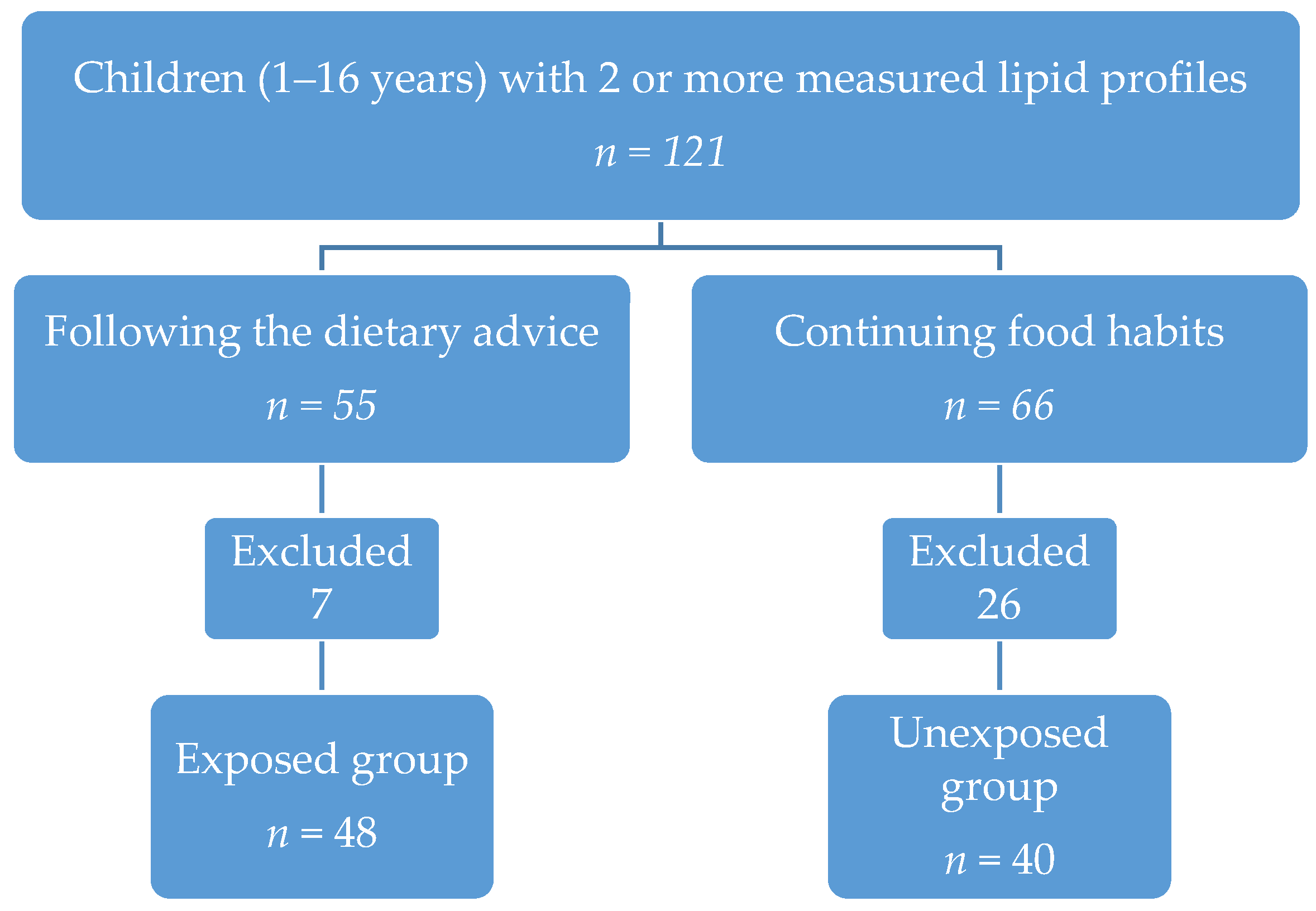
2. Materials and Methods
3. Results
3.1. Baseline Data
3.2. Changes within Groups
4. Discussion
5. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Field, C.J.; Johnson, I.R.; Schley, P.D. Nutrients and their role in host resistance to infection. J. Leukocyte Biol. 2002, 71, 16–32. [Google Scholar] [PubMed]
- Jimenez, C.; Leets, I.; Puche, R.; Anzola, E.; Montilla, R.; Parra, C.; Aguilera, A.; Garcia-Casal, M.N. A single dose of vitamin A improves haemoglobin concentration, retinol status and phagocytic function of neutrophils in preschool children. Brit. J. Nutr. 2010, 103, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Maggini, S.; Wenzlaff, S.; Hornig, D. Essential role of vitamin C and zinc in child immunity and health. J. Int. Med. Res. 2010, 38, 386–414. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Zinc: Role in immunity, oxidative stress and chronic inflammation. Curr. Opin. Clin. Nutr. 2009, 12, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Wintergerst, E.S.; Maggini, S.; Hornig, D.H. Immune-enhancing role of vitamin C and zinc and effect on clinical conditions. Ann. Nutr. Metab. 2006, 50, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Cherayil, B.J. Iron and immunity: Immunological consequences of iron deficiency and overload. Arch. Immunol. Ther. Exp. 2010, 58, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Ekiz, C.; Agaoglu, L.; Karakas, Z.; Gurel, N.; Yalcin, I. The effect of iron deficiency anemia on the function of the immune system. Hematol. J. 2005, 5, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Munow, M.; van der Gaag, E.J. Ailing Toddlers: Is There a Relation between Behavior and Health? Book of Abstracts 27th Annual Meeting of the European Society for Pediatric Infectious Diseases; 2009; p. 764. Available online: http://www.scirp.org/%28S%28351jmbntvnsjt1aadkposzje%29%29/reference/ReferencesPapers.aspx?ReferenceID=962833 (accessed on 18 May 2017).
- National Institute for Public Health and the Environment (RIVM)/the Kingdom of the Netherlands. Dutch Food Composition Database 2014. Available online: http://nevo-online.rivm.nl/ (accessed on 12 May 2014).
- German, J.B. Dietary lipids from an evolutionary perspective: Sources, structures and functions. Matern. Child Nutr. 2011, 7, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Batovska, D.; Todorova, I.; Tsvetkova, I.; Najdenski, H. Antibacterial study of the medium chain fatty acids and their 1-monoglycerides: Individual effects and synergistic relationships. Pol. J. Microbiol. 2009, 58, 43–47. [Google Scholar] [PubMed]
- Dietary Reference Intakes: Energy, Proteins, Fats and Digestible Carbohydrates. Health Council Neth. Available online: https://www.narcis.nl/publication/RecordID/oai:cris.maastrichtuniversity.nl:publications%2Fdc7e056b-a54d-471d-a496-ec334fd5ad1e (accessed on 13 June 2013).
- Brink, E.J.; Breedveld, B.C.; Peters, J.A.C. Aanbevelingen Voor Vitamines, Mineralen en Spoorelementen. Factsheet The Netherlands Nutrition Centre. Available online: http://www.voedingscentrum.nl/Assets/Uploads/voedingscentrum/Documents/Professionals/Pers/Factsheets/Factsheet%20Aanbevelingen%20voor%20vitamines,%20mineralen%20en%20spoorelementen.pdf (accessed on 15 December 2016).
- Ten Velde, L.G.H.; Leegsma, J.; van der Gaag, E.J. Recurrent upper respiratory tract infections in children;the influence of green vegetables, beef, whole milk and butter. Food Nutr. Sci. 2013, 4, 71–77. [Google Scholar] [CrossRef]
- Steenbruggen, T.G.; Hoekstra, S.J.; van der Gaag, E.J. Could a change in diet revitalize children who suffer from unresolved fatigue? Nutrients 2015, 7, 1965–1977. [Google Scholar] [CrossRef] [PubMed]
- Shrapnel, W.S.; Calvert, G.D.; Nestle, P.J.; Truswell, A.S. Diet and coronary heart disease. Natl. Heart Found. Aust. Med. J. Aust. 1992, 156, S9–S16. [Google Scholar]
- Gidding, S.S.; Dennison, B.A.; Birch, L.L.; Daniels, S.R.; Gillman, M.W.; Lichtenstein, A.H.; Rattay, K.T.; Steinberger, J.; Stettler, N.; van Horn, L. Dietary recommendations for children and adolescents: A guide for practitioners. Pediatrics 2006, 117, 544–559. [Google Scholar] [CrossRef] [PubMed]
- The Netherlands Nutrition Centre. Verzadigd vet. Available online: http://www.voedingscentrum.nl/encyclopedie/verzadigd-vet.aspx (accessed on 15 May 2016).
- Muskiet, F.A.J.; Muskiet, M.H.A.; Kuipers, R.S. Het faillissement van de verzadigd vethypothese van cardiovasculaire ziektes. Ned. Tijdschr. Klin. Chem. Labgeneesk 2012, 37, 192–211. (In Dutch) [Google Scholar]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am. J. Clin. Nutr. 2010, 91, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Kratz, M.; Baars, T.; Guyenet, S. The relationship between high-fat dairy consumption and obesity, cardiovascular, and metabolic disease. Eur. J. Nutr. 2013, 52, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Kinosian, B.; Glick, H.; Garland, G. Cholesterol and coronary heart disease predicting risks by levels and ratios. Ann. Intern. Med. 1994, 121, 641–647. [Google Scholar] [CrossRef] [PubMed]
- The Netherlands Nutrition Centre. Cholesterol. Available online: http://www.voedingscentrum.nl/encyclopedie/cholesterol.aspx (accessed on 16 May 2016).
- Department of Health and Human Services. National Heart Lung and Blood Institute. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents. Available online: https://www.nhlbi.nih.gov/files/docs/guidelines/peds_guidelines_full.pdf (accessed on 15 January 2017).
- The Netherlands National Health Care Institute. Farmacotherapeutisch kompas. Available online: https://www.farmacotherapeutischkompas.nl/ (accessed on 5 January 2014).
- U.S. Department of Health and Human Services. National Center for Health Statistics, Z-Score Data Files. Available online: https://www.cdc.gov/growthcharts/zscore.htm (accessed on 14 February 2014).
- Kubo, T.; Takahashi, K.; Furujo, M.; Hyodo, Y.; Tsuchiya, H.; Hattori, M.; Fujinaga, S.; Urayama, K. Usefulness of non-fasting lipid parameters in children. J. Pediatr. Endocr. Metab. 2017, 30, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Mensink, R.P.; Zock, P.L.; Kester, A.D.; Katan, M.B. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: A meta-analysis of 60 controlled trials. Am. J. Clin. Nutr. 2003, 77, 1146–1155. [Google Scholar] [PubMed]
- Huth, P.J.; Park, K.M. Influence of dairy product and milk fat consumption on cardiovascular disease risk: A review of the evidence. Adv. Nutr. 2012, 3, 266–285. [Google Scholar] [CrossRef] [PubMed]
- Lewington, S.; Whitlock, G.; Clarke, R.; Sherliker, P.; Emberson, J.; Halsey, J.; Qizilbash, N.; Peto, R.; Collins, R. Blood cholesterol and vascular mortality by age, sex, and blood pressure: A meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet 2007, 370, 1829–1839. [Google Scholar] [CrossRef] [PubMed]
- Skeaf, C.M.; Miller, J. Dietary fat and coronary heart disease: Summary of evidence from prospective cohort and randomized controlled trails. Ann. Nutr. Metab. 2009, 55, 173–201. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, M.U.; O’Reilly, E.J.; Heitmann, B.L.; Pereira, M.A.; Bälter, K.; Fraser, G.E.; Goldbourt, U.; Hallmans, G.; Knekt, P.; Liu, S. Major types of dietary fat and risk of coronary heart disease: A pooled analysis of 11 cohort studies. Am. J. Clin. Nutr. 2009, 89, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Musunuru, K. Atherogenic dyslipidemia: Cardiovascular risk and dietary intervention. Lipids 2010, 45, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Yakoob, M.Y.; Shi, P.; Willet, W.C.; Rexrode, K.M.; Campos, H.; Orav, E.J.; Hu, F.B.; Mozaffarian, D. Circulating Biomarkers of dairy fat and risk of incident diabetes mellitus among men and women in the United States in two large prospective cohorts. Circulation 2016, 133, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, C.E.; Hibbeln, J.R.; Majchrzak, S.F.; Davis, J.M. N-6 fatty acid-specific and mixed polyunsaturate dietary interventions have different effects on CHD risk: A meta-analysis of randomised controlled trials. Brit. J. Nutr. 2010, 104, 1586–1600. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the Omega-6/Omega-3 fatty-acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F. Health: Edible advice. Nature 2010, 468, S10–S12. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.H.; Kristal, A.R.; Standford, J.L. Fruit and vegetable intakes and prostate cancer risk. J. Natl. Cancer Inst. 2000, 92, 61–68. [Google Scholar] [CrossRef]
- Ambrosone, C.B.; McCann, S.E.; Freudenheim, J.L.; Marshall, J.R.; Zhang, Y.; Shields, P.G. Breast cancer risk in premenopausal women is inversely associated with consumption of broccoli, a source of isothiocyanates, but is not modified by GST genotype. J. Nutr. 2004, 134, 1134–1138. [Google Scholar] [PubMed]
- Zhang, P.Y.; Xu, X.; Li, X.C. Cardiovascular diseases: Oxidative damage and antioxidant protection. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 3091–3096. [Google Scholar] [PubMed]
- The Netherlands Nutrition Centre. Hoeveel en wat kan ik per dag eten? Available online: http://www.voedingscentrum.nl/nl/schijf-van-vijf/eet-niet-teveel-en-beweeg/hoe-eet-ik-niet-te-veel.aspx (accessed on 21 March 2014).
- Haug, A.; Høstmark, A.T.; Harstad, O.M. Bovine milk in human nutrition—A review. Lipids Health Dis. 2007, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Snijder, M.B.; van der Heijden, A.A.W.A.; van Dam, R.M.; Stehouwer, C.D.A.; Hiddink, G.J.; Nijpels, G.; Heine, R.J.; Bouter, L.M.; Dekker, J.M. Is higher dairy consumption associated with lower body weight and fewer metabolic disturbances? Am. J. Clin. Nutr. 2007, 85, 989–995. [Google Scholar] [PubMed]
- Rautiainen, S.; Wang, L.; Lee, I.M.; Manson, J.E.; Buring, J.E.; Sesso, H.D. Dairy consumption in association with weight change and risk of becoming overweight or obese in middle-aged and older woman: A prospective cohort study. Am. J. Clin. Nutr. 2016, 103, 979–988. [Google Scholar] [CrossRef] [PubMed]
| Food Product | Nutrients per 100 Grams | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vitamin A (ug) | Vitamin D (ug) | Vitamin E (mg) | Iron (mg) | Zinc (mg) | Calorie (kcal) | Saturated Fats (g) | Total Unsaturated fats (g) | N-3 Fats (g) | Linoleic Acid (N-6 Fat) (g) | |
| Spinach cooked | 652 | - | 3.5 | 2.4 | 1.20 | 25 | 0.1 | 0.7 | 0.5 | 0.1 |
| Broccoli cooked | 116 | - | 2.5 | 0.9 | 0.62 | 27 | 0.1 | 0.2 | 0.1 | - |
| Cauliflower cooked | 0 | - | 0.1 | 0.3 | 0.26 | 23 | 0.1 | 0.2 | 0.2 | - |
| Chicory cooked | 1 | - | 0.2 | 0.2 | 0.17 | 17 | - | 0.1 | - | 0.1 |
| Beef > 10% fat | 68 | 0.5 | 2.4 | 2.8 | 5.84 | 277 | 6.2 | 10.5 | 0.2 | 2.9 |
| Chicken breast | 18 | 0.1 | 1.1 | 0.7 | 0.74 | 158 | 1.4 | 1.8 | 0.1 | 0.8 |
| Pork 10%–19% fat | 25 | 0.6 | 1.1 | 1.0 | 2.65 | 378 | 5.4 | 10.1 | 0.2 | 3.2 |
| Butter | 903 | 1.2 | 2.5 | 0.1 | 0.09 | 737 | 52.9 | 19.9 | 0.5 | 1.3 |
| Margarine | 800 | 7.5 | 9.5 | 0.1 | - | 349 | 8.5 | 34.5 | 5.9 | 19 |
| Whole milk | 36 | - | 0.1 | - | 0.46 | 62 | 2.2 | 0.8 | - | 0.1 |
| Skimmed milk | 1 | - | - | - | 0.46 | 35 | 0.1 | - | - | - |
| Adequate intake or recommended dietary allowance/day for children [12,13] | ♂/♀ 2–5 years: 350 ug | ♂/♀ 4–8 years: 10 ug | ♂/♀ 2–5 years: 5 mg | ♂/♀ 2–5 years: 8 mg | ♂/♀ 2–5 years: 6 mg | 4–8 years: ♂1720 kcal ♀1552 kcal | ♂/♀ 4–8 years: 10 En% | ♂/♀ all ages: 8–38 En% | ♂/♀ 4–8 years: 0.15–0.2 g | ♂/♀ 4–8 years: 2 En% |
| Exposed Group | (n = 55) | Unexposed Group | (n = 66) |
|---|---|---|---|
| Incomplete lipid profile | 2 | Incomplete lipid profile | 5 |
| Familiar hypercholesterolemia | 2 | Familiar hypercholesterolemia | 3 |
| Obesity | 1 | Obesity | 13 |
| Age < 1 year or > 16 years | 1 | Age < 1 year or > 16 years | 1 |
| Diabetes mellitus | 0 | Diabetes mellitus | 3 |
| Metabolic disorder | 0 | Metabolic disorder | 1 |
| Medication | 0 | Medication | 0 |
| Dropouts | 1 | Dropouts | 0 |
| Exposed group | (n = 48) | Unexposed group | (n = 40) |
| Characteristic | Unexposed Group n = 40 | Exposed Group n = 48 | p-Value |
|---|---|---|---|
| Gender (n, %)MenWomen | 24 (60%) 16 (40%) | 25 (52%) 23 (48%) | 0.457 |
| Age (years) (median, IQR) | 4.7 (2.3–9.0) | 2.6 (1.6–8.0) | 0.102 |
| Follow-up (months)(median, IQR) | 5.0 (4.0–8.0) | 4.5 (4.0–8.8) | 0.744 |
| BMI (median, IQR) | 15.9 (15.1–17.5) | 16.7 (15.4–18.5) | 0.408 |
| Measurements | Unexposed Group n = 40 | Exposed Group n = 48 | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | Change (95%-CI/IQR)) | p-Value | Baseline | Follow-up | Change (95%-CI) | p-Value | |
| Total cholesterol (mmol/L) (median, IQR) | 4.05 (3.83–4.70) | 4.20 (3.70–4.68) | −0.06 a (−0.11–0.22) | 0.581 d | 4.20 (3.5–5.0) | 4.35 (3.7–4.7) | −0.03 a (−0.25–0.18) | 0.738 c |
| HDL-cholesterol (mmol/L) (median, IQR) | 1.35 (0.93–1.59) | 1.30 (0.95–1.57) | −0.01 b (−0.19–0.12) | 0.842 c | 1.17 (0.88–1.48) | 1.35 (1.12–1.53) | 0.14 a (0.04–0.24) | 0.009 d |
| Cholesterol/HDL (mmol/L) (median, IQR) | 3.45 (2.57–4.70) | 3.40 (2.53–4.45) | 0,00 b (−0.35–0.38) | 0.883 d | 3.75 (3.00–4.95) | 3.15 (2.80–4.95) | −0.30 b (−1.2–0.17) | < 0.001 c |
| Triglycerides (mmol/L) (median, IQR) | 0.96 (0.70–1.93) | 1.00 (0.80–1.47) | 0.05 b (−0.38–0.30) | 0.821 d | 1.10 (0.80–1.67) | 1.05 (0.80–1.50) | −0.07 a (−0.31–0.16) | 0.469 d |
| LDL-cholesterol (mmol/L) (median, IQR) | 2.30 (2.00–2.80) | 2.30 (1.90–2.88) | 0.00 a (−0.15–0.13) | 0.852 c | 2.55 (1.70–3.00) | 2.40 (1.93–2.80) | −0.10 b (−0.60–0.30) | 0.384 c |
| Non-HDL cholesterol (mmol/L) (median, IQR) | 3.01 (2.54–3.49) | 2.83 (2.40–3.39) | −0.06 a (−0.21–0.08) | 0.384 c | 3.14 (2.56–3.61) | 2.98 (2.45–3.28) | −0.17 a (–0.34—0.01) | 0.044 d |
| BMI (median, IQR) | 15.9 (15.1–17.5) | 15.8 (15.1–17.5) | 0.24 a (-0.05-0.54) | 0.178 d | 16.7 (15.4–18.5) | 16.0 (14.9–18.0) | 0.00 b (−0.63–0.30) | 0.719 d |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van der Gaag, E.J.; Wieffer, R.; Van der Kraats, J. Advising Consumption of Green Vegetables, Beef, and Full-Fat Dairy Products Has No Adverse Effects on the Lipid Profiles in Children. Nutrients 2017, 9, 518. https://doi.org/10.3390/nu9050518
Van der Gaag EJ, Wieffer R, Van der Kraats J. Advising Consumption of Green Vegetables, Beef, and Full-Fat Dairy Products Has No Adverse Effects on the Lipid Profiles in Children. Nutrients. 2017; 9(5):518. https://doi.org/10.3390/nu9050518
Chicago/Turabian StyleVan der Gaag, Ellen José, Romy Wieffer, and Judith Van der Kraats. 2017. "Advising Consumption of Green Vegetables, Beef, and Full-Fat Dairy Products Has No Adverse Effects on the Lipid Profiles in Children" Nutrients 9, no. 5: 518. https://doi.org/10.3390/nu9050518




